Abstract
TCL1, the overexpression of which may result in T-cell leukemia, is normally expressed in early embryonic tissues, the ovary, and lymphoid lineage cells. Our analysis of mouse B-lineage cells indicates that Tcl1 expression is initiated in pro-B cells and persists in splenic marginal zone and follicular B cells. T-lineage Tcl1 expression begins in thymocyte progenitors, continues in CD4+CD8+ thymocytes, and is extinguished in mature T cells. In Tcl1-deficient mice, we found B lymphopoiesis to be compromised at the pre-B cell stage and T-cell lymphopoiesis to be impaired at the CD4+CD8+ thymocyte stage. A corresponding increase was observed in thymocyte susceptibility to anti-CD3ϵ–induced apoptosis. Reduced numbers of splenic follicular and germinal center B cells were accompanied by impaired production of immunoglobulin G1 (IgG1) and IgG2b antibodies in response to a T-dependent antigen. The marginal zone B cells and T-cell–independent antibody responses were also diminished in Tcl1-/- mice. This analysis indicates a significant role for Tcl1, a coactivator of Akt signaling, in normal T- and B-cell development and function.
Introduction
T-cell prolymphocytic leukemias (T-PLLs) in humans often have chromosomal translocations that juxtapose the T-cell receptor (TCR) α/δ or β locus to the proximity of the T-cell leukemia/lymphoma-1 gene (TCL1) located in the 14q32.1 region or, less frequently, to lay near its mature T-cell proliferation 1 (MTCP1) gene homolog in the Xq23 region.1-4 The ensuing aberrant influence of a TCR enhancer element results in overexpression of the TCL1 or MTCP1 genes.5,6 Overexpression of either TCL1 or MTCP1 in transgenic mouse models employing a T-cell–specific promoter may also result in a T-cell leukemia that resembles human T-PLL.7,8 In addition to the implicit TCL1 involvement in this T-cell malignancy, a variety of B-lineage tumor cell lines, ranging from pre-B cell to mature B-cell phenotype, have also been shown to express high Tcl1 levels.9-11 Moreover, TCL1 overexpression under the control of B-lineage–specific enhancer and promoter elements has been shown to promote B-cell chronic lymphocytic leukemia12 and B-cell lymphomas in mice.13 An important clue to the role of TCL1 in the leukomogenesis process is provided by the functional linkage of Tcl1 to Akt kinase, an intracellular component that participates in the transduction of antiapoptotic and proliferative signals.14,15 In the Akt signaling cascade, Tcl1 acts as an Akt cofactor to enhance kinase activity and nuclear translocation.16,17 Tcl1 binding to Akt also facilitates the formation of Akt-Tcl1 hetero-oligomers.18 The resultant (trans)phosphorylation of Akt1 at Ser473 may thus amplify the phosphatidylinositol 3 (PI3)–Akt1 pathway to contribute a survival advantage.19
Normally, TCL1 expression is tightly regulated, being confined to lymphoid and germinal cells in humans and mice. In human B-lineage cells, TCL1 expression is initiated in pro-B cells, peaks in the pre-B cells, and persists in immunoglobulin M (IgM)–bearing B cells.5 High expression levels of TCL1 transcripts have been found in the mantle zone B cells in the spleen, whereas TCL1 expression is down-regulated in germinal center and marginal zone B cells, and is extinguished in terminally differentiated plasma cells.9-11 In human T-lineage cells, TCL1 expression is seen in the intrathymic CD4-CD8- subpopulation, but not in mature T cells. In the present study, we observed a similar pattern for Tcl1 expression in mouse T- and B-lineage cells. In order to gain insight into the physiologic role(s) that Tcl1 may have in T and B lymphopoiesis, we have examined both pathways of lymphocyte development and their cooperative function in antibody responses of Tcl1-deficient mice. These mice are shown to have modestly compromised T and B lymphopoiesis due either to impaired cellular proliferation or enhanced apoptosis.
Materials and methods
Mice, cell preparation, cell counting, and statistical analysis
Tcl1-/- and Tcl1+/- mice were generated as described previously.20 Bone marrow (BM) cells were obtained by flushing the cavities of both femoral and tibial bones with media. Thymus and spleen samples were minced between frosted ends of glass slides. Cells suspended in media were then filtered through fine metal screens or Nylon membranes (Fisher, Pittsburgh, PA) to remove cellular debris. Erythrocytes were lysed in a 0.1 M ammonium chloride buffer at pH 7.4 (Sigma-Aldrich, St Louis, MO) for 1 to 2 minutes at room temperature and the cells were washed in fluorescence-activated cell sorter (FACS) buffer (1% fetal calf serum in phosphate-buffered saline [PBS]) before enumeration of the nucleated cells by light microscopy. For isolation of peripheral blood mononuclear cells (PBMCs), blood collected from Tcl1+/+,Tcl1+/-, and Tcl1-/- mice was layered over lymphocyte separation medium (density 1.078 g/mL; Cellgro, Mediatech, Herndon, VA) and centrifuged to remove leukocytes and erythrocytes. Since there were no differences between wild-type and heterozygous mice in lymphoid organ cellularity, we grouped wild-type and heterozygous mouse data together. The total number of cells in each population was estimated by enumerating the nucleated cells by light microscopy and determining the percentage for each population by immunofluorescence. The statistical significance of population differences was calculated by a Student 2-tailed t test using the Excel program.
Antibodies
Peridinin chlorophyll protein (PerCP)–labeled anti-B220, allophycocyanin (APC)–labeled anti-B220, fluorescein isothiocyanate (FITC)–labeled anti-S7/CD43, phycoerythrin (PE)–labeled anti-S7/CD43, FITC-labeled anti-CD4, PE-labeled anti-CD4, FITC-labeled anti-CD8, PE-labeled anti-CD8, FITC-labeled anti-CD25, PE-Cy5–labeled anti-CD44, PE-labeled anti-IgD, FITC-labeled anti-IgD, biotin-labeled anti-IgM; PE-labeled anti-CD5, PE-labeled anti-B220, and PE-CyChrome (Cy5)–labeled anti-CD19 were obtained from PharMingen (San Diego, CA). FITC-labeled goat anti–mouse IgM, biotin-labeled goat anti–rat Ig, streptavidin (SA)–Cy-Chrome, SA-PE, and SA-APC were obtained from Southern Biotechnology Associates (Birmingham, AL). FITC-labeled anti–peanut agglutinin (PNA) was obtained from Vector Laboratories (Burlingham, CA).
Flow cytometric analysis and cell sorting
Cells (106) were incubated with FcR blocker (PharMingen) before staining with FITC-, PE-, PerCP-, PE-Cy5–, or APC-labeled monoclonal antibodies against cell surface antigens. After washing, the stained cells were analyzed using a FACS Calibur instrument (Becton-Dickinson, Franklin Lakes, NJ) and WINMDI 2.8 software (The Scripps Research Institute Cytometry Software Page, http://facs.Scripps.edu/software.html). Lymphocyte subpopulations were purified by differential immunofluorescence cell sorting with a MoFlo instrument (Cytomation, Fort Collins, CO).
Analysis of Tcl1 gene family transcripts
RNA was isolated from FACS-sorted subpopulations (96% to 99% purity) of lymphocytes from tissue samples using the TRI Reagent (Molecular Research Center, Cincinnati, OH) and dissolved in 20 μL RNase-free distilled water (Ambion, Austin, TX). First-strand cDNA was synthesized from the isolated RNA (10 μL) using oligo(dT)15 primers and RNase H- reverse transcriptase (Superscript II; GIBCO/BRL, Carlsbad, CA) in a total volume of 40 μL. cDNA (2 μL) was used for polymerase chain reaction (PCR). The first round of PCR for Tcl1 amplification was carried out with 5′orf (5′-ATGGCTACCCAGCGGGCACAC-3′) and 3′ orf (5′-GTTATTCATCGTTGGACTCCGAG-3′) primers. After denaturation at 94°C for 4 minutes, 30 cycles of PCR were performed with the following conditions: 94°C for 1 minute, 61°C for 1 minute, 72°C for 30 seconds. First PCR reaction (2 μL) was used in a nested PCR of 30 cycles using 5′ sense (5′-ACACCCCAACCGCCTGTGGATC-3′) and 3′ reverse (5′-GATATGGTACAGGATCTGCCAATAC-3′) primers. As a cDNA quality control, murine β-actin cDNA was amplified (single round of PCR) under the same PCR conditions except for an annealing temperature of 58°C using 5′ oligo (CCTAAGGCCAACCGTGAAAAG) and 3′ oligo (5′-TCTTCATGGTGCTAGG-AGCCA-3′) primers. To verfiy the murine Tcl1 cDNA PCR product, a Southern blot analysis was performed with 5 μL of 10 times diluted nested PCR products using a specific internal Tcl1 probe (5′-GGGAGAAGCACGTGTACTTGGATGAG-3′). The β-actin cDNA internal probe was 5′-CACCCCAGCCATGTACGTAGCCATCC-3′. PCR products were separated on an agarose gel and transferred to a Nylon membrane (Amersham, Buckinghamshire, United Kingdom). The gene-specific probes were labeled using 20 U of T4 polynucleotide kinase in 40 μL labeling mixture (1480 Bq [40 μCi] of γ-[32P]adenosine triphosphate (ATP) and 1X kinase buffer (Gibco BRL). PCR primers used for evaluation of the Tcl1b1-b5 genes were: Tcl1-b1 forward: GCA GCT TTT GAT CCC CTG GGG C and reverse: 5′ GAG AAC GGT CAG GAC CCA AAC C with annealing of 70° C; Tcl1b2 forward: TGC AGG TTT TTA TCC TCC GA and reverse: CCT TTT ACT CCA GCA TCA GGA TC with annealing at 55°C; Tc11 B4 forward: AGT CCC GAC TCT CTC AAG ACT TT and reverse: CAA AGG CAC AAA GTG AGC AAG AG with an annealing temperature of 60°C; Tcl1B5 forward: CTG TGT CTG TTG ATC CCC AG and reverse: TCA TCC TCG CCT ATT ATT ATG TC with annealing at 55 °C. All the reactions were denatured at 94°C for 30 seconds; annealed at the specific temperature for 30 seconds, and elongated at 72°C for 1 minute.
Anti-CD3 antibody treatment and immunizations
Mice, 8 to 12 weeks old, received a single intraperitoneal injection of 25 μg anti-CD3ϵ antibody. The hamster anti–mouse CD3ϵ antibody (clone 145-2C11) was kindly provided by Dr Chander Raman (University of Alabama at Birmingham [UAB]). Mice, 11 to 13 weeks old, were immunized intravenously with 2 × 108 to 4 × 108 sheep red blood cells (SRBCs; Colorado Serum Company, Denver, CO) to elicit SRBC-specific antibody immune responses, or immunized intraperitoneally to evaluate germinal center (GC) responses. Pre-immune blood samples were obtained 2 days before immunization, and blood samples were drawn one week after immunization. Spleens were obtained 4 days after an intraperitoneal immunization to evaluate GC formation. To evaluate T-independent antigen responsiveness, mice (11 to 16 weeks old) were immunized intraperitoneally with 1 × 108 heat-inactivated S pneumoniae organisms (a gift from Dr John Kearney, UAB).
In vitro proliferation and survival assays
Cell suspensions of spleen and thymus samples were depleted of red cells and macrophages and cultured at 1 × 105 cells per well in 96-well plates in RPMI medium 1640 with 10% fetal calf serum (FCS). Splenocytes were cultured with 50 μg/mL Escherichia coli lipopolysaccharide (LPS; Calbiochem, San Diego, CA) for 2, 4, and 9 days. Before each checkpoint, BrdU (Sigma-Aldrich) was added at a concentration of 30 ng/mL and incubation continued for 6 hours at 37°C/5% CO2. Monoclonal antibody anti-BRdU-FITC–conjugated (Becton Dickinson) staining and propidium iodide labeling were used to identify nonapoptotic cells undergoing DNA synthesis by flow cytometry.
Immunoglobulin and antibody measurement
Serum Ig levels were measured by enzyme-linked immunosorbent assay (ELISA) using polyvinyl chloride microtiter plates (Dynex Technologies, Chantilly, VA) and goat anti–mouse Ig antibodies labeled with alkaline phosphatase (AP; Southern Biotechnology Associates, Birmingham, AL). A standard regression analysis curve was used to calculate relative Ig concentrations in individual samples based on optical density (OD) measurements at 405 nm in duplicate wells using p-nitrophenyl phosphate as AP substrate (Sigma-Aldrich). Plastic wells were coated with goat anti–mouse immunoglobulins (1 μg/mL) overnight at 4°C and then blocked with 1% bovine serum albumin in PBS. Duplicates of a diluted serum sample were added and Ig isotypes were measured in serum samples by employing goat anti–mouse isotype-specific antibodies conjugated with AP. Isotype-specific anti-SRBC antibodies were measured by fixing SRBCs onto the plastic plate.21 Anti-phosphocholine (PC)–specific antibodies were similarly measured in plastic wells coated with PC-BSA (a gift from Dr John Kearney, UAB).
Results
Tcl1 expression in normal lymphoid tissues
In an analysis of the lymphoid tissues from wild-type mice, Tcl1 transcripts could be detected by nested PCR in bone marrow, thymus, spleen, lymph nodes, and peripheral blood lymphocytes, but not in lymphocytes from the Peyer patches or intestinal epithelium. To evaluate Tcl1 expression as a function of lymphocyte differentiation, the levels of Tcl1 transcripts were assessed in purified subpopulations of bone marrow, thymus, and splenic lymphocytes. In the bone marrow, Tcl1 transcripts were detected at low levels in the pro-B fraction of B220+CD43+IgM- cells, and in slightly increased levels in pre-B cells (B220+CD43-IgM-) and immature B cells (B220+IgM+IgD-) (Figure 1, top panels). Low levels of Tcl1 protein were also detectable in these cells by using a monoclonal antibody (Supplemented Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).20
Expression of Tcl1 mRNA in flow-sorted lymphocyte subpopulations from bone marrow, thymus, spleen, peripheral blood, and lymph nodes of wild-type mice. For each lymphocyte subpopulation, 5 × 105 cells were used for total RNA isolation. Pro-B cells were defined as B220+CD43+IgM-, pre-B cells as B220+CD43-IgM-, immature B as B220+IgM+IgD-, mature B as B220+IgM+IgD+. The double-positive (DP) thymocytes were defined as CD4/8 double-positive. The CD4/8 double-negative (DN) thymocytes were further subdivided based on differential CD25 and CD44 expression: DN-A, CD4-CD8-CD44+CD25-; DN-B, CD4-CD8-CD44+CD25+; DN-C, CD4-CD8-CD44-CD25+; DN-D, CD4-CD8-CD44-CD25-. Splenic B cells were divided into 3 subpopulations based on expression of the B220, CD21, and CD23 cell-surface markers: MZ, marginal zone B cells (B220+CD21hiCD23int), FO, follicular B cells (B220+CD21intCD23hi); NF, newly formed B cells (B220+CD21loCD23lo). Peripheral blood mononuclear cells are designated PBMCs.
Expression of Tcl1 mRNA in flow-sorted lymphocyte subpopulations from bone marrow, thymus, spleen, peripheral blood, and lymph nodes of wild-type mice. For each lymphocyte subpopulation, 5 × 105 cells were used for total RNA isolation. Pro-B cells were defined as B220+CD43+IgM-, pre-B cells as B220+CD43-IgM-, immature B as B220+IgM+IgD-, mature B as B220+IgM+IgD+. The double-positive (DP) thymocytes were defined as CD4/8 double-positive. The CD4/8 double-negative (DN) thymocytes were further subdivided based on differential CD25 and CD44 expression: DN-A, CD4-CD8-CD44+CD25-; DN-B, CD4-CD8-CD44+CD25+; DN-C, CD4-CD8-CD44-CD25+; DN-D, CD4-CD8-CD44-CD25-. Splenic B cells were divided into 3 subpopulations based on expression of the B220, CD21, and CD23 cell-surface markers: MZ, marginal zone B cells (B220+CD21hiCD23int), FO, follicular B cells (B220+CD21intCD23hi); NF, newly formed B cells (B220+CD21loCD23lo). Peripheral blood mononuclear cells are designated PBMCs.
When thymocyte subpopulations were examined, the levels of Tcl1 transcripts were highest in double-negative cells and in double-positive thymocytes. No signal was detected in CD4 single-positive thymocytes, and only a faint Tcl1 band was visible in the CD8 single-positive thymocytes, perhaps reflecting the presence of the immature CD8 single-positive cells in this population. To determine more precisely at which thymocyte progenitor stage Tcl1 expression appears, the CD4/CD8 double-negative thymocytes were sorted on the basis of differential CD44 and CD25 expression.22 Tcl1 transcripts were expressed in easily detectable levels only in the late-stage subpopulation of thymocyte progenitors (CD4-CD8-CD44-CD25-) that precedes the CD4+CD8+ double-positive differentiation stage (Figure 1, bottom panels). Tcl1 expression is thus preferentially expressed during the intermediate intrathymic differentiation stages when T cells are undergoing proliferation and clonal selection.
For analysis of Tcl1 expression by splenic B cells, marginal zone (MZ) B cells (B220+CD21highCD23low), follicular B cells (B220+CD21intCD23high), and newly formed B cells (B220+CD21lowCD23low) were sorted.23,24 Tcl1 mRNA could be detected in all of these B-cell subpopulations, although MZ B cells appeared to express Tcl1 in higher levels than follicular B cells (Figure 1). Lymph node B-cell subpopulations clearly expressed Tcl1 transcripts. Tcl1 transcripts were found only in trace levels in splenic CD4+ and CD8+ T cells, and not at all in lymph node T cells. Low levels of Tcl1 transcripts were detected in circulating B cells, but were not seen in other types of circulating lymphocytes. The overall pattern of Tcl1 expression in the mouse during T- and B-linage differentiation thus closely resembles that observed in humans.5,9-11,25,26
Impaired T- and B-cell lymphopoiesis in Tcl1-deficient mice
Although the Tcl1-/- mice have a fertilty defect that is manifested by reduced litter size,20 the Tcl1-/- newborns appeared normal and continued to develop normally. Histologic examination of the lymphoid organs of the Tcl1-/- mice did not reveal obvious developmental abnormalities, nor did we observe significant differences in organ size and total body weight (data not shown). However, reduced numbers of lymphocytes were found in the bone marrow, thymus, and spleen (Table 1). Both the percentages (wild-type and heterozygous mice, 29.1% ± 5.2% vs Tcl1-/-, 20.7% ± 5.3%; P = .004) and numbers (wild-type and heterozygous mice, 147.5 × 105 ± 13.3 × 105 vs Tcl1-/-, 99 × 105± 8.7 × 105; P = .0007) of B220+ B-lineage cells were significantly decreased in the bone marrow of Tcl1-/- mice. The numbers of myeloid-lineage cells in bone marrow were comparable in Tcl1-/- and wild-type control mice (Tcl1+/+ or Tcl1+/-), although slightly increased percentages of Mac-1+ myeloid cells were observed in the Tcl1-/- mice (Tcl1-/-, 72.0% ± 3.3% vs wild-type and heterozygous mice, 64.5% ± 6.9%). A 50% reduction in the numbers of cells in the pre-B and immature B-cell subpopulations (P values of .0008 and .0009, respectively) was evident in the Tcl1-/- mice (Figure 2). Mature B-cell numbers were also lower in the bone marrow of Tcl1-/- mice, but the reduction was not significant (P > .05). Impaired generation of B-lineage cells in Tcl1-/- mice was thus manifested primarily at the pre-B and immature B-cell stages in differentiation.
Cellularity of hematopoietic and lymphoid tissues in Tcl1-/-- deficient and -nondeficient mice
. | Mean no. nucleated cells, × 106, ± SE . | . | |
|---|---|---|---|
| Organ . | Tcl1+/+ and Tcl1+/- . | Tcl1-/- . | |
| Bone marrow* | 51.1 ± 4.0§ | 38.4 ± 1.4§ | |
| Spleen† | 110.4 ± 6.3 | 82.5 ± 5.1§ | |
| Thymus‡ | 116.8 ± 6.9 | 84.1 ± 7.1§ | |
. | Mean no. nucleated cells, × 106, ± SE . | . | |
|---|---|---|---|
| Organ . | Tcl1+/+ and Tcl1+/- . | Tcl1-/- . | |
| Bone marrow* | 51.1 ± 4.0§ | 38.4 ± 1.4§ | |
| Spleen† | 110.4 ± 6.3 | 82.5 ± 5.1§ | |
| Thymus‡ | 116.8 ± 6.9 | 84.1 ± 7.1§ | |
Cells were harvested from both femoral and tibial bones of 7 to 9 mice per group, at 6 to 9 weeks of age
Twelve mice per group, ages 7 to 11 weeks
Results expressed as mean
P < .01
Hematopoietic and lymphoid cell subpopulations in Tcl1-/--deficient and wild-type (Tcl1+/+ and Tcl1+/-) mice. Analysis of bone marrow B-lineage cells in Tcl1-deficient and wild-type mice was conducted by 3-color flow cytometric analysis of cells from both femoral and tibial bones (mean ± one standard error). Splenic marginal zone (MZ) cells were defined as CD19+CD21hiCD23int, follicular cells (FO) as CD19+CD21intCD23hi, and newly formed (NF) cells as CD19+CD21loCD23lo. Both B220 and CD19 antibodies stained splenic B cells similarly when used in combination with CD21 and CD23 antibodies. Each group included 7 to 10 mice 6 to12 weeks of age. Results expressed as mean plus or minus one standard error. Asterisks indicate statistically significant differences (P < .01, as assessed by Student t test) between Tcl1-deficient and wild-type mice.
Hematopoietic and lymphoid cell subpopulations in Tcl1-/--deficient and wild-type (Tcl1+/+ and Tcl1+/-) mice. Analysis of bone marrow B-lineage cells in Tcl1-deficient and wild-type mice was conducted by 3-color flow cytometric analysis of cells from both femoral and tibial bones (mean ± one standard error). Splenic marginal zone (MZ) cells were defined as CD19+CD21hiCD23int, follicular cells (FO) as CD19+CD21intCD23hi, and newly formed (NF) cells as CD19+CD21loCD23lo. Both B220 and CD19 antibodies stained splenic B cells similarly when used in combination with CD21 and CD23 antibodies. Each group included 7 to 10 mice 6 to12 weeks of age. Results expressed as mean plus or minus one standard error. Asterisks indicate statistically significant differences (P < .01, as assessed by Student t test) between Tcl1-deficient and wild-type mice.
Analysis of thymocyte differentiation in the Tcl1-/- mice indicated a significant reduction in the numbers of immature CD4-/CD8- thymocytes and of intermediate CD4+/CD8+ thymocytes (Figure 1). The CD4+ and CD8+ single-positive subpopulations of mature thymocytes were also slightly lower than normal, but these reductions were not statistically significant. When the CD44 and CD25 cell-surface antigens were used to subdivide the CD4-/CD8- thymocytes, the hypocellularity noted for this progenitor population was primarily attributable to reduced numbers of cells in the CD4-CD8-CD44-CD25- subpopulation, the stage at which pre-TCR expression occurs (Figure 2). The impaired thymopoiesis in Tcl1-/- mice thus is manifested principally during the pre-T and intermediate thymocyte stages in T-cell differentiation.
A significant reduction was observed in the numbers of splenic follicular and MZ subpopulations of B cells and in the numbers of splenic CD4+ and CD8+ T cells in young adult Tcl1-/- mice (Figure 2). Notably, these data indicate that Tcl1 deficiency does not lead to a complete differentiation block in either lymphoid lineage.
Hypersensitivity of TCR-bearing thymocytes to CD3 ligation in Tcl1-/- mice
Since earlier studies suggest roles for the human TCL1 gene product in cellular proliferation7,8 and in antiapoptotic signaling,10,16,17 we tested the effects of apoptotic and proliferative stimuli under in vivo and in vitro conditions. When the rate of spontaneous apoptosis was assessed by measuring cell viability in thymocytes cultured in media or for thymocytes stimulated with dexamethasone, significant differences were not observed for the wild-type and null mice (data not shown). We therefore examined the effect of TCR/CD3 ligation-induced apoptosis as a surrogate model for the negative clonal selection that occurs during thymocyte development.27,28 When an anti-CD3ϵ antibody was injected into Tcl1-/- mice, the CD4+/CD8+ subpopulation comprised only 20% of the thymocytes 48 hours later, whereas 51% of the double-positive thymocytes survived this treatment in wild-type mice. A 56.5-fold reduction in the numbers of CD4+/CD8+ thymocytes and a 5-fold reduction in single-positive CD4+ thymocyte subpopulation occurred in the Tcl1-/- mice treated with the anti-CD3ϵ antibody. These results contrasted with a 15.8-fold reduction in CD4+/CD8+ thymocytes and a 2.1-fold in the CD4+ thymocytes after corresponding treatment of control mice (Table 2). The results of these experiments indicate increased susceptibility of the CD4+/CD8+ thymocytes and, to a lesser extent, of the CD4+ thymocytes to CD3 ligation-induced apoptosis in Tcl1-/- mice.
Comparison of anti-CD3 effects on thymocytes in Tcl1-/--deficient versus -nondeficient mice
. | Tcl1+/+ and Tcl1+/- . | . | . | Tcl1-/- . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Mean cell no. × 105, ± SE . | . | . | Mean cell no. × 105, ± SE . | . | . | ||||
| Thymocyte population . | Control . | Anti-CD3* . | Fold decrease . | Control . | Anti-CD3 . | Fold decrease . | ||||
| CD4-CD8- | 33.4 ± 2.8† | 9.0 ± 1.7 | 3.6 | 24.1 ± 2.1 | 15.9 ± 3.6 | 1.5 | ||||
| CD4+CD8+ | 1019 ± 65.1 | 64.4 ± 15.5 | 15.8 | 679 ± 104.1 | 12.1 ± 7.4 | 56.5 | ||||
| CD4+CD8- | 61.1 ± 6.0 | 29.4 ± 2.5 | 2.1 | 50.7 ± 6.5 | 9.6 ± 2.4 | 5.3 | ||||
| CD4-CD8+ | 26.3 ± 2.5 | 12.6 ± 1.0 | 2.1 | 23.7 ± 3.1 | 5.9 ± 1.4 | 4.0 | ||||
| Total | 1115 ± 80.4 | 115.4 ± 19.5 | 9.6 | 801 ± 101 | 43.5 ± 11.5 | 18.4 | ||||
. | Tcl1+/+ and Tcl1+/- . | . | . | Tcl1-/- . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Mean cell no. × 105, ± SE . | . | . | Mean cell no. × 105, ± SE . | . | . | ||||
| Thymocyte population . | Control . | Anti-CD3* . | Fold decrease . | Control . | Anti-CD3 . | Fold decrease . | ||||
| CD4-CD8- | 33.4 ± 2.8† | 9.0 ± 1.7 | 3.6 | 24.1 ± 2.1 | 15.9 ± 3.6 | 1.5 | ||||
| CD4+CD8+ | 1019 ± 65.1 | 64.4 ± 15.5 | 15.8 | 679 ± 104.1 | 12.1 ± 7.4 | 56.5 | ||||
| CD4+CD8- | 61.1 ± 6.0 | 29.4 ± 2.5 | 2.1 | 50.7 ± 6.5 | 9.6 ± 2.4 | 5.3 | ||||
| CD4-CD8+ | 26.3 ± 2.5 | 12.6 ± 1.0 | 2.1 | 23.7 ± 3.1 | 5.9 ± 1.4 | 4.0 | ||||
| Total | 1115 ± 80.4 | 115.4 ± 19.5 | 9.6 | 801 ± 101 | 43.5 ± 11.5 | 18.4 | ||||
Treated animals received 25 mg anti-CD3ϵ antibody intraperitoneally 2 days before analysis
Results for 6 mice, 8 to 12 weeks old, in each group
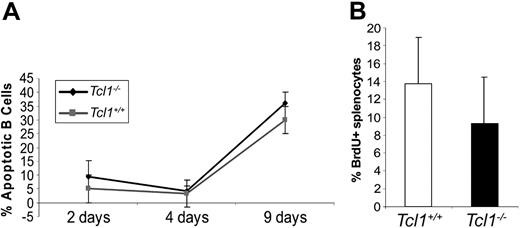
Splenic B cells derived from young mice were also assayed for apoptosis after stimulation with LPS at 2, 4, and 9 days (Figure 3). A slight increase (around 5%) in the number of apoptotic cells, measured by PI incorporation, was observed for Tcl1-/- splenocytes compared with Tcl1+/+ splenocytes. A modest, but significant, difference was instead observed in the percentage of B220+ splenocytes when cell proliferation was measured after LPS stimulation (Tcl1+/+ 13.75 ± 6.2 vs Tcl1-/- 9.36 ± 5.1; P = .046; wild-type = 10; N ≥ 7 in each group). Since TCL1 is known to activate AKT, we examined the possibility that the phosphorylation level and kinase activity of Akt could be altered in splenocytes or thymocytes of null mice. We could not observe any difference in the rate of Akt phosphorylation and kinase activation (not shown), a result that may reflect an alternative mechanism of Akt activation in the absence of Tcl1.
Apoptotic (A) and proliferation (B) rate in cultured Tcl1-/- and Tcl1+/+ splenocytes stimulated with LPS. See “Results” for a detailed description.
Apoptotic (A) and proliferation (B) rate in cultured Tcl1-/- and Tcl1+/+ splenocytes stimulated with LPS. See “Results” for a detailed description.
Immunoglobulin levels, antibody responses, and expression of Tcl1b1-b5 in Tcl1-/- mice
Serum IgG1 and IgG2b levels in Tcl1-/- mice were approximately 40% lower in Tcl1-/- mice than in control mice, whereas other immunoglobulin isotypes were not significantly affected (Table 3). To assess antibody responsiveness to a thymus-dependent antigen, mice were immunized intravenously with SRBCs and bled one week later. The levels of SRBC-specific IgG1 and IgG2b antibodies in Tcl1-/- mice were approximately 40% of those in the control mice (Figure 4). Tcl1-/- and control mice produced comparable levels of IgG2a, IgG3 (Figure 4A), and IgM anti-SRBC antibodies (not shown). Since isotype switching occurs primarily within the germinal centers,29 germinal center formation was evaluated after SRBC immunization by enumerating the PNA-reactive B cells in the spleen.30 Although clear histologic differences in germinal center reactions were not evident, there were fewer PNA-staining cells. Four days after intraperitoneal immunization, the frequency of PNA-positive cells was significantly lower in Tcl1-/- mice than in wild-type mice (3.1% ± 0.13% vs 3.9% ± 0.34%, n = 4; P = .04).
Serum immunoglobulin levels in Tcl1-/--deficient and -nondeficient mice
Ig isotype . | Tcl1+/+ and Tcl1+/-, μg/mL, mean ± SE . | Tcl1-/-, μg/mL, mean ± SE . | P . |
|---|---|---|---|
| IgG1 | 482.6 ± 33.6 | 262.8 ± 37.8 | .0001 |
| IgG2a | 270.2 ± 51.2 | 321.9 ± 37.8 | .439 |
| IgG2b | 945.9 ± 94.2 | 556.6 ± 81.8 | .0063 |
| IgG3 | 243.1 ± 24.8 | 172.4 ± 23.1 | .046 |
| IgM | 280.6 ± 32.0 | 204.0 ± 21.1 | .056 |
| IgA | 160.5 ± 30.9 | 135.6 ± 23.9 | .517 |
Ig isotype . | Tcl1+/+ and Tcl1+/-, μg/mL, mean ± SE . | Tcl1-/-, μg/mL, mean ± SE . | P . |
|---|---|---|---|
| IgG1 | 482.6 ± 33.6 | 262.8 ± 37.8 | .0001 |
| IgG2a | 270.2 ± 51.2 | 321.9 ± 37.8 | .439 |
| IgG2b | 945.9 ± 94.2 | 556.6 ± 81.8 | .0063 |
| IgG3 | 243.1 ± 24.8 | 172.4 ± 23.1 | .046 |
| IgM | 280.6 ± 32.0 | 204.0 ± 21.1 | .056 |
| IgA | 160.5 ± 30.9 | 135.6 ± 23.9 | .517 |
Results are from 12 to 14 mice per group, ages 8 to 13 weeks.
Evaluation of immune responses to T-dependent and T-independent antigens. (A) Analysis of anti–sheep red blood cells (SRBCs) antibody response. Tcl1-/- mice and Tcl+/+/Tcl+/-control mice immunized intravenously with SRBCs were bled at day 7 and day 15, and the serum samples analyzed for SRBC-specific IgM and IgG subclasses by ELISA (relative units/mL). Day 7 data only is shown here since antibody levels were similar at both time points. *Differences between Tcl1-deficient and wild-type mice were statistically significant (P < .02) by Student t test analysis. (B) Analysis of anti-phosphocholine (PC) antibody responsiveness. Seven 11- to 16-week-old mice in the experimental and control groups were immunized intraperitoneally with 1 × 108S pneumoniae and serum samples obtained one week later were analyzed for PC-specific IgM antibodies by ELISA (relative units/mL). *Differences between Tcl1-deficient and wild-type mice were statistically significant (P < .02) by Student t test analysis.
Evaluation of immune responses to T-dependent and T-independent antigens. (A) Analysis of anti–sheep red blood cells (SRBCs) antibody response. Tcl1-/- mice and Tcl+/+/Tcl+/-control mice immunized intravenously with SRBCs were bled at day 7 and day 15, and the serum samples analyzed for SRBC-specific IgM and IgG subclasses by ELISA (relative units/mL). Day 7 data only is shown here since antibody levels were similar at both time points. *Differences between Tcl1-deficient and wild-type mice were statistically significant (P < .02) by Student t test analysis. (B) Analysis of anti-phosphocholine (PC) antibody responsiveness. Seven 11- to 16-week-old mice in the experimental and control groups were immunized intraperitoneally with 1 × 108S pneumoniae and serum samples obtained one week later were analyzed for PC-specific IgM antibodies by ELISA (relative units/mL). *Differences between Tcl1-deficient and wild-type mice were statistically significant (P < .02) by Student t test analysis.
Examination of the T-independent antibody response to the immunodominant phosphocholine epitope of S pneumoniae31,32 indicated that the levels of IgM anti-PC–specific antibodies produced in Tcl1-/- mice were approximately 45% of those observed in control mice one week after immunization (Figure 4B). The compromised T-independent antibody response and reduction in the MZ subpopulation suggested an impairment in the B1 subset33 of Tcl1-/- mice. In accordance with this inference, a 40% reduction of circulating B cells with the B1 phenotype, IgMhigh, IgDlow, CD5+ B220+, was observed in Tcl1-/- mice (2.77 ± 0.27 vs 1.50 ± 0.36, n = 7; P = .03), thereby indicating a role for Tcl1 in the development of this subset of B cells.
Since other members of the TCL1 family gene have been described in humans and mice,34-36 we explored the possibility that these Tcl1 relatives could compensate in part for the effects of null Tcl1 alleles and thereby account for the modest immunodeficiency observed in the Tcl1-/- mice. When transcription of the Tcl1b1-5 genes was evaluated in bone marrow, thymus, and spleen cells of Tcl1-/- mice by seminested reverse transcriptase (RT)–PCR, we were unable to amplify Tcl1b1, b2, b4, and b5 transcripts from any of the lymphoid organs, whereas these transcripts were detectable in the analysis of a control egg library (data not shown), thereby suggesting that the Tcl1-/- phenotype is not affected by a compensatory expression of neighboring Tcl1b genes.
Discussion
Since the recognition of TCL1 involvement in the pathogenesis of T-cell leukemias, TCL1 has also been shown to be expressed in B-cell tumor lines and during normal T and B lymphopoiesis in humans. The present analysis of Tcl1 expression in wild-type mice and of the immune system alterations in Tcl1-/- mice indicates a significant role for Tcl1 in the generation of both T and B lymphocytes. In wild-type mice, Tcl1 expression was found to be up-regulated during the early stages in lymphocyte differentiation when immature T- and B-lineage cells are undergoing proliferation and are highly sensitive to receptor-mediated apoptosis.37,38 In Tcl1-/- mice, the numbers of thymocytes and bone marrow B-lineage cells were significantly reduced. The differentiation stages most affected by the Tcl1 deficiency were the precursor subpopulations of T- and B-lineage cells that are undergoing positive and negative clonal selection via their antigen receptors.39,40
The impairment in T and B lymphopoiesis that we observed in Tcl1-/- mice is also accompanied by functional deficits in immune responses. Following immunization with a T-dependent immunogen, the Tcl1-/- mice exhibited a reduction in numbers of germinal center B cells and impaired IgG1 and IgG2b antibody responses. Serum IgG1 and IgG2b levels were also significantly reduced in Tcl1-/- mice, whereas the levels of other immunoglobulin isotypes were not. This reduction in these switch isotypes was associated with reduced numbers of CD4+ helper T cells and follicular B cells in the spleen. Tcl1-/- mice also displayed significantly impaired antibody responses to a T-independent antigen, the phosphocholine determinant of S pneumoniae. Reduced numbers of MZ B cells in the Tcl1-/- mice further attest a Tcl1 role in the response to polysaccharide antigens during bacterial infection.24 Together with the reduced numbers of B1 cells in these mice, these findings indicate a role for Tcl1 in natural immunity. Conversely, overexpression of the human TCL1 gene under the control of an Ig enhancer causes an expansion of B1 cells in transgenic mice that later undergo neoplastic transformation of their CD5+ B cells.12
The impaired T- and B-cell generation observed in the Tcl1-/- mice was relatively modest. This could reflect the relatively low levels at which Tcl1 is expressed in mouse lymphocytes relative to human lymphocytes.34,41 We also considered the possibility that the relatively modest phenotype seen in the Tcl1-/--deficient mice might represent a functional compensation by Tcl1 gene relatives. However, expression of the Tclb1-b5 genes could not be detected in the lymphoid cells from either Tcl1-null or wild-type mice, thereby suggesting these genes exert their function in nonlymphoid cells. Tcl1b2 expression has been reported in lymphoid tissues, such as the spleen,36 but our analysis of isolated lymphocytes, the use of different primer pairs, and the study of different mouse strains could account for our failure to detect this transcript in lymphocytes of the Tcl1-/- mice.
The impaired generation of early T- and B-lineage cells in Tcl1-/- mice may reflect an increased susceptibility to receptor-induced apoptosis. In support of this hypothesis, we observed an increased vulnerability of the CD4+/CD8+ thymocytes and, to a lesser extent, of the CD4+ and CD8+ thymocytes to receptor-mediated apoptosis when Tcl1-/- mice were treated with an anti-CD3ϵ antibody.27,28 This finding is concordant with a recent report indicating that Tcl1 overexpression in a human cell line markedly enhances cell survival following treatment with the apoptosis-inducing agents, anti-CD3, anti-Fas, and dexamethasone.17 It is not clear why the single-positive thymocytes that do not express Tcl1 at easily detectable levels were hypersensitive to CD3 ligation in Tcl1-/- mice. It is possible that undetectable levels of Tcl1 expression in a subpopulation of the thymic CD4 cells may have a role in their survival.
Human TCL1 can bind to the pleckstrin domain of AKT with resultant enhancement of the AKT kinase activity and nuclear translocation.16,17 Mutational and structural TCL1 analyses indicate its interaction with AKT facilitates the formation of AKT-TCL1 oligomers.18 In the presence of TCL1, AKT Ser-473 phosphorylation may result from the transphosphorylation of other AKT molecules in the oligomeric complexes. Enhanced phosphorylation of both threonine residues (308/309/305 in Akt1/2/3, respectively) and serine residues (473/474/472) ensures maximal kinase activity.19 Tcl1 may thus facilitate a structural amplification loop in the PI3-kinase Akt pathway.18 However, following apoptotic or proliferative stimuli we did not observe changes in Akt or downstream targets that would have been predicted in this mouse model. This might reflect the very low expression levels of Tc11 in the mouse relative to humans, or there could be an alternative mechanism for Tcl1-Akt interaction to compensate for the deficiency of Tcl1 in mice. Noteworthy in this regard, there are 7 mouse othologs and 3 human TCL1 family genes. Moreover, previous studies16,18,42 and our own work do not clarify whether or not murine Tcl1 binds to Akt. Although the crystal structures for human and murine Tcl1 are similar, the sequence conservation is only 50% and replacement of single key residues in a way that would allow overall structural integrity could still disrupt TCL1/AKT-binding interactions.43
On the other hand, the phenotypical changes here described are consistent with those observed in other murine models of Akt transgenic or null mice. Akt has been functionally linked to B- and T-cell development in studies that have demonstrated the activation of Akt in response to both BCR- and TCR-mediated signaling.14,44,45 Additional insight into this developmental role has been obtained in studies of animal models wherein Akt is overexpressed46 or underexpressed.47 Akt1-/- mice display increased thymocyte apoptosis and hypersensitivity to apoptosis induction by gamma irradiation and dexamathasone treatment.47 Conversely, immune system alteration in Akt transgenics is manifested by B- and T-cell hypercellularity, enhanced immunoglobulin levels, especially IgG2a and IgA, and increased resistance to FAS-mediated apoptosis induced by CD3 stimulation.46 Transgenic mice that overexpress either Tcl1 or its MTCP1 homologue also have exaggerated T- and B-cell proliferation and they develop leukemias.7,8,12,13 By contrast, Tcl1-deficient mice are shown in the present study to manifest precisely the opposite effects of those seen in Akt transgenic mice. It may therefore be of interest to cross these animal models in future studies to explore the coordinate roles of the Tcl1 and Akt genes in BCR/TCR-mediated signaling and clonal selection.
Prepublished online as Blood First Edition Paper, October 12, 2004; DOI 10.1182/blood-2004-04-1453.
Supported by Associazione Italiana Ricerca sul Cancro, Telethon grant no. D.102, Ministero Italiano della Universitá e della Ricerca Scientifica (MIUR) Piano Nationale Ricerca (PNR) Oncologia, Ministero della Sanita', and National Institutes of Health grants CA76259 and AI39816. S.-M.K. was a Howard Hughes Medical Institute Research Associate, and M.D.C. is a Howard Hughes Medical Investigator.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mauro Helmer Citterich and Barbara Arredi for technical assistance, and John Kearney, Flavius Martin, Manuela Rosado, and Rita Carsetti for helpful discussions.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal