Abstract
Although widespread skeletal dissemination is a critical step in the progression of myeloma, little is known regarding mechanisms that control metastasis of this cancer. Heparanase-1 (heparanase), an enzyme that cleaves heparan sulfate chains, is expressed at high levels in some patients with myeloma and promotes metastasis of some tumor types (eg, breast, lymphoma). Using a severe combined immunodeficient (SCID) mouse model, we demonstrate that enhanced expression of heparanase by myeloma cells dramatically up-regulates their spontaneous metastasis to bone. This occurs from primary tumors growing subcutaneously and also from primary tumors established in bone. Interestingly, tumors formed by subcutaneous injection of cells metastasize not only to bone, but also to other sites including spleen, liver, and lung. In contrast, tumors formed by injection of cells directly into bone exhibit a restricted pattern of metastasis that includes dissemination of tumor to other bones but not to extramedullary sites. In addition, expression of heparanase by myeloma cells (1) accelerates the initial growth of the primary tumor, (2) increases whole-body tumor burden as compared with controls, and (3) enhances both the number and size of microvessels within the primary tumor. These studies describe a novel experimental animal model for examining the spontaneous metastasis of bone-homing tumors and indicate that heparanase is a critical determinant of myeloma dissemination and growth in vivo.
Introduction
Although the study of enzymes that cleave heparan sulfate is in its infancy, recent findings imply that heparanases play a critical role in promoting tumor growth and metastasis. In humans, heparanase-1 (heparanase) appears to be the dominant, if not the only, heparanase that can cleave extracellular heparan sulfate.1-4 Although other heparanases are likely present in many cells, their activity appears to be localized to intracellular compartments.5,6 Heparanase expression is rare in normal tissues, but becomes evident in many human tumors where it significantly increases both the angiogenic and metastatic potential of tumor cells.7 Elevated heparanase expression in humans has been correlated with advanced progression and metastasis of tumors of the breast,8 colon,9 ovary,10 bladder,11 pancreas,12 and acute myeloid leukemia.13 High levels of heparanase are associated with a shorter postoperative survival time compared with cancer patients with low levels of heparanase.12,14 However, heparanase is not universally expressed by tumors. Reverse transcription–polymerase chain reaction (RT-PCR) failed to detect heparanase in tumor cells of 33 patients with chronic lymphoblastic leukemia or of 7 patients with non-Hodgkin lymphoma, and the enzyme was rarely detected in tumor cells of chronic myeloid leukemia.13 In experimental models, pancreatic cells engineered to express heparanase exhibit increased invasiveness in Boyden chamber assays as compared with controls,12 and in mice heparanase promotes metastasis of melanoma and T-lymphoma cells.1,15,16
The mechanism of heparanase function in tumors is under intense investigation, and it appears that this enzyme may have multiple effects. For example, degradation of heparan sulfate likely facilitates tumor cell motility and budding of new blood vessels, an event that requires remodeling of the heparan sulfate–rich basement membrane. Heparan sulfate proteoglycans within the tumor microenvironment can serve as a reservoir of heparin-binding growth factors and chemokines, and the activity of heparanase may act to release these factors for use by tumor cells. Moreover, it is important to note that active heparanase does not completely digest the heparan sulfate chains it attacks; rather, it selectively cleaves the glycosidic bonds of heparan sulfate chains at only a few specific sites, producing fragments that are only 10 to 20 sugar residues long.17,18 These fragments generated by heparanase, or by enzymes that cleave heparan sulfate in a manner similar to that of heparanase, have been shown to enhance the ability of heparan sulfate to potentiate the activity of bound growth factors.18,19 Thus, degradation of heparan sulfate surrounding the tumor may remove physical barriers and enhance signaling events that spark metastasis.
The mechanisms regulating tumor cell metastasis to bone are poorly understood and a role for heparanase in bone metastasis has not been previously defined. We recently discovered that heparanase is expressed by myeloma tumor cells and that high levels of heparanase activity are present in the bone marrow plasma of some patients.20 The presence of heparanase in myeloma may be particularly important because these tumor cells express high levels of the heparan sulfate proteoglycan syndecan-1 on their cell surfaces and within the bone marrow stroma.21 Moreover, syndecan-1 accumulates in the sera of patients with myeloma, and high levels of circulating syndecan-1 are an indicator of poor prognosis.22,23 Thus, the cleavage of syndecan-1 heparan sulfate by heparanase in myeloma may provide a rich source of biologically active fragments of heparan sulfate that enhance tumor growth and metastasis. In the present work, experiments were performed to determine the direct effects of heparanase expression on myeloma tumor behavior in vivo. We discovered that heparanase has a dramatic impact on myeloma cells by accelerating their growth and promoting their metastasis to bone. Furthermore, we demonstrate that expression of heparanase enhances both the number and size of blood vessels within the tumor, thereby providing a potential explanation for the tumor-promoting effects of this enzyme.
Patients, materials, and methods
Cells and transfections
CAG cells were established from a bone marrow aspirate of a patient with myeloma at our center as previously described.24 Heparanase cDNA (HPSE1)2 was subcloned in the sense direction into pIRES2-EGFP vector (Clontech, Palo Alto, CA), which allowed both the heparanase gene and the enhanced green fluorescent protein (EGFP) to be translated from a single bicistronic mRNA. The pIRES2-EGFP/HPSE1 construct (10 μg DNA), or as a control the pIRES2-EGFP vector only, was transfected into the CAG cells using Lipofectin (Invitrogen, Carlsbad, CA) and Opti-MEM I (Gibco-BRL, Rockville, MD), per the manufacturer's instructions. Once cell numbers reached 2.0 × 107, both the heparanase transfectants and control transfectants were sorted by flow cytometry using EGFP expression. Following a minimum of 3 sorts, experiments were performed using populations of cells that had a minimum of 60% EGFP-positive cells.
For growth assays, the 3[4,5-dimethylthiazol-2-y]-2,5-diphenyltetrazolium (MTT) assay was performed as previously described.25 Apoptosis was determined using a FragEL DNA Fragmentation Detection Kit (Calbiochem, San Diego, CA) on cytospin slides.
Protein isolation
Cells in culture (1 × 106) were pelleted by centrifugation, washed with 2 mL phosphate-buffered saline (PBS), then resuspended in 200 μL cold lysis buffer (50 mM Tris; pH 7.5, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 0.5% Triton X-100, 0.2 mM phenylmethylsulfonic acid, 10 μg/mL leupeptin) and incubated on ice for 30 minutes. Lysates were centrifuged at 13 000 g, 4°C for 20 minutes, and supernatants frozen.
Tumor tissues harvested from mice were homogenized in 15-mL centrifuge tubes containing cold lysis buffer (1 mL/100 mg tissue weight) using a polytron. After a 30-minute incubation on ice, lysates were centrifuged at 11 000 g for 20 minutes and supernatants frozen.
Western blot analysis
Proteins isolated from cell lines and tumor tissues were quantified by a BCA Protein Assay Reagent Kit (Pierce, Rockford, IL). For heparanase detection, equal amounts of protein (50 μg/lane) were loaded onto 4% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane and probed with affinity-purified rabbit antibody against heparanase26 and a horseradish peroxidase–conjugated donkey anti-rabbit immunoglobulin G (IgG; Nitrocellulose; Schlercher & Schuell, Keene, NH). For syndecan-1 detection, samples were run on 4% SDS-PAGE, transferred to a positively charged nylon membrane (NytranSPC; Schlercher & Schuell) and probed with mouse anti–human syndecan-1 (antibody B-B4; Serotec, Raleigh, NC) and a horseradish peroxidase–conjugated secondary antibody. Immunoreactive bands were detected using chemiluminescence (ECL; Amersham Biosciences, Piscat-away, NJ). Initially, gels for Western blotting of syndecan-1 were loaded with equal amounts of protein, but signals were not easily visible in tumors formed by the heparanase-transfected cells. In subsequent gels, 4 times higher protein levels were loaded in samples from heparanase-transfected cells than from controls to generate a visible signal. The necessity for loading higher levels is likely due to the fact that tumors from heparanase-transfected cells have a lower heparan sulfate content (and thus a lower total charge density per unit of core protein) and therefore do not stick to the cationic filter as well as the syndecan that was not degraded by heparanase.
Mouse models and their analysis
For the subcutaneous model, neocontrols or heparanase-transfected cells (1.0 × 106) were suspended in 100 μL PBS and injected subcutaneously into the left flanks of severe combined immunodeficient (SCID) mice. For the tibial injection model, SCID mice were deeply anesthetized with a 1:1:4.6 solution of xylazine/ketamine/PBS (administered intraperitoneally at 0.033-mL mixture/10 g body weight). The mice were placed in a prone position and 10 μL (20 000 cells) was injected slowly into the right tibia using a 50 μL syringe and 28-gauge needle.
The levels of human immunoglobulin kappa light chain were determined by enzyme-linked immunosorbant assay (ELISA) as previously described.27 All samples were analyzed in duplicate simultaneously to preclude interassay variability. The standard curve was linear, between 0.35 ng/mL and 300 ng/mL, and samples were diluted to concentrations within this range.
For histology, tissues were fixed in 10% formalin (pH 7.0), and embedded in paraffin. Bones were decalcified with 10% EDTA prior to embedding. For immunohistochemistry, antigen retrieval on slides was performed using citrate buffer solution (pH 6.0; Zymed, San Francisco, CA) in a steamer for 20 minutes, followed by blocking of endogenous peroxide with 3% hydrogen peroxide for 15 minutes. After blocking for 20 minutes with normal serum, primary antibodies (either mouse monoclonal antibody against heparanase2 or biotinylated anti–human kappa light chain) were applied, followed by the appropriate biotinylated secondary antibody for anti-heparanase. Vector ABC solution (Vector Laboratories, Burlingame, CA) was added to enhance sensitivity of detection followed by DAB solution (3,3′-diaminobenzidine). All microscopy was performed using an Olympus BH2 microscope (Olympus Optical, Tokyo, Japan) equipped with a Splan 20PL 20 ×/0.46 objective lens (Olympus Optical). Digital images were captured with a SPOT 1.3.0 camera and SPOT RT software v. 2.2.1 (Diagnostic Instruments, Sterling Heights, MI).
To detect osteoclasts, tartarate-resistant acid phosphatase (TRAP) staining was performed on sections of bone using an acid phosphatase kit (Sigma, St Louis, MO) followed by Harris hematoxylin as a nuclear counterstain.
Vascular count and vascular area measurement were made using histomorphometry software (Osteomeasure, Atlanta, GA). Sections of tumor tissue were carefully scanned at low power (×40 and ×100) to identify areas with the highest number of CD34-stained microvessels (hot spots). After regions of highest vascularization were identified, the microvessel count in a sufficiently extended field (×200; field size, 0.378 mm2) was measured (average of 5 fields per tumor section). The number of vessels with an area more than 0.0001 mm2 per field was also enumerated.
Results
Elevated expression of heparanase promotes enhanced growth of myeloma cells in vivo
Initial studies with CAG myeloma cells were undertaken to examine their behavior following injection into the lateral tail-vein of SCID mice. At 5 to 6 weeks after injection of wild-type CAG cells, 10 of 11 mice exhibited human kappa light chain in the circulation (mean kappa level = 5.73 μg/mL ± 0.91 μg/mL), and 9 of 11 developed hind limb paralysis (not shown). Upon termination of the experiment, all 10 of the mice that were kappa light chain–positive had detectable tumor cells within the bone marrow as determined by CD45/CD38 flow cytometry analysis (mean percentage marrow plasmacytosis = 27.2 ± 6.8 standard error of the mean [SEM]). Macroscopic tumors outside of the bone marrow were not detected on visual inspection at necropsy, suggesting that the CAG cells, like most myeloma cell lines that are injected intravenously, home to and grow within the bone marrow.
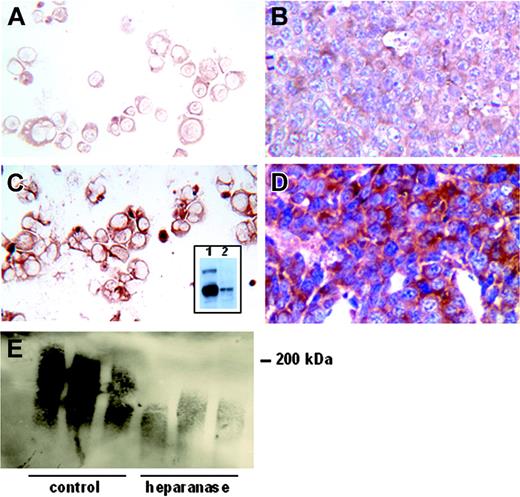
Although intravenous injection of the tumor cells indicates that these cells, once in circulation, have the capacity to colonize within bone, these experiments do not examine the capability of tumor cells to escape from their primary site of growth, enter the circulation, and metastasize throughout the skeleton (spontaneously metastasize). Because widespread dissemination of myeloma cells is an important feature of this disease, we sought a model in which to examine this metastatic cascade. In preliminary experiments we found that CAG cells would grow when injected subcutaneously in SCID mice, but would not readily metastasize (not shown). Because heparanase has been shown to promote metastasis in some tumors,17 and we have shown that some patients with myeloma express relatively high levels of heparanase,20 we studied the behavior of CAG cells following their transfection with a cDNA for human heparanase. Cells transfected with heparanase express high levels of the active 50-kDa form of the enzyme (Figure 1C, inset), and we have previously demonstrated that they exhibit a 4-fold increase in the level of heparanase enzyme activity as compared with control transfectants.20 Immunohistochemistry confirms that CAG cells transfected with heparanase express high levels of the heparanase protein as compared with controls (Figure 1A,C). Moreover, this high level of enzyme expression is retained in primary tumors that form following subcutaneous injection of the cells into SCID mice (Figure 1B,D). Heparanase activity within the growing tumors was confirmed by Western blots showing a reduction in the size of syndecan-1 extracted from the tumors bearing heparanase as compared with control tumors (Figure 1E). This is an important finding because it indicates that the amount of heparan sulfate on the tumor cells is dramatically reduced by the expression of heparanase, and that fragments of the cleaved heparan sulfate are likely released into the tumor microenvironment.
CAG myeloma cells transfected with the cDNA for human heparanase express high levels of the enzyme when growing either in vitro or in vivo. Neotransfected cells exhibit a low level of immunostaining for heparanase in vitro (A, cytospins) or in vivo (B, tissue section of a primary tumor). In contrast, heparanase-transfected cells stain intensely for heparanase in vitro (C, cytospin) and in vivo (D, primary tumor). (Original magnification, × 200.) The inset in panel C is a Western blot of extracts from CAG cells growing in culture. The presence of a low level of active heparanase (50 kDa) in the control-transfected cells (lane 2) and a high level of the active heparanase at 50 kDa in the heparanase-transfected cells (lane 1, bottom band) is apparent. A low level of the inactive form of the enzyme (65 kDa) is also present in the heparanase-transfected cells. Western blotting of syndecan-1 extracted from subcutaneous tumors demonstrates that the proteoglycan is dramatically reduced in size in the presence of elevated heparanase (E). Shown are the syndecans present in tumors from 3 mice bearing control-transfected cells and from 3 mice bearing heparanase-transfected cells (heparanase).
CAG myeloma cells transfected with the cDNA for human heparanase express high levels of the enzyme when growing either in vitro or in vivo. Neotransfected cells exhibit a low level of immunostaining for heparanase in vitro (A, cytospins) or in vivo (B, tissue section of a primary tumor). In contrast, heparanase-transfected cells stain intensely for heparanase in vitro (C, cytospin) and in vivo (D, primary tumor). (Original magnification, × 200.) The inset in panel C is a Western blot of extracts from CAG cells growing in culture. The presence of a low level of active heparanase (50 kDa) in the control-transfected cells (lane 2) and a high level of the active heparanase at 50 kDa in the heparanase-transfected cells (lane 1, bottom band) is apparent. A low level of the inactive form of the enzyme (65 kDa) is also present in the heparanase-transfected cells. Western blotting of syndecan-1 extracted from subcutaneous tumors demonstrates that the proteoglycan is dramatically reduced in size in the presence of elevated heparanase (E). Shown are the syndecans present in tumors from 3 mice bearing control-transfected cells and from 3 mice bearing heparanase-transfected cells (heparanase).
Importantly, although the transfected CAG cells express high levels of heparanase, the levels expressed do not appear to be substantially different from those found in human myeloma patients. The level of heparanase detected by immunohistochemistry in the primary tumors formed by CAG cells (Figure 1) is similar to that seen in tumor cells in the bone marrow of patients with myeloma.20 Moreover, levels of heparanase enzyme activity of the heparanase-transfected CAG cells are in the same range as heparanase activity measured from the serum harvested from the marrow of patients with myeloma (data not shown). Thus, the levels of heparanase in the transfected CAG cells mimics what is seen in some patients with myeloma.
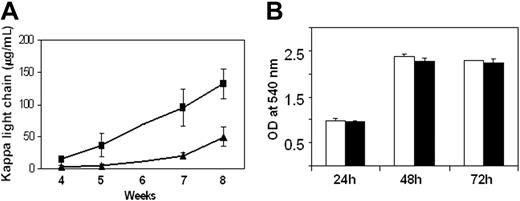
Following subcutaneous injection of heparanase-transfected or control cells into SCID mice, we noticed that the cells with elevated heparanase expression formed palpable tumors more rapidly than did control cells. Visual inspection of the subcutaneous tumors also revealed that in early stages of primary tumor growth, those tumors formed by heparanase-transfected cells were growing faster than controls (not shown). To quantify this, sera were collected from mice at intervals throughout the experiment and analyzed for the presence of human immunoglobulin kappa light chain as a measure of whole animal tumor burden. By week 5, tumors formed by heparanase transfectants clearly show enhanced growth as compared with controls (Figure 2A). By week 8, this growth difference is substantial and highly statistically significant. To determine whether enhanced tumor growth was due to an intrinsic ability of the heparanase-transfected cells to grow faster, we compared their growth in vitro. In contrast to what was observed in vivo, heparanase-transfected and control cells in culture grew at nearly identical rates (Figure 2B). In addition, the rate of apoptosis as measured by DNA fragmentation was nearly identical for the heparanase-transfected and control cells (3.8% and 4.0%, respectively, at 72 hours after plating cells). This indicates that rather than there being an intrinsic difference in growth or death rates of the transfected and control cells, interactions between the tumor cells and the host are likely responsible for the enhanced growth in vivo of the heparanase-transfected cells.
Heparanase expression promotes tumor growth in vivo. (A) Following injection of heparanase-transfected (▪) or control-transfected (▴) CAG myeloma cells into SCID mice, sera were harvested at specific intervals and analyzed for the level of human immunoglobulin kappa light chain as a measure of tumor burden (n = 10 for heparanase-transfected, n = 7 for control-transfected; P < .002 at week 8). Values represent the mean plus or minus SD of each group of animals as derived from duplicate samples from each animal within the group. Sera were not collected at week 6. (B) Heparanase-transfected (▪) and control-transfected (□) cells exhibit identical growth rates in vitro. Cells were plated at equal cell numbers and analyzed by MTT assay at 24, 48, and 72 hours after plating. Values represent means of duplicate samples plus or minus SD. OD indicates optical density.
Heparanase expression promotes tumor growth in vivo. (A) Following injection of heparanase-transfected (▪) or control-transfected (▴) CAG myeloma cells into SCID mice, sera were harvested at specific intervals and analyzed for the level of human immunoglobulin kappa light chain as a measure of tumor burden (n = 10 for heparanase-transfected, n = 7 for control-transfected; P < .002 at week 8). Values represent the mean plus or minus SD of each group of animals as derived from duplicate samples from each animal within the group. Sera were not collected at week 6. (B) Heparanase-transfected (▪) and control-transfected (□) cells exhibit identical growth rates in vitro. Cells were plated at equal cell numbers and analyzed by MTT assay at 24, 48, and 72 hours after plating. Values represent means of duplicate samples plus or minus SD. OD indicates optical density.
Elevated expression of heparanase promotes spontaneous metastasis of myeloma cells to bone
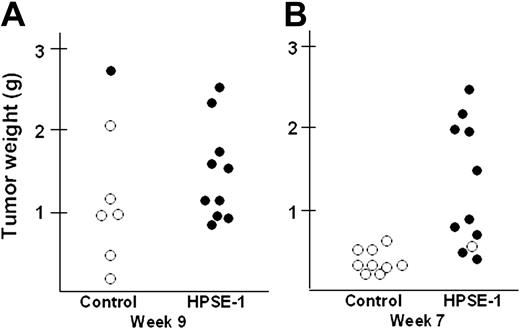
Interestingly, although the primary tumors formed by the heparanase-transfected cells were palpable earlier and seemed to grow faster initially than controls, following growth for 9 weeks the tumors from the 2 groups appeared, upon visual inspection, to be approximately the same size (not shown). This observation was confirmed following excision and weighing of the primary tumors. The mean weight of primary tumors formed from heparanase-transfected cells (n = 10) was 1.50 g ± 0.18 g; of those from control-transfected cells (n = 7), 1.24 g ± 0.34 g (P = .522). Because the whole animal tumor burden of the heparanase primary tumors was twice that of controls (based on sera kappa levels shown in Figure 2A), this indicated the presence of occult tumors within the mice bearing the heparanase-transfected cells.
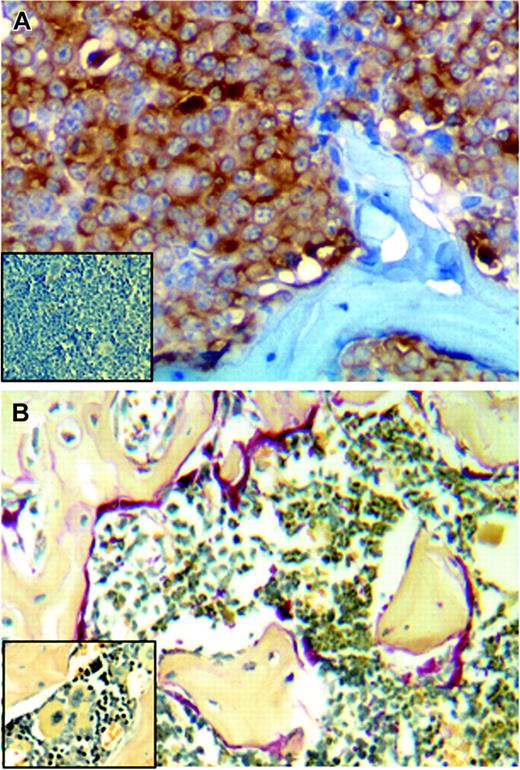
To determine whether the tumor cells had metastasized to bone, the preferred site of myeloma tumor growth in humans, following sacrifice of the animals the femurs from the mice were fixed, decalcified, and stained for human kappa light chain. Extensive tumor infiltration within the femur was detected in animals injected subcutaneously with heparanase-transfected cells (Figure 3A), and staining with TRAP reveals extensive osteoclast activity at the bone surface, thus mimicking the natural history of myeloma in humans (Figure 3B). Figure 4A shows a plot of the weight of the primary tumor from each animal and whether or not the tumor was metastatic to bone (open circle = nonmetastatic to bone; filled circle = metastatic to bone). All of the animals bearing heparanase-transfected tumor cells had metastases within the bone, whereas only one of the 7 control animals had detectable metastases within bone. Interestingly, metastasis of the tumor to bone does not appear to be dependent on the size of the primary tumor, because 5 of the primary tumors from control animals were as large as those from the heparanase-transfected animals, yet only 1 of the 5 exhibited metastasis to the bone (Figure 4A). These results suggest that, in this model, metastasis may be an early event that is not associated with tumor size. To examine this, the in vivo protocol was repeated and animals were killed after only 7 weeks following injection of tumor cells. Upon sacrifice, 10 of 11 animals bearing heparanase-transfected cells had tumor in the bone, whereas none of the 9 control animals had metastasis to bone (Figure 4B). At 7 weeks, 6 of the heparanase-bearing tumors were under one gram in weight and growing at a rate similar to that of the tumors in control animals. However, 5 of those animals with heparanase-bearing tumors had detectable bone marrow metastases. This supports the idea that heparanase-transfected cells are metastatic well before tumors grow to a large size, and that the difference in metastatic growth of heparanase cells versus controls is not simply a function of the rate of tumor growth.
Tumors formed by heparanase-transfected cells metastasize to bone and enhance osteoclastogenesis. (A) Immunostaining for human kappa light chain (brown) detects CAG tumor cells present in the femur of an animal injected subcutaneously with heparanase-transfected cells. (Inset) Cells stained for kappa light chain are not detected in the femur taken from a mouse injected subcutaneously with the control-transfected CAG cells. (B) Extensive osteoclast activity is detected by TRAP staining (red) in femurs where heparanase-transfected CAG cells have metastasized. (Inset) Little osteoclast activity is detected in control animals that lack bone metastases. (Original magnification, × 200.)
Tumors formed by heparanase-transfected cells metastasize to bone and enhance osteoclastogenesis. (A) Immunostaining for human kappa light chain (brown) detects CAG tumor cells present in the femur of an animal injected subcutaneously with heparanase-transfected cells. (Inset) Cells stained for kappa light chain are not detected in the femur taken from a mouse injected subcutaneously with the control-transfected CAG cells. (B) Extensive osteoclast activity is detected by TRAP staining (red) in femurs where heparanase-transfected CAG cells have metastasized. (Inset) Little osteoclast activity is detected in control animals that lack bone metastases. (Original magnification, × 200.)
Heparanase promotes growth and metastasis of myeloma tumor cells in vivo. Control-transfected (Control) or heparanase-transfected cells (HPSE-1) were injected at a single site subcutaneously in SCID mice. At 9 weeks (A) or 7 weeks (B) after injection, animals were euthanized, the primary tumors harvested and weighed, and the femurs analyzed for metastasis by immunohistochemistry as shown in Figure 3A. Plots in panels A and B show the wet weight of the primary tumor from each mouse and whether or not that mouse had detectable metastases in the femur (○ = nonmetastatic; • = metastatic).
Heparanase promotes growth and metastasis of myeloma tumor cells in vivo. Control-transfected (Control) or heparanase-transfected cells (HPSE-1) were injected at a single site subcutaneously in SCID mice. At 9 weeks (A) or 7 weeks (B) after injection, animals were euthanized, the primary tumors harvested and weighed, and the femurs analyzed for metastasis by immunohistochemistry as shown in Figure 3A. Plots in panels A and B show the wet weight of the primary tumor from each mouse and whether or not that mouse had detectable metastases in the femur (○ = nonmetastatic; • = metastatic).
At necropsy, animals from the 9-week experiment were analyzed to determine whether bone was the sole site of tumor metastases. Macroscopically, the only obvious metastasis was within the intestinal mesentery of 70% of the animals bearing tumors of heparanase-transfected cells. Microscopic examination of tissue sections revealed that these mesenteric tumors were predominantly within the lymph nodes draining the subcutaneous tumor. Heparanase-bearing tumors also had micrometastases within the liver (4 of 9 animals), spleen (5 of 9 animals), and lung (4 of 9 animals), but not in the kidney. In contrast, none of the animals bearing tumor from control-transfected cells had detectable macroscopic or microscopic evidence of metastasis.
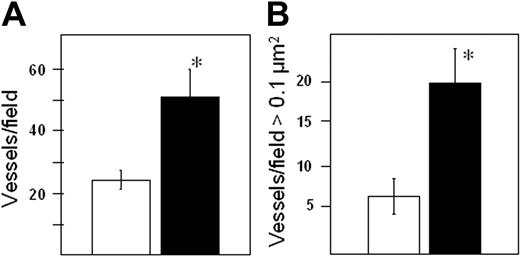
Expression of heparanase enhances microvessel density in myeloma tumors
Using biopsies from patients with myeloma, we previously demonstrated that high heparanase activity in the bone marrow is associated with enhanced tumor microvessel density.20 Limited studies also indicated that transfection of myeloma cells with heparanase promoted enhanced microvessel density. To further evaluate this, following 7 weeks of tumor growth in vivo, the number of vessels in the primary tumors of animals bearing either heparanase-transfected or control-transfected cells was determined. Results indicate a significantly higher average number of vessels per field in the heparanase-transfected tumors as compared with controls (Figure 5A). Furthermore, upon visual inspection of the stained tissue sections, we noted the tumors of heparanase-transfected cells had vessels that appeared to be larger on average than vessels in tumors from control animals. Analysis of the average area per vessel confirmed this observation (average area per vessel in heparanase transfectants = 0.125/μm2 ± 0.015; controls = 0.083 μm2 ± 0.013; P = .026). But more striking is the observation that tumors formed from heparanase-transfected cells have more than 3 times the number of vessels of a size more than 0.1 μm2 as compared with control tumors (Figure 5B). Importantly, the differences in vascularity between the control and heparanase-expressing tumors are not due to differences in tumor size, because when the vascularity of only those tumors smaller than one gram are compared, the quantitative differences between microvessel density and vessel size are still apparent (mean microvessel density of heparanase-expressing and control tumors less than one gram = 49.75 vs 23.75, respectively; mean number of vessels > 0.1 μm2 of heparanase-expressing and control tumors = 22.5 vs 6.50, respectively). These results indicate that elevated levels of heparanase enhance both the number and size of blood vessels within the myeloma tumor independently of tumor size.
Heparanase expression enhances both the number and size of tumor blood vessels. Seven weeks after subcutaneous injection of tumor cells, tissue sections from primary tumors were stained with antibody to CD34. Both vessel density (A) and the number of large microvessels (B) are significantly higher in tumors expressing high levels of heparanase (▪) as compared with controls (□). *P = .0009 for vessel density and P = .0005 for vessel size. Vessel size is plotted as the number of vessels per field greater than 0.1 μm2. Error bars represent the mean plus or minus SD.
Heparanase expression enhances both the number and size of tumor blood vessels. Seven weeks after subcutaneous injection of tumor cells, tissue sections from primary tumors were stained with antibody to CD34. Both vessel density (A) and the number of large microvessels (B) are significantly higher in tumors expressing high levels of heparanase (▪) as compared with controls (□). *P = .0009 for vessel density and P = .0005 for vessel size. Vessel size is plotted as the number of vessels per field greater than 0.1 μm2. Error bars represent the mean plus or minus SD.
Expression of heparanase promotes growth within bone and bone-to-bone metastasis
Subcutaneous injection of CAG myeloma cells is a convenient model because it is easy to monitor and quantify growth of the primary tumor as related to metastases. However, because the primary site of myeloma is presumably within the bone, we performed further studies to assess the role of heparanase in myeloma by injecting tumor cells directly into the tibia of SCID mice. Six weeks after injection of the cells, animals were euthanized, sera were collected, and the femurs contralateral to the injected tibia were harvested. Fluorescence imaging to detect GFP within the injected tibias indicates that tumors were established in the tibias of all animals including those injected with control-transfected cells (not shown). Analysis of kappa light chain levels indicates that the overall tumor burden was greater in animals bearing heparanase-transfected cells than in controls (Figure 6). Metastasis to contralateral legs was greatly enhanced by heparanase expression. All animals injected with heparanase-transfected cells had metastatic tumor cells in the contralateral femur (10 of 10 animals) as compared with controls, where only 2 of 7 animals exhibited metastases (Figure 6). In 2 of the animals bearing heparanase-transfected cell tumors, metastasis occurred despite their systemic kappa levels being relatively low (approximately 1 μg/mL), supporting the idea that heparanase can have an important effect on tumor behavior at early stages of tumor growth. These results indicate that elevated heparanase has a positive impact on both growth and metastasis of myeloma tumors growing within the bone microenvironment and that primary tumor size is not a determinant of metastatic capacity.
Heparanase promotes bone-to-bone metastasis. Six weeks after injection of heparanase-transfected or control-transfected CAG myeloma cells into the tibia of SCID mice, sera were harvested and analyzed for the level of human immunoglobulin kappa light chain as a measure of tumor burden. Plots show the kappa light chain level for each individual mouse. The mean kappa chain level for the heparanase group was significantly higher than that of the control group (heparanase group, 6.13 μg/mL ± 1.33 μg/mL, control group 2.36 μg/mL ± 1.02 μg/mL [P = .04]), although several animals within the heparanase group had low tumor burden. Values are means plus or minus SEM. Metastases of tumor to distant bone were assessed by immunostaining for the presence of kappa light chain in the femur contralateral to the injected tibia. • denotes animals with bone metastases; ○, those without detectable bone metastases.
Heparanase promotes bone-to-bone metastasis. Six weeks after injection of heparanase-transfected or control-transfected CAG myeloma cells into the tibia of SCID mice, sera were harvested and analyzed for the level of human immunoglobulin kappa light chain as a measure of tumor burden. Plots show the kappa light chain level for each individual mouse. The mean kappa chain level for the heparanase group was significantly higher than that of the control group (heparanase group, 6.13 μg/mL ± 1.33 μg/mL, control group 2.36 μg/mL ± 1.02 μg/mL [P = .04]), although several animals within the heparanase group had low tumor burden. Values are means plus or minus SEM. Metastases of tumor to distant bone were assessed by immunostaining for the presence of kappa light chain in the femur contralateral to the injected tibia. • denotes animals with bone metastases; ○, those without detectable bone metastases.
Histologic analysis of lung, liver, spleen, and kidney revealed no metastases were present in these organs in either the animals bearing tumors formed from control-transfected, or those with tumors formed from heparanase-transfected, cells. This is in contrast to what was found in the animals following subcutaneous injection, where dissemination was widespread. Thus, once within bone, the metastasis promoted by heparanase expression appears to be selective for bone. This is similar to the metastatic pattern seen in most human myeloma patients.
Discussion
The present study demonstrates that heparanase can have a profound influence on the metastasis of myeloma tumors, resulting in high tumor burden. In 3 separate experiments that included 2 different sites of tumor cell injection (subcutaneous or intratibial), 30 of 31 mice having tumors that expressed elevated levels of heparanase metastasized to bone. In contrast, control cells that exhibit low levels of heparanase activity were poorly metastatic to bone over the time course of these experiments, with only 3 of 23 mice having detectable metastases within bone, despite having tumors of similar size at the primary site. Given that we have previously demonstrated that heparanase activity is detected in the plasma isolated from the bone marrow of most patients with myeloma,20 and that we now show that heparanase enhances both growth and metastasis of myeloma tumor cells, it is possible that this enzyme plays a key role in determining the progression of this cancer.
Previous studies on myeloma metastases have used models in which tumor cells are either injected intravenously or directly into bone.28-30 Following intravenous injection, as with most cell lines, we find that CAG cells home to bone and preferentially grow within the bone marrow. However, this model does not address the initial events of metastasis, in which the tumor cell must exit the primary tumor and enter the vasculature. By using the subcutaneous injection model we demonstrate that in the absence of elevated heparanase expression, tumors fail to readily metastasize to bone (Figure 4). However, when these cells are engineered to express heparanase, they readily metastasize to bone. This suggests that the tumor cells lack the capacity to escape from the primary tumor site. It is possible that heparanase plays an important role in early events of myeloma metastasis, events that include tumor cell migration and intravasation into the circulation. This is consistent with previous work demonstrating a direct effect of heparanase on tumor cell invasion in vitro.12,31 Another possibility is that heparanase enhances metastasis by promoting survival of tumor cells at the metastatic site. The precise role of heparanase in promoting metastasis to bone awaits further investigation.
Interestingly, the expression of heparanase by the CAG cells does not have an impact on tumor growth in vitro (Figure 2B). This suggests that the accelerated growth of the heparanase-transfected cells in vivo is due to effects of heparanase on the tumor microenvironment rather than a direct effect on the tumor cells themselves. Growth of primary myeloma tumor cells in vivo is highly dependent on interactions with the host marrow stroma,28,32 and heparanase may be modulating these interactions.
The direct injection of tumor cells into the mouse tibia reveals several important features of these tumor cells and heparanase. First, it demonstrates that the expression of heparanase provides a growth advantage to myeloma cells within the bone marrow microenvironment (Figure 6). Thus, heparanase enriches the microenvironment, possibly by regulating the availability of heparan sulfate–bound growth factors. Second, the fact that control cells grew in all of the tibias injected indicates that the bone can support CAG myeloma cell growth in the absence of enhanced heparanase expression. This is an important observation because it indicates that in the subcutaneous injection model, where metastasis of control cells was poor (only one metastasis detected in a total of 16 control animals), the poor metastasis was not due to the inability of CAG cells to survive within the bone marrow. Rather, it suggests that heparanase plays a role in ensuring either intravasation into the vasculature or extravasation from the vasculature, or both. Third, we demonstrate with the tibia injection model that when a myeloma tumor arises within bone, the expression of heparanase promotes its metastasis to other bones. Thus, heparanase expression is implicated as a critical component of the events that lead to seeding of myeloma tumors throughout the skeleton.
Following metastasis of tumor to bone, we observed enhanced osteoclastogenesis (Figure 3B). This is consistent with observations in patients with myeloma, where bone destruction is an important manifestation of the disease.33,34 Interestingly, it was recently shown that osteoclasts support myeloma tumor growth in vivo.35 We do not know if the enhanced expression of heparanase directly contributes to increased osteoclastogenesis in the animals or if this is the result of other factors produced by the tumor. It is noteworthy that osteoclastogenesis is stimulated by hepatocyte growth factor (HGF) and that HGF signaling activity is enhanced by syndecan-1 heparan sulfate.36,37 Because heparanase can enhance growth factor signaling,18 it could directly impact osteoclast development via the HGF pathway.
These observations lead to the critical question: How is heparanase regulating the growth and metastatic properties of myeloma cells? First, it is known that heparan sulfates play an important role in regulating myeloma tumors.38 The heparan sulfate–bearing proteoglycan syndecan-1 is highly expressed on myeloma cells where it acts, via its heparan sulfate chains, to mediate cell-cell adhesion, adhesion of cells to collagen, and inhibition of cell invasion through collagen gels.39-43 Thus, heparanase, by degrading heparan sulfate at the myeloma cell surface, could render cells less adhesive and more motile, thereby facilitating tumor cell invasion. This observation is supported by data demonstrating that heparanase can cleave heparan sulfate on the surface of tumor cells growing in vitro31 and by the finding that transgenic animals overexpressing heparanase have a reduction in heparan sulfate size.44 Importantly, in this study we show for the first time that syndecan-1 within a tumor can be extensively remodeled by heparanase with a resulting dramatic reduction in overall proteoglycan size due to cleavage of heparan sulfate (Figure 1E). Interestingly, we find that these same cells growing in vitro show only minor changes in syndecan-1 size on Western blots (not shown). This suggests that the heparanase is more active within the tumor microenvironment in vivo than in vitro, possibly due to a lower pH in vivo.
Perhaps even more important than changes in cell adhesion is the fact that many of the signaling or signaling-related molecules in the bone marrow milieu that drive myeloma growth (eg, interleukin 6 [IL-6], IL-7, IL-8, HGF, fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor [VEGF]) have the capacity to bind to heparan sulfate. The myeloma tumor microenvironment is continually bathed in heparan sulfate due to the syndecan-1 that is shed from the myeloma cell surface and accumulates in the marrow stroma as well as in the serum of patients.21,22 Moreover, soluble syndecan-1 is an independent predictor of poor prognosis in patients with myeloma, and we have shown that soluble syndecan-1 can actively promote growth and metastasis of myeloma tumors growing with human bone implanted in SCID mice.23,27
Heparanase could play a decisive role in regulating the availability and activity of the heparan sulfate–bound growth factors that control the tumor growth and metastasis. For example, heparanase can directly promote angiogenesis by releasing heparin-binding angiogenic growth factors, such as FGF-2 and VEGF, that are trapped within the extracellular matrix.45,46 This idea is supported by the finding that heparanase increases the angiogenic response to tumors and that a significant positive correlation has been found between enhanced heparanase mRNA expression and tumor vascularity.14,47 In agreement with these data are our results demonstrating that elevation of heparanase in myeloma cells enhances both the number and size of tumor microvessels (Figure 5). Such an effect would contribute to both the growth and metastasis of the tumor cells. These effects may also be explained by a role for heparanase that goes beyond it acting to simply remodel extracellular matrix or release growth factors bound to the extracellular matrix. There is evidence that the fragments of heparan sulfate released by heparanase maintain bioactivity and may in fact be more active than the native heparan sulfate chain from which they are derived.47 In a landmark study, it was discovered that the intact heparan sulfate chains present on shed syndecan-1 potently inhibit FGF-2 mitogenicity.18 However, subsequent degradation of the intact heparan sulfate with heparanase (which cleaves the poorly sulfated regions along the heparan sulfate chain) generated heparan sulfate fragments that markedly activate FGF-2 mitogenicity.18 Importantly, these “activating fragments” of heparan sulfate are present in wound fluids18 and probably cancer as well. Similarly, it was recently demonstrated that the action of bacterial heparinase I (which cleaves heparan sulfate in regions similar to those cleaved by human heparanase) yields biologically active fragments that promote growth and metastasis of melanoma cell lines.19 In contrast, the action of a different enzyme, bacterial heparinase III, which produces heparan sulfate fragments distinct from those of bacterial heparinase I, inhibits growth and metastasis of these tumors.19,48 Thus, encoded within intact heparan sulfate chains are cryptic structural elements that have the power to either positively or negatively impact the behavior of cancer cells, and the action of heparanase liberates these cryptic fragments with resulting biologic consequences.19
In summary, expression of heparanase leads to enhanced metastasis of myeloma tumors to bone with a resulting substantial increase in tumor burden. We envision that intact heparan sulfate from secreted proteoglycans (eg, perlecan) or shed proteoglycans (eg, syndecans) becomes incorporated into the extracellular matrix, where it traps numerous growth and chemotactic factors and stores them in an inactive form. Tumor cells growing within the extracellular matrix produce heparanase that cleaves heparan sulfate, thereby releasing growth factors in an active form. These, in concert with the biologically active heparan sulfate fragments, support further tumor growth and progression. In addition to direct effects on the tumor cells, degradation of heparan sulfates within the bone microenvironment may disrupt the balance of growth and inhibitory factors that together help to regulate bone formation and bone resorption. Enhanced cross-talk between tumor cells and bone, perhaps mediated in part by the release of activated heparan sulfate fragments, could support lytic bone disease associated with tumor progression.33
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-06-2141.
Supported by grants CA55819 and CA103054 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Heparanase promotes bone-to-bone metastasis. Six weeks after injection of heparanase-transfected or control-transfected CAG myeloma cells into the tibia of SCID mice, sera were harvested and analyzed for the level of human immunoglobulin kappa light chain as a measure of tumor burden. Plots show the kappa light chain level for each individual mouse. The mean kappa chain level for the heparanase group was significantly higher than that of the control group (heparanase group, 6.13 μg/mL ± 1.33 μg/mL, control group 2.36 μg/mL ± 1.02 μg/mL [P = .04]), although several animals within the heparanase group had low tumor burden. Values are means plus or minus SEM. Metastases of tumor to distant bone were assessed by immunostaining for the presence of kappa light chain in the femur contralateral to the injected tibia. • denotes animals with bone metastases; ○, those without detectable bone metastases.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-06-2141/6/m_zh80030573450006.jpeg?Expires=1770099489&Signature=TSijqsGQ5FsaAAFXASbpodXOXXBnyzVAC4pYR4vtuUil7EVjE9YdGN2eHuz9DPi7oLCWDORY5RZUw9gsMPAbyQLaLF2mpc4jEBHOGrKuy7kMGg3e2VSSEZGteBTgK71o8dTWfmGeft~qcn5YWKtXkocMV-d0QqgA-3-YvE0puXX1GtyWJaHYFV1hN8Ljxxl2uHPaSrFPSRTEMH0Ce7~LkBJY5v2MTRQEz-RzVRsA5lBWcLx76NNd~21HFrdZJJkfaDrMDCWd0UiZZRRPUWv6xpeA-gzSymIkDPgaQfAIpqt3VxVYXmP~bHjqQKYgkqKwA8YkHnuP~aqIwWtpskbPWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal