Abstract
Kaposi sarcoma (KS) is an angioproliferative tumor derived from endothelial cells in which tumor cells form aberrant vascular structures. Ephrin B2 and ephrin B4 (EphB4) are artery- and vein-specific proteins, respectively, with critical roles in vessel maturation. We investigated whether the disorganized KS vasculature was due to unbalanced expression of ephrin B2 and EphB4. Secondly, we wished to determine if human herpesvirus type 8 (HHV-8), the viral agent associated with KS, regulates ephrin B2 and EphB4. An arterial phenotype was observed in KS tissue and cell lines, as shown by abundant expression of ephrin B2 with little or no EphB4. Infection of venous endothelial cells with HHV-8 resulted in a phenotype switch from EphB4 to ephrin B2, similar to that seen with vascular endothelial growth factor (VEGF). The HHV-8 effect on ephrin B2 expression was reproduced with the HHV-8-specific viral G-protein-coupled receptor. We also showed that ephrin B2 expression is required for KS cell viability by knock down with siRNA. KS is the first example of a human tumor with a predominantly arterial phenotype. This predominance can be attributed to expression of HHV-8 proteins and their downstream effects. Ephrin B2 is thus an important novel factor in KS biology and a potential target for therapy.
Introduction
Kaposi sarcoma (KS) manifests as a multifocal angioproliferative disease, most commonly of the skin and mucus membranes with subsequent spread to visceral organs.1 Hallmarks of the disease are angiogenesis, edema, infiltration of lymphomononuclear cells, and growth of spindle-shaped tumor cells. Pathologically, established lesions exhibit an extensive vascular network of slitlike spaces. Abnormal spindle-shaped endothelial cells (tumor cells) line the tumor vessels, which also lack basement membranes. Defective vasculature results in an accumulation of the blood components including albumin and red and mononuclear cells in the lesions.1 The KS tumor is endothelial in origin; the tumor cells express many endothelial markers, including lectin binding sites for Ulex europeaus agglutinin-1 (UEA-1), CD34, EN-4, pathologische anatomie Leiden endothelium (PAL-E),2 and the endothelial cell-specific tyrosine kinase receptors vascular endothelial growth factor receptor 1 (VEGFR-1; fetal liver tyrosine kinase 1 [Flt-1]), VEGFR-2 (fetal liver kinase-1/kinase domain receptor [Flk-1/KDR]), VEGFR-3 (Flt-4), Tie-1, and Tie-2 (Masood et al3 ; R.M. and P.S.G., unpublished data, 2004). KS cells coexpress lymphatic endothelial cell-related proteins including lymphatic vessel endothelial hyaluronan receptor (LYVE) and podoplanin.4
The gammaherpesvirus human herpesvirus type 8 (HHV-8) is considered the etiologic agent for the disease. In 1994 sequences of this herpes virus were identified in KS tumor tissue,5 and subsequent molecular epidemiology studies have shown that nearly all KS tumors contain viral genome. Seroepidemiology studies show that HIV-infected patients with KS have the highest prevalence of HHV-8 and secondly that those with HIV infection but no KS have increased risk of development of KS over the ensuing years if they are also seropositive for HHV-8.6 Direct evidence for the role of HHV-8 in KS is the transformation of bone marrow endothelial cells after infection with HHV-8.7 A number of HHV-8-encoded genes could contribute to cellular transformation (reviewed in Mihalcea et al8 ). However, the most evidence has accumulated for the viral G-protein-coupled receptor (vGPCR) in this role.9
Vascular endothelium is functionally and phenotypically divided into 2 distinct populations. Venous endothelial cells express the tyrosine kinase receptor EphB4, whereas arterial endothelial cells express its membrane-bound ligand ephrin B2.10-12 Cell-cell contact is required for binding and activation of Eph's by clustered membrane-attached ephrins.13 Full activation of Eph dimers is accomplished by autophosphorylation of juxtamembrane tyrosine residues.14,15 In addition to forward signaling through the Eph's, reverse signaling can occur through phosphorylation of the ephrin Bs.16-18
Targeted gene knockouts of either ephrin B2 or EphB4 in mice show similar phenotypes with disrupted vessel maturation and early embryonic lethality.11,12 Artery and vein phenotypes are determined very early in development, at the time of mesoderm differentiation to the vascular lineage.19 The ephrin B2/EphB4 ligand/receptor pair is important in vasculogenesis in the embryo and is also essential for defining the boundaries between arterial and venous domains10 that persist in the adult.20 It has been shown in vitro that EphB4-mediated forward signaling restricts intermingling of cells and supports cellular segregation, whereas reverse signaling from ephrin B2 stimulates migration and sprouting angiogenesis.21,22
The current model for artery/vein differentiation is that venous differentiation is the default pathway. VEGF appears to be a permissive signal for attaining the arterial phenotype through up-regulation of the Notch receptor and its ligand delta-like 4 (Dll-4) in arterial endothelial cells.23,24
We investigated whether KS tumor cells are derived from arterial or venous endothelium. In addition, we investigated whether HHV-8 has an effect on expression of arterial or venous markers in a model of KS. KS tumor cells were found to express the ephrin B2 arterial marker. Further, ephrin B2 expression was induced by HHV-8 and also by the HHV-8-specific vGPCR in KS and endothelial cell lines. Ephrin B2 is a potential target for treatment of KS because inhibition of ephrin B2 expression or signaling was detrimental to KS cell viability and function.
Patients, materials, and methods
Cell lines and reagents
Human umbilical vascular endothelial cells (HUVECs) were from Clonetics (San Diego, CA) and were maintained in endothelial growth medium 2 (EGM-2; Clonetics). EGM-2 is supplied as endothelial basal medium 2 (EBM-2) with supplements for fresh addition: fetal calf serum (FCS) to 2%, human epidermal growth factor (hEGF), hydrocortisone, VEGF, basic fibroblast growth factor (bFGF), recombinant insulin-like growth factor 1 (IGF-1), ascorbic acid, heparin, gentamicin, and Amphotericin-B. T1 human fibroblast line was from Dr Peter Jones (University of Southern California [USC]). BC-1 (HHV-8-positive, Epstein-Barr virus [EBV]-positive) and BC-3 (HHV-8-positive, EBV-negative) human pleural effusion lymphoma cell lines25 and monoclonal antibodies to latency-associated nuclear antigen 1 (LANA1) and HHV-8 lytic-phase ORF59 protein (ORF59) were the kind gift of Dr Dharam Ablashi (Advanced Biotechnologies, Columbia, MD). The KS-SLK cell line was isolated from a patient with classic KS.26 SCC-25 and SCC-15 cell lines were from the American Type Culture Collection (ATCC; Manassas VA). Antibodies to EphB4, ephrin B2, CD148, platelet endothelial cell adhesion molecule 1 (PECAM-1; all polyclonal), and VEGFR-2 (monoclonal) were from Santa Cruz Biotechnology (Santa Cruz, CA). VEGFR-3/Fc and monoclonal antibodies to human VEGF, VEGF-C, interleukin-6 (IL-6), IL-8, and oncostatin-M were from R&D Systems (Minneapolis, MN). Antibodies to p44/42 mitogen-activated protein kinase (MAPK; polyclonal) and phospho-p44/42 MAPK (monoclonal) were from New England Biolabs (Beverly, MA), and antiphosphotyrosine monoclonal antibody was from Upstate (Charlotte, VA). Expression vectors pCEFL-KS-associated herpesvirus-GPCR (pCEFL-KSHV-GPCR) and pCEFL were the kind gift of Dr Enrique Mesri (Cornell University, NewYork, NY). Expression vectors for HHV-8 LANAand LANAΔ440, which lacks the nuclear localization domain, were kindly provided by Dr Matthew Rettig, Veteran's Administration Greater LosAngeles Healthcare System.
Collection and preparation of human tissue
Human cutaneous KS biopsies were obtained under local anesthesia with informed consent from patients at the Los Angeles County (LAC)/USC Medical Center, using an institutional review board (IRB)-approved consent form. Biopsies were processed for either total RNA, paraffin blocks, or frozen tissue blocks in optimum cutting temperature (OCT). Total RNA was extracted by homogenization in RNAzol (Tel-Test, Friendswood, TX). cDNAs were synthesized by reverse transcriptase using a random hexamer primer (Superscript II; Invitrogen, Carlsbad, CA).
Preparation of digoxigenin-labeled RNA probes and in situ hybridization
Ephrin B2 and EphB4 polymerase chain reaction (PCR) products from the primers shown in Table 1 for in situ hybridization (ISH) were cloned into pGEM-T Easy Vector System (Promega, Madison, WI). DNA sequencing confirmed the orientation and authenticity of the inserts. Antisense or sense digoxigenin (DIG)-labeled RNA probes were created by run-off transcription from T7 or SP6 promoters using a DIG RNA labeling kit (Roche, Indianapolis, IN). RNA probes were quantitated by spot assay according to the manufacturer's instructions.
Primers
Genes . | Primer sequences . | Product size, bp . |
|---|---|---|
| ISH probe primers | ||
| Ephrin B2 | 5′-TCC GTG TGG AGT ACT GCT G-3′ | 296 |
| 5′-TCT GGT TTG GCA CAG TTG AG-3′ | ||
| EphB4 | 5′-CTT TGG AAG AGA CCC TGC TG-3′ | 297 |
| 5′-AGA CGG TGA AGG TCT CCT TG-3′ | ||
| RT-PCR primers | ||
| Ephrin B2 | 5′-AGA CAA GAG CCA TGA AGA TC-3′ | 200 |
| 5′-GGA TCC CAC TTC GGA CCC GAG-3′ | ||
| EphB4 | 5′-TCA GGT CAC TGC ATT GAA CGG G-3′ | 400 |
| 5′-AAC TCG CTC TCA TCC AGT T-3′ | ||
| vGPCR | 5′-ATG GCG GCC GAG GAT TTC CTA ACC-3′ | 434 |
| 5′-AGG TAC CTC ACT AGA CTG ACG CAC-3′ | ||
| LANA1 | 5′-TCC CCC TAG ATG TGA CTT CG-3′ | 406 |
| 5′-ATC CTC CTC GTC ATC CTC CT-3′ | ||
| GAPDH | 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ | 983 |
| 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′ | ||
| β-actin | 5′-GTG GGG CGC CCC AGG CAC CA-3′ | 546 |
| 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′ |
Genes . | Primer sequences . | Product size, bp . |
|---|---|---|
| ISH probe primers | ||
| Ephrin B2 | 5′-TCC GTG TGG AGT ACT GCT G-3′ | 296 |
| 5′-TCT GGT TTG GCA CAG TTG AG-3′ | ||
| EphB4 | 5′-CTT TGG AAG AGA CCC TGC TG-3′ | 297 |
| 5′-AGA CGG TGA AGG TCT CCT TG-3′ | ||
| RT-PCR primers | ||
| Ephrin B2 | 5′-AGA CAA GAG CCA TGA AGA TC-3′ | 200 |
| 5′-GGA TCC CAC TTC GGA CCC GAG-3′ | ||
| EphB4 | 5′-TCA GGT CAC TGC ATT GAA CGG G-3′ | 400 |
| 5′-AAC TCG CTC TCA TCC AGT T-3′ | ||
| vGPCR | 5′-ATG GCG GCC GAG GAT TTC CTA ACC-3′ | 434 |
| 5′-AGG TAC CTC ACT AGA CTG ACG CAC-3′ | ||
| LANA1 | 5′-TCC CCC TAG ATG TGA CTT CG-3′ | 406 |
| 5′-ATC CTC CTC GTC ATC CTC CT-3′ | ||
| GAPDH | 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ | 983 |
| 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′ | ||
| β-actin | 5′-GTG GGG CGC CCC AGG CAC CA-3′ | 546 |
| 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′ |
Frozen sections were subject to ISH essentially as previously described,27 except that the hybridization step was overnight at 42°C with 25 ng antisense or sense RNAprobes. Hybridization signal was detected using alkaline-phosphatase-conjugated anti-DIG antibodies (Roche) according to the manufacturer's instructions. Cells were visualized by counterstaining of nucleic acids with Nuclear Fast Red (Vector Laboratories, Burlingame, CA).
Coculture of HUVECs and BC-1
HUVECs were grown to 50% to 70% confluence in EGM-2 on gelatin-coated chamber slides (Nalge Nunc International, Naperville, IL). Coculture with BC-1 or BC-3 was essentially as described by Sakurada et al.28 Briefly, BC-1 or BC-3 cells were pretreated with TPA (12-O-tetradecanoyl-phorbol 13-acetate; 20 ng/mL) to induce virus for 48 hours and then added to the HUVEC culture at a ratio of 10:1 for cocultivation for 2 days. The HUVECs were washed extensively with phosphate-buffered saline (PBS) to remove the attached BC-1 or BC-3 cells.
Preparation of cDNA and RT-PCR
The TITANIUM One-Step reverse transcriptase-polymerase chain reaction (RT-PCR) kit (Clontech, Palo Alto, CA) was used for RT-PCR from 1 × 105 cells. Primer pairs for amplification of EphB4, ephrin B2, and β-actin are shown in Table 1. Gene-specific amplification consisted of 30 cycles of denaturation at 94°C for 30 seconds, primer annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. PCR products were separated on 1.5% agarose gels and stained with ethidium bromide.
Cell viability assay
KS-SLK cells were seeded at a density of 1 × 104 per well in 48-well plates on day 0 in appropriate growth medium containing 2% FCS. On the following day, the medium was changed and cells were transfected with 0, 10, or 100 nM siRNA. Forty-eight hours after transfection, viability was assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) as previously described.29 A similar procedure was followed for studies with neutralizing antibody and VEGFR-3/Fc. The following day, plating medium was replaced with 100 ng/mL antibody or recombinant Fc fusion protein and viability assessed 48 hours later with MTT.
Immunofluorescence studies
Cells cultured on chamber slides or frozen sections of KS biopsy material were fixed in 4% paraformaldehyde and preincubated with blocking buffer (0.2% Triton-X100, 1% bovine serum albumin [BSA] in PBS) for 20 minutes, followed by incubation with antibodies to EphB4, ephrin B2, CD148, LANA1 or ORF59 (1:100 dilution in PBS) in blocking buffer at 4°C for 16 hours. Slides were then incubated with the appropriate fluorescein- or rhodamine-conjugated secondary antibodies (Sigma-Aldrich, St Louis, MO). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole dihydrochloride hydrate). Images were obtained using a Olympus AX70 fluorescence microscope and Spot v2.2.2 (Diagnostic Instruments, Sterling Heights, MI) digital imaging system.
Immunofluorescence detection of ephrin B2 with EphB4-Fc
Frozen sections fixed in 4% paraformaldehyde and blocked with 20% FCS were incubated with 5 μg/mL EphB4/Fc (R&D Systems) for 1 hour at room temperature. Sections were then incubated with 10 μg/mL rabbit anti-human immunoglobulin G-fluorescein isothiocyanate (IgG-FITC) in PBS (Jackson ImmunoResearch Laboratories, West Grove, PA) at room temperature for 1 hour. Nuclei were counterstained with DAPI and sections mounted. Human IgG Fc (Jackson ImmunoResearch Laboratories) was used as the negative control.
Western blot
Crude cell lysates were prepared, quantitated, fractionated, and transferred to membranes as described previously.29 Membranes were blocked with 5% nonfat milk prior to incubation with antibody to ephrin B2 (1:5000 dilution) at 4°C for 16 hours. Secondary antibody (1:5000 dilution) conjugated with horseradish peroxidase was applied for 1 hour at 25°C. The membranes were developed using the SuperSignal West Femto Maximum sensitivity chemiluminescent substrate (Pierce, Rockford, IL) according to the manufacturer's instructions. Membranes were stripped using Restore Western Blot Stripping Buffer (Pierce) and reprobed with EphB4 or β-actin.
Synthesis of ephrin B2 and EphB4 siRNA by in vitro transcription
The Silencer siRNA construction kit (Ambion, Austin, TX) was used to synthesize siRNA to ephrin B2 and EphB4. Briefly, three 21-bp target sequences comprising 19-bp downstream of a 5′-AA dinucleotide were identified in the ephrin B2 cDNA (accession number NM_004093) with no significant homology to other sequences in GenBank. Sense and antisense siRNA 29-mer DNA oligonucleotide templates were synthesized at the USC Norris Microchemical Core Facility. Antisense template corresponded to the target sequence followed by 8-bp addition (5′-CCTGTCTC-3′)atthe 3′ end complementary to the T7 promoter primer provided with the Silencer SiRNA Construction Kit. Sense template comprised 5′-AA, followed by the complement of the target 19 bp, then theT7 8-bp sequence. siRNA was generated by individual in vitro transcription of the 2 templates, hybridized to create dsRNA, which was further processed to remove leader sequences and DNA template. The resulting siRNAs were purified according to the manufacturer's instructions. End-product double-stranded 21-mer siRNAs are shown in Table 2. Similarly, an EphB4 and scrambled green fluorescence protein (GFP) siRNAs were synthesized.
siRNAs
Primers . | Primer sequences . |
|---|---|
| Ephrin B2 siRNA254 | 5′-GCAGACAGAUGCACUAUUAUU-3′ |
| 3′-UUCGUCUGUCUACGUGAUAAU-5′ | |
| Ephrin B2 siRNA63 | 5′-CUGCGAUUUCCAAAUCGAUUU-3′ |
| 3′-UUGACGCUAAAGGUUUAGCUA-5′ | |
| Ephrin B2 siRNA137 | 5′-GGACUGGUACUAUACCCACUU-3′ |
| 3′-UUCCUGACCAUGAUAUGGGUG-5′ | |
| EphB4 siRNA50 | 5′-GAGACCCUGCUGAACACAAUU-3′ |
| 3′-UUCUCUGGGACGACUUGUGUU-5′ | |
| Scrambled GFP siRNA | 5′-CGCUGACCCUGAAGUUCAUUU-3′ |
| 3′-UUGCGACUGGGACUUCAAGUA-5′ |
Primers . | Primer sequences . |
|---|---|
| Ephrin B2 siRNA254 | 5′-GCAGACAGAUGCACUAUUAUU-3′ |
| 3′-UUCGUCUGUCUACGUGAUAAU-5′ | |
| Ephrin B2 siRNA63 | 5′-CUGCGAUUUCCAAAUCGAUUU-3′ |
| 3′-UUGACGCUAAAGGUUUAGCUA-5′ | |
| Ephrin B2 siRNA137 | 5′-GGACUGGUACUAUACCCACUU-3′ |
| 3′-UUCCUGACCAUGAUAUGGGUG-5′ | |
| EphB4 siRNA50 | 5′-GAGACCCUGCUGAACACAAUU-3′ |
| 3′-UUCUCUGGGACGACUUGUGUU-5′ | |
| Scrambled GFP siRNA | 5′-CGCUGACCCUGAAGUUCAUUU-3′ |
| 3′-UUGCGACUGGGACUUCAAGUA-5′ |
Ephrin B2 or EphB4 knockdown with siRNA in HUVECs grown with or without VEGF
Duplicate cultures of HUVECs were seeded on 8-well chamber slides coated with fibronectin and grown overnight in EGM-2. Sixteen hours later, medium was replaced with basal medium (EBM-2) supplemented with 5% fetal calf serum (FCS) and EGM-2 BulletKit supplements bFGF, hEGF, and recombinant insulin-like growth factor 1 (R3-IGF-1) at the concentrations provided by the manufacturer in half of the wells or 5% FCS and 10 ng/mL recombinant human VEGF (rhVEGF; R&D Systems) in the remaining wells. After 2 hours incubation at 37°C, all cells were transfected using Lipofectamine 2000 (1 μg/mL; Invitrogen) and 10 nM specific siRNAs in Opti-MEM-1 serum-free medium (Invitrogen). Following transfection for 2 hours in Opti-MEM-1, EBM-2 medium containing growth supplements was replaced in the appropriate wells. After 48 hours, the cells were stained with crystal violet and immediately photographed at × 10 magnification.
Construction of ephrin B2 reporter plasmids
Human ephrin B2 5′-flanking DNA from -2491 to -11 with respect to the translation start site was amplified from BACPAC clone RP11-297I6 (BacPac Resources, Children's Hospital, Oakland, CA) using theAdvantage GC Genomic PCR kit (Clontech) to overcome the large tracts of GC-rich sequence in the target area. Primers were designed to contain MluI sites for cloning into pGL3Basic Vector (Promega). Orientation of the resulting clones was confirmed by restriction digest analysis. The correct clone was designated pEFNB2-2491/-11luc. Digestion of this clone with either KpnIor SacI followed by recircularization yielded pEFNB2-1242/-11luc and pEFNB2-577/-11luc, respectively. Plasmid DNAs used for transient transfections were purified using a Mega Prep kit (QIAGEN, Valencia, CA).
Transient transfection and reporter assays
HUVECs (0.8 × 104 cells/well in 24-well plates) maintained in EGM-2 media were transiently cotransfected with 0.5 μg/well ephrin B2 promoter-luciferase constructs together with 50 ng/well either pCEFL or pCEFL-KSHV-GPCR, using Superfect reagent (QIAGEN) according to the manufacturer's instructions. Cells were harvested 48 hours after transfection and lysed with Luciferase cell lysis buffer (Promega). Luciferase activity was assayed using the Luciferase Assay System (Promega) according to the manufacturer's instructions. Luciferase was normalized to protein because pCEFL-vGPCR induced the expression of β-galactosidase from pCMV-Sport-βgal (Invitrogen).
Apoptosis enzyme-linked immunosorbent assay (ELISA) and caspase activation assays
Apoptosis was detected using the Cell Death Detection ELISAPlus Kit (Roche) following the manufacturer's instructions 48 hours after siRNA transfection of 2500 KS-SLK cells/well. For caspase activation assays, 2.5 × 106 cells were transfected with siRNAs. Forty-eight hours after transfection, activation of caspases 8 and 9 were determined on cytosolic extracts using the caspase 8 and caspase 9 colorimetric assays (R&D Systems) according to the manufacturer's instructions.
Results
Ephrin B2 but not EphB4 transcripts in KS tumors
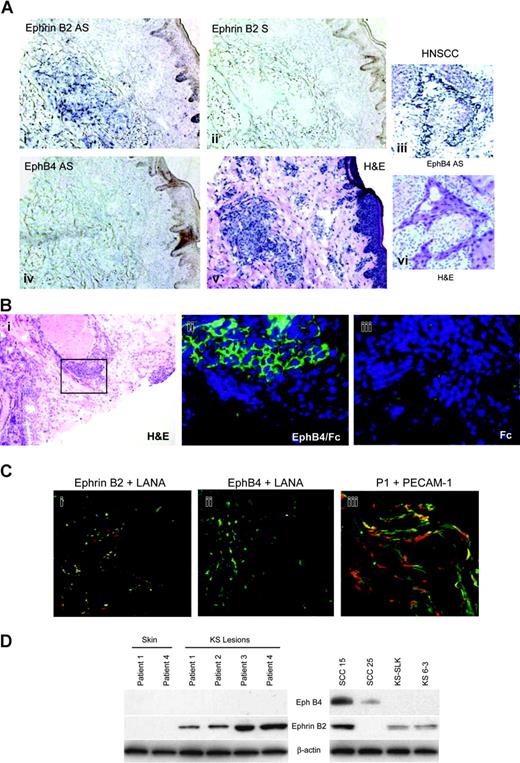
The highly vascular nature of KS lesions and the probable endothelial cell origin of the tumor cells prompted investigation of expression of EphB4 and ephrin B2, which are markers for venous and arterial endothelial cells, respectively. Ephrin B2 but not EphB4 transcripts were detected in tumor cells of KS biopsies by in situ hybridization (Figure 1A). Comparison of the positive signal with ephrin B2 antisense probe and tumor cells as shown by hematoxylin and eosin (H&E) staining shows that ephrin B2 expression is limited to the areas of the biopsy that contain tumor cells. Specificity of the EphB4 antisense probe was confirmed by positive signal for transcripts in head and neck squamous cell carcinoma (HNSCC), which we have shown expresses EphB4.30
Ephrin B2 but not EphB4 is expressed in KS biopsy tissue. (A) ISH with antisense probes for ephrin B2 and EphB4 with corresponding H&E-stained section to show tumor architecture. Dark blue color in the ISH indicates positive reaction for ephrin B2. No signal for EphB4 was detected in the KS biopsy. For contrast, ISH signal for EphB4 is strong in HNSCC tumor cells. Ephrin B2 was also detected in KS using EphB4-alkaline phosphatase (AP) fusion protein (iv). AS indicates antisense; S, sense. (B) Detection of ephrin B2 with EphB4/Fc fusion protein. Adjacent sections were stained with H&E (i) to show tumor architecture; the black rectangle indicates the area shown in the EphB4/Fc-treated section (ii) detected with FITC-labeled anti-human Fc antibody as described in “Patients, materials, and methods.” As a control, an adjacent section was treated with human Fc fragment (iii). Specific signal arising from EphB4/Fc binding to the section is seen only in areas of tumor cells. (C) Coexpression of ephrin B2 and the HHV-8 latency protein LANA1. (i-ii) Double-label confocal immunofluorescence microscopy with antibodies to ephrin B2 (red), LANA1 (green), or EphB4 (red) of frozen KS biopsy material directly demonstrates coexpression of LANA1 and ephrin B2 in KS biopsy. Coexpression is seen as yellow color. (iii) Double-label confocal image of biopsy with antibodies to PECAM-1 (green) in cells with nuclear propidium iodide stain (red), demonstrating the vascular nature of the tumor. (D) Western blot of protein extracts of KS biopsies, normal skin, and cell lines. Membranes were sequentially probed with ephrin B2, EphB4, and β-actin monoclonal antibodies. Specific bands were detected at 120 kDa for EphB4, 37 kDa for ephrin B2, and 40 kDa for β-actin. Oral squamous cell carcinoma cell lines SCC-15 an SCC-25 are included as positive controls for EphB4 probing. Membranes were probed with β-actin as a control for loading and transfer of protein.
Ephrin B2 but not EphB4 is expressed in KS biopsy tissue. (A) ISH with antisense probes for ephrin B2 and EphB4 with corresponding H&E-stained section to show tumor architecture. Dark blue color in the ISH indicates positive reaction for ephrin B2. No signal for EphB4 was detected in the KS biopsy. For contrast, ISH signal for EphB4 is strong in HNSCC tumor cells. Ephrin B2 was also detected in KS using EphB4-alkaline phosphatase (AP) fusion protein (iv). AS indicates antisense; S, sense. (B) Detection of ephrin B2 with EphB4/Fc fusion protein. Adjacent sections were stained with H&E (i) to show tumor architecture; the black rectangle indicates the area shown in the EphB4/Fc-treated section (ii) detected with FITC-labeled anti-human Fc antibody as described in “Patients, materials, and methods.” As a control, an adjacent section was treated with human Fc fragment (iii). Specific signal arising from EphB4/Fc binding to the section is seen only in areas of tumor cells. (C) Coexpression of ephrin B2 and the HHV-8 latency protein LANA1. (i-ii) Double-label confocal immunofluorescence microscopy with antibodies to ephrin B2 (red), LANA1 (green), or EphB4 (red) of frozen KS biopsy material directly demonstrates coexpression of LANA1 and ephrin B2 in KS biopsy. Coexpression is seen as yellow color. (iii) Double-label confocal image of biopsy with antibodies to PECAM-1 (green) in cells with nuclear propidium iodide stain (red), demonstrating the vascular nature of the tumor. (D) Western blot of protein extracts of KS biopsies, normal skin, and cell lines. Membranes were sequentially probed with ephrin B2, EphB4, and β-actin monoclonal antibodies. Specific bands were detected at 120 kDa for EphB4, 37 kDa for ephrin B2, and 40 kDa for β-actin. Oral squamous cell carcinoma cell lines SCC-15 an SCC-25 are included as positive controls for EphB4 probing. Membranes were probed with β-actin as a control for loading and transfer of protein.
EphB4 binding sites in KS tumors
Additional evidence for the expression of ephrin B2 in KS tumor tissue is afforded by the localization of EphB4/Fc signal to tumor cells detected by FITC-conjugated anti-human Fc antibody. Because ephrin B2 is the only ligand for EphB4, this reagent is specific for the expression of ephrin B2 (Figure 1Bi). An adjacent section treated only with human Fc and the secondary reagents shows no specific signal.
Coexpression of ephrin B2 and HHV-8 proteins in KS tumors
In other studies, 2-color confocal microscopy demonstrated the presence of the HHV-8 latency protein LANA1 in the ephrin B2-positive cells (Figure 1Ci), indicating that it is the tumor cells, not tumor vessels, that are expressing this arterial marker. Staining of tumor biopsy with the vessel-specific PECAM-1/CD31 antibody revealed the highly vascular nature of this tumor (Figure 1Ciii).
Ephrin B2 but not EphB4 is present in KS biopsies and cell lines
Western blots on KS biopsy lysates confirmed the expression of ephrin B2 but not EphB4. Matched normal skin was obtained for 2 of the cases and these expressed neither ephrin B2 nor EphB4 (Figure 1D). In addition, the immortalized KS cell line KS-SLK and a primary isolate, KS 6-3, showed the same pattern of expression as the biopsy material. The EphB4 antibody could detect protein, as shown by signal in squamous cell carcinomas SCC-15 and SCC-25.
Arterial phenotype switch in venous endothelial cells infected with HHV-8
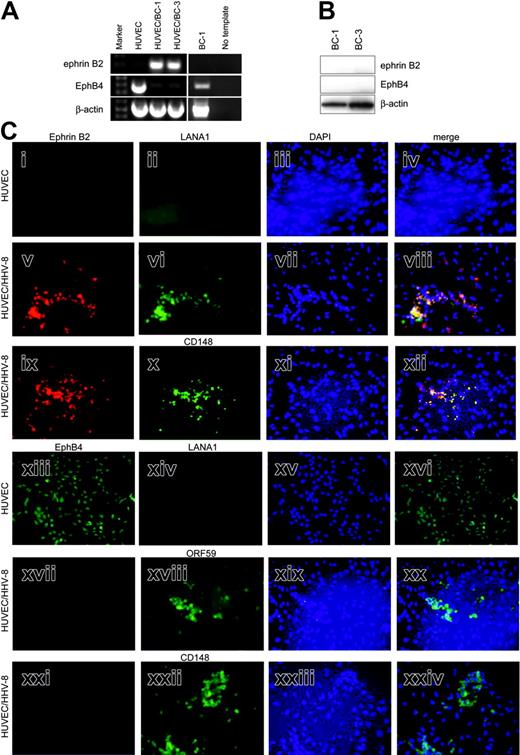
We next asked whether HHV-8, the presumed etiologic agent for KS, itself induced expression of ephrin B2 and repressed EphB4 expression in endothelial cells. Coculture of HUVECs and BC-1 lymphoma cells, which are productively infected with HHV-8, results in effective infection of the endothelial cells.28 The attached monolayers of endothelial cells remaining after extensive washing were examined for ephrin B2 and EphB4 by RT-PCR and immunofluorescence. HUVECs express EphB4 venous marker strongly at the RNA level but not ephrin B2 (Figure 2A). In contrast, HHV-8-infected cultures (HUVEC/BC-1 and HUVEC/BC-3) express ephrin B2, whereas EphB4 transcripts are almost absent. Ephrin B2 signal in infected cell cultures is not due to residual BC-1 cells, as they do not express ephrin B2 and only express low levels of EphB4 mRNA (Figure 2A). No evidence of ephrin B2 and EphB4 protein in BC-1 and BC-3 cell lines was found by Western blot (Figure 2B). Positive controls for the antibodies run on the same gel are shown in Figure 1D (SCC-15).
HHV-8 induces arterial marker expression in venous endothelial cells. (A) RT-PCR of HUVECs, 2 HHV-8-infected cultures (HUVEC/BC-1 and HUVEC/BC-3), and BC-1 for ephrin B2 and EphB4. Ephrin B2 product (200 bp) is seen in HUVEC/BC-1 and HUVEC/BC-3, and EphB4 product (400 bp) is seen in HUVECs and BC-1. No template lane had exactly the same reaction components and conditions, except template cDNA was omitted. This is included to show the specificity of the amplified products. β-Actin RT-PCR is also shown as a control for amount and integrity of input RNA. (B) Western blot of protein extracts of BC-1 and BC-3 HHV-8-positive cell lines. Membranes were sequentially probed with ephrin B2, EphB4, and β-actin monoclonal antibodies. Specific bands were detected at 120 kDa for EphB4, 37 kDa for ephrin B2, and 40 kDa for β-actin. (C) Immunofluorescence of cultures of HUVECs and HUVEC/BC-1 for artery/vein markers and viral proteins. Cultures were grown on chamber slides and processed for immunofluorescence detection of ephrin B2 (i, v, ix), EphB4 (xiii, xvii, xxi), CD148 (x, xxii), and the HHV-8 proteins LANA1 (ii, vi, xiii) or ORF59 (xviii) as described in “Patients, materials, and methods.” Yellow color in the merged images of the same field demonstrate coexpression of ephrin B2 and LANA or ephrin B2 and CD148. The positions of viable cells were revealed by nuclear staining with DAPI (blue) in the third column (iii, vii, xi, xv, xix, xxiii). Photomicrographs are of representative fields.
HHV-8 induces arterial marker expression in venous endothelial cells. (A) RT-PCR of HUVECs, 2 HHV-8-infected cultures (HUVEC/BC-1 and HUVEC/BC-3), and BC-1 for ephrin B2 and EphB4. Ephrin B2 product (200 bp) is seen in HUVEC/BC-1 and HUVEC/BC-3, and EphB4 product (400 bp) is seen in HUVECs and BC-1. No template lane had exactly the same reaction components and conditions, except template cDNA was omitted. This is included to show the specificity of the amplified products. β-Actin RT-PCR is also shown as a control for amount and integrity of input RNA. (B) Western blot of protein extracts of BC-1 and BC-3 HHV-8-positive cell lines. Membranes were sequentially probed with ephrin B2, EphB4, and β-actin monoclonal antibodies. Specific bands were detected at 120 kDa for EphB4, 37 kDa for ephrin B2, and 40 kDa for β-actin. (C) Immunofluorescence of cultures of HUVECs and HUVEC/BC-1 for artery/vein markers and viral proteins. Cultures were grown on chamber slides and processed for immunofluorescence detection of ephrin B2 (i, v, ix), EphB4 (xiii, xvii, xxi), CD148 (x, xxii), and the HHV-8 proteins LANA1 (ii, vi, xiii) or ORF59 (xviii) as described in “Patients, materials, and methods.” Yellow color in the merged images of the same field demonstrate coexpression of ephrin B2 and LANA or ephrin B2 and CD148. The positions of viable cells were revealed by nuclear staining with DAPI (blue) in the third column (iii, vii, xi, xv, xix, xxiii). Photomicrographs are of representative fields.
Coexpression of arterial markers and HHV-8 proteins in infected endothelial cells
Immunofluorescence analysis of cultures of HUVECs and HUVEC/HHV-8 for artery/vein markers and viral proteins was undertaken to determine whether changes in protein expression mirrored that seen in the RNA. In addition, cellular localization of the proteins could be determined. Consistent with the RT-PCR data, HUVECs are ephrin B2 negative and EphB4 positive (Figure 2Ci,xiii). As expected, they do not express any HHV-8 LANA1 (Figure 2Cii,xiv). Coculture of BC-1 cells, which are productively infected with HHV-8, resulted in infection of HUVECs as shown by presence of viral proteins LANA1 and ORF59 (Figure 2Cvi,xviii). HHV-8-infected HUVECs now express ephrin B2 but not EphB4 (Figure 2Cv,xvii,xxi, respectively). Expression of ephrin B2 and LANA1 cocluster is shown by yellow signal in the merged image (Figure 2Cviii). HHV-8-infected HUVECs positive for ephrin B2 and negative for EphB4 also express the arterial marker CD14819 (Figure 2Cx,xxii). Expression of ephrin B2 and CD148 cocluster is shown by yellow signal in the merged image (Figure 2Cxii). Uninfected HUVECs expressing EphB4 were negative for CD148 (not shown).
HHV-8 vGPCR induces ephrin B2 protein
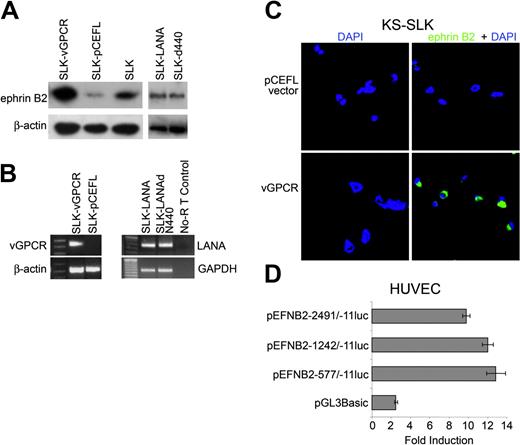
To test whether individual viral proteins could induce the expression of ephrin B2 seen with the whole virus, KS-SLK cells were stably transfected with HHV-8 LANA, LANAΔ440 (which lacks the nuclear localization domain), or vGPCR. Western blot of stable clones showed overexpression of ephrin B2 in KS-SLK cells transfected with vGPCR compared with LANA, LANAΔ440, or vector alone (pCEFL; Figure 3A). Expression of HHV-8 proteins in stable clones was confirmed by RT-PCR (Figure 3B). Transient transfection of KS-SLK cells with vGPCR resulted in induction of ephrin B2 expression as detected by immunofluorescence (Figure 3C) compared with vector alone (pCEFL). EphB4 was not induced in KS-SLK-vGPCR compared with KS-SLK-pCEFL, and negligible EphB4 expression in KS-SLK cells (data not shown) precluded determination of whether it was repressed in the KS-SLK-vGPCR transfectants.
HHV-8 induces arterial marker expression in Kaposi sarcoma cells. (A) Western blot for ephrin B2 on various cell lysates. SLK-vGPCR is a stable clone of SLK expressing the HHV-8 vGPCR, and SLK-pCEFL is control stable clone transfected with empty expression vector. SLK cells transfected with LANA or LANAΔ440 are SLK-LANA and SLK-Δ440, respectively. Quantity of protein loading and transfer was determined by reprobing the membranes with β-actin monoclonal antibody. (B) RT-PCR of stable cell lines shown in panel A, demonstrating expression of the relevant viral mRNAs. Housekeeping mRNA to demonstrate equivalent input mRNA and loading was β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Specific bands of the expected sizes (Table 1) were obtained with all gene-specific primer pairs. (C) Transient transfection of KS-SLK cells with expression vector pCEFL-KSHV-GPCR resulted in the expression of ephrin B2 as shown by immunofluorescence staining with FITC (green), whereas the control vector pCEFL had no effect. KS-SLK cells (0.8 × 105/well) were transfected with 0.8 μg DNA using Lipofectamine 2000. Twenty-four hours later cells were fixed and stained with ephrin B2 polyclonal antibody and FITC-conjugated secondary antibody as described in “Patients, materials, and methods.” (D) Transient transfection of HUVECs with vGPCR induces transcription from ephrin B2 luciferase constructs. HUVECs (8 × 103) in 24-well plates were transfected using Superfect with 0.8 μg/well ephrin B2 promoter constructs containing sequences from -2941 to -11 with respect to the translation start site, or 2 5′ deletions as indicated, together with 80 ng/well pCEFL or pvGPCR-CEFL. Luciferase was determined 48 hours after transfection. pGL3Basic is promoterless luciferase control vector. Luciferase was normalized to protein since GPCR induced expression of the cotransfected β-galactosidase. Graphed is induction ratio (vGPCR/pCEFL) of mean ± SEM of 6 replicates. Shown is 1 of 3 similar experiments.
HHV-8 induces arterial marker expression in Kaposi sarcoma cells. (A) Western blot for ephrin B2 on various cell lysates. SLK-vGPCR is a stable clone of SLK expressing the HHV-8 vGPCR, and SLK-pCEFL is control stable clone transfected with empty expression vector. SLK cells transfected with LANA or LANAΔ440 are SLK-LANA and SLK-Δ440, respectively. Quantity of protein loading and transfer was determined by reprobing the membranes with β-actin monoclonal antibody. (B) RT-PCR of stable cell lines shown in panel A, demonstrating expression of the relevant viral mRNAs. Housekeeping mRNA to demonstrate equivalent input mRNA and loading was β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Specific bands of the expected sizes (Table 1) were obtained with all gene-specific primer pairs. (C) Transient transfection of KS-SLK cells with expression vector pCEFL-KSHV-GPCR resulted in the expression of ephrin B2 as shown by immunofluorescence staining with FITC (green), whereas the control vector pCEFL had no effect. KS-SLK cells (0.8 × 105/well) were transfected with 0.8 μg DNA using Lipofectamine 2000. Twenty-four hours later cells were fixed and stained with ephrin B2 polyclonal antibody and FITC-conjugated secondary antibody as described in “Patients, materials, and methods.” (D) Transient transfection of HUVECs with vGPCR induces transcription from ephrin B2 luciferase constructs. HUVECs (8 × 103) in 24-well plates were transfected using Superfect with 0.8 μg/well ephrin B2 promoter constructs containing sequences from -2941 to -11 with respect to the translation start site, or 2 5′ deletions as indicated, together with 80 ng/well pCEFL or pvGPCR-CEFL. Luciferase was determined 48 hours after transfection. pGL3Basic is promoterless luciferase control vector. Luciferase was normalized to protein since GPCR induced expression of the cotransfected β-galactosidase. Graphed is induction ratio (vGPCR/pCEFL) of mean ± SEM of 6 replicates. Shown is 1 of 3 similar experiments.
Transcriptional regulation of ephrin B2 by HHV-8 vGPCR
Since we had shown that HHV-8 induced expression of ephrin B2 in HUVECs, we next asked if this could be mediated by a transcriptional effect. Ephrin B2 5′-flanking DNA-luciferase reporter plasmids were constructed as described in “Patients, materials, and methods” and transiently transfected into HUVECs. Ephrin B2 5′-flanking DNA sequences -2491/-11 have minimal activity in HUVECs, consistent with ephrin B2 being an arterial not venous marker. However, we have noted that HUVECs in culture do express some ephrin B2 RNA. Cotransfection of HHV-8 vGPCR induces ephrin B2 transcription approximately 10-fold above background compared with the control expression vector pCEFL (Figure 3D). Roughly equal induction was seen with ephrin B2 sequences -2491/-11, -1242/-11, or -577/-11, which indicates that elements between -577 and -11 are sufficient to mediate the response to vGPCR.
Expression of ephrin B2 is regulated by KS growth factors
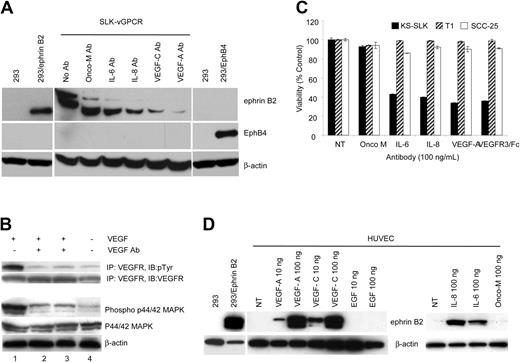
We next asked whether known KS growth factors could be involved in the vGPCR-mediated induction of ephrin B2 expression. KS-SLK-vGPCR stable transfectants were treated with neutralizing antibodies to oncostatin-M, IL-6, IL-8, VEGF, or VEGF-C for 36 hours. Neutralization of VEGF almost completely blocked expression of ephrin B2 in KS-SLK-vGPCR cells (Figure 4A). A lesser but significant decrease in ephrin B2 was seen with the IL-8, IL-6, and VEGF-C antibody treatments. No appreciable effect was seen with neutralization of oncostatin-M. The biochemical activity of the VEGF neutralizing antibody in these cells was verified by inhibition of VEGFR-2 and p44/42 MAPK phosphorylation (Figure 4B). The biologic activity of antibody treatment was illustrated by decreased cell viability in SLK cells but not in unrelated T1 fibroblasts or squamous cell carcinoma SCC-25 (Figure 4C). A soluble form of the VEGF-C receptor, VEGFR-3/Fc, was used to interfere with VEGF-C stimulation of the cells in lieu of the antibody, which is not neutralizing. VEGF, VEGF-C, IL-8, and IL-6 induced expression of ephrin B2 in HUVECs, whereas EGF and oncostatin-M did not (Figure 4D), thus providing additional evidence that these KS growth factors are integral to the induction of ephrin B2.
Ephrin B2 regulation by KS growth factors. (A) Inhibition of ephrin B2 in KS-SLK-vGPCR stable cell lines by neutralizing antibodies. Cells were cultured in full growth medium and exposed to antibodies as indicated in the figure (100 ng/mL) for 36 hours before collection and lysis for Western blot. Membranes were sequentially probed with antibody to ephrin B2, EphB4, and β-actin. Cell line 293 does not express ephrin B2 or EphB4 and serves as a negative control. Positive controls are 293 transiently transfected with either full-length ephrin B2 or EphB4 expression vectors (293/ephrin B2 and 293/EphB4, respectively). MAb indicates oncostatin M monoclonal antibody. (B) Immunoblots (IB) of protein extracts of KS-SLK cells were serum-starved for overnight and then treated with VEGF alone (25 ng/mL; lane 1), VEGF and VEGF antibody (50 ng/mL) premixed for 10 minutes and 20 minutes (lanes 2 and 3, respectively), or media alone (lane 4) for 20 minutes. (Top) Equal amounts of proteins from these samples were immunoprecipitated (IP) with VEGFR-2 antibody (50 ng/mL) on protein G sepharose and probed for antiphosphotyrosine monoclonal antibody (pTyr). The same blot was stripped and then probed for VEGFR-2. (Bottom) Lysates were also loaded directly and sequentially probed for p44/42 MAP kinase, phospho-p44/42 MAP kinase, and β-actin. (C) Cell viability assay in KS-SLK, T1 fibroblasts, and SCC-25 squamous cell carcinoma cells treated with neutralizing antibodies used in panel A. Note that VEGF-C antibody is not verified as neutralizing, instead VEGFR3/Fc chimera was used as a soluble decoy receptor to block VEGF-C binding to cells. Viability was measured by MTT after 48-hour exposure to antibodies or Fc fusion. Shown are means ± SEM of triplicate determinations. (D) Induction of ephrin B2 in HUVECs. Cells were cultured in EBM-2 (endothelial basal medium) supplemented with 5% FCS. This medium lacks the additional growth factors provided for EGM-2 (endothelial growth medium; see “Patients, materials, and methods” for details) in which HUVECs are routinely cultured. Individual growth factors were added as indicated and the cells harvested after 36 hours. Quantity of protein loading and transfer was determined by reprobing the membranes β-actin monoclonal antibody. NT indicates no treatment; and Onco-M, oncostatin-M.
Ephrin B2 regulation by KS growth factors. (A) Inhibition of ephrin B2 in KS-SLK-vGPCR stable cell lines by neutralizing antibodies. Cells were cultured in full growth medium and exposed to antibodies as indicated in the figure (100 ng/mL) for 36 hours before collection and lysis for Western blot. Membranes were sequentially probed with antibody to ephrin B2, EphB4, and β-actin. Cell line 293 does not express ephrin B2 or EphB4 and serves as a negative control. Positive controls are 293 transiently transfected with either full-length ephrin B2 or EphB4 expression vectors (293/ephrin B2 and 293/EphB4, respectively). MAb indicates oncostatin M monoclonal antibody. (B) Immunoblots (IB) of protein extracts of KS-SLK cells were serum-starved for overnight and then treated with VEGF alone (25 ng/mL; lane 1), VEGF and VEGF antibody (50 ng/mL) premixed for 10 minutes and 20 minutes (lanes 2 and 3, respectively), or media alone (lane 4) for 20 minutes. (Top) Equal amounts of proteins from these samples were immunoprecipitated (IP) with VEGFR-2 antibody (50 ng/mL) on protein G sepharose and probed for antiphosphotyrosine monoclonal antibody (pTyr). The same blot was stripped and then probed for VEGFR-2. (Bottom) Lysates were also loaded directly and sequentially probed for p44/42 MAP kinase, phospho-p44/42 MAP kinase, and β-actin. (C) Cell viability assay in KS-SLK, T1 fibroblasts, and SCC-25 squamous cell carcinoma cells treated with neutralizing antibodies used in panel A. Note that VEGF-C antibody is not verified as neutralizing, instead VEGFR3/Fc chimera was used as a soluble decoy receptor to block VEGF-C binding to cells. Viability was measured by MTT after 48-hour exposure to antibodies or Fc fusion. Shown are means ± SEM of triplicate determinations. (D) Induction of ephrin B2 in HUVECs. Cells were cultured in EBM-2 (endothelial basal medium) supplemented with 5% FCS. This medium lacks the additional growth factors provided for EGM-2 (endothelial growth medium; see “Patients, materials, and methods” for details) in which HUVECs are routinely cultured. Individual growth factors were added as indicated and the cells harvested after 36 hours. Quantity of protein loading and transfer was determined by reprobing the membranes β-actin monoclonal antibody. NT indicates no treatment; and Onco-M, oncostatin-M.
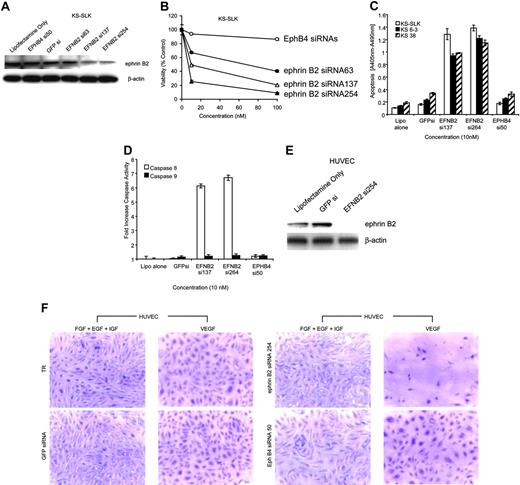
Ephrin B2 is necessary for KS and EC viability
Three ephrin B2 siRNAs were synthesized and transfected into KS-SLK cells. Forty-eight-hour treatment with ephrin B2 siRNAs 137 or 254 knocked down ephrin B2 protein by about 70% and inhibited KS-SLK cell viability, whereas ephrin B2 siRNA 63, EphB4 siRNA 50, or scrambled GFP siRNA had little or no effect (Figure 5A-B). Additionally, apoptosis was increased with respect to both mock-treated (Lipofectamine 2000 alone) and control siRNAs (GFPsi and EphB4 si50) with the 2 active ephrin B2 siRNAs (si137 and si254) in KS-SLK and 2 early passage KS cell isolates to a similar degree (Figure 5C). The mechanism of apoptosis was determined to be via the extrinsic or death-receptor-mediated pathway as shown by increase in activation of caspase 8. Under the same conditions, caspase 9 (mitochondrial or intrinsic pathway) was not activated (Figure 5D). KS-SLK cells express high levels of ephrin B2 and these results show that maintenance of ephrin B2 expression is integral to cell viability in this setting.
Ephrin B2 knockdown with specific siRNA inhibits viability in KS cells and HUVECs grown in the presence of VEGF but not IGF, EGF, or bFGF. (A) Ephrin B2 siRNAs block ephrin B2 expression in KS-SLK cells. Cells were transfected with various siRNA to ephrin (EFN) B2 and controls. After 48 hours the cells were harvested and crude cell lysates fractionated on 4% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blot was performed with monoclonal antibody to ephrin B2 generated in-house. The membrane was stripped and reprobed with β-actin monoclonal antibody (Sigma) to illustrate equivalent loading and transfer. (B) Three-day cell viability assay of KS-SLK cultures in the presence of ephrin B2 and EphB4 siRNAs. Cells (1 × 105/well) in 24-well plates were treated with 0, 10, and 100 ng/mL siRNAs as indicated on the graph. Viability of cultures was determined by MTT assay as described in “Patients, materials, and methods.” Shown are the mean ± standard deviation of duplicate samples. (C) ELISA of cytosolic nucleosomes 48 hours after transfection of cells with siRNA as indicated. KS 6-3 and KS 38 are early passage isolates generated in our laboratory from AIDS-KS patient biopsies. (D) Colorimetric assay of caspase 8 (extrinsic cell death) and caspase 9 (intrinsic cell death) activity in KS-SLK cells treated as in panel C. Means ± standard deviation of triplicate determinations are shown for both panels C and D. (E) Ephrin B2 siRNA blocks VEGF-mediated induction of ephrin B2 in HUVECs. HUVECs cultured in endothelial basal medium (EBM-2) supplemented with 5% FCS and 10 ng/mL VEGF were transfected with the indicated siRNAs. Cell lysates were prepared as in panel A and subjected to sequential Western blot with ephrin B2 and β-actin. (F) HUVECs were seeded on 8-well chamber slides coated with fibronectin. The HUVECs were grown overnight in endothelial growth medium (EGM-2), which contains all growth supplements (see “Patients, materials, and methods”). On the following day, the medium was replaced with EBM-2 supplemented with 5% FCS and either VEGF (10 ng/mL) alone or EGF, FGF, and IGF in combination as indicated. After 2 hours of incubation at 37°C, the cells were transfected using Lipofectamine 2000 (Invitrogen) in Opti-MEM medium containing 10 nM of siRNA to ephrin B2, EphB4, or green fluorescence protein (GFP) as control. The cells were returned to EBM-2 supplemented as described in “Patients, materials, and methods” with VEGF alone or EGF, FGF, and IGF in combination, following the 2-hour transfection in Opti-MEM-1. After a further 48 hours, the cells were stained with crystal violet. Digital images were captured immediately after staining using a Nikon Coolpix 5000 with microscope adaptor. Original magnification × 10. TR indicates transfection reagent.
Ephrin B2 knockdown with specific siRNA inhibits viability in KS cells and HUVECs grown in the presence of VEGF but not IGF, EGF, or bFGF. (A) Ephrin B2 siRNAs block ephrin B2 expression in KS-SLK cells. Cells were transfected with various siRNA to ephrin (EFN) B2 and controls. After 48 hours the cells were harvested and crude cell lysates fractionated on 4% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blot was performed with monoclonal antibody to ephrin B2 generated in-house. The membrane was stripped and reprobed with β-actin monoclonal antibody (Sigma) to illustrate equivalent loading and transfer. (B) Three-day cell viability assay of KS-SLK cultures in the presence of ephrin B2 and EphB4 siRNAs. Cells (1 × 105/well) in 24-well plates were treated with 0, 10, and 100 ng/mL siRNAs as indicated on the graph. Viability of cultures was determined by MTT assay as described in “Patients, materials, and methods.” Shown are the mean ± standard deviation of duplicate samples. (C) ELISA of cytosolic nucleosomes 48 hours after transfection of cells with siRNA as indicated. KS 6-3 and KS 38 are early passage isolates generated in our laboratory from AIDS-KS patient biopsies. (D) Colorimetric assay of caspase 8 (extrinsic cell death) and caspase 9 (intrinsic cell death) activity in KS-SLK cells treated as in panel C. Means ± standard deviation of triplicate determinations are shown for both panels C and D. (E) Ephrin B2 siRNA blocks VEGF-mediated induction of ephrin B2 in HUVECs. HUVECs cultured in endothelial basal medium (EBM-2) supplemented with 5% FCS and 10 ng/mL VEGF were transfected with the indicated siRNAs. Cell lysates were prepared as in panel A and subjected to sequential Western blot with ephrin B2 and β-actin. (F) HUVECs were seeded on 8-well chamber slides coated with fibronectin. The HUVECs were grown overnight in endothelial growth medium (EGM-2), which contains all growth supplements (see “Patients, materials, and methods”). On the following day, the medium was replaced with EBM-2 supplemented with 5% FCS and either VEGF (10 ng/mL) alone or EGF, FGF, and IGF in combination as indicated. After 2 hours of incubation at 37°C, the cells were transfected using Lipofectamine 2000 (Invitrogen) in Opti-MEM medium containing 10 nM of siRNA to ephrin B2, EphB4, or green fluorescence protein (GFP) as control. The cells were returned to EBM-2 supplemented as described in “Patients, materials, and methods” with VEGF alone or EGF, FGF, and IGF in combination, following the 2-hour transfection in Opti-MEM-1. After a further 48 hours, the cells were stained with crystal violet. Digital images were captured immediately after staining using a Nikon Coolpix 5000 with microscope adaptor. Original magnification × 10. TR indicates transfection reagent.
HUVECs do not express ephrin B2 (Figure 2A), except when stimulated by VEGF as shown in Figure 4B. Ephrin B2 siRNA 254 knocked down expression of ephrin B2 in VEGF-stimulated HUVECs (Figure 5E) and dramatically reduced the number of HUVECs cultured with VEGF as the sole growth factor (Figure 5F). In contrast, no appreciable effect was seen when HUVECs were cultured with a combination of IGF, EGF, and bFGF. As controls, EphB4 siRNA 50 and GFP siRNA had no detrimental effect on HUVECs in either culture condition.
Discussion
KS is a vascular proliferative disorder with tumor cells derived from endothelial cells. Recent identification of phenotypic markers that identify the arterial and venous endothelium prompted us to investigate their expression pattern in KS. Cell lines and primary tumor tissues expressed only the arterial endothelial marker ephrin B2. Expression of EphB4, which is the ephrin B2 cognate receptor and marker for venous endothelial cells, was notably lacking. It is known that ephrin B2 expression represses EphB4 and vice versa,19 therefore the observed findings are consistent with this reciprocal regulation and show this regulation is intact in KS cells. The arterial phenotype of KS may be broader than just ephrin B2 expression since KS cells also express CD148, which is normally expressed in arterial endothelial cells.31
The normal capillary network of the microvasculature is composed of equal compartments of arterial capillaries expressing ephrin B2 and ephB4-expressing venous capillaries, which form regular junctions with each other.10,32 Since KS lesions consist of proliferative microvasculature, one would expect that the distribution of ephrin B2- and EphB4-expressing cells would be nearly equal. Our finding of a preponderance of ephrin B2 expression is consistent with a clonal population of neoplastic KS cells, expressing ephrin B2 and certain other arterial markers, that has evaded the normal constraints of artery-vein demarcation in the capillary bed.
HHV-8 is associated with the development of KS. Nearly all KS cells in situ express latency proteins,33 and some cells express lytic-phase proteins.34 The role of HHV-8 in the induction of ephrin B2 was tested by infection of EphB4-positive venous endothelial cells. Ephrin B2 was indeed induced and EphB4 down-regulated in the clusters of cells showing viral infection. This supports the role of HHV-8 in regulation of ephrin B2 and suggests that the presence of HHV-8 in most KS tumor cells causes the overwhelming expression of ephrin B2 and lack of EphB4.
HHV-8 could induce expression of ephrin B2 and certain other artery-specific genes via one or more of the viral proteins. Two HHV-8 genes were assessed for their effect on ephrin B2 expression. LANA1 is a latency-phase protein present in nearly all KS cells in situ, which makes it a candidate for ephrin B2 induction. LANA1, however, failed to induce ephrin B2. The effect of vGPCR expression was tested due to its ability to transform cells and induce KS-like lesions when expressed in endothelial cells of the mouse.9 We found that ectopic expression of the lytic protein vGPCR in both stable and transient systems induced ephrin B2 expression.
The obvious question is whether constitutive expression of vGPCR is required in KS tumor cells to maintain ephrin B2-positive phenotype. Widespread expression of ephrin B2 in KS tumor tissue and the fact that vGPCR expression is limited to rare cells35 argues that it is not necessary. It is possible that vGPCR induces paracrine effects via regulation of cellular genes. However, induction of ephrin B2 by HHV-8 is likely not limited to vGPCR and would include the activities of other viral proteins. KS cell lines widely used for investigation of KS lack HHV-8, consistent with the observation that KS primary cultures lose HHV-8 within a few passages. Lack of HHV-8 and continued expression of ephrin B2 suggest that the phenotype becomes fixed and independent of continued need for the presence of HHV-8 and is maintained by clonal expansion of transformed cells.
The transient expression of the lytic cycle vGPCR transcript36 makes it unlikely that vGPCR directly transforms spindle cells in vivo. However, vGPCR up-regulates the expression of genes involved in KS pathogenesis, including growth-related oncogene α (GROα), IL-6, VEGF-C, and the vitamin D receptor (VDR) in the vGPCR-transfected classic KS cell line KS-SLK.37 In addition, IL-6, IL-8, RANTES (regulated on activation, normal T-expressed, and secreted), granulocyte-macrophage colony-stimulating factor (GM-CSF), and VEGF are up-regulated secondary to the regulation of nuclear factor-κB (NFκB) in KSIMM cells or HUVECs transfected with vGPCR.38 vGPCR is able to use multiple cellular pathways to alter endothelial cells; it enhances expression of VEGF as through p38 and MAPK stimulation of hypoxia-inducible factor 1α (HIF-1α)39 ; and promotes endothelial cell survival in a phosphatidylinositol 3 kinase (PI3K)/AKT-dependent manner.40 In addition, HHV-8 induces VEGFR-2 thus conferring increased sensitivity to VEGF.7,41 In the context of ephrin B2 expression, it is notable that vGPCR induces expression of VEGF and VEGFR-2, since VEGF is necessary for ephrin B2 expression and maintenance of the arterial phenotype.30,42 Consistent with this we show that VEGF is required for up-regulation of ephrin B2 in KS cells expressing vGPCR. However, we also document the contribution of other KS growth factors, notably VEGF-C, IL-8, and IL-6, to ephrin B2 expression. Neutralization of these endogenously expressed factors by antibodies or soluble receptors all decreased ephrin B2 expression and viability in KS-SLK cells expressing vGPCR. Conversely, VEGF, VEGF-C, IL-8, and IL-6 were all able to induce ephrin B2 expression in venous endothelial cells. These data support the indirect/paracrine effects of vGPCR in inducing ephrin B2 and provide the first demonstration of the involvement of VEGF-C, IL-8, and IL-6 in arterial marker gene expression. KS cell lines express high levels of VEGF, VEGF-C, IL-8, IL-6, and their cognate receptors. It is thus likely that the ephrin B2 phenotype is maintained via constitutive activation of these receptors. The role of other KS growth factors, such as tumor necrosis factor α (TNF-α), bFGF, and IL-1β, in altering ephrin B2 and EphB4 expression is under investigation.
In the context of HIV infection, the activation of VEGF receptors by HIV Tat protein43 would complement this phenotype. In addition, HIV induces several cytokines that are known to enhance VEGF expression, including IL-1β and TNF-α in selected cell types.44 HHV-8 and HIV are thus likely to induce the observed phenotype in AIDS-KS via viral and cellular proteins. Whether HIV Tat can itself induce ephrin B2 expression in our system and whether it potentiates the demonstrated effect of HHV-8 vGPCR is the subject of ongoing investigations.
We showed that ephrin B2 expression is necessary for survival of KS cells. Knock down of ephrin B2 expression by specific siRNA reduced KS cell viability. Further, ephrin B2 knockdown resulted in loss of endothelial cell viability when cultured in VEGF (conditions that induce the expression of ephrin B2) but not of the same cells cultured in the absence of VEGF and presence of IGF, EGF, and bFGF. This indicates that once the arterial phenotype is expressed, the continued presence of ephrin B2 is required for cell viability. This is the first demonstration of a direct requirement for the expression of ephrin B2 for cell viability. Ablation of ephrin B2 expression activated caspase 8 and led to apoptosis. Caspase 8 activation is indicative of cell death receptor-mediated apoptosis, which is initiated by the formation of a death-inducing signaling complex (DISC) at the cell membrane (reviewed in Curtin and Cotter45 ). Examples of death receptors are Fas/CD95/Apo1, TNF-R1, and TNF-related apoptosis-inducing ligand receptor 1 (TRAIL-R1). Notably, both Fas and ephrin B2 have been shown to bind the protein tyrosine phosphatase (PTP)-basophil/basophil-like (PTB-BL), which is a central cytoskeletal scaffolding protein (reviewed in Erdman46 ). This raises the intriguing possibility that high-level expression of ephrin B2 in KS inhibits the formation of Fas DISC hexamers through interaction with PTB-BL, thus tipping the balance away from apoptosis. By ablation of ephrin B2 in this setting, we may have swung the balance in favor of DISC formation and allowed apoptosis. This possibility is under formal investigation.
It is also known that reverse signaling from ephrin B2 stimulates migration and sprouting angiogenesis.21,22 This suggests that ephrin B2 expression in KS has functional consequences for KS pathology and further that ephrin B2 is a valid therapeutic target in this disease.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-03-0933.
Supported by research grant from National Cancer Institute 1R01 CA 79218 (P.S.G.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal