Hepcidin, a recently discovered regulator of organismal iron homeostasis, causes iron sequestration in hepatocytes and its levels are decreased in hereditary hemochromatosis caused by mutations of transferrin receptor 2.
Iron represents a quandary for organisms. Virtually all life forms are exploiting this metal's unique chemical properties, namely its capacity to either accept or donate electrons, that are essential for crucial biochemical reactions. Hence, iron, as an essential part of hemoglobins, cytochromes, and innumerable redox enzymes, plays a key role in many vital biologic processes. However, iron is virtually insoluble under conditions occurring in our cells and body fluids; moreover, it is potentially toxic due to its capacity to promote the formation of extremely toxic oxygen-free radicals. Hence, all living systems evolved sophisticated mechanisms to maintain appropriate iron levels in their cells and within their organisms. In humans, and likely other mammals, the absence of a physiologic excretion mechanism requires organismal iron homeostasis to be regulated by iron absorption. Occurring primarily in the upper duodenum, this regulation has been totally elusive until very recently. Since 2001 an increasing body of evidence has accumulated that hepcidin, a 25–amino acid peptide made in hepatocytes,1 is a long-anticipated humoral factor that controls organismal iron homeostasis.
Pigeon et al2 were the first to document the link between hepcidin and iron metabolism by showing that iron overload in mice was associated with an increase in hepcidin mRNA levels in the liver. Moreover, these authors demonstrated that not only iron but also treatment of mice with lipopolysaccharide increased hepcidin mRNA expression in the liver. Somewhat later, Nicolas et al3 provided compelling evidence that genetically based defects in hepcidin expression in mice caused iron overload, whereas transgenic mice, generated to overexpress hepcidin in their liver, became severely iron deficient, causing fatal anemia shortly after birth. Collectively, these reports strongly suggested that hepcidin expression is stimulated by high iron levels in the organism and that this peptide may block iron export from “iron donor” cells, such as the duodenal epithelial cells, the appropriate cells of the placenta, and, possibly, macrophages. Moreover, hepcidin was unequivocally demonstrated to play an important role in the pathogenesis of anemia of inflammation (AI).4,5 Nemeth et al4 demonstrated that interleukin 6 (IL-6)–stimulated production of hepcidin in hepatocytes is responsible for many symptoms of AI, including hypoferremia.FIG1
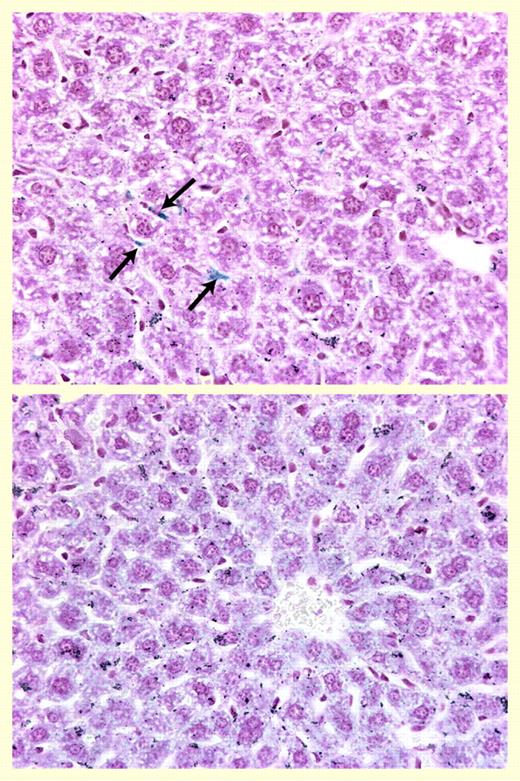
Hepatic iron staining. See the complete figure in the article beginning on page 1797.
Hepatic iron staining. See the complete figure in the article beginning on page 1797.
This issue of Blood contains an article that significantly enhances our knowledge regarding in vivo effects of hepcidin and its role in the pathogenesis of AI. Rivera and colleagues examined the acute and chronic effects of human hepcidin on various aspects of iron metabolism and hematologic parameters in mice. The authors showed that the administration of a single large dose of human hepcidin caused, just within 4 hours, a significant decrease in plasma iron levels. To investigate the chronic effects, the authors developed an astute strategy involving the implantation of hepcidin-producing hepatoma cells into nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice. This approach revealed that hypoferremia was maintained during chronic overproduction of this peptide by hepcidin-producing tumor cells. Importantly, mice with hepcidin-producing tumors developed anemia even when they were maintained on the high-iron diet. Furthermore, liver iron stores were significantly increased by hepcidin and the iron was present predominantly in hepatocytes (Figure 1). This is an important finding that rectifies a general but undocumented belief that in AI, iron is predominantly sequestered in macrophages. This is the first study demonstrating that hepcidin peptide has a direct role in iron metabolism; previous studies were either based on genetic manipulations or reported hepcidin effects were only marginal.
Recent research also revealed that hepcidin, or more precisely, the lack of it, plays an important role in the pathophysiology of several types of hereditary hemochromatoses. Although, as mentioned above, hepcidin levels are increased by iron overload,2 the expression of this peptide is decreased or inappropriately low (considering the degree of iron overload) in patients with “classical” HFE-related hemochromatosis (reviewed in this issue of Blood by Nemeth et al). Moreover, as could be predicted, hepcidin-inactivating mutations result in severe iron overload in a subset of patients with juvenile hemochromatosis (reviewed by Nemeth et al in this issue of Blood). Furthermore, another type of juvenile hemochromatosis, caused by mutations in the recently discovered hemojuvelin gene, that seems to be an important regulator of hepcidin expression is also associated with low or undetectable urinary hepcidin levels (reviewed by Nemeth and colleagues in this issue of Blood).
Until now it was not known what the hepcidin levels are in other hereditary hemochromatoses (eg, those caused by mutations of transferrin receptor 2 [TfR2], a protein showing a high homology to “classical” TfR but whose function is elusive). In the study in this issue of Blood, Nemeth and colleagues measured urinary hepcidin levels in 10 patients homozygous for TfR2 mutations; all these individuals had increased transferrin saturation. These authors found low or undetectable urinary hepcidin levels in most patients, except for 2 who had concomitant inflammatory conditions. Hence, this important study has revealed that TfR2 is a regulator of hepcidin production in response to iron. Since hepcidin is deficient in HFE-related, juvenile, and TfR2-related hemochromatoses, it would seem of considerable interest to examine hepcidin levels in patients whose hemochromatosis is caused by mutations of ferroportin,6 a protein that is involved in iron export from cells. The outcome of such investigation cannot be predicted with confidence but it is tempting to speculate that individuals with ferroportin mutations would be able to maintain the appropriate hepcidin response to iron overload, since ferroportin operates downstream of hepcidin. This was demonstrated in a recent elegant study by Nemeth et al7 who documented that hepcidin binds to ferroportin overexpressed in human embryonic kidney (HEK) cells and that this binding triggers ferroportin internalization and consequent degradation, leading to decreased export of cellular iron. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal