Abstract
In the Non-Hodgkin Lymphoma–Berlin-Frankfurt-Münster 95 (NHL-BFM95) study, we tested by randomization whether for patients with B-cell neoplasms methotrexate as intravenous infusion over 4 hours (MTX-4h) is not inferior to, but less toxic than, a 24-hour intravenous infusion (MTX-24h). Second, we investigated against the historical control of study NHL-BFM90, whether for patients with moderate tumor mass MTX can be reduced from 5 g/m2 to 1 g/m2. Patients received 2 5-day therapy courses in risk group R1 (resected), 4 in R2 (lactate dehydrogenase [LDH] < 500 U/L), 5 in R3 (LDH > 500 to < 1000 U/L) and 6 in R4 (LDH > 1000 U/L and/or central nervous system [CNS] disease). Courses contained MTX 1 g/m2 in R1 + R2 and 5 g/m2 in R3 + R4. Of 505 patients (April 1996 to March 2001), 364 were randomized to receive MTX-4h or MTX-24h. Failure-free survival (pFFS, 1 year) for arm MTX-4h versus MTX-24h, respectively, was 95% ± 5% (n = 20) versus 100% (n = 19) in R1, 94% ± 2% (n = 88) versus 96% ± 2% (n = 95) in R2, and 77% ± 5% (n = 62) versus 93% ± 3% (n = 69) in R3 ± R4 (per-protocol analysis). Incidence of mucositis grade III/IV was significantly lower with MTX-4h in all risk groups. For patients in R2, event-free survival (pEFS) was 95% ± 2% (n = 222) in NHL-BFM95 (MTX 1 g/m2) and 97% ± 1% (n = 154) in NHL-BFM90 (MTX 5 g/m2). In conclusion, MTX-4h was less toxic than MTX-24h. MTX-4h was noninferior to MTX-24h for limited stage B-cell non-Hodgkin lymphoma (B-NHL) but not for advanced disease. For limited disease, MTX 1 g/m2 is noninferior to 5 g/m2.
Introduction
Favorable results were reported from previous studies on childhood and adolescent B-cell non-Hodgkin lymphoma (B-NHL) and acute B-cell leukemia (B-AL) with 2 to 8 short intensive therapy courses depending on stage and tumor mass.1-8 The toxicity of these protocols was considerable, however. Therefore, the Non-Hodgkin Lymphoma–Berlin-Frankfurt-Münster 95 (NHL-BFM95) study aimed at reducing treatment-related toxicity without jeopardizing treatment outcome. In our previous trials, increasing the dose of methotrexate (MTX) from 0.5 g/m2 to 5 g/m2 (administered over 24 hours) resulted in significant improvement of the probability of event-free survival (pEFS) for patients with B-AL as well as for patients with B-NHL stage III with higher tumor load.4,9 However, high-dose MTX particularly contributed to the toxicity of the treatment, namely orointestinal mucositis, which may increase the risk of sepsis and toxic death.4
High-dose MTX combined with racemic tetrahydrofolic acid (leucovorin) is a key component of the majority of treatment regimens currently used for childhood and adolescent B-cell neoplasms.1-6,8 However, dose and administration schedule of MTX varies considerably between therapy protocols. Dosages of MTX range from 0.5 g/m2 to 8.0 g/m2; duration of continuous intravenous infusion of MTX ranges from 1 hour to 24 hours, resulting in very different pharmacokinetic profiles. Furthermore, time of application of the first dose of leucovorin varies between 24 hours6 and 42 hours4 after the start of MTX infusion, which introduces potentially significant differences regarding the duration of MTX exposure of healthy and malignant cells.
All mentioned parameters—dosage, administration schedule, drug exposure time—may influence efficacy and toxicity of MTX therapy.10-14 Controlled clinical trials investigating the impact of these parameters on treatment efficacy and toxicity are still lacking. Therefore, in order to optimize the MTX therapy in the treatment of children and adolescents with B-cell neoplasms, we investigated 2 questions in study NHL-BFM95. First, we tested in a randomized trial whether the incidence of severe orointestinal mucositis can be reduced by shortening the duration of intravenous infusion of high-dose MTX from 24 hours to 4 hours without impairment of the probability of failure-free survival. Second, we tested whether for patients with unresected B-NHL of moderate tumor mass (lactate dehydrogenase [LDH] < 500 U/L), representing approximately 45% of B-NHL patients, the dose of MTX can be reduced to 1 g/m2 without lowering the probability of EFS of 95% or higher that the patients achieved in our previous study NHL-BFM90, with therapy courses including MTX 5 g/m2.4
Patients, materials, and methods
Patients
Children and adolescents up to 18 years of age newly diagnosed with mature B-cell NHL or B-AL were eligible for trial NHL-BFM95. From April 1996 to March 2001, 566 patients were registered from 84 clinics in Austria, Germany, and Switzerland after informed consent according to the Declaration of Helsinki. Approval of the study was obtained from the ethical committee of the principal investigator (A.R.) and the participating investigators. Due to the following criteria, 61 patients were excluded: previous treatment (n = 4), no therapy (n = 3), NHL as a second malignancy (n = 4), severe immunodeficiency (n = 14, 4 failures), preexisting disease prohibiting protocol therapy (n = 1), AIDS-related NHL (n = 1, 1 failure), posttransplantation NHL (n = 11, 3 failures), treatment according to a different protocol due to decision of 2 participating clinics (n = 21, one failure), or erroneous diagnosis (n = 2). There were 505 patients eligible for the trial.
Diagnosis
Staging
The St Jude staging system was used.17 Staging included physical examination, peripheral blood and bone marrow (BM) aspiration smears, cerebrospinal fluid (CSF) analyses, ultrasonography, X-ray, computed tomography (CT) and/or magnetic resonance imaging (MRI), and skeletal scintigraphy. Initial central nervous system (CNS) disease was diagnosed if one of the following was present: lymphoma cells in the CSF, cerebral infiltrates on cranial CT or MRI, or cranial nerve palsy that was not caused by an extradural mass. The total serum LDH activity was measured as a parameter for the tumor mass.
Stratification of treatment intensity
Patients were stratified into 4 risk groups according to stage, resection status, pretherapeutic serum LDH, and presence of CNS disease (Figure 1).
Treatment strategy. Patients were stratified into 4 risk groups: R1, R2, R3, and R4. The composition of therapy courses is given in Table 1. V indicates cytoreductive prephase.
Treatment strategy. Patients were stratified into 4 risk groups: R1, R2, R3, and R4. The composition of therapy courses is given in Table 1. V indicates cytoreductive prephase.
Chemotherapy
The treatment strategy is depicted in Figure 1. The composition of therapy courses is given in Table 1. Patients in risk groups R2, R3, and R4 received a 5-day cytoreductive prephase before the first course A (AA) was administered. By November 15, 1997, an amendment was introduced. All patients in R3 + R4 had to receive urate oxidase 3 × 50 U/kg per day intravenously during the first days of cytoreductive chemotherapy.18-20 Conditions for starting the second and subsequent courses were as follows: platelet levels higher than 50 × 109/L and neutrophil counts higher than 0.5 × 109/L after the nadir of postchemotherapeutic cytopenia. For patients of risk groups R3 and R4, granulocyte colony-stimulating factor 5 μg/kg per day subcutaneously was recommended after the first 2 therapy courses. In CNS-positive patients, a device for intraventricular application of chemotherapy was implanted before the second course. MTX 3 mg and prednisolone 2.5 mg were administered intraventricularly on days 2, 3, 4, and 5, and cytarabine 30 mg was given on day 6 of courses AA and BB. In course CC, MTX 3 mg and prednisolone 2.5 mg were administered on days 3, 4, 5, and 6; cytarabine 30 mg was given on day 7.
Therapy courses
. | . | Day . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug . | Dose . | 1 . | 2 . | 3 . | 4 . | 5 . | ||||
| Prephase V | ||||||||||
| Dexamethasone orally/IV | mg/m2¶ | 5 | 5 | 10 | 10 | 10 | ||||
| Cyclophosphamide IV 1 h | 200 mg/m2 | x | x | |||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
| Course A | ||||||||||
| Dexamethasone orally/IV | 10 mg/m2¶ | x | x | x | x | x | ||||
| Vincristine IV | 1.5 mg/m2∥ | x | ||||||||
| Ifosfamide IV 1 h | 800 mg/m2 | x | x | x | x | x | ||||
| Cytarabine IV 1 h | 150 mg/m2 | x - x** | x - x** | |||||||
| Etoposide IV 1 h | 100 mg/m2 | x | x | |||||||
| Methotrexate IV† | 1 g/m2 | x | ||||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
| Course B | ||||||||||
| Dexamethasone orally/IV | 10 mg/m2¶ | x | x | x | x | x | ||||
| Vincristine IV | 1.5 mg/m2∥ | x | ||||||||
| Cyclophosphamide IV 1 h | 200 mg/m2 | x | x | x | x | x | ||||
| Doxorubicin IV 1 h | 25 mg/m2 | x | x | |||||||
| Methotrexate IV† | 1 g/m2 | x | ||||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
| Courses AAठand BBठ| ||||||||||
| Methotrexate IV† | 5 g/m2 | x | ||||||||
| Methotrexate* IT | 6 mg | x | x | |||||||
| Cytarabine* IT | 15 mg | x | x | |||||||
| Prednisolone* IT | 5 mg | x | x | |||||||
| Course CC‡ | ||||||||||
| Dexamethasone orally/IV | 20 mg/m2¶ | x | x | x | x | x | ||||
| Vindesine IV | 3 mg/m2†† | x | ||||||||
| Cytarabine IV 3 h | 3 g/m2 | x - x** | x - x** | |||||||
| Etoposide IV 2 h | 100 mg/m2 | x - x** | x - x** | x** | ||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
. | . | Day . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug . | Dose . | 1 . | 2 . | 3 . | 4 . | 5 . | ||||
| Prephase V | ||||||||||
| Dexamethasone orally/IV | mg/m2¶ | 5 | 5 | 10 | 10 | 10 | ||||
| Cyclophosphamide IV 1 h | 200 mg/m2 | x | x | |||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
| Course A | ||||||||||
| Dexamethasone orally/IV | 10 mg/m2¶ | x | x | x | x | x | ||||
| Vincristine IV | 1.5 mg/m2∥ | x | ||||||||
| Ifosfamide IV 1 h | 800 mg/m2 | x | x | x | x | x | ||||
| Cytarabine IV 1 h | 150 mg/m2 | x - x** | x - x** | |||||||
| Etoposide IV 1 h | 100 mg/m2 | x | x | |||||||
| Methotrexate IV† | 1 g/m2 | x | ||||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
| Course B | ||||||||||
| Dexamethasone orally/IV | 10 mg/m2¶ | x | x | x | x | x | ||||
| Vincristine IV | 1.5 mg/m2∥ | x | ||||||||
| Cyclophosphamide IV 1 h | 200 mg/m2 | x | x | x | x | x | ||||
| Doxorubicin IV 1 h | 25 mg/m2 | x | x | |||||||
| Methotrexate IV† | 1 g/m2 | x | ||||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
| Courses AAठand BBठ| ||||||||||
| Methotrexate IV† | 5 g/m2 | x | ||||||||
| Methotrexate* IT | 6 mg | x | x | |||||||
| Cytarabine* IT | 15 mg | x | x | |||||||
| Prednisolone* IT | 5 mg | x | x | |||||||
| Course CC‡ | ||||||||||
| Dexamethasone orally/IV | 20 mg/m2¶ | x | x | x | x | x | ||||
| Vindesine IV | 3 mg/m2†† | x | ||||||||
| Cytarabine IV 3 h | 3 g/m2 | x - x** | x - x** | |||||||
| Etoposide IV 2 h | 100 mg/m2 | x - x** | x - x** | x** | ||||||
| Methotrexate* IT | 12 mg | x | ||||||||
| Cytarabine* IT | 30 mg | x | ||||||||
| Prednisolone* IT | 10 mg | x | ||||||||
IV indicates intravenously; h, hour; IT, intrathecally.
Doses were adjusted for children younger than 3 years. In courses A, B, AA, and BB, intrathecal therapy was administered 24 hours after beginning of MTX intravenous infusion.
Patients were randomized to receive MTX as continuous intravenous infusion either over 24 hours or over 4 hours. In the 24-hour arm, 10% of the MTX dose was given within 0.5 hours, 90% of dose intravenously over 23.5 hours. Racemic folinic acid (leucovorin) intravenously 15 mg/m2 at hours 42, 48, and 54 after beginning of MTX. In courses AA and BB, the dose of leucovorin at hour 42 was 30 mg/m2 intravenously. Adjustment of leucovorin dose in case of impaired MTX excretion as previously described.4
For CNS-positive patients, chemotherapy was applied intraventricularly as described in “Chemotherapy.”
Courses AA and BB are the same as A and B, respectively, with the exceptions listed.
Subdivided in 3 doses.
Maximum dose was 2 mg; vincristine was not given in patients of branch R1.
Doses are 12 hours apart.
Maximum dose was 5 mg.
For patients in risk groups R3 + R4 who had residual tumor after the fifth course of therapy, a second-look operation was performed. If viable lymphoma tissue was detected, megadose chemotherapy with autologous stem cell rescue (autologous stem cell transplantation [ASCT]) was performed as previously described.4 If no viable lymphoma tissue was found, therapy was continued with the last course CC in risk group R4, while patients in risk group R3 did not receive any further therapy. For patients in risk group R2, no intervention was foreseen in case of a persistent tumor remnant during or after therapy.
Randomization of MTX infusion schedule
Patients were randomized to receive MTX as continuous intravenous infusion either over 24 hours (MTX-24h) or over 4 hours (MTX-4h). The dose of MTX was 1 g/m2 in courses A and B, (risk groups R1 + R2) and was 5 g/m2 in courses AA and BB (risk groups R3 + R4). Intrathecal therapy was given at hour 24 after the beginning of the MTX infusion in both randomized arms. The MTX serum concentration was measured at hours 24, 42, and 48 from the start of the MTX infusion. In both randomized arms, leucovorin 15 mg/m2 was given intravenously at hours 42, 48, and 54 after the beginning of MTX. In courses AA and BB, the dose of leucovorin at hour 42 was 30 mg/m2 intravenously. In case of impaired MTX excretion, intensified leucovorin rescue was carried out as previously described.4
Response criteria
The response to the treatment was evaluated after each course of therapy. Follow-up studies were performed at 4- to 6-week intervals during the first 1.5 years. In patients with BM and/or CNS involvement, follow-up punctures of BM and/or CSF were performed only until the BM or the CNS, respectively, was cleared from blasts. Tumor failure was defined as a recurrence of lymphoma proven by biopsy, or regrowth of an incompletely resolved tumor, or persistence of BM blasts after the second course of therapy. Isolated BM relapse was based on 25% or more blasts in the BM. Isolated CNS relapse was based on the appearance of blasts in the CSF.
Study design and statistical analysis
Event-free survival (EFS) was calculated from the day of diagnosis to an event (tumor failure, death for any reason, second malignancy) or to the date of last follow-up contact. Failure-free survival (FFS) was calculated from the day of diagnosis to treatment failure (tumor failure, death due to therapy) or to the date of last follow-up contact, while patients who experienced second malignancy were censored at the time of that event. Analyses of EFS and FFS were performed using the Kaplan and Meier method with differences compared by the log-rank test.21,22 The 95% confidence interval (CI) for the Kaplan-Meier estimate of EFS and FFS was calculated using standard errors according to Greenwood.23 In order to take into account prognostic factors when comparing FFS of patient subgroups, Cox regression analysis was used.24 The statistical analysis was carried out using the SAS statistical program (SAS-PC, Version 6.12; SAS Institute, Cary, NC). Follow-up data were updated as of June 1, 2003. Trial NHL-BFM95 was supervised by an external data safety and monitoring committee (DMC).
The first aim of study NHL-BFM95 was to test whether the incidence of orointestinal mucositis grades III or IV can be reduced by shortening the duration of intravenous infusion of high-dose MTX from 24 hours to 4 hours, without impairment of the probability of FFS (pFFS). Patients were randomized to receive either MTX-24h or MTX-4h. Randomization was stratified by risk group. For safety reasons, the first question to be answered was: Is MTX-4h noninferior to MTX-24h? The end point of that analysis was the one-sided 95% CI for the difference of a one-year pFFS between the 2 arms. This test was planned as a per-protocol analysis; that is, only randomized patients who received therapy according to their randomized arm were analyzed.25 Analysis was performed separately for branch R1, R2, and the combined branches R3 + R4. MTX-4h was accepted to be noninferior to MTX-24h if the lower limit of the one-sided 95% CI for the difference of pFFS between randomized patients treated with MTX-4h and those treated with MTX-24h did not exceed –11% in risk group R2 and –17% in the combined risk groups R3 + R4. The expected total number of randomized patients was 405 with a risk group distribution of 17%, 43%, 13%, and 27% for risk groups R1, R2, R3, and R4, respectively. With these expected numbers of patients for risk group R2, the power to prove noninferiority of MTX-4h was estimated to be 0.80 if the pFFS is 0.95 for the MTX-24h arm (type I error = 5%, n = 87 per arm). For the combined risk groups R3 + R4, the test power was estimated to be 0.80 if the pFFS is 0.80 for the MTX-24h arm (type I error = 5%, n = 81 per arm). There was no planned test for risk group R1 because of the small number of patients and the extremely low number of expected events.4 After the test for noninferiority, the second part of the study question was addressed: Is it possible to significantly reduce orointestinal toxicity by reducing the MTX infusion time from 24 hours to 4 hours? The end point of this analysis was the incidence of the maximum grade of mucositis per randomized arm. The analysis (Wilcoxon rank-sum test) was performed separately for risk group R1 (2 MTX-containing courses with MTX 1 g/m2), risk group R2 (4 courses with MTX 1 g/m2), and together for risk groups R3 + R4 (4 MTX-containing courses with MTX 5 g/m2) with type I error equal to 5% for each test. For patients receiving courses including MTX 5 g/m2 as intravenous infusion over 24 hours, an incidence of mucositis grade III or IV of 72% was expected based on observations in the preceding study NHL-BFM90. There were 2 interim analyses and a final analysis planned when 33%, 66%, and 100% of the expected total number of randomized patients would have a potential follow-up of at least one year. The overall alpha 0.05 was corrected according to O'Brien and Fleming for the first interim (P = .0005), the second interim (P = .014), and the final analysis (P = .045).26
The second aim of study NHL-BFM95 was to investigate, against the historical control of study NHL-BFM90, whether for patients in risk group R2 the dose of MTX can be reduced from 5 g/m2 in study NHL-BFM90 to 1 g/m2 in study NHL-BFM95 without impairment of pEFS. The end point of that analysis was the one-sided 95% CI for the difference of a 3-year pEFS between patients in risk group R2 in studies NHL-BFM90 and NHL-BFM95. Treatment with MTX 1 g/m2 was accepted to be noninferior to the therapy with MTX 5 g/m2 if the lower limit of the one-sided 95% CI for the difference in pEFS of patients in risk group R2 in study NHL-BFM95 and study NHL-BFM90 did not exceed –7%. This test was planned as a per-protocol analysis; that is, only patients allocated to risk group R2 who received R2 therapy were included in that analysis.25
Results
Patient characteristics
Of the 505 eligible patients, 119 were girls and 386 were boys. The median age was 9.3 years (range, 1.4-19.7 years). The diagnoses of patients are given in Table 2. Table 3 lists the distribution of patients according to risk groups and stages: 48, 233, 82, and 142 patients were assigned to risk group R1, R2, R3, and R4, respectively. Of the 40 patients with CNS disease, 27 had CSF blasts, 9 had an intraparenchymal mass, and 4 patients had a cranial nerve palsy only. Of the boys, 11 had testicular disease. The median pretreatment LDH was 371 U/L (range, 57-46 340 U/L).
Diagnosis, treatment results
. | . | . | Patients suffering from tumor failure . | Site of tumor failure (any involvement*) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | No. of patients (%) . | pEFS, 3 y, % . | . | Local . | BM . | CNS . | Testes . | New site . | ||||
| Burkitt lymphoma | 283 (56) | 93 ± 2 | 14 | 8 | 6 | 6 | 2 | 5 | ||||
| B-AL | 79 (16) | 77 ± 5 | 12 | 2 | 9 | 4 | 0 | 2 | ||||
| Diffuse large B-cell lymphoma | ||||||||||||
| Centroblastic | 81 (16) | 93 ± 3 | 4 | 3 | 0 | 0 | 0 | 2 | ||||
| Immunoblastic | 7 | NA | 1 | 1 | 0 | 0 | 0 | 1 | ||||
| T-cell rich B-cell lymphoma | 9 | NA | 1 | 1 | 0 | 0 | 0 | 0 | ||||
| Primary mediastinal large B-cell lymphoma | 15 (3) | 53 ± 13† | 7 | 7 | 0 | 0 | 0 | 3 | ||||
| B-NHL, not further specified | 31 (6) | 100 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| All | 505 (100) | 89 ± 1 | 39 | 22 | 15 | 10 | 2 | 13 | ||||
. | . | . | Patients suffering from tumor failure . | Site of tumor failure (any involvement*) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | No. of patients (%) . | pEFS, 3 y, % . | . | Local . | BM . | CNS . | Testes . | New site . | ||||
| Burkitt lymphoma | 283 (56) | 93 ± 2 | 14 | 8 | 6 | 6 | 2 | 5 | ||||
| B-AL | 79 (16) | 77 ± 5 | 12 | 2 | 9 | 4 | 0 | 2 | ||||
| Diffuse large B-cell lymphoma | ||||||||||||
| Centroblastic | 81 (16) | 93 ± 3 | 4 | 3 | 0 | 0 | 0 | 2 | ||||
| Immunoblastic | 7 | NA | 1 | 1 | 0 | 0 | 0 | 1 | ||||
| T-cell rich B-cell lymphoma | 9 | NA | 1 | 1 | 0 | 0 | 0 | 0 | ||||
| Primary mediastinal large B-cell lymphoma | 15 (3) | 53 ± 13† | 7 | 7 | 0 | 0 | 0 | 3 | ||||
| B-NHL, not further specified | 31 (6) | 100 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| All | 505 (100) | 89 ± 1 | 39 | 22 | 15 | 10 | 2 | 13 | ||||
Note: multiple sites can be involved in one patient.
P < .05 (log-rank test) for all comparisons.
Risk groups and stages
. | . | Stage, no. patients (%) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk group . | No. patients (%) . | I . | II . | III . | IV CNS- . | IV CNS+ . | B-AL CNS- . | B-AL CNS+ . | ||||||
| R1 | 48 (10) | 18 | 30 | 0 | 0 | 0 | 0 | 0 | ||||||
| R2 | 233 (46) | 35 | 89 | 109 | 0 | 0 | 0 | 0 | ||||||
| R3 | 82 (16) | 0 | 0 | 65 | 8 | 0 | 9 | 0 | ||||||
| R4 | 142 (28) | 0 | 0 | 47 | 8 | 17 | 47 | 23 | ||||||
| All | 505 (100) | 53 (10) | 119 (24) | 221 (44) | 16 (3) | 17 (3) | 56 (11) | 23 (5) | ||||||
. | . | Stage, no. patients (%) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk group . | No. patients (%) . | I . | II . | III . | IV CNS- . | IV CNS+ . | B-AL CNS- . | B-AL CNS+ . | ||||||
| R1 | 48 (10) | 18 | 30 | 0 | 0 | 0 | 0 | 0 | ||||||
| R2 | 233 (46) | 35 | 89 | 109 | 0 | 0 | 0 | 0 | ||||||
| R3 | 82 (16) | 0 | 0 | 65 | 8 | 0 | 9 | 0 | ||||||
| R4 | 142 (28) | 0 | 0 | 47 | 8 | 17 | 47 | 23 | ||||||
| All | 505 (100) | 53 (10) | 119 (24) | 221 (44) | 16 (3) | 17 (3) | 56 (11) | 23 (5) | ||||||
Event-free survival
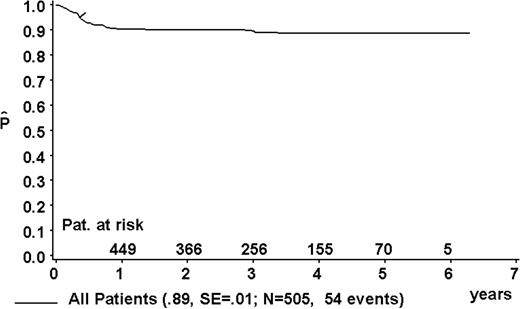
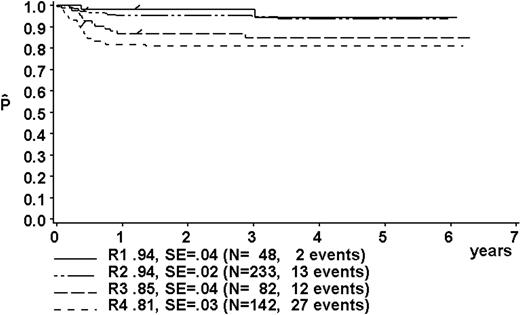
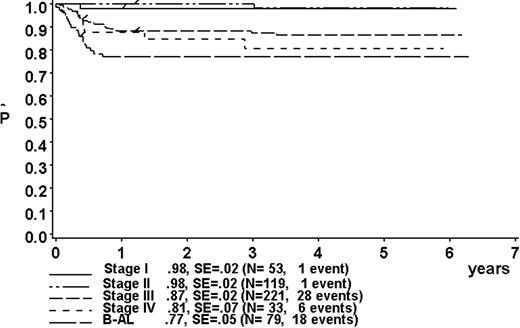
At a median follow-up of 3.3 years (range, 0.4-6.3 years), the 3-year pEFS was 89% ± 1% (SE) for the total group (Figure 2). The 3-year pEFS was 94% ± 4%, 94% ± 2%, 85% ± 4%, and 81% ± 3% for patients in risk groups R1, R2, R3, and R4, respectively (Figure 3). pEFS according to stage is depicted in Figure 4. There was no statistically significant difference between pEFS of patients with Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL). Patients with primary mediastinal large B-cell lymphoma had an inferior outcome, however (Table 2).
Kaplan-Meier estimate (P) of EFS at 3 years for the total group. SE indicates standard error.
Kaplan-Meier estimate (P) of EFS at 3 years for the total group. SE indicates standard error.
Kaplan-Meier estimate (P) of EFS at 3 years according to risk groups R1, R2, R3, and R4. SE indicates standard error.
Kaplan-Meier estimate (P) of EFS at 3 years according to risk groups R1, R2, R3, and R4. SE indicates standard error.
Kaplan-Meier estimate (P) of EFS at 3 years according to stage. SE indicates standard error.
Kaplan-Meier estimate (P) of EFS at 3 years according to stage. SE indicates standard error.
Events
Table 4 summarizes adverse events according to risk group and according to stage. One child in risk group R4 died early of tumor lysis syndrome. A total of 10 children died of sepsis (n = 6), invasive mycosis (n = 3), or meningitis (n = 1) after the first (n = 6), the third (n = 1), and the fourth (n = 3) course of therapy. There were 39 children who suffered from tumor failure. Local manifestations were the most frequent site of tumor failure followed by BM and new sites (Table 2). While on therapy, 11 patients suffered from tumor failure; 1 of them had persistent blasts after the second course of therapy. In 27 patients, tumor failure occurred after completion of chemotherapy within one year from diagnosis. There was one relapse 1.3 years after diagnosis. There were 3 patients who developed a second malignancy. There were 2 patients with BL who developed a second BL of different clonality 2.9 and 3.4 years after the first disease. One patient with DLBCL suffered a malignant melanoma. One BL patient suffered from a late recurrence 3.3 years after the first diagnosis. There was no material available for analysis regarding clonal identity or difference between the first and second malignant growths.
Adverse events according to risk groups and stages
. | Risk group, no. . | . | . | . | . | Stage, no. . | . | . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | All . | R1 . | R2 . | R3 . | R4 . | I . | II . | III . | IV CNS- . | IV CNS+ . | B-AL CNS- . | B-AL CNS+ . | ||||||||||
| No. patients | 505 | 48 | 233 | 82 | 142 | 53 | 119 | 221 | 16 | 17 | 56 | 23 | ||||||||||
| Early death of tumor lysis syndrome, | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||||||||||
| Death unrelated to tumor | 10 | 0 | 1 | 2 | 7 | 0 | 0 | 4 | 1 | 0 | 4 | 1 | ||||||||||
| Tumor failure | 39 | 1 § | 10 | 9 | 19 | 16 | 0 | 22 | 0 | 4 | 5 | 7 | ||||||||||
| Late event* | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||||||||
| Second malignancy† | 3 | 1 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | ||||||||||
| Lost to follow-up‡ | 14 | 0 | 11 | 2 | 1 | 2 | 3 | 8 | 0 | 0 | 0 | 1 | ||||||||||
. | Risk group, no. . | . | . | . | . | Stage, no. . | . | . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | All . | R1 . | R2 . | R3 . | R4 . | I . | II . | III . | IV CNS- . | IV CNS+ . | B-AL CNS- . | B-AL CNS+ . | ||||||||||
| No. patients | 505 | 48 | 233 | 82 | 142 | 53 | 119 | 221 | 16 | 17 | 56 | 23 | ||||||||||
| Early death of tumor lysis syndrome, | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||||||||||
| Death unrelated to tumor | 10 | 0 | 1 | 2 | 7 | 0 | 0 | 4 | 1 | 0 | 4 | 1 | ||||||||||
| Tumor failure | 39 | 1 § | 10 | 9 | 19 | 16 | 0 | 22 | 0 | 4 | 5 | 7 | ||||||||||
| Late event* | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||||||||
| Second malignancy† | 3 | 1 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | ||||||||||
| Lost to follow-up‡ | 14 | 0 | 11 | 2 | 1 | 2 | 3 | 8 | 0 | 0 | 0 | 1 | ||||||||||
Not possible to perform a successful molecular study on clonal differences between the first and the second malignant growths.
Secondary NHL in 2 cases, malignant melanoma in 1 patient.
After event-free follow-up duration of 0.4 to 5.4 years (median, 2.75 years).
Primary in parailiac nodes, combined local and bone marrow relapse 3 months after completion of front-line therapy. The patient is in complete second remission for 34 months following salvage therapy including megadose chemotherapy with autologous stem cell rescue.
CNS disease
The 3-year pEFS for the 40 CNS-positive patients was 69% ± 7%. One patient died of infection (Table 4). Of the remaining 39 patients, 11 suffered from progression, 7 of them within the CNS.
Second-look surgery and autologous blood stem cell transplantation
Of the patients, 15 in risk group R3 and 16 in R4 received second-look surgery due to residual tumor after the fifth course. The histologic examination revealed viable residual tumor in only one patient of R4. This patient underwent ASCT and remained free of relapse (follow-up, 42 months). Another 4 patients (1 in R3, 3 in R4) underwent ASCT after completion of chemotherapy without undergoing second-look surgery. Although not foreseen in the protocol, 17 patients in risk group R2 underwent second-look surgery or biopsy after the fourth course of therapy due to a persistent tumor remnant. In 2 of these 17 cases, vital residual tumor was found. ASCT was performed in both patients, but one patient died of progressive disease thereafter.
Randomized trial of methotrexate as intravenous infusion over 4 hours versus 24 hours
Of the 505 patients, 364 were randomized to receive either MTX-4h (n = 180) or MTX-24h (n = 184) (Table 5). Of the patients randomized to receive MTX-4h, 10 chose to receive MTX-24h. One patient randomized to receive MTX-24h chose to receive MTX-4h. In 63 cases (12%), patients/guardians did not give consent for randomization, preferring instead to choose the treatment arm. For the following reason, 20 patients were not randomized: In the first and second interim analysis, there was a trend toward inferior results in the randomized arm MTX-4h in risk groups R3 + R4. Therefore, randomization was temporarily halted in a blinded fashion, pending approval and recommendation whether to stop or to continue the trial. In these periods, all requests of the participating clinics for randomization of patients were answered with the allocation MTX-24h.
Randomization
. | Risk group, no. . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | R1 . | R2 . | R3 . | R4 . | Total . | ||||
| No. of patients | 48 | 233 | 82 | 142 | 505 | ||||
| Early death before randomization | 0 | 0 | 0 | 1 | 1 | ||||
| No. patients randomized in arm MTX-4h | 21 | 93 | 26 | 40 | 180 | ||||
| Refused, MTX-24h given | 1 | 5 | 1 | 3 | 10 | ||||
| No. patients randomized in arm MTX-24h | 19 | 96 | 31 | 38 | 184 | ||||
| Refused, MTX-4h given | 0 | 1 | 0 | 0 | 1 | ||||
| Not randomized* | |||||||||
| MTX-4h chosen | 2 | 7 | 1 | 3 | 13 | ||||
| MTX-24h chosen | 3 | 33 | 4 | 10 | 50 | ||||
| Randomization halted† | 3 | 4 | 3 | 10 | 20 | ||||
| Randomization stopped in R3 + R4 | |||||||||
| MTX-24h given‡ | NA | NA | 17 | 40 | 57 | ||||
. | Risk group, no. . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | R1 . | R2 . | R3 . | R4 . | Total . | ||||
| No. of patients | 48 | 233 | 82 | 142 | 505 | ||||
| Early death before randomization | 0 | 0 | 0 | 1 | 1 | ||||
| No. patients randomized in arm MTX-4h | 21 | 93 | 26 | 40 | 180 | ||||
| Refused, MTX-24h given | 1 | 5 | 1 | 3 | 10 | ||||
| No. patients randomized in arm MTX-24h | 19 | 96 | 31 | 38 | 184 | ||||
| Refused, MTX-4h given | 0 | 1 | 0 | 0 | 1 | ||||
| Not randomized* | |||||||||
| MTX-4h chosen | 2 | 7 | 1 | 3 | 13 | ||||
| MTX-24h chosen | 3 | 33 | 4 | 10 | 50 | ||||
| Randomization halted† | 3 | 4 | 3 | 10 | 20 | ||||
| Randomization stopped in R3 + R4 | |||||||||
| MTX-24h given‡ | NA | NA | 17 | 40 | 57 | ||||
NA indicates not applicable.
Not randomized due to patients/guardians refusal or by physicians in charge.
Because there was a trend for inferior results in the randomized arm MTX-4h, after the first and second interim analysis randomization was halted in blinded fashion for the time period until approval and recommendation of the external data safety and monitoring committee and the study committee regarding continuation or stopping of the trial. During this period, all requests for randomization of patients were answered with the allocation MTX-24 h.
In risk groups R3 and R4, randomization was stopped after the second interim analysis.
In the second interim analysis in the combined risk groups R3 + R4, the incidence of tumor failure was 5 times higher in the randomized arm MTX-4h than in the randomized arm MTX-24h, while in both arms 2 patients had died of toxicity. Because of this unexpected and alarming observation, in accordance with the DMC we changed from a test for noninferiority in pFFS to a log-rank test for difference. Because for a test of difference, an intent-to-treat analysis is the most conservative approach (not a per-protocol analysis), we also changed from the per-protocol analysis set of patients to an intent-to-treat set.25 pFFS at one year was 91% ± 4% for 56 patients with a minimal potential follow-up of one year and randomized to receive MTX-24h. In contrast, the one-year pFFS was 75% ± 6% for 49 patients with a minimal potential follow-up of one year and randomized to receive MTX-4h (P = .03). Therefore, after consultation with the DMC, the randomization was stopped for patients in risk groups R3 + R4 after the second interim analysis. The 57 patients thereafter enrolled in risk group R3 (n = 17) and risk group R4 (n = 40) and received MTX-24h. For patients in risk groups R1 and R2, however, randomization was continued until the end of the planned accrual period.
The final analysis was performed after a median follow-up of 3.3 years (range, 0.4-6.3 years). Table 6 shows the distribution of patient characteristics per randomized arm and according to risk group. The MTX serum concentrations at 24, 42, and 48 hours after the start of the MTX intravenous infusion were higher in patients receiving MTX-24h compared with those receiving MTX-4h (Table 7). There was no statistically significant difference of intervals between 2 subsequent courses in either randomization group (Table 8).
Characteristics of randomized patients
. | R1 . | . | R2 . | . | R3 + R4 . | . | All . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | ||||
| No. patients | 21 | 19 | 93 | 96 | 66 | 69 | 180 | 184 | ||||
| No. male | 18 | 13 | 68 | 72 | 54 | 52 | 140 | 137 | ||||
| No. female | 3 | 6 | 25 | 24 | 12 | 17 | 40 | 47 | ||||
| Median age, y | 10.9 | 9.8 | 9.0 | 9.3 | 8.6 | 7.7 | 8.7 | 8.8 | ||||
| Age 10 years or older, no. | 12 | 8 | 40 | 43 | 24 | 19 | 76 | 70 | ||||
| Burkitt, no. | 16 | 12 | 53 | 67 | 53 | 55 | 122 | 134 | ||||
| DLBL, no. | 4 | 6 | 27 | 23 | 5 | 6 | 36 | 35 | ||||
| Other, no. | 1 | 1 | 13 | 6 | 8 | 8 | 22 | 15 | ||||
| Stage, no. | ||||||||||||
| I | 9 | 6 | 12 | 12 | 0 | 0 | 21 | 18 | ||||
| II | 12 | 13 | 36 | 40 | 0 | 0 | 48 | 53 | ||||
| III | 0 | 0 | 45 | 44 | 39 | 32 | 84 | 76 | ||||
| IV, CNS- | 0 | 0 | 0 | 0 | 5 | 8 | 5 | 8 | ||||
| IV, CNS+ | 0 | 0 | 0 | 0 | 2 | 9 | 2 | 9 | ||||
| B-AL, CNS- | 0 | 0 | 0 | 0 | 13 | 17 | 13 | 17 | ||||
| B-AL, CNS+ | 0 | 0 | 0 | 0 | 7 | 3 | 7 | 3 | ||||
| Median LDH, U/L | 206 | 212 | 248 | 234 | 1,036 | 883 | 348 | 308 | ||||
| LDH 1000 U/L or more, no. | 0 | 0 | 0 | 0 | 34 | 31 | 34 | 31 | ||||
| Vital tumor at SL-OP, no. | NA | NA | 1* | 1† | 1‡ | 0 | 2 | 1 | ||||
| Risk group R4 patients, no. | NA | NA | NA | NA | 40 | 38 | 40 | 38 | ||||
. | R1 . | . | R2 . | . | R3 + R4 . | . | All . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | ||||
| No. patients | 21 | 19 | 93 | 96 | 66 | 69 | 180 | 184 | ||||
| No. male | 18 | 13 | 68 | 72 | 54 | 52 | 140 | 137 | ||||
| No. female | 3 | 6 | 25 | 24 | 12 | 17 | 40 | 47 | ||||
| Median age, y | 10.9 | 9.8 | 9.0 | 9.3 | 8.6 | 7.7 | 8.7 | 8.8 | ||||
| Age 10 years or older, no. | 12 | 8 | 40 | 43 | 24 | 19 | 76 | 70 | ||||
| Burkitt, no. | 16 | 12 | 53 | 67 | 53 | 55 | 122 | 134 | ||||
| DLBL, no. | 4 | 6 | 27 | 23 | 5 | 6 | 36 | 35 | ||||
| Other, no. | 1 | 1 | 13 | 6 | 8 | 8 | 22 | 15 | ||||
| Stage, no. | ||||||||||||
| I | 9 | 6 | 12 | 12 | 0 | 0 | 21 | 18 | ||||
| II | 12 | 13 | 36 | 40 | 0 | 0 | 48 | 53 | ||||
| III | 0 | 0 | 45 | 44 | 39 | 32 | 84 | 76 | ||||
| IV, CNS- | 0 | 0 | 0 | 0 | 5 | 8 | 5 | 8 | ||||
| IV, CNS+ | 0 | 0 | 0 | 0 | 2 | 9 | 2 | 9 | ||||
| B-AL, CNS- | 0 | 0 | 0 | 0 | 13 | 17 | 13 | 17 | ||||
| B-AL, CNS+ | 0 | 0 | 0 | 0 | 7 | 3 | 7 | 3 | ||||
| Median LDH, U/L | 206 | 212 | 248 | 234 | 1,036 | 883 | 348 | 308 | ||||
| LDH 1000 U/L or more, no. | 0 | 0 | 0 | 0 | 34 | 31 | 34 | 31 | ||||
| Vital tumor at SL-OP, no. | NA | NA | 1* | 1† | 1‡ | 0 | 2 | 1 | ||||
| Risk group R4 patients, no. | NA | NA | NA | NA | 40 | 38 | 40 | 38 | ||||
NA indicates not applicable; SL-OP, second look—operation.
Alive disease-free after autologous stem cell transplantation.
Died of progressive disease after autologous stem cell transplantation.
Alive disease-free after autologous stem cell transplantation.
Serum methotrexate concentrations of randomized patients
. | R1 + R2 . | . | R3 + R4 . | . | ||
|---|---|---|---|---|---|---|
| . | 1 g/m2 MTX . | . | 5 g/m2 MTX . | . | ||
. | 4 h* . | 24 h* . | 4 h* . | 24 h* . | ||
| No. MTX courses given | 305 | 318 | 199 | 235 | ||
| MTX serum concentration at hour 24,† μM, median (range) | 0.32 (0.1-75.9) | 8.17 (0.1-107.0) | 0.88 (0.1-31.0) | 46.40 (0.3-344.9) | ||
| MTX serum concentration at hour 42 † μM, median (range) | 0.09 (0-2.6) | 0.18 (0-1.0) | 0.18 (0-13.0) | 0.43 (0-6.5) | ||
| MTX serum concentration at hour 48,† μM, median (range) | 0.06 (0-1.2) | 0.1 (0-0.7) | 0.13 (0-4.9) | 0.24 (0-5.0) | ||
. | R1 + R2 . | . | R3 + R4 . | . | ||
|---|---|---|---|---|---|---|
| . | 1 g/m2 MTX . | . | 5 g/m2 MTX . | . | ||
. | 4 h* . | 24 h* . | 4 h* . | 24 h* . | ||
| No. MTX courses given | 305 | 318 | 199 | 235 | ||
| MTX serum concentration at hour 24,† μM, median (range) | 0.32 (0.1-75.9) | 8.17 (0.1-107.0) | 0.88 (0.1-31.0) | 46.40 (0.3-344.9) | ||
| MTX serum concentration at hour 42 † μM, median (range) | 0.09 (0-2.6) | 0.18 (0-1.0) | 0.18 (0-13.0) | 0.43 (0-6.5) | ||
| MTX serum concentration at hour 48,† μM, median (range) | 0.06 (0-1.2) | 0.1 (0-0.7) | 0.13 (0-4.9) | 0.24 (0-5.0) | ||
Indicates the number of hours over which the infusion was given.
After the beginning of MTX intravenous infusion.
Number of days between beginning of 2 subsequent courses in randomized patients (MTX intravenous infusion as given)
. | R1 . | . | . | . | R2 . | . | . | . | R3/R4 . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | MTX-4h . | . | MTX-24h . | . | MTX-4h . | . | MTX-24h . | . | MTX-4h . | . | MTX-24h . | . | |||||||||
. | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | |||||||||
| Interval course* | |||||||||||||||||||||
| 1 to 2 | 22 (19-34) | 20 | 22 (18-26) | 20 | 22 (15-49) | 86 | 22 (14-31) | 95 | 21 (16-32) | 58 | 21 (15-41) | 69 | |||||||||
| 2 to 3 | NA | NA | NA | NA | 22 (15-42) | 86 | 22 (17-43) | 95 | 21 (14-29) | 57 | 22 (17-51) | 68 | |||||||||
| 3 to 4 | NA | NA | NA | NA | 23 (16-43) | 84 | 23 (18-36) | 94 | 22 (16-38) | 56 | 22 (17-36) | 68 | |||||||||
| 4 to 5 | NA | NA | NA | NA | NA | NA | NA | NA | 23 (15-43) | 55 | 22 (18-43) | 64 | |||||||||
| 5 to 6 | NA | NA | NA | NA | NA | NA | NA | NA | 22 (17-43) | 27† | 23 (18-41) | 37† | |||||||||
. | R1 . | . | . | . | R2 . | . | . | . | R3/R4 . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | MTX-4h . | . | MTX-24h . | . | MTX-4h . | . | MTX-24h . | . | MTX-4h . | . | MTX-24h . | . | |||||||||
. | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | Median d (range) . | No. patients . | |||||||||
| Interval course* | |||||||||||||||||||||
| 1 to 2 | 22 (19-34) | 20 | 22 (18-26) | 20 | 22 (15-49) | 86 | 22 (14-31) | 95 | 21 (16-32) | 58 | 21 (15-41) | 69 | |||||||||
| 2 to 3 | NA | NA | NA | NA | 22 (15-42) | 86 | 22 (17-43) | 95 | 21 (14-29) | 57 | 22 (17-51) | 68 | |||||||||
| 3 to 4 | NA | NA | NA | NA | 23 (16-43) | 84 | 23 (18-36) | 94 | 22 (16-38) | 56 | 22 (17-36) | 68 | |||||||||
| 4 to 5 | NA | NA | NA | NA | NA | NA | NA | NA | 23 (15-43) | 55 | 22 (18-43) | 64 | |||||||||
| 5 to 6 | NA | NA | NA | NA | NA | NA | NA | NA | 22 (17-43) | 27† | 23 (18-41) | 37† | |||||||||
NA indicates not applicable.
The difference of the interval of the beginning of 2 subsequent courses was statistically not significant between randomized arms in either risk group.
Interval between day one of 2 subsequent courses.
Patients of risk group R3 received only 5 courses.
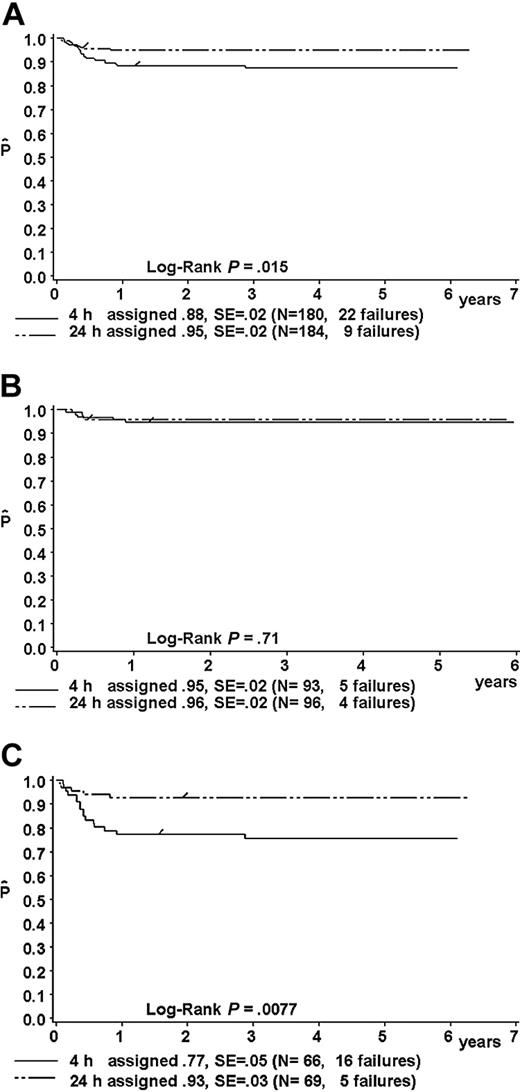
Intent-to-treat analysis
Results of the intent-to-treat analysis are given in Table 9 and in Figure 5A-C. The one-year pFFS for patients randomized to receive MTX-4h versus MTX-24h was as follows: in the total group, 88% ± 2% (n = 180) versus 95% ± 2% (n = 184) (P = .015; Figure 5A); in risk group R1, 95% ± 5% (n = 21) versus 100% (n = 19) (P = 0.34); in risk group R2, 95% ± 2% (n = 93) versus 96% ± 2% (n = 96) (P = .71) (hazard ratio, 1.28; 95% CI, 0.34-4.78) (Figure 5B); and in the combined risk groups R3 + R4 77% ± 5% (n = 66) versus 93% ± 3% (n = 69) (P = .0077; hazard ratio, 3.58; 95% CI, 1.31-9.79) (Figure 5C).
Treatment results of randomized patients
. | R1 . | . | R2 . | . | R3 + R4 . | . | All . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | ||||
| No. patients | 21 | 19 | 93 | 96 | 66 | 69 | 180 | 184 | ||||
| Toxic death, no. | 0 | 0 | 1 | 0 | 3 | 3 | 4 | 3 | ||||
| Tumor failure, no. (no. alive after rescue) | 1 (1) | 0 | 4 (2) | 4 (0) | 12 (2) | 2 (0) | 17 (5) | 6 (0) | ||||
| Second malignancy, no. | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | ||||
| Late event, no. | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||||
| pFFS at 1 y, ± SE, % | 95 ± 5 | 100 | 95 ± 2 | 96 ± 2 | 77 ± 5 | 93 ± 3 | 88 ± 2 | 95 ± 2 | ||||
. | R1 . | . | R2 . | . | R3 + R4 . | . | All . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | MTX-4h . | MTX-24h . | ||||
| No. patients | 21 | 19 | 93 | 96 | 66 | 69 | 180 | 184 | ||||
| Toxic death, no. | 0 | 0 | 1 | 0 | 3 | 3 | 4 | 3 | ||||
| Tumor failure, no. (no. alive after rescue) | 1 (1) | 0 | 4 (2) | 4 (0) | 12 (2) | 2 (0) | 17 (5) | 6 (0) | ||||
| Second malignancy, no. | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | ||||
| Late event, no. | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||||
| pFFS at 1 y, ± SE, % | 95 ± 5 | 100 | 95 ± 2 | 96 ± 2 | 77 ± 5 | 93 ± 3 | 88 ± 2 | 95 ± 2 | ||||
Intent-to-treat analysis. Log-rank P = .34 for R1, .72 for R2, .0077 for R3 + R4, and .015 for all.
Kaplan-Meier estimate (P) of a one-year failure-free survival of patients randomized to receive MTX as intravenous infusion either over 4 hours or over 24 hours. Intent-to-treat analysis. (A) For the whole group; (B) for patients in risk group R2; and (C) for patients in combined risk groups R3 + R4. SE indicates standard error.
Kaplan-Meier estimate (P) of a one-year failure-free survival of patients randomized to receive MTX as intravenous infusion either over 4 hours or over 24 hours. Intent-to-treat analysis. (A) For the whole group; (B) for patients in risk group R2; and (C) for patients in combined risk groups R3 + R4. SE indicates standard error.
In univariate analysis of patients of the combined risk groups R3 + R4, female sex (P = .03) and age older than 10 years (P = .01) were associated with inferior pFFS. In a Cox regression model with the covariables sex, age younger than 10 years versus 10 years or older, and risk group R3 versus R4, the hazard ratio of the randomized arm MTX-4h was 3.59 (95% CI, 1.30-9.93; P = .014; Table 10). This result is very close to the univariate analysis.
Cox regression analysis for randomized patients of risk groups R3 + R4
. | Hazard ratio (95% CI) . | P. chi . |
|---|---|---|
| Randomization arm MTX 4 hours | 3.59 (1.30-9.93) | .014 |
| Female | 2.84 (1.16-6.92) | .022 |
| Age 10 years or older | 2.62 (1.09-6.29) | .031 |
| Risk group R4 | 1.32 (0.54-3.20) | .545 |
. | Hazard ratio (95% CI) . | P. chi . |
|---|---|---|
| Randomization arm MTX 4 hours | 3.59 (1.30-9.93) | .014 |
| Female | 2.84 (1.16-6.92) | .022 |
| Age 10 years or older | 2.62 (1.09-6.29) | .031 |
| Risk group R4 | 1.32 (0.54-3.20) | .545 |
Per-protocol analysis to test for noninferiority of the randomized arm MTX-4h
Excluded from the per-protocol analysis were 11 patients who rejected the randomized arm but instead chose to receive the alternative MTX schedule (Table 5). In risk group R2 (MTX dose, 1 g/m2), the one-year pFFS was 94% ± 2% for 88 randomized patients who received MTX-4h compared with 96% ± 2% for 95 randomized patients who received MTX-24h (hazard ratio, 1.34; 95% CI, 0.36-4.99). The lower limit of the one-sided 95.5% CI for the difference (pFFS MTX-4h – pFFS MTX-24h, alpha corrected for multiple testing) was –6.8%. This was above the predetermined loss of –11%, which was considered still to be acceptable.
In the combined risk groups R3 + R4 (MTX dose, 5 g/m2), the one-year pFFS was 77% ± 5% for 62 randomized patients who received MTX-4h (12 tumor failures) compared with 93% ± 3% for 69 randomized patients who received MTX-24h (2 tumor failures). In both arms, 3 patients each died of toxicity. The hazard ratio was 3.56 (95% CI, 1.29-9.82). The lower limit of the one-sided 95% CI for the difference (pFFS MTX-4h – pFFS MTX-24h) was –25%, which was far lower than the predetermined level of –17%. This means that the possible loss in the efficacy is too high.
Toxicities with MTX-4h or MTX-24h
In all risk groups, the incidence of the maximum grade of mucositis was significantly lower in randomized patients who received MTX-4h compared with randomized patients who received MTX-24h (Table 11). The frequencies of main toxicities grades III/IV per total number of courses administered are given as a descriptive analysis in Table 12.
Incidence of the maximum grade of mucositis according to patients receiving MTX as 4-hour intravenous infusion compared with patients receiving MTX as 24-hour intravenous infusion
. | R1 . | . | R2 . | . | R3 + R4 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | 1 g/m2 MTX . | . | 1 g/m2 MTX . | . | 5 g/m2 MTX . | . | |||
. | 4 h* . | 24 h* . | 4 h* . | 24 h* . | 4 h* . | 24 h* . | |||
| No. MTX-containing courses | 2 | 2 | 4 | 4 | 4 | 4 | |||
| No. of patients evaluable | 20 | 20 | 86 | 94 | 60 | 71 | |||
| % patients with maximal mucositis grade | |||||||||
| 0 | 35 | 30 | 26 | 15 | 8 | 1 | |||
| I | 45 | 15 | 27 | 13 | 15 | 4 | |||
| II | 15 | 15 | 26 | 28 | 20 | 16 | |||
| III | 5 | 25 | 16 | 22 | 32 | 36 | |||
| IV | 0 | 15 | 6 | 22 | 25 | 43 | |||
. | R1 . | . | R2 . | . | R3 + R4 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | 1 g/m2 MTX . | . | 1 g/m2 MTX . | . | 5 g/m2 MTX . | . | |||
. | 4 h* . | 24 h* . | 4 h* . | 24 h* . | 4 h* . | 24 h* . | |||
| No. MTX-containing courses | 2 | 2 | 4 | 4 | 4 | 4 | |||
| No. of patients evaluable | 20 | 20 | 86 | 94 | 60 | 71 | |||
| % patients with maximal mucositis grade | |||||||||
| 0 | 35 | 30 | 26 | 15 | 8 | 1 | |||
| I | 45 | 15 | 27 | 13 | 15 | 4 | |||
| II | 15 | 15 | 26 | 28 | 20 | 16 | |||
| III | 5 | 25 | 16 | 22 | 32 | 36 | |||
| IV | 0 | 15 | 6 | 22 | 25 | 43 | |||
Per protocol analysis as MTX given. Wilcoxon test P = .06 for R1, .0002 for R2, and .0027 for R3 + R4.
Indicates the number of hours over which the infusion was given.
Percent courses with toxicity grade III/IV (NCI-CTC) of randomized patients (MTX IV infusion as given)
. | R1 + R2 . | . | . | . | R3 + R4 . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Courses A/B . | . | . | . | Courses AA/BB . | . | . | . | . | . | ||||||
| . | 1 g/m2 MTX . | . | . | . | 5 g/m2 MTX . | . | . | . | . | . | ||||||
| . | 4 h*† . | . | 24 h*‡ . | . | 4 h*§ . | . | 24 h*∥ . | . | CC¶ . | . | ||||||
. | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | ||||||
| Performance status | 3 | 1 | 8 | 1 | 11 | 5 | 23 | 6 | 10 | 2 | ||||||
| Hemoglobin | 21 | 8 | 28 | 9 | 40 | 13 | 39 | 24 | 56 | 17 | ||||||
| WBC | 36 | 37 | 25 | 49 | 26 | 48 | 17 | 69 | 9 | 87 | ||||||
| Platelets | 21 | 10 | 18 | 12 | 20 | 28 | 18 | 44 | 12 | 75 | ||||||
| Infection | 2 | 0 | 3 | 0 | 3 | 1 | 9 | 2 | 9 | 1 | ||||||
| Mucositis | 5 | 1 | 11 | 7 | 16 | 9 | 25 | 19 | 7 | 4 | ||||||
| Diarrhea | 1 | 1 | 0 | 0 | 2 | 3 | 5 | 3 | 3 | 2 | ||||||
| Bilirubin | 1 | 0 | 1 | 0 | 3 | 1 | 3 | 1 | 3 | 1 | ||||||
| Central neurotoxicity | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ||||||
. | R1 + R2 . | . | . | . | R3 + R4 . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Courses A/B . | . | . | . | Courses AA/BB . | . | . | . | . | . | ||||||
| . | 1 g/m2 MTX . | . | . | . | 5 g/m2 MTX . | . | . | . | . | . | ||||||
| . | 4 h*† . | . | 24 h*‡ . | . | 4 h*§ . | . | 24 h*∥ . | . | CC¶ . | . | ||||||
. | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | Grade III toxicity % . | Grade IV toxicity % . | ||||||
| Performance status | 3 | 1 | 8 | 1 | 11 | 5 | 23 | 6 | 10 | 2 | ||||||
| Hemoglobin | 21 | 8 | 28 | 9 | 40 | 13 | 39 | 24 | 56 | 17 | ||||||
| WBC | 36 | 37 | 25 | 49 | 26 | 48 | 17 | 69 | 9 | 87 | ||||||
| Platelets | 21 | 10 | 18 | 12 | 20 | 28 | 18 | 44 | 12 | 75 | ||||||
| Infection | 2 | 0 | 3 | 0 | 3 | 1 | 9 | 2 | 9 | 1 | ||||||
| Mucositis | 5 | 1 | 11 | 7 | 16 | 9 | 25 | 19 | 7 | 4 | ||||||
| Diarrhea | 1 | 1 | 0 | 0 | 2 | 3 | 5 | 3 | 3 | 2 | ||||||
| Bilirubin | 1 | 0 | 1 | 0 | 3 | 1 | 3 | 1 | 3 | 1 | ||||||
| Central neurotoxicity | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ||||||
NCI-CTC indicates National Cancer Institute-Common Toxicity Criteria.
Hepatotoxicity grade III/IV was 0% for all courses.
Indicates the number of hours over which the infusion was given.
374 courses recorded.
409 courses recorded.
235 courses recorded.
286 courses recorded.
165 courses recorded.
Comparison of pEFS for patients in risk group R2 against the historical control group in study NHL-BFM90
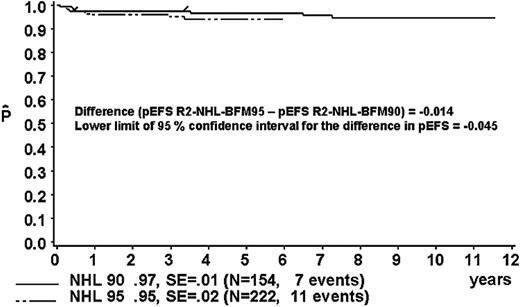
The proportion of patients in risk group R2 was 40% in study NHL-BFM90 and 46% in study NHL-BFM95 (Table 13). In studies BFM90 and BFM95, 13 and 11 patients, respectively, received treatment differing from risk group R2. Thus, 154 and 222 R2 patients of studies NHL-BFM90 and NHL-BFM95, respectively, were evaluable for comparison. In study BFM90, the 3-year pEFS was 97% ± 1% for the 154 patients of risk group R2 who received R2 therapy and was 95 ± 2% for the 222 patients in study BFM95 (Figure 6). The lower limit of the one-sided 95% CI for the difference in pEFS of risk group R2 in study NHL-BFM95 (MTX dose, 1 g/m2) minus pEFS of risk group R2 in study NHL-BFM95 (MTX dose, 5 g/m2) was –4.5%. The distribution of patient characteristics was comparable in studies NHL-BFM90 and NHL-BFM95 with one exception. In study NHL-BFM95, the percentage of BL patients was lower than in study NHL-BFM90, while the proportion of patients with DLBCL was higher. The 3-year pEFS for patients with BL or DLBCL was comparable in both studies, however (Table 13).
Characteristics and treatment results of patients of risk group R2 in studies NHL-BFM90 and NHL-BFM95
. | NHL-BFM90 patients . | NHL-BFM95 patients . |
|---|---|---|
| No. patients in risk group R2 | 167 | 233 |
| % of study population | 40 | 46 |
| No. patients receiving R2 therapy | 154 | 222 |
| Male, % | 75 | 75 |
| Female, % | 25 | 25 |
| Age, y, median (range) | 9.6 (1.9-17.9) | 9.6 (2.5-19.7) |
| Burkitt, % | 69 | 62 |
| DLBCL, % | 17 | 28 |
| PMLBL, % | 4 | 2 |
| Other, % | 10 | 7 |
| Stage, % | ||
| I | 14 | 16 |
| II | 44 | 40 |
| III | 42 | 45 |
| Mediastinal tumor, no. patients | 13 | 12 |
| LDH, U/L, median (range) | 238 (80-3583) | 229 (57-654) |
| Events, no. | ||
| Death unrelated to tumor | 3 | 1 |
| Tumor failure | 2 | 10 |
| Second malignancy | 3 | 2 |
| pEFS, 3 years, % | 97 ± 1 | 95 ± 2 |
. | NHL-BFM90 patients . | NHL-BFM95 patients . |
|---|---|---|
| No. patients in risk group R2 | 167 | 233 |
| % of study population | 40 | 46 |
| No. patients receiving R2 therapy | 154 | 222 |
| Male, % | 75 | 75 |
| Female, % | 25 | 25 |
| Age, y, median (range) | 9.6 (1.9-17.9) | 9.6 (2.5-19.7) |
| Burkitt, % | 69 | 62 |
| DLBCL, % | 17 | 28 |
| PMLBL, % | 4 | 2 |
| Other, % | 10 | 7 |
| Stage, % | ||
| I | 14 | 16 |
| II | 44 | 40 |
| III | 42 | 45 |
| Mediastinal tumor, no. patients | 13 | 12 |
| LDH, U/L, median (range) | 238 (80-3583) | 229 (57-654) |
| Events, no. | ||
| Death unrelated to tumor | 3 | 1 |
| Tumor failure | 2 | 10 |
| Second malignancy | 3 | 2 |
| pEFS, 3 years, % | 97 ± 1 | 95 ± 2 |
DLBCL indicates diffuse large B-cell lymphoma; and PMLBL, primary mediastinal (thymic) large B-cell lymphoma.
Kaplan-Meier estimate (P) of EFS at 3 years for patients of risk group R2 in study NHL-BFM95 and in the preceding trial NHL-BFM90. SE indicates standard error.
Kaplan-Meier estimate (P) of EFS at 3 years for patients of risk group R2 in study NHL-BFM95 and in the preceding trial NHL-BFM90. SE indicates standard error.
Discussion
Favorable results have been reported from previous studies on childhood and adolescent B-cell neoplasms.1-8 The toxicity of these protocols was considerable, however. Therefore, study NHL-BFM95 aimed at reducing treatment-related toxicity without jeopardizing treatment outcome. The 3-year pEFS for the 505 eligible patients was 89% ± 1% and thus comparable with the results of previous studies.1-8 The treatment proved equally efficacious for all subtypes of childhood and adolescent B-NHL, with the possible exception of primary mediastinal large B-cell lymphoma. Recent reports support the unique nature of this large cell lymphoma, which may need a specifically adapted treatment (eg, the high local failure rate [Table 2] may ask for a role of local radiotherapy).27
High-dose MTX is a key component of most protocols for childhood and adolescent B-cell neoplasms. However, the dose of MTX, the administration schedule, as well as the leucovorin rescue vary considerably between these protocols. All these parameters, especially the duration of the continuous intravenous infusion of MTX, may have a substantial influence on efficacy and toxicity.10-14
In our previous trials, orointestinal toxicity appeared to be the most important therapy-associated toxicity, mainly attributable to high-dose MTX given as continuous intravenous infusion over 24 hours.4 Therefore, the first aim of study NHL-BFM95 was to test in a randomized trial whether the incidence of orointestinal mucositis grade III/IV can be reduced by shortening the time of intravenous infusion of high-dose MTX from 24 hours to 4 hours, without impairment of the probability of FFS. Indeed, the present study showed an impressive reduction of severe orointestinal mucositis in patients having received MTX-4h compared with MTX-24h in all therapy branches.
However, in terms of efficacy, significant differences were observed regarding outcome and MTX infusion regimen in different risk groups. Regarding pFFS, the 4-hour infusion of high-dose MTX was noninferior to the 24-hour infusion for patients in risk groups R1 and R2 (55% of the total patient population). In risk groups R3 + R4, however, pFFS was significantly worse for patients having received MTX as a 4-hour infusion. The second interim analysis of the trial showed a 5-fold excess of tumor failure if MTX was administered over 4 hours compared with a 24-hour infusion. As a consequence of this alarming finding, randomization was omitted for patients in risk groups R3 + R4 after the second interim analysis. In the final analysis, pFFS of patients randomized to receive MTX 5 g/m2 as infusion over 4 hours was significantly (P = .008) lower compared with patients randomized to receive MTX 5 g/m2 as infusion over 24 hours. This difference in pFFS was not attributable to differences in patient characteristics between the 2 randomized arms. In a multivariate Cox regression analysis (including the covariables risk group, age, and sex), randomization in therapy arm MTX-4h was a significant risk factor for tumor failure. The leucovorin rescue as well as the application of all other chemotherapeutic agents were identical in both randomized arms. We conclude, therefore, that the difference in treatment efficacy between the 2 randomized arms for patients in risk groups R3 + R4 can be attributed to the different schedule of intravenous MTX infusion.
The observed difference of the impact of MTX administration schedules on efficacy for patients in risk groups R3 + R4 compared with those in risk groups R1 and R2 is intriguing. Patients in risk groups R3 + R4 differ from those in risk groups R1 and R2 primarily with respect to the higher tumor mass, which, in turn, might be associated with a higher proportion of multiple resistant cells within the tumor cell population.28 Because cytotoxicity of MTX is time dependent,12 longer exposure times of these resistant cells during a 24-hour infusion of MTX might be associated with improved clearance of these cells and, thus, might explain the higher pFFS of patients with high tumor load and long MTX infusion. In fact, this observation may be interpreted as strong evidence for the efficacy of MTX as such in the treatment of B-cell neoplasms of childhood and adolescents. According to the Goldie Coldman postulation,28 the lower tumor mass of the patients in risk groups R1 and R2 might be associated with a lower proportion of multiple resistant cells within the tumor cell population. Hence, these cells might be eradicated with a less efficacious schedule of MTX. On the other hand, the noninferiority of the short MTX infusion regimen compared with the long infusion regimen in terms of pFFS for patients in risk groups R1 and R2 may reflect that the impact of MTX on the overall therapeutic success may be of lesser importance in patients with lower tumor load compared with those with higher tumor masses.
Comparison of treatment outcome of patients in risk group R2 in the present trial NHL-BFM95 versus the previous study NHL-BFM90 supports the following hypothesis: pEFS for patients in risk group R2 did not differ between trials NHL-BFM90 and NHL-BFM95 despite a reduction of the dose of MTX to 1 g/m2 in study NHL-BFM95 compared with 5 g/m2 in the preceding trial NHL-BFM90. Furthermore, for this patient subgroup, intensification of chemotherapy in case of incomplete tumor regression after 2 therapy courses as performed in study NHL-BFM90 seems to be dispensable with the exception of single cases with very poor response to treatment.4
Our data suggest that a favorable balance of efficacy and toxicity is possible for patients with B-NHL stage I, II, and III (LDH < 500 U/L) (55% of all cases of B-cell neoplasms of childhood and adolescence). They have a pFFS of 95% with only 2 (R1) or 4 (R2) 5-day courses of chemotherapy including MTX 1 g/m2 as intravenous infusion over 4 hours. Mucositis grade III/IV was observed after only 6% of 374 courses, while infections grade III were observed after only 2% of 374 courses. However, further reduction of therapy in this patient subgroup may be crucial as long as no proven salvage regimen for relapsed patients is available. Surprisingly all 3 second malignancies occurred in risk groups R1 and R2 with less intense chemotherapy.
For patients with advanced disease, however, FFS rates comparable with the excellent results in the French study LMB 896 were obtained only with the courses including MTX 5 g/m2 as intravenous infusion over 24 hours. In study LMB 89, however, high-dose MTX was given as intravenous infusion over only 3 or 4 hours depending on treatment branch. Although almost the same drugs were used in the LMB and the BFM strategy, the weight of some drugs such as anthracyclines, etoposide, and alkylating agents differed considerably. This may have outweighed the effect of the short infusion time of MTX in the LMB regimen. This assumption is supported by the observation that the acute toxicity profiles of the COPADM courses used in study LMB 89, including MTX 3 g/m2 and 8 g/m2 as infusion over 3 and 4 hours, respectively, are similar to those of the courses AA and BB in study NHL-BFM95, including MTX 5 g/m2 as infusion over 24 hours.6
Interestingly, in study NHL-BFM95, the pFFS of patients in the combined risk groups R3 + R4 who received MTX 5 g/m2 as intravenous infusion over 24 hours was higher compared with the corresponding patients in study NHL-BFM90 (risk group R3 of study NHL-BFM90 was subdivided into risk groups R3 + R4 by LDH < or > 1000 U/L in study NHL-BFM95).4 In trial NHL-BFM95, all patients in risk groups R3 + R4 received course CC including high-dose cytarabine and etoposide. High-dose cytarabine/etoposide was described as effective even in relapsed B-NHL patients.29 For comparison, in our preceding study NHL-BFM90 only 30% of the corresponding patients received course CC. Moreover, the dose of cytarabine and etoposide was increased in course CC of study NHL-BFM95.
To our knowledge, study NHL-BFM95 is the first randomized controlled trial describing the impact of different MTX administration regimens on patient outcome and toxicity in the therapy of NHL. The results and conclusions from this trial may help to further optimize treatment not only for children and adolescents with B-cell NHL/AL, but also for adults suffering from highly aggressive B-cell neoplasms. Furthermore, experience from this trial shows that changes in administration schedules of well-established drugs may have profound and unexpected impact on both efficacy and side effects of chemotherapy.
Appendix 1: Study committee of trial NHL-BFM95
W. Dörffel, Berlin; W. Ebell, Berlin; N. Graf; Homburg; H. Gadner, Vienna; G. Henze, Berlin; G. Janka-Schaub, Hamburg; T. Klingebiel, Frankfurt; St Müller-Weihrich, Munich; I. Mutz, Leoben; H. J. R. Parwaresch, Kiel; A. Reiter, Giessen; H. Riehm, Hannover; G. Schellong, Münster; M. Schrappe, Hannover; F. Zintl, Jena.
Appendix 2: Reference laboratories
Histopathology and immunohistochemistry: R. Parwaresch, Lymph Node Registry founded by the Society of German Pathologists, Institute of Hematopathology, University Kiel; A. Feller, Institute of Pathology, University Luebeck; M. L. Hansmann, Institute of Pathology, University of Frankfurt; P. Möller, Institute of Pathology, University of Ulm; H. K. Müller-Hermelink, Institute of Pathology, University Wuerzburg; H. Stein, Institute of Pathology, Free University Berlin; I. Simonitsch, Institute of Pathology, University Vienna, Austria.
Immunophenotyping: W.-D. Ludwig, Berlin; W. Knapp, Vienna; F. Niggli, Zürich.
Cytomorphology: A. Reiter, Giessen; W. Haas, Wien; F. Niggli, Zürich.
Appendix 3: Principal investigators
R. Mertens (Aachen); R. Angst (Aarau); A. Gnekow (Augsburg); R. Dickerhoff (St Augustin); P. Imbach (Basel); G. F. Wuendisch (Bayreuth); W. Dörffel (Berlin-Buch); G. Henze (Berlin, Charité); U. Bode (Bonn); W. Eberl (Braunschweig); H. J. Spaar (Bremen); I. Krause (Chemnitz); J.-D. Thaben (Coburg); D. Möbius (Cottbus); W. Wiesel (Datteln); B. Ausserer (Dornbirn); H. Breu (Dortmund); G. Weißbach (Dresden, University); W. Kotte (Dresden); U. Göbel (Düsseldorf); W. Weinmann (Erfurt): J. D. Beck (Erlangen); W. Havers (Essen); G. Müller (Feldkirch); B. Kornhuber (Frankfurt); C. Niemeyer (Freiburg); F. Lampert (Gießen); M. Lakomek (Göttingen); C. Urban (Graz); H. Reddemann (Greifswald); S. Burdach (Halle); G. Janka-Schaub (Hamburg); H. Riehm/K. Welte (Hannover); B. Selle/A. Kulozik (Heidelberg); N. Graf (Homburg); C. Tautz (Herdecke); F. M. Fink (Innsbruck); F. Zintl (Jena); G. Nessler (Karlsruhe); H. Wehinger (Kassel); R. Schneppenheim (Kiel); H. Meßner (Klagenfurt); M. Rister (Koblenz); F. Berthold (Köln); W. Sternschulte (Köln); C. Schulte-Wissermann (Krefeld); M. Domula (Leipzig); I. Mutz (Leoben); G. Schmitt (Linz); O. Stoellinger (Linz); L. Nobile (Locarno); P. Bucsky (Lübeck); H. Ruetschle (Ludwigshafen); U. Caflisch (Luzern); U. Mittler (Magdeburg); P. Gutjahr (Mainz); O. Sauer (Mannheim); C. Eschenbach (Marburg); W. Tillmann (Minden); S. Müller-Weihrich (Muenchen, Technical University); C. Bender-Götze (Muenchen); R. J. Haas (Muenchen); H. Jürgens (Münster); O. Schofer (Neunkirchen); A. Jobke (Nürnberg); U. Schwarzer (Nürnberg); G. Eggers (Rostock); R. Geib-König (Saarbrücken); H. Grienberger (Salzburg); H. Haas (Schwarzach); F. J. Göbel (Siegen); R. Ploier (Steyr); J. Treuner (Stuttgart); R. Schumacher (Schwerin); A. Feldges (St Gallen); H. Rau (Trier); D. Niethammer (Tübingen); K. M. Debatin (Ulm); D. Franke (Vechta); H. Gadner (Wien, St Anna Kinderspital); F. Haschke (Wien); J. Weber (Wiesbaden); A. Dohrn (Wuppertal); J. Kühl (Würzburg); F. Niggli (Zurich).
Appendix 4: Pathologists
H. Mittermeyer (Aachen); B. Stamm (Aarau); R. Backmann (Augsburg); H. Ohnacker (Basel); M. Stolte (Bayreuth); H. Stein (Berlin); W. Schneider (Berlin); H. J. Foedisch, (Bonn); R. Donhuisen (Braunschweig); F. K. Koessling (Bremen); J. O. Habeck (Chemnitz); P. Stosiek (Cottbus); E. W. Schwarze (Dortmund); M. Müller (Dresden); H. E. Gabbert (Düsseldorf); V. Becker (Erlangen); L. D. Leder (Essen); M. L. Hansmann (Frankfurt); H. E. Schäfer (Freiburg); W. Schultz (Gießen); E. Kunze (Göttingen); C. Schmid (Graz); G. Lorenz (Greifswald); F. W. Rath (Halle); U. Helmchen (Hamburg); F. Kreipe (Hannover); F. Otto (Heidelberg); K. Remberger (Homburg); G. Mikuz (Innsbruck); D. Katenkamp (Jena); R. Wagner (Kaiserslautern); W. Gusek (Karlsruhe); O. Klinge (Kassel); R. Parwaresch (Kiel); W. Wagner (Klagenfurt); F. deLeon (Koblenz); H. P. Dienes (Köln); O. M. Gokel (Krefeld); C. Wittekind (Leipzig); G. Leitner (Leoben); M.
Weber (Linz); E. Pedrinis (Locarno); K. Wegener (Ludwigshafen); A. Feller (Lübeck); J. O. Grebbers (Luzern); A. Roesser (Magdeburg); W. Thoenes (Mainz); U. Bleyl (Mannheim); C. Thomas (Marburg); E. Jehn (Minden); K. Wurster (Muenchen, Technical University); U. Löhrs (Muenchen); W. Böcker (Münster); P. H. Wünsch (Nürnberg); F. Hofstädter (Regensburg); H. Nizze (Rostock); H. Mitschke (Saarbrücken); O. Dietze (Salzburg); A. Hittmair (Schwarzach); G. Wittstock (Schwerin); D. Kunde (Siegen); A. Diener (St Gallen); J. Feichtinger (Steyr); A. Bosse (Stuttgart); H. Maeusle (Trier); P. Kaiserling (Tübingen); O. Haferkamp (Ulm); M. Respondek (Vechta); T. Radasckiewicz (Wien); W. Remmle (Wiesbaden); G. Fischer (Wilhelmshaven); H. K. Müller-Hermelink (Würzburg); T. Stallmach (Zurich).
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-03-0973.
Supported by the Deutsche Krebshilfe, Bonn, grant no. M 109/91/Re1.
Complete lists of the principal investigators and pathologists of the BFM Group appear in “Appendix 3” and “Appendix 4,” respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work is dedicated to our colleague Stephan Mueller-Weihrich, MD, who died too early.
We acknowledge the expert work of Edelgard Odenwald, Beatrice Puttkammer (cytomorphology), Ulrike Meyer, and U. Regelsberger (data management).
We wish to thank the members of the external data safety and monitoring committee for their most valuable work: Catherine Patte, MD, Villejuif/France; Maria-Gracia Valsecchi, PhD, Milano/Italy; Ian Magrath, MD, PhD, Bethesda/USA; and Joerg Michaelis, MD, Mainz/Germany.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal