Abstract
Interferon (IFN) induces expression of proapoptotic genes and has been used in the clinical treatment of multiple myeloma. The promyelocytic leukemia (PML) gene is an IFN-induced target that encodes a tumor suppressor protein. PML protein is typically localized within discrete speckled nuclear structures termed PML nuclear bodies (NBs). Multiple myeloma cells demonstrate differential responses to IFN treatment, the mechanism of which is largely unknown. Herein, we show that growth inhibition effects of IFN-α in myeloma cells correlate with PML NBs and tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) induction, whereas known IFN targets including signal transducer and activator of transcription-1 (STAT1), STAT3, p38, and Daxx cannot account for these differential responses. RNAi silencing of PML blocks IFN-α–induced apoptosis in myeloma cells and correspondingly down-regulates TRAIL expression. Similarly, stable expression of a dominant negative TRAIL receptor DR5 partially blocks IFN-induced cell death. These results demonstrate that PML and TRAIL play important roles in IFN-induced apoptosis and identify TRAIL as a novel downstream transcriptional target of PML. Identification of PML and PML NBs as effectors of IFN responses provides insights into mechanisms by which tumor cells exhibit resistance to this class of agents and may prove useful in assessing treatment regimens.
Introduction
Multiple myeloma (MM) is an incurable form of cancer characterized by the accumulation and proliferation of malignant plasma cells in the bone marrow. Molecular mechanisms leading to the development of this disease are still poorly understood although a variety of lesions have been described in both patient samples and myeloma cell lines. Currently there is no clear picture as to the requirements for initiation or progression of this disease although multiple events such as chromosomal abnormalities and oncogene expression are likely involved.1,2
Standard treatment of MM involves combination chemotherapy with or without bone marrow transplantation. More recently, a series of new treatment regimens have been initiated, including agents such as thalidomide, proteasome inhibitors, and interferons (IFNs), which have all been used with varying success.1,3,4 Because the mechanisms by which such agents inhibit tumor growth are poorly understood, it is probably not surprising that optimal regimens and combinations have not been defined. Among these compounds, IFNs have been used for the treatment of multiple forms of cancer, including MM3 as well as other diseases.
IFNs are pleiotropic cytokines that were first identified as antiviral agents secreted by infected mammalian cells but have later been shown to also be important regulators of cell growth (reviewed by Stark et al5 ). Following binding to cell type–specific receptors, IFNs regulate target genes through activation of Jak/signal transducer and activator of transcription (Jak/STAT) signaling pathways. It has been shown in MM that IFN-α activates STAT3 as well as a transcription factor complex consisting of STAT1, STAT2, and IFN regulatory factors 1 and 2.6-8 One of the consequences of IFN treatment is induction of apoptosis through up-regulation of tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) as demonstrated in the human multiple myeloma cell line U266.9 The death ligand TRAIL triggers apoptosis through activation of its cognate receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), which, in turn, recruit adapter proteins and activate caspases.10 TRAIL has been shown to effectively induce apoptosis and overcome drug resistance in several types of transformed cells, including MM but not most normal cells.11-13 The mechanism by which IFN induces TRAIL in MM cells is currently unknown.
Another target of IFNs that is specifically up-regulated at the transcriptional level is the promyelocytic leukemia (PML) gene.14,15 Multiple isoforms of PML have been identified, most of which are found in the nucleus where they form PML nuclear bodies (PML NBs).16 Microscopy studies have shown that there are typically 10 to 20 PML NBs in most normal cells, varying in size between 0.2 and 1.0 μm. More than 50 different proteins have been found to localize to PML NBs, but they are conditionally present and include several different classes of proteins such as transcription factors and cofactors, oncoproteins, and ribosomal proteins.17 Posttranslational modifications such as small ubiquitin-like modifier–ylation (SUMOylation) and phosphorylation of PML have been reported to be important for formation and maturation of PML NBs.18 The exact molecular function of PML and PML NBs is still largely unclear, but a biologic role for PML in cell proliferation, apoptosis, and regulation of transcription is well established.19
PML has previously attracted intense interest in cancer biology due to its role in the pathogenesis of acute promyelocytic leukemia (APL).20-22 In APL the PML gene is fused to the retinoic acid receptor α gene (RARα) as a result of a t(15;17) chromosomal translocation. This translocation results in expression of a PML-RARα fusion protein leading to disruption of the PML NBs and delocalization of PML NB components. The disruption of PML NBs leads to a growth advantage for leukemic blast cells.21 Degradation of PML-RARα and induction of the remaining wild-type PML allele by treatment with As2O3, retinoic acid, or IFN results in reformation of PML NBs and complete remission of the disease.23 Furthermore, a role for PML as a tumor suppressor has been suggested by studies on mice with impaired PML function. Investigations of PML-RARα transgenic and PML-/- mice have demonstrated that PML inactivation results in impaired induction of apoptosis by multiple apoptotic pathways,24 and PML-/- mice are highly susceptible to developing tumors when challenged by carcinogens.25 Recently, comprehensive studies have shown that PML protein is frequently lost in human cancers of various histologic origins.26 These observations demonstrate that PML is crucial for critical tumor-suppressive pathways although its mechanism of action is largely unknown.
To date, a functional role for PML in MM has not been addressed. Herein, we describe the IFN-α–mediated induction of PML NBs in MM cells and the impaired regulation of the PML gene in a subset of lines that correlates with nonresponsiveness to IFN-induced apoptosis. TRAIL induction is directly involved in the cytotoxic effect of IFN against MM. Furthermore, induction of PML appears important for enhanced expression of TRAIL and subsequent activation of death receptors and apoptosis.
Materials and methods
Cell lines
Human myeloma cell lines were kindly provided by Dr W. Michael Kuehl (National Cancer Institute [NCI], Bethesda, MD). All lines were maintained in RPMI 1640 (Biofluids, Rockville, MD) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 50 μg/mL streptomycin (Invitrogen, Carlsbad, CA). In addition, human interleukin-6 (IL-6) was added at 10 ng/mL for IL-6–dependent myeloma lines ANBL6, XG1, and XG2.
Western blot and immunofluorescence analysis
For Western blot analysis, lysates were prepared as previously described.27 Thirty-five micrograms of total protein per sample was fractionated on 4% to 12% NuPAGE gels (Invitrogen) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). Antibodies used for Western blot analysis were rabbit polyclonal anti-PML, polyclonal anti-STAT3 (K15), polyclonal anti-Daxx (M112), goat anti-p38 (Santa Cruz Biotechnology, Santa Cruz, CA); anti–p-84/91STAT1 (E23), rabbit anti–p-Tyr701-STAT1, anti–p-Tyr705-STAT3, anti–p-Thr180/Tyr182-p38 (Cell Signaling, Beverly, MA); anti-GAPDH (Biodesign International, Saco, ME); and goat anti–human TRAIL (R&D Systems, Minneapolis, MN).
Cells were prepared for confocal immunofluorescence studies as previously described.28 Confocal fluorescent images were collected with a Bio-Rad (Hercules, CA) MRC 1024 confocal scan head mounted on a Nikon Optiphot microscope with a 60 × planapochromat lens in the Confocal Core Facility, Center for Cancer Research, NCI. A krypton-argon gas laser provided excitation at 488 nm. Emission filters of 522/532 nm were used for sequentially collecting green fluorescence. Z sections were collected at 0.5-μm intervals for each cell. Image files were collected and analyzed using Bio-Rad LaserSharp software.
Cell staining and analysis by laser scanning cytometry (LSC)
Cells were washed twice with phosphate-buffered saline (PBS) and resuspended in PBS at approximately 2 × 105 cells per milliliter. Cells were then added to poly-l-lysine–coated chambers (Nalge Nunc, Naperville, IL) for 15 minutes to allow attachment, fixed with 70% ethanol at -20°C for 20 minutes, followed by 15 minutes of incubation at room temperature. Chambers were then rinsed twice with PBS and subjected to blocking using 5% bovine serum albumin (BSA) in PBS. Fixed cells were incubated for 1 hour at room temperature with monoclonal anti-PML antibody (Santa Cruz Biotechnology), washed 3 times with PBS containing 0.1% Tween 20, and then incubated in Alexa 488–conjugated goat antimouse secondary antibody (Molecular Probes, Eugene, OR) for 30 minutes at room temperature. After washing 3 times with PBS containing 0.1% Tween 20, cells were treated with PBS containing 5 μg/mL propidium iodine (PI) and 100 μg/mL RNase for 30 minutes at 37°C. Slides were washed with PBS and mounting medium (5 μg/mL PI, 75% glycerol in PBS) added before placement of coverslips. Slides were stored at 4°C prior to scanning. PML NB fluorescence was measured by LSC (CompuCyte, Cambridge, MA) in the fluorescence-activated cell sorting (FACS) core facility of the Center for Cancer Research, NCI. Slides were scanned using an argon laser at 5 mW and 40 × 10.75 NA objectives. Green and red channels were used for Alexa 488 and PI, respectively; cells were contoured on PI fluorescence and scored for PML NBs in the green channel. All samples were scanned using the same photomultiplier tube (PMT) settings. At least 3000 to 5000 cells were measured per sample, and data were analyzed using WinCyte software (CompuCyte).
siRNA transfection
Small interfering RNA (siRNA) duplexes were obtained from Dharmacon (Lafayette, CO). Small interfering PML (siPML) corresponds in nucleotide sequence to positions 398 to 418 of the PML coding region relative to the first nucleotide of the start codon and is present in all PML isoforms. Luciferase GL2 duplex (siluc) was used as a negative control. Transfection was performed using an Amaxa electroporation apparatus (Amaxa, Cologne, Germany). Transfection efficiency was monitored using cyanine 3 (cy3)–conjugated siluc and FACS analysis. A series of optimization transfections were first performed according to the manufacturer's instructions. The program (C16) and solution (T) that gave the best efficiency and least cell death were chosen for later studies. One day before transfection, cells were diluted in fresh growth medium and 5 × 106 cells were mixed with 2 μM siRNA in 100 μL solution T and transfected using program C16. All analyses were performed 36 hours after transfection. Using these conditions, transfection efficiencies above 70% were consistently obtained.
Proliferation assay
Cell proliferation assays were performed using the CellTiter 96 Aqueous One Solution Cell Proliferation MTS Assay (Promega, Madison, WI) following the manufacturer's instructions as previously described.27 Proliferation (percent of untreated cells) was calculated using the following equation: (100 + 100 × [abs. 490 nm treated cells - abs. 490 nm untreated cells]/absorbance (abs) 490 nm untreated cells). This calculation assigns a value of 100% proliferation to untreated cells.
Trypan blue viability assay
Viability of cells was assessed by trypan blue (TB) staining followed by counting unstained cells. Viability (percent of untreated) was calculated similarly as described above for proliferation.
Caspase-3 assay
Caspase-3 activity was detected using ApoAlert Colorimetric Assay Kits (BD Biosciences, Palo Alto, CA) as described in the user manuals. Protein content was determined by BCA Protein Assay kit (Pierce, Rockford, IL). Four hundred micrograms of total protein per sample in duplicate was used. All experiments were repeated at least 3 times.
Terminal deoxynucleotide transferase (TdT) assay
Cells were washed with cold PBS and fixed in -20°C methanol. After removal of methanol, cells were incubated in terminal deoxynucleotide transferase (TdT) TUNEL Label solution (Roche, Mannheim, Germany) and TUNEL Enzyme (Roche) for 30 minutes at 37°C. Cells were then washed and resuspended in PBS containing Hoechst. Terminal deoxynucleotidyl transferase nick-end labeling (TUNEL)–positive cells were quantitated using flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA).
Preparation of IFN-α–stimulated culture supernatants
Supernatants were prepared as previously described.29 Briefly, cells (2 × 105/mL) treated with or without IFN-α for 60 hours were resuspended in complete RPMI 1640 medium (5 × 106/mL), incubated for an additional 6 hours, and harvested by centrifugation. Supernatant was passed through 0.22-μm filters (Millipore) and assayed for cytoxicity. Blocking of secreted TRAIL was performed by adding 10 mg/mL anti-TRAIL antibodies (R&D Systems).
RT-PCR
Total RNA was isolated using TRIzol Reagent (Invitrogen). RNA was then treated with DNA-free kit (Ambion, Austin, TX) to remove contaminating genomic DNA. Purity of RNA samples was confirmed by agarose gel analysis. Reverse transcriptase–polymerase chain reaction (RT-PCR) of TRAIL expression was conducted using TITANIUM ONE-Step RT-PCR kit (BD Biosicences) according to the user manual. TRAIL primer set was as follows: sense, 5′-GGCTATGATGGAGGTCCAGG-3′; antisense, 5′-GGTCCATGTCTATCAAGTGCTC-3′. PML primer set was as follows: sense, 5′-TTCTGGTGCTTTGAGTGCGAG-3′; antisense, 5′-TCACTGTGGCTGCTGTCAAG-3′. Samples were subjected to 25 cycles of amplification under the following conditions: 94°C for 30 seconds, 55°C (TRAIL) or 57°C (PML and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) for 30 seconds, and 68°C for 1 minute, with a final extension at 68°C for 7 minutes. Reaction mixtures were separated on 1% agarose gels. For control reactions, human GAPDH amplimer set (BD Biosciences) was used.
Cloning and expression of dominant-negative DR5 in myeloma cells
Dominant-negative DR5 (DR5-DN) was constructed by introducing an SmaI fragment (residues 1 to 275 of wild-type DR5) of pCMV-SPORT6/DR5 (Open Biosystems, Huntsville, AL) into retroviral construct pMSCVhyg vector (BD Biosciences). Myeloma cell line 8226 was first infected with murine ecotropic retroviral receptor (gift from Dr Weguo Zhang, Department of Immunology, Duke University, Durham, NC) as previously described.27 Following DR5-DN infection, stable clones were generated by limited dilution. DR5-DN–positive clones were selected using DR5 antibody (Santa Cruz Biotechnology).
Results
PML is induced in IFN-α–sensitive human MM lines
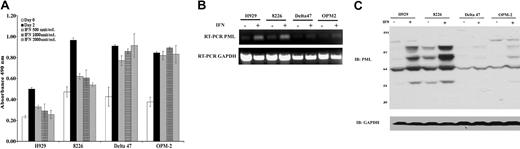
Although IFN-α has been shown to induce apoptosis in hematopoietic cells including MM, the mechanism of action by which it facilitates cell death in MM is currently unclear. Because the PML gene has been associated with IFN-mediated apoptotic pathways in other cell types, the current studies were undertaken to evaluate the role of PML in IFN-α–induced apoptosis of human MM cells. Among 9 MM lines tested, 5 demonstrated growth inhibition that correlated with PML induction upon IFN-α treatment. The 4 nonresponsive lines failed to evidence PML induction (Table 1). From this set, 2 IFN-α–responsive lines, H929 and 8226, and 2 nonresponsive lines, Delta47 and OPM2, were selected for further studies. As shown in Figure 1A, IFN-α inhibited proliferation starting at concentrations as low as 500 U/mL in H929 and 8226 cells but did not inhibit growth of Delta47 and OPM2 even at the highest concentration of 2000 U/mL. The growth-inhibitory activity correlated with strong PML mRNA and protein induction (Figure 1B-C). Multiple forms of PML with varying molecular weight were induced that likely correspond to different isoforms and posttranslational modifications of the PML protein.
PML and TRAIL induction following IFN-α treatment of MM lines
MM cell line . | Growth inhibition response . | Induction of PML . | TRAIL protein expression . |
|---|---|---|---|
| OPM1 | No | No | No |
| OPM2 | No | No | No |
| XG1 | No | No | No |
| Delta47 | No | No | No |
| ANBL6 | Yes | Yes | Yes |
| MM144 | Yes | Yes | Yes |
| H929 | Yes | Yes | Yes |
| 8226 | Yes | Yes | Yes |
| XG2 | Yes | Yes | Yes |
MM cell line . | Growth inhibition response . | Induction of PML . | TRAIL protein expression . |
|---|---|---|---|
| OPM1 | No | No | No |
| OPM2 | No | No | No |
| XG1 | No | No | No |
| Delta47 | No | No | No |
| ANBL6 | Yes | Yes | Yes |
| MM144 | Yes | Yes | Yes |
| H929 | Yes | Yes | Yes |
| 8226 | Yes | Yes | Yes |
| XG2 | Yes | Yes | Yes |
MM cell lines were treated with or without 2000 U/mL IFN-α for 48 hours. Growth inhibition was measured by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphenyl)-2H-tetrazolium (MTS) assay as described in “Materials and methods.” PML induction was assessed by Western blot and confocal image analysis. TRAIL was detected by Western blotting.
IFN-α–induced growth inhibition in MM correlates with enhanced PML expression. (A) Proliferation of MM cell lines H929, 8226, Delta47, and OPM2 following treatment with indicated amounts of IFN-α for 48 hours was determined by MTS assay as described in “Materials and methods.” Data are the average of 3 independent experiments performed in triplicate (±SD). (B) RT-PCR analysis of transcriptional induction of PML mRNA in the indicated MM cell lines with (+) or without (-) IFN-α (2000 U/mL) treatment for 48 hours. The amplified fragment is located at 5′-end and appears in all PML isoforms. The level of GAPDH serves as loading control. (C) Western blot analysis of PML protein isoforms using rabbit polyclonal anti-PML with (+) or without (-) IFN-α (2000 U/mL) treatment for 48 hours. The level of GAPDH serves as loading control.
IFN-α–induced growth inhibition in MM correlates with enhanced PML expression. (A) Proliferation of MM cell lines H929, 8226, Delta47, and OPM2 following treatment with indicated amounts of IFN-α for 48 hours was determined by MTS assay as described in “Materials and methods.” Data are the average of 3 independent experiments performed in triplicate (±SD). (B) RT-PCR analysis of transcriptional induction of PML mRNA in the indicated MM cell lines with (+) or without (-) IFN-α (2000 U/mL) treatment for 48 hours. The amplified fragment is located at 5′-end and appears in all PML isoforms. The level of GAPDH serves as loading control. (C) Western blot analysis of PML protein isoforms using rabbit polyclonal anti-PML with (+) or without (-) IFN-α (2000 U/mL) treatment for 48 hours. The level of GAPDH serves as loading control.
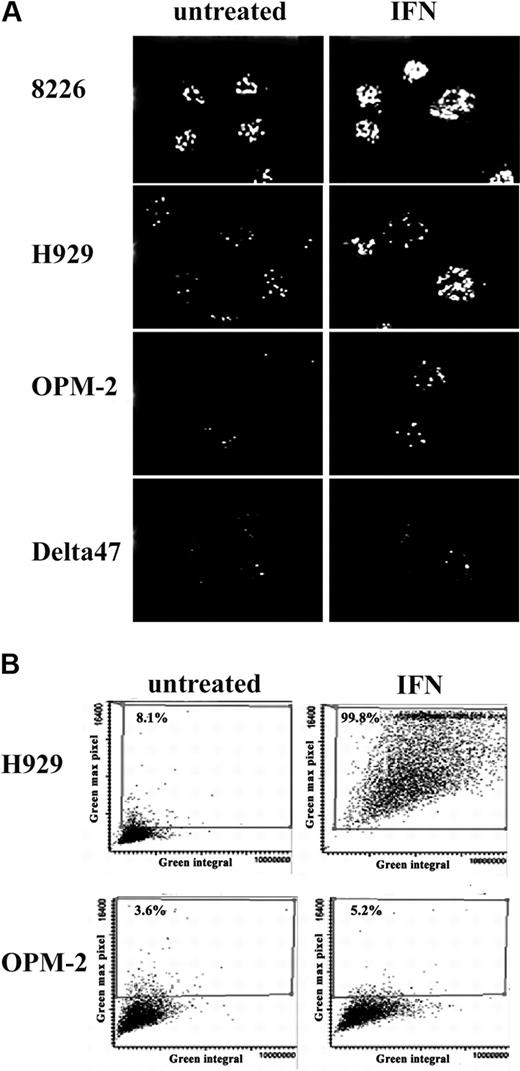
Immunofluorescence analysis of the IFN-α–treated cells showed a marked increase in both the number and size of PML NBs appearing as typical discrete speckled structures within the nuclei of H929 and 8226 cells but not in Delta47 and OPM2 (Figure 2A). Notably, H929 and 8226 also have higher basal levels of PML NBs than OPM2 and Delta47. Induction of PML was quantitated using LSC to analyze 3000 to 5000 cells. Figure 2B shows scattergrams representing bivariate distributions of integrated green fluorescence versus maximal pixel of green fluorescence. In untreated cultures, most cells were characterized by low values of integrated as well as maximal pixel fluorescence. Upon IFN-α treatment of H929, most cells exhibited increased fluorescence values. This increase was even more obvious when expressed as maximal pixel rather than as an integral of fluorescence intensity and indicated increased expression in nearly 100% of cells. In contrast, no significant increase in fluorescence intensity was observed in OPM2 cells after IFN-α treatment. These results indicate that the inhibition of proliferation induced by IFN-α in myeloma cell lines correlates with PML protein induction and NB formation.
Confocal image and LSC analysis of PML NBs. (A) PML nuclear bodies induced by IFN-α (2000 U/mL) were visualized by immunostaining using monoclonal anti-PML. Pictures are a projection of confocal Z sections. (B) IFN-α–treated (2000 U/mL) and untreated H929 and OPM2 cells were immunostained with mouse monoclonal anti-PML followed by incubation with Alexa 488–conjugated secondary antibody. LSC analysis was performed as described in “Materials and methods”; 3000 cells per sample were scanned. PML NBs are represented by scattergrams of 2 parameters of LSC measurement. Green fluorescence integral represents overall fluorescent intensity within the nucleus, and green maximal pixels represent maximal fluorescent signal (brightest cells).
Confocal image and LSC analysis of PML NBs. (A) PML nuclear bodies induced by IFN-α (2000 U/mL) were visualized by immunostaining using monoclonal anti-PML. Pictures are a projection of confocal Z sections. (B) IFN-α–treated (2000 U/mL) and untreated H929 and OPM2 cells were immunostained with mouse monoclonal anti-PML followed by incubation with Alexa 488–conjugated secondary antibody. LSC analysis was performed as described in “Materials and methods”; 3000 cells per sample were scanned. PML NBs are represented by scattergrams of 2 parameters of LSC measurement. Green fluorescence integral represents overall fluorescent intensity within the nucleus, and green maximal pixels represent maximal fluorescent signal (brightest cells).
Downstream targets of IFN-α
In an attempt to biochemically define the link between IFN-α treatment and PML induction, a series of known downstream targets of IFN-α were analyzed. Both STAT1 and STAT3 have been shown to be activated by IFN-α in other cell types.5 IFN-α treatment resulted in an increase in STAT1 protein and phosphorylated STAT1 in both responsive and nonresponsive lines (Figure 3). Similarly, in both responsive and nonresponsive lines STAT3 was readily phosphorylated although protein levels were unchanged. Moreover, coimmunostaining of either STAT1 or STAT3 with PML revealed no colocalization with PML NBs (data not shown). Therefore, activation of STAT1 and STAT3 is not likely to be sufficient for PML induction or the differential growth inhibition exhibited by IFN-α–responsive and nonresponsive lines.
STAT1, STAT3, Daxx, and p38 do not correlate with IFN-α effects on responsive and nonresponsive cell lines. Western blot analysis of STAT1, STAT3, Daxx, and p38 MAP kinase determined after 48 hours in the presence (+) or absence (-) of IFN-α (2000 U/mL).
STAT1, STAT3, Daxx, and p38 do not correlate with IFN-α effects on responsive and nonresponsive cell lines. Western blot analysis of STAT1, STAT3, Daxx, and p38 MAP kinase determined after 48 hours in the presence (+) or absence (-) of IFN-α (2000 U/mL).
Other downstream IFN targets include members of the mitogen-activated protein (MAP) kinase family, particularly p38, which has been reported to play an important role in IFN signaling and to be involved in activation of the PML promoter.30 However, p38 was found to be constitutively phosphorylated in all of the MM cell lines tested, and IFN-α treatment did not enhance phosphorylation.
IFN-induced apoptosis in B cells has been reported to occur through a STAT1-independent pathway involving a downstream element designated Daxx.31 Daxx is of particular interest in that association with PML has been demonstrated and a role in apoptosis, although controversial, has been proposed.32,33 In myeloma lines, Daxx protein was not induced by IFN and no difference was seen between responsive and nonresponsive cells (Figure 3). Additionally, RT-PCR of H929 cells treated with IFN-α for various times (4 hours, 8 hours, 16 hours, 24 hours, and 48 hours) showed no induction of Daxx mRNA, and cellular protein localization was also unchanged (data not shown). Thus, IFN-α–induced apoptosis in multiple myeloma is Daxx independent. Taken together, STAT1, STAT3, the p38 pathway, and Daxx induction are not sufficient for IFN-α–induced apoptosis in MM cells, suggesting an additional pathway associated with this phenotype. PML is the only IFN-induced gene detected in this study that is restricted to responsive cell lines.
RNAi silencing of PML establishes an essential role in IFN-induced apoptosis in MM
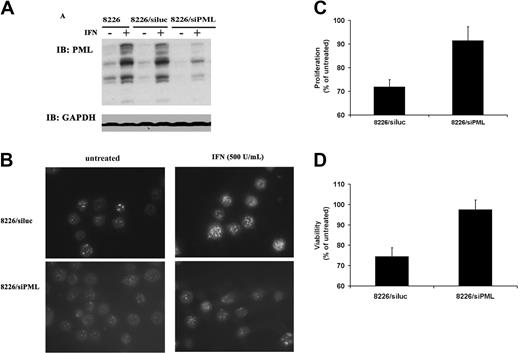
To clarify the role of PML in IFN-α–induced apoptosis of MM cells, an oligonucleotide corresponding to the published RNAi sequence of PML (siPML)34 was used to inhibit protein expression. PML duplex had no effect on cell survival in the absence of IFN-α (data not shown). RNAi treatment of 8226 cells markedly reduced the IFN-α–induced levels of PML protein (Figure 4A). PML NB formation was also correspondingly decreased as readily visualized in representative microscopy of cells immunostained for PML (Figure 4B). LSC quantitation revealed that IFN-α–treated control cells transfected with siRNA corresponding to the luciferase gene (siluc) exhibited an induction of 37%, which was reduced to 17% in the siPML-treated cells (Table 2). The biologic consequences of the reduction in PML expression are reflected in a decrease in the ability of IFN-α to inhibit cell growth as measured by MTS assay (Figure 4C). IFN-α stimulation reduced proliferation to 72% in 8226/siluc-treated cells but to a much lesser extent (91%) in the 8226/siPML group when compared with untreated controls. Similar results are also seen in a trypan blue viability assay (Figure 4D).
RNAi leads to specific down-regulation of PML and a corresponding loss of IFN-α–mediated growth inhibition. (A) Immunoblot analysis of PML induction by IFN (500 U/mL) in parental 8226 cells (left), 8226 cells transfected with control siRNA targeting luciferase (siluc, middle), and 8226 cells transfected with siRNA targeting PML (siPML, right). Protein loading control was measured by GAPDH blotting. (B) Fluorescence microscopy image of PML NB induction. Cells in left panels were untreated and, in right panels, treated with IFN-α. (C) MTS proliferation assay comparing the effect of IFN-α on 8226 cells transfected with siluc or siPML. Data are expressed as percentage of optical density (OD) value of IFN-treated versus untreated cells as described in “Materials and methods,” with untreated cells representing 100% proliferation, and are averaged values of 5 independent experiments performed in triplicate (± SD). (D) Trypan blue viability assay of the same experiment as in panel C. The data are average of 3 independent experiments performed in triplicate (± SD).
RNAi leads to specific down-regulation of PML and a corresponding loss of IFN-α–mediated growth inhibition. (A) Immunoblot analysis of PML induction by IFN (500 U/mL) in parental 8226 cells (left), 8226 cells transfected with control siRNA targeting luciferase (siluc, middle), and 8226 cells transfected with siRNA targeting PML (siPML, right). Protein loading control was measured by GAPDH blotting. (B) Fluorescence microscopy image of PML NB induction. Cells in left panels were untreated and, in right panels, treated with IFN-α. (C) MTS proliferation assay comparing the effect of IFN-α on 8226 cells transfected with siluc or siPML. Data are expressed as percentage of optical density (OD) value of IFN-treated versus untreated cells as described in “Materials and methods,” with untreated cells representing 100% proliferation, and are averaged values of 5 independent experiments performed in triplicate (± SD). (D) Trypan blue viability assay of the same experiment as in panel C. The data are average of 3 independent experiments performed in triplicate (± SD).
LSC quantitation of PML NBs in 8226 cells transfected with siPML and siluc
. | Untreated, % . | IFN-α, 500 U/mL, % . |
|---|---|---|
| 8226/siluc | 6.0 | 37.1 |
| 8226/siPML | 5.3 | 17.0 |
. | Untreated, % . | IFN-α, 500 U/mL, % . |
|---|---|---|
| 8226/siluc | 6.0 | 37.1 |
| 8226/siPML | 5.3 | 17.0 |
Five thousand cells per sample were scanned by LSC as described in “Materials and methods.” Percentage represents maximal green pixel value.
PML mediates IFN-α–induced apoptosis through TRAIL
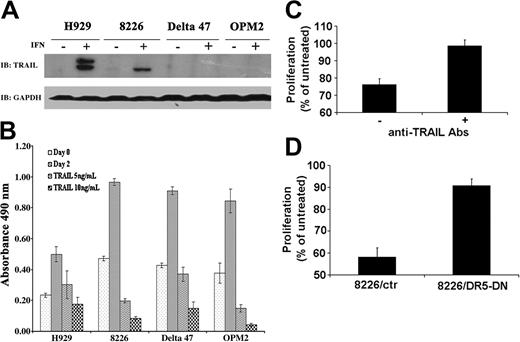
The molecular mechanisms of PML's cellular function(s) remain unclear. However, there is increasing evidence suggesting a role for PML in transcriptional regulation (reviewed by Salomoni and Pandolfi19 and Zhong et al35 ). To address the issue of the molecular mechanism of PML action in IFN-α–induced apoptosis of MM cells, we next examined the status of TRAIL, a major IFN-α–induced apoptotic factor known to be transcriptionally regulated. TRAIL protein was induced only in MM lines sensitive to IFN-α–mediated apoptosis (Table 1, Figure 5A). Interestingly, exogenous TRAIL was effective at inhibiting the growth of both IFN-α–responsive and nonresponsive cell types, indicating that the TRAIL death receptor pathway is intact in all MM lines tested (Figure 5B). To further assess the role of TRAIL in the IFN-α response, we analyzed TRAIL secretion from IFN-α–treated responsive human myeloma cells. Treatment of the IFN-α–nonresponsive cell line Delta47 with supernatant from IFN-α–treated H929 cells resulted in growth inhibition of the Delta47 cells. This inhibition was reversed by the addition of anti-TRAIL antibody (Figure 5C). Supernatant from IFN-α–treated responsive myeloma cells therefore has a growth-inhibitory effect on nonresponsive cells that is mainly mediated by secreted TRAIL. The role of TRAIL in mediating the growth-inhibitory response of IFN-α was further confirmed by stably expressing a dominant negative death receptor 5 (DR5-DN) construct containing a deletion of the cytoplasmic death domain36 in the 8226 cell line. Expression of DR5-DN not only blocks TRAIL-induced cell death in 8226 cells (data not shown) but also rescues 8226 from IFN-α–induced growth inhibition (Figure 5D). These studies demonstrate that TRAIL mediates most, if not all, of the IFN-α response exhibited by myeloma cells.
PML up-regulation correlates with TRAIL induction in MM lines. (A) TRAIL is induced only in IFN-responsive cell lines H929 and 8226. Western blot analysis of TRAIL protein following treatment of indicated lines with or without IFN-α for 48 hours (2000 U/mL). GAPDH expression is included as protein loading control. (B) TRAIL inhibits proliferation of both IFN-responsive and nonresponsive cell lines. MM cell lines were treated with the indicated amount of TRAIL and proliferation measured by MTS assay. (C) Biologically active TRAIL is secreted from IFN-α–responsive cell lines. Supernatant from IFN-α–treated and untreated H929 cells was added to IFN-α–nonresponsive Delta47 cells with or without anti-TRAIL antibodies (10 mg/mL) and proliferation measured by MTS assay. Data are presented as percent proliferation of cells treated with supernatant from untreated H929 cells as described in “Materials and methods.” Data are average of 3 independent experiments done in triplicate (± SD). (D) Dominant negative TRAIL receptor blocks IFN-α growth inhibition. The 8226 cells transfected with either vector control (8226/ctr) or DR5-DN construct (8226/DR5-DN) were stimulated with IFN-α 500 U/mL, and proliferation was measured by MTS assay. Proliferation was calculated as described in “Materials and methods.” A representative experiment (1 of 3 independent experiments) performed in triplicate (± SD) is shown.
PML up-regulation correlates with TRAIL induction in MM lines. (A) TRAIL is induced only in IFN-responsive cell lines H929 and 8226. Western blot analysis of TRAIL protein following treatment of indicated lines with or without IFN-α for 48 hours (2000 U/mL). GAPDH expression is included as protein loading control. (B) TRAIL inhibits proliferation of both IFN-responsive and nonresponsive cell lines. MM cell lines were treated with the indicated amount of TRAIL and proliferation measured by MTS assay. (C) Biologically active TRAIL is secreted from IFN-α–responsive cell lines. Supernatant from IFN-α–treated and untreated H929 cells was added to IFN-α–nonresponsive Delta47 cells with or without anti-TRAIL antibodies (10 mg/mL) and proliferation measured by MTS assay. Data are presented as percent proliferation of cells treated with supernatant from untreated H929 cells as described in “Materials and methods.” Data are average of 3 independent experiments done in triplicate (± SD). (D) Dominant negative TRAIL receptor blocks IFN-α growth inhibition. The 8226 cells transfected with either vector control (8226/ctr) or DR5-DN construct (8226/DR5-DN) were stimulated with IFN-α 500 U/mL, and proliferation was measured by MTS assay. Proliferation was calculated as described in “Materials and methods.” A representative experiment (1 of 3 independent experiments) performed in triplicate (± SD) is shown.
To address the question of whether TRAIL is transcriptionally regulated by, and downstream of, PML, TRAIL protein and mRNA levels were analyzed in cells treated with control and siPML duplexes. As seen in Figure 6, both IFN-α–induced TRAIL protein and mRNA expression were reduced in cells treated with siPML, whereas control treated cells were unaffected. These results strongly indicate that TRAIL is a downstream target of PML in IFN-α–induced apoptosis of MM cells.
siPML down-regulates TRAIL. (A) RNAi silencing of PML inhibits induction of TRAIL protein by IFN-α. The 8226 cells transfected with siluc or siPML were treated with IFN-α 500 U/mL for 48 hours and analyzed for TRAIL protein expression by immunoblotting. GAPDH protein level serves as loading control. (B) RNAi silencing of PML inhibits induction of TRAIL mRNA by IFN-α. RT-PCR of cells treated as in panel A using primers specific for TRAIL and GAPDH as a control.
siPML down-regulates TRAIL. (A) RNAi silencing of PML inhibits induction of TRAIL protein by IFN-α. The 8226 cells transfected with siluc or siPML were treated with IFN-α 500 U/mL for 48 hours and analyzed for TRAIL protein expression by immunoblotting. GAPDH protein level serves as loading control. (B) RNAi silencing of PML inhibits induction of TRAIL mRNA by IFN-α. RT-PCR of cells treated as in panel A using primers specific for TRAIL and GAPDH as a control.
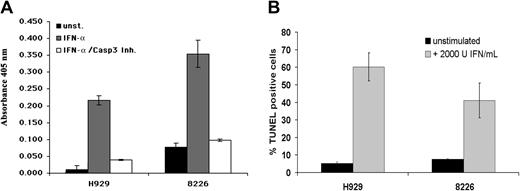
Because the biologic consequences of TRAIL activation are mediated by the activation of caspases leading to apoptosis, we performed experiments to verify that IFN stimulation induces apoptosis as opposed to stasis in the MM cell lines used in this study. As expected, IFN-α induced caspase-3 activity in the responsive MM cell lines H929 and 8226, and this activity could be inhibited by a caspase-3–specific inhibitor (Figure 7A). Furthermore, internucleosomal fragmentation was detected in about 60% of the H929 cells and 40% of the 8226 cells by TdT assay (Figure 7B). Thus, IFN-α induces apoptosis in responsive MM cell lines, and this process is mediated by PML, which is necessary for subsequent induction of TRAIL.
IFN-αinduces cell death in MM lines by activation of caspases. (A) IFN-α stimulation induces apoptosis in MM cell lines. IFN-α (2000 U/mL) activates caspase-3 in the MM cell lines H929 and 8226, and inhibitor of caspase-3 blocks IFN-α–induced caspase-3 activity and cell death. (B) IFN-α (2000 U/mL) induces apoptosis in the MM cell lines H929 and 8226 as assayed by internucleosomal fragmentation using a TdT assay. Data are presented as average of 3 independent experiments (± SD).
IFN-αinduces cell death in MM lines by activation of caspases. (A) IFN-α stimulation induces apoptosis in MM cell lines. IFN-α (2000 U/mL) activates caspase-3 in the MM cell lines H929 and 8226, and inhibitor of caspase-3 blocks IFN-α–induced caspase-3 activity and cell death. (B) IFN-α (2000 U/mL) induces apoptosis in the MM cell lines H929 and 8226 as assayed by internucleosomal fragmentation using a TdT assay. Data are presented as average of 3 independent experiments (± SD).
Discussion
IFNs have been used with varying effectiveness as proapoptotic agents in the treatment of multiple myeloma.3 Although only a small subset of patients appears to derive clinical benefit from IFN treatment, these results do not preclude the possibility that IFNs, in combination with newer agents such as proteasome inhibitors or thalidomide, may prove to be clinically effective. The precise mechanism by which IFNs exert antitumor activity remains unclear, but recent studies have indicated that IFN induces up-regulation of TRAIL as a critical downstream target in this apoptotic pathway.9 Moreover, it has been shown that IFN-α–stimulated neutrophils and monocytes release a soluble form of TRAIL that has an apoptotic effect on leukemic cells,37 suggesting a novel mechanism by which IFN-α might exert antitumor activity. The mechanism(s) of TRAIL regulation and the identity of other elements in this pathway remain to be elucidated. Herein, we present evidence that PML plays a major role in IFN-induced apoptosis and contributes to the regulation of TRAIL in MM cell lines.
Interest in investigation of the possible involvement of PML in the IFN response in human myeloma was stimulated by the important role suggested for PML as a tumor suppressor in human cancers and as an essential mediator of various proapoptotic stimuli.23,24,38,39 Initial studies demonstrated that PML protein and mRNA are induced by IFN-α only in MM cell lines responsive to IFN-α treatment wherein both the number and size of PML NBs increase (Figure 1, Table 1). Cell lines that are not responsive to IFN-α treatment do not show PML induction. Multiple isoforms of PML are induced, varying in size from about 50 to 150 kDa. These isoforms may be generated by alternative splicing and/or posttranslational modification. Furthermore, IFN responsiveness and PML induction appear not to be dependent on elements associated with these processes in other systems (Figure 3), including STATs 1 and 3,14,15 p38 MAPK,30,40 and Daxx,31,32,41 nor with the p44/42 MAPK or phosphatidylinositol-3 kinase (PI-3K) pathways as determined by use of corresponding inhibitors (not shown). Colocalization of PML with previously reported binding partners such as STAT3 and Daxx was also not observed, although the present studies were limited to endogenously rather than overexpressed proteins.31,32,41
Although PML appears to act as a tumor suppressor, the mechanisms by which it achieves this function remain unclear. PML NBs have been suggested to be sites of transcriptional regulation,35 and IFN-α stimulation has been reported to induce more than 300 genes.42,43 Among these is the TRAIL gene, which has also been described as an important mediator of IFN-α–induced apoptosis in human myeloma.9,42,44,45 Herein, it was observed that, similar to PML induction, TRAIL is induced by IFN-α only in responsive MM cell lines. That recombinant TRAIL kills both IFN-responsive and nonresponsive cells with equal efficiency indicates that the failure of IFN-α to induce apoptosis in certain lines is not due to TRAIL receptor defects. Rather, these observations suggest a failure in the induction of PML and TRAIL in response to IFN-α with TRAIL downstream of PML. TRAIL has also been reported to be secreted from cells stimulated with IFN,29 and the current studies also provide evidence that TRAIL is secreted upon IFN stimulation of responsive MM cell lines. The ability of secreted TRAIL to kill IFN-nonresponsive MM lines indicates that TRAIL may be important for paracrine and autocrine effects of IFN-α treatment in human myeloma. The functional significance of TRAIL as the biologic mediator of IFN-α–induced growth inhibition was further confirmed by expressing a dominant negative DR5 construct in 8226 cells (IFN-α responsive) to prevent TRAIL/TRAIL receptor signaling. Dominant negative expression resulted in a rescue of IFN-α–induced growth inhibition. These findings are consistent with studies indicating that apoptosis induced by IFN-α in the myeloma cell line U266 was partially blocked by dominant negative DR5.9
To confirm a role for PML in IFN-α–induced apoptosis in myeloma, RNAi was used to specifically down-regulate PML expression.34 Treatment of an IFN-α–responsive MM cell line with siPML led to a partial rescue of the growth-inhibitory effect of IFN-α and correlated with the reduction in level of PML protein levels and formation of PML NBs. Importantly, reduced PML expression resulted in down-regulation of both TRAIL mRNA and protein levels. Therefore, differential PML and subsequent TRAIL induction in MM lines appears to determine the response versus nonresponse phenotype to IFN-α.
PML expression is not lost in IFN-nonresponsive MM cell lines, but rather the amount of PML is much higher in IFN-responsive cell lines particularly after IFN treatment. This observation indicates that PML's function in IFN signaling may be concentration dependent and that certain minimal levels of PML protein are required for transcriptional induction of the TRAIL gene, although other mechanisms contributing to IFN nonresponsiveness cannot be ruled out. Consistent with this interpretation is the suggestion that PML is haploinsufficient for some of its tumor-suppressive properties,26,39 as has been reported for other tumor suppressors.46 The present observations, in conjunction with other similar studies (as reviewed by Zhong et al35 ), point toward a mechanism whereby PML protein levels regulate the availability and localization of transcription factors and can through this scenario affect downstream gene activation. This model for the role of PML in regulating transcription is consistent with the current understanding of the role of the PML NB as a nuclear depot47,48 wherein a variety of transcription factors and other proteins are assembled in complexes the stoichiometry of which is likely to be highly dependent on PML levels
As mentioned previously, the binding partners of PML appear to be quite diverse in different cell types. To understand its role in transcriptional control, a major focus in the future will therefore be to identify transcription factors PML interacts with upon IFN stimulation and define which of these subsequently regulate activation of the TRAIL gene. The availability of both IFN-responsive and nonresponsive lines may provide an opportunity by using techniques such as array analysis to identify PML-regulated target genes and, subsequently, additional tumor suppressive pathways that are regulated by PML upon IFN treatment. Finally, this study provides new insights in the understanding of IFN responsiveness that may be useful in evaluating the potential use of this agent in patient treatment.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-04-1614.
Supported in part by the Norwegian Research Council (Ø.D., O.S.G.) and The Norwegian Cancer Society (O.S.G).
C.C., Ø.D., O.S.G., and S.R. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Myung Kim (Laboratory of Biochemical Genetics; National Heart, Lung, and Blood Institute; National Institutes of Health) and Dr Stan Lipkowitz (Laboratory of Cellular and Molecular Biology, NCI, National Institutes of Health) for helpful discussion and suggestion.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal