The review article “Targeting the Multidrug Resistance-1 Transporter in AML” by Mahadevan and List1 is thorough and thought-provoking, and we support its principal conclusions that pglycoprotein (pgp) remains one of the most powerful prognostic factors in adult acute myeloid leukemia (AML) and that future trials of pgp modulators are called for. Since the preselection of patients with pgp-positive AML would target those who are most likely to benefit and would also improve the predictive power of modulator trials, it follows that pgp phenotyping should be carried out at diagnosis. However, Mahadevan and List were unable to indicate a suitable method for pgp measurement in the laboratory. They referred right back to the consensus recommendations of 19962 and to the French report of 1997,3 both of which highlighted discrepancies in methodology and analysis. Meanwhile, Broxterman and colleagues4 published a report of a flow cytometric assay of functional pgp, which was reproducible in 2 centers in The Netherlands. In the United Kingdom, where pgp is being measured on AML trial patients in more than one laboratory, we built on the lessons of the Dutch and the French groups and decided to use a single standard operating procedure at participating laboratories. The Dutch protocol was adopted with the minor modification of adding CD45 staining to identify blasts.5,6
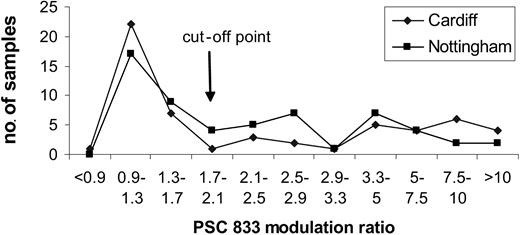
Most samples were sent to one of 2 centers, Cardiff or Nottingham. Thirteen quality control samples have been shared between the 2 centers since our collaboration started in 1999. Discordant results were noted in 1 of 13 samples; 8 had negative or low values (less than 1.7), 2 had intermediate values (1.7-3.39), and 2 had high values (3.4). Furthermore, there was a remarkable similarity in the distributions of data. Figure 1 illustrates the distributions obtained on 120 samples, the first 60 analyzed at each center. At one stage in the trial a third center also participated in the functional pgp analysis. A Kolmogoroff-Smirnoff test showed no significant difference in the distribution of PSC-833 modulation ratios at each of the 3 centers.
In contrast to the functional assays, antibody assays using MRK-16, also performed according to a standard operating procedure, showed significant differences in distribution between the 3 centers. While Mahadevan and List rightly pointed out that functional assays do not yield greater prognostic significance than antibody measurement in clinical trials, we have found that an important advantage of the functional assay lies in its greater sensitivity and reproducibility. We acknowledge that discrepancies do occur between phenotypic and functional pgp results, which are not fully understood.7,8 However, in the United Kingdom LRF AML 14 trial, an additional advantage of using a pgp functional assay was that the agent being used in the trial, PSC-833, could also be used in the assay.
Using the Dutch protocol over several years has confirmed to us that this methodology is robust and could now be passed to the safe and reliable hands of hospital immunophenotyping laboratories. From receiving a marrow sample, the assay takes about 4 hours and has been undertaken with as few as 3 × 106 cells. Using this protocol, future trial organizers can have the choice of whether to give pgp modulators to an unsorted cohort or to pgp-positive patients only.
These studies were funded by the Leukaemia Research Fund. We acknowledge the support of the NCRN Leukaemia Working Party.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal