Abstract
Coexpression of the homeodomain protein Meis1 and either HoxA7 or HoxA9 is characteristic of many acute myelogenous leukemias. Although Meis1 can be overexpressed in bone marrow long-term repopulating cells, it is incapable of mediating their transformation. Although overexpressing HoxA9 alone transforms murine bone marrow cells, concurrent Meis1 overexpression greatly accelerates oncogenesis. Meis1-HoxA9 cooperation suppresses several myeloid differentiation pathways. We now report that Meis1 overexpression strongly induces apoptosis in a variety of cell types in vitro through a caspase-dependent process. Meis1 requires a functional homeodomain and Pbx-interaction motif to induce apoptosis. Coexpressing HoxA9 with Meis1 suppresses this apoptosis and provides protection from several apoptosis inducers. Pbx1, another Meis1 cofactor, also induces apoptosis; however, coexpressing HoxA9 is incapable of rescuing Pbx-mediated apoptosis. This resistance to apoptotic stimuli, coupled with the previously reported ability to suppress multiple myeloid differentiation pathways, would provide a strong selective advantage to Meis1-HoxA9 coexpressing cells in vivo, leading to leukemogenesis.
Introduction
Proliferation, though necessary for the development of an organism, can have negative consequences if uncontrolled. Apoptosis can protect against uncontrolled proliferation. There are 2 basic interpretations of apoptosis.1 One is that apoptosis results from conflicting growth-promoting and growth-suppressing signals.2 Another interpretation is that apoptosis represents a “default” cellular pathway actively suppressed by specific survival signals during proliferation.3 Oncogenes can trigger proliferation and apoptosis. The c-myc proto-oncogene cotransforms cells with a variety of proteins (eg, Max and activated H-ras), activating proliferation pathway gene transcription.4-6 However, c-myc also potently induces apoptosis.7,8 Similarly, the E2F1 transcription factor mediates proliferative and apoptotic signals.9,10 Thus, the end point of oncogene activation may depend on reception of an apoptosis-suppressing signal. This may explain why many tumors demonstrate mutations in apoptosis-regulating genes.
BXH-2 recombinant inbred mice express an ecotropic murine leukemia virus (MuLV), causing myeloid leukemia at nearly 100% incidence, with death by 8 months.11 Meis1 is a common proviral integration site in nearly 15% of these leukemias.12 Of those tumors containing an integration at the Meis1 locus, approximately 98% have a cointegration at either HoxA7 or HoxA9.13 The Meis1 protein is a member of the TALE family of homeodomain (HD) proteins.12,14 The Meis1 mRNA is alternatively spliced, yielding at least 3 known isoforms.12,15 Meis1 is the prototypical member of a family of related proteins.16-19 Sequence-based analysis of this family reveals a conserved N-terminal bipartite Meinox domain that mediates protein–protein interactions.15,20 Increased Meis1 expression, in conjunction with HoxA7 or HoxA9, is found in a variety of human tumor types.21-24 Meis1 and HoxA9 are downstream targets of MLL fusion proteins, and enforced Meis and HoxA9 expression can replace MLL fusion protein function.25
Meis1 binds DNA alone or binds as a cofactor for Pbx or Hox proteins.26-29 Pbx also functions as a Hox protein cofactor,30-33 increasing the selectivity and specificity of Hox protein binding.34,35 Meis1 increases the DNA-binding stability of Meis–Hox complexes.27,36 Meis, Hox, and Pbx also interact by forming trimers in the presence or absence of DNA.37-40
This array of interactions produces varying effects on proliferation and differentiation. Hox overexpression is sufficient to transform Rat-1 cells, promote soft agar colony formation, and form tumors in mice.41 A general property of Hox overexpression is increased proliferation42,43 or alteration of differentiation.44,45 Pbx overexpression alone is insufficient for cellular transformation.46 Pbx cooperates with Hox to transform in vitro47 but not in vivo.48,49
Meis1 overexpression has been reported in murine bone marrow long-term repopulating cells48-50 but not in cell lines. Meis1 cannot transform alone, but it cotransforms with Hox proteins, reducing the latency period for tumor development.48,49,51 Meis1 cooperates with HoxA9 to suppress hematopoietic cell differentiation.52,53 Meis1 and Pbx interact in mediating cellular localization of Pbx-Meis complexes in Drosophila and vertebrate cells. Meis binding to Pbx causes a conformational change in Pbx, unmasking a putative nuclear localization signal54 while blocking a putative nuclear export signal.55 In the absence of Pbx, Meis proteins are predominantly cytoplasmic, requiring interaction with Pbx for nuclear localization.56
Pbx1 was originally described as part of a chimeric protein resulting from the (t1;19) translocation in a number of human acute myelogenous leukemias.57,58 Chimeric E2A-Pbx1 is another example of an oncogene stimulating proliferation and apoptosis. E2A-Pbx1 transgenic mice exhibit increased lymphocyte proliferation and lymphoma development with massive apoptosis in the premalignant phase of these lymphomas.59 Inducible overexpression of E2A-Pbx1 in human Reh lymphoblastoma cells causes massive apoptosis.60 This apoptosis is not caused by the E2A moiety and requires the Pbx1 HD.
We report the effects of transient Meis1 overexpression in human and murine cell lines of myeloid, lymphoid, and fibroblast origin. We found that Meis1 overexpression consistently resulted in massive apoptosis, approaching 100% of transfected cells within 3 days. Meis1 expression changed the levels of proteins important in apoptosis control and execution. The Meis1 HD and Pbx-interaction motif were required for apoptosis induction. Further, we found that overexpressing full-length Pbx1b also induced apoptosis. Coexpressing HoxA9 abrogated Meis-mediated apoptosis but not Pbx-mediated apoptosis. Finally, we found that Meis- and Pbx-mediated apoptosis was caspase dependent because inhibiting caspase 3 or caspase 8 resulted in apoptotic levels indistinguishable from those of control cells.
Materials and methods
DNA constructs
For inducible protein expression, the murine Meis1b open reading frame (ORF) was polymerase chain reaction (PCR)–amplified from a pET28-Meis1b plasmid using vector-specific primers (Novagen, Madison, WI). The murine Pbx1b, Pbx3a, and Pbx3b ORFs were amplified from pSP-73 DNA vectors containing these cDNAs. Amplified ORFs were subcloned into the multiple cloning site of the pInd expression vector (Invitrogen, Carlsbad, CA) and were sequenced to confirm correct orientation.
For transient protein expression, the murine Meis1a, Meis1b, Meis1d, and HoxA9 ORFs were PCR-amplified from pET28 plasmids containing these cDNAs using vector-specific primers (Novagen). The murine Pbx1b, Pbx3a, and Pbx3b ORFs were amplified from pSP-73 DNA vectors containing these cDNAs. Amplified ORFs were subcloned into the multiple cloning sites of the pIRES2-EGFP bicistronic expression vector (Clontech, Palo Alto, CA). The Meis1bΔM2 deletion construct was generated by overlap extension PCR61 using the following primers for 30 cycles of first-round amplification on a Meis1b template: Meis1For, 5′-GATCTCTAGAATGGCGCAAAGGTACGAC-3′; Meis1Rev, 5′-GATCCAATTGCTACTGAGCGTGAATGTC-3′; M2sense, 5′-GGATAACTTGATGATTCAAGTACACGAATTATGTGACAA-3′; and M2antisense, 5′-TTGTCACATAATTCGTGTACTTGAATCATCAAGTTATCC-3′. Products were gel purified and used in a second-stage reaction in equimolar amounts for 30 cycles. The final product was gel purified and cloned into the pspFlag vector. To generate the mammalian expression vector, the insert was digested with EcoRI and was subcloned into the pIRES-EGFP expression vector. All clones were sequenced to confirm correct orientation.
Cell culture and calcium-phosphate transfection
Reh human lymphoblastoma cells (American Type Culture Collection [ATCC], Manassas, VA), wild-type cells, and caspase 8-/- Jurkat T cells (ATCC) were maintained in RPMI 1640 medium, 10% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY), HL-60 cells (ATCC) in Iscove modified Dulbecco medium (IMDM), 10% FBS, 32Dcl3 cells in IMDM, 10% FBS, 10% WEHI-conditioned medium, and NIH3T3 cells (ATCC) in Dulbecco modified Eagle medium (DMEM), 10% FBS. All nonadherent cultures were maintained at a density of 1 × 106 cells/mL. Ten micrograms DNA was transfected into 5 × 106 cells using standard calcium-phosphate transfection procedure.62 For cotransfections, 10 μg (5 μg each construct) was used. For single transfections, DNA amounts were kept constant by adding 5 μg empty, undigested-vector DNA. Media were changed 12 hours after transfection. Transfection efficiency was monitored by parallel transfection with pSV-β galactosidase vector (Promega, Madison, WI) and was stained for β-galactosidase expression.62
Expression analysis
Protein expression was analyzed by Western blot.62 Nuclear and cytoplasmic extracts were isolated as previously described63 and were quantitated by Bradford assay (Bio-Rad, Hercules, CA). Fifty micrograms nuclear or cytoplasmic proteins was separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels in Tris-glycine buffer. Proteins were transferred onto Hybond-P polyvinylidene difluoride (PVDF) membrane (Amersham, Arlington Heights, IL). Blots were incubated with primary antibody raised to an N-terminal Meis1 epitope (78-5) at 1:250 dilution and then with secondary antibody at 1:40 000 dilution. Bands were visualized with enhanced luminescence (Pierce, Rockford, IL).
Ecdysone-inducible expression
To generate ecdysone receptor-expressing cells, the pVgRXR plasmid (Invitrogen) encoding this receptor was transfected by calcium-phosphate transfection, and stable transfectants were selected using 200 μg/mL Zeocin (Cayla, Toulouse, France). The Meis1/pInd vector (Invitrogen) was transfected into RXR-expressing cells and was selected with 400 μg/mL G418. Expression was induced using 1 to 10 μM ponasterone A (Invitrogen).
Kinexus protein assay
The Kinexus Apoptosis Screen is a proprietary procedure of Kinexus (Vancouver, BC, Canada) for analyzing multiple protein expression.64 Briefly, total protein was extracted from cells in standard RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 2 mM EDTA [ethylenediaminetetraacetic acid], 10 mM NaF, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 20 μg/mL aprotinin). Aliquots from mock and experimental samples were size fractionated on separate SDS-PAGE gels and transferred to PVDF membranes, and each lane was probed with mixtures of up to 3 specific antibodies. After secondary antibody exposure, blots were visualized using enhanced chemiluminescence. Band intensities were measured using a Bio-Rad FluorS Max Multi-Imager, and signal intensities were quantitated using Quantity One software (BioRad, Hercules, CA). Mock-transfected sample protein levels were arbitrarily set to 0 and were compared with intensities from the experimental (Meis1b) sample.
Caspase inhibitors and apoptosis inducers
z-VAD-fmk, z-DEVD-fmk, z-IETD-fmk, z-LEHD-fmk, and z-VEHD-fmk (Calbiochem, San Diego, CA) and etoposide, staurosporine, and valinomycin (Sigma, St Louis, MO) were solubilized to working concentrations of 1 mM in dimethyl sulfoxide (DMSO). Appropriate volumes were added to each transfected culture to yield the desired concentration. To control for solvent effects, an aliquot of DMSO equivalent to the highest experimental concentration used was added to mock- and vector-transfected samples.
DAPI staining
Cells were centrifuged, media aspirated, and washed in 1 × phosphate-buffered saline (PBS; Gibco-BRL) and were fixed in 70% ethanol for 20 minutes. Cells were washed in 1 × PBS, and DAPI (4′, 6-diamino-2-phenylindol-dihydrochloride) was added to a final concentration of 1 μg/mL in 1 × PBS. Cells were incubated in the dark for 12 minutes, then washed and resuspended in 1 × PBS. Cells were visualized at 40 × magnification, 0.75 numerical aperture, under epifluorescence illumination with 330 to 380 excitation UV filter using a Nikon (Tokyo, Japan) Eclipse E600 microscope, and photomicrographs were taken using a 2.3.1 camera and Spot RT Software, v3.4.4 (Diagnostic Instruments, Los Angeles, CA).
Trypan blue assay
Cells were pelleted and resuspended in 1 mL media. Aliquots were diluted 1:1 in trypan blue (Gibco-BRL). Living and dead cells were counted by hemocytometer at 24-hour intervals for 5 days after transfection. At least 500 cells was scored per sample per day. Cell death ratios were determined and normalized to mock-transfected cells. Trials consisted of 3 replicates per construct or combination of constructs. Trials were repeated 3 times. Weighted means and standard deviations were calculated.
Results
Selection against Meis1 overexpression in stable cell lines
Because Meis1 expression is up-regulated in a subset of murine and human myeloid leukemias, we examined the effect of Meis1 overexpression in mammalian cell lines. We were unable to generate stable transfectants using standard mammalian expression vectors containing Meis1 cDNAs (data not shown). We hypothesized that Meis1 overexpression was toxic to cells. Therefore, we used 2 different inducible expression systems: the pMRE zinc–inducible vector65 and the pInd ecdysone–inducible vector (Invitrogen). Although antibiotic-resistant clones were obtained after transfection with both inducible expression vectors, no clone tested demonstrated increased or inducible Meis1 expression (data not shown). Given that inducible expression systems can produce low background expression, we conjectured that even low-level ectopic Meis1 expression is toxic to cells and is selected against.
Transient overexpression of Meis1a and Meis1b mediates apoptosis in Reh lymphoblastoma cells
To test whether Meis1 overexpression is selected against, Reh cells were analyzed for apoptosis after transient transfection of Meis1 expression constructs. The data demonstrate that Meis1a- and Meis1b-transfected cultures exhibited significantly higher apoptosis levels than vector-transfected cells, with apoptotic cells accumulating through the first 3 days after transfection (Figure 1A). The percentages of dead cells gradually declined because of the proliferation of nontransfected cells (data not shown). Transfection efficiency was assessed by parallel transfection with a lacZ-containing construct. By day 3, the percentage of apoptosis approached 100% of lacZ-positive cells in control cultures (data not shown). Meis1a and Meis1b produced indistinguishable levels of apoptosis in all experiments performed. Morphologically, affected cells displayed cytoplasmic blebbing characteristic of apoptotic cells. Selection of Meis1-transfected cells yielded no stable transfectants from any trials conducted.
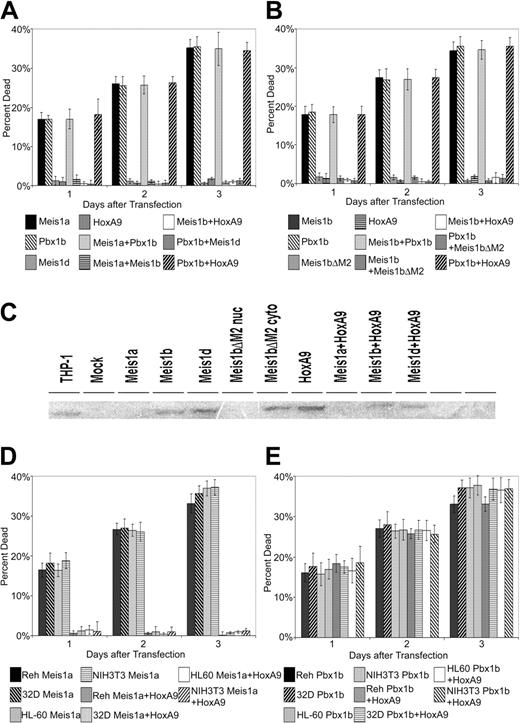
Overexpression of Meis1 and Pbx proteins induces apoptosis. Human Reh cells were transiently transfected with constructs expressing murine Meis1a or Meis1b or the murine Pbx proteins Pbx1b, Pbx3a, and Pbx3b by calcium-phosphate transfection. Cells were counted by trypan blue–dye exclusion assay at 1-day intervals after transfection. (A) Percentages of cell death were assayed at 1, 2, and 3 days after transfection for cells transiently transfected with Meis1a or Meis1b isoforms or with empty vector. Percentages of mock-transfected cultures were arbitrarily set to 0 to normalize data. Results are pooled means and standard deviations of 3 trials consisting of 3 replicate samples. (B) Percentages of cell death for cells transiently transfected with Pbx1b, Pbx3a, Pbx3b, or empty vector. (A-B) Transfection efficiency, 39%. (C-E) DAPI staining of transiently transfected cells. (C) Vector-transfected cells display uniform nuclear staining. (D-E) Murine Meis1b- and Pbx1b-transfected cells exhibit staining of condensed nuclear material, characteristic of apoptosis (arrows).
Overexpression of Meis1 and Pbx proteins induces apoptosis. Human Reh cells were transiently transfected with constructs expressing murine Meis1a or Meis1b or the murine Pbx proteins Pbx1b, Pbx3a, and Pbx3b by calcium-phosphate transfection. Cells were counted by trypan blue–dye exclusion assay at 1-day intervals after transfection. (A) Percentages of cell death were assayed at 1, 2, and 3 days after transfection for cells transiently transfected with Meis1a or Meis1b isoforms or with empty vector. Percentages of mock-transfected cultures were arbitrarily set to 0 to normalize data. Results are pooled means and standard deviations of 3 trials consisting of 3 replicate samples. (B) Percentages of cell death for cells transiently transfected with Pbx1b, Pbx3a, Pbx3b, or empty vector. (A-B) Transfection efficiency, 39%. (C-E) DAPI staining of transiently transfected cells. (C) Vector-transfected cells display uniform nuclear staining. (D-E) Murine Meis1b- and Pbx1b-transfected cells exhibit staining of condensed nuclear material, characteristic of apoptosis (arrows).
Given that Meis1 and Pbx family members can interact, and given that the E2A-Pbx1 fusion protein mediates apoptosis in Reh cells, we examined the ability of Pbx family members to induce apoptosis. The Pbx1b, Pbx3a, and Pbx3b isoforms were transfected into Reh cells. All 3 Pbx family members induced apoptosis comparable to that observed after Meis1 overexpression (Figure 1B). As with Meis-induced apoptosis, cell death levels peaked at nearly 40% by day 3. Antibiotic selection of Pbx-overexpressing cells yielded no stable transfectants. DAPI nuclear staining showed uniform staining of normal rounded nuclei in mock-transfected cells (Figure 1C), whereas Meis1b- and Pbx1b-transfected cells exhibited nuclear condensation characteristic of apoptosis (Figure 1D-E).
HoxA9 can abrogate Meis1a-mediated but not Pbx1b-mediated apoptosis
Because Hox proteins cooperatively bind DNA with Meis and Pbx proteins and because approximately 98% of BXH-2 lymphomas with a proviral integration at the Meis1 locus also have an integration at either the HoxA7 or the HoxA9 locus, we examined the effect of coexpressing HoxA9 on Meis1- and Pbx-mediated apoptosis. Meis1a or Pbx1b constructs were transfected alone or in combination with HoxA9 constructs. Empty vector was cotransfected along with single Meis, Pbx, or Hox constructs to control for total transfected DNA amounts. HoxA9 abrogated apoptosis when coexpressed with Meis1a (Figure 2A). Only low apoptosis levels comparable to those of vector-transfected cultures were observed. Cells transfected with Meis1a alone demonstrated high levels of apoptosis, approaching 40% by day 3. Interestingly, HoxA9 expression had no effect on Pbx1b-induced apoptosis (Figure 2B). This ability of HoxA9 to prevent Meis1-mediated apoptosis was proportional to the amount of HoxA9 transfected (Figure 2C), with apoptosis levels decreasing 12-fold, from 36% to 3%, as transfected Hox DNA increased from 1 to 5 μg and was specific for Meis1-induced apoptosis. In contrast to Pbx1b and HoxA9 cotransfected cultures, we were able to obtain stable clones from cultures cotransfected with Meis1a and HoxA9.
Coexpression of HoxA9 abrogates Meis1a-mediated but not Pbx1b-mediated apoptosis. (A-B) Effect of coexpression of murine HoxA9 with murine Meis1a (A) or murine Pbx1b (B). Total amounts of DNA were kept constant by transfecting empty vector with Meis1a- or Pbx1b-transfected cultures. Results are pooled means and standard deviations of 3 separate experiments consisting of 3 replicate samples. (A-B) Transfection efficiency, 39%. (C) Effects of increasing amounts of HoxA9 on Meis1b-transfected cells. Cells were transiently transfected with 5 μg murine Meis1b DNA alone or with 1 to 5 μg murine HoxA9 DNA. Total amounts of transfected DNA were kept constant using empty vector with single transfections. Transfection efficiency, 35%. (D) Resistance of Meis1b+HoxA9–expressing cells to induction of apoptosis by etoposide. Cells were transiently transfected with empty vector or murine Meis1b or were cotransfected with murine Meis1b and murine HoxA9. After 24 hours, 17 μM etoposide was added to each culture. Cell death was assayed at 1-day intervals after etoposide treatment. Transfection efficiency, 37%
Coexpression of HoxA9 abrogates Meis1a-mediated but not Pbx1b-mediated apoptosis. (A-B) Effect of coexpression of murine HoxA9 with murine Meis1a (A) or murine Pbx1b (B). Total amounts of DNA were kept constant by transfecting empty vector with Meis1a- or Pbx1b-transfected cultures. Results are pooled means and standard deviations of 3 separate experiments consisting of 3 replicate samples. (A-B) Transfection efficiency, 39%. (C) Effects of increasing amounts of HoxA9 on Meis1b-transfected cells. Cells were transiently transfected with 5 μg murine Meis1b DNA alone or with 1 to 5 μg murine HoxA9 DNA. Total amounts of transfected DNA were kept constant using empty vector with single transfections. Transfection efficiency, 35%. (D) Resistance of Meis1b+HoxA9–expressing cells to induction of apoptosis by etoposide. Cells were transiently transfected with empty vector or murine Meis1b or were cotransfected with murine Meis1b and murine HoxA9. After 24 hours, 17 μM etoposide was added to each culture. Cell death was assayed at 1-day intervals after etoposide treatment. Transfection efficiency, 37%
Coexpression of Meis1b and HoxA9 provides resistance to multiple apoptosis inducers
Based on the data in Figure 2A, we wanted to determine whether Meis1b/HoxA9 coexpression provides more generalized protection against proapoptotic stimuli. We exposed cotransfected cultures to concentrations of 3 apoptosis inducers (etoposide, staurosporine, and valinomycin) that produce massive apoptosis. Exposure of Reh cells transfected with either Meis1b or HoxA9 alone resulted in approximately 90% of cells undergoing apoptosis, whereas cells cotransfected with Meis1b plus HoxA9 demonstrated greatly reduced levels of apoptosis for etoposide (Figure 2D), staurosporine, and valinomycin (data not shown), comparable to those seen in nonexposed cultures.
Apoptosis-inducing ability of Meis1 requires the presence of the HD
E2A-Pbx1–induced apoptosis has previously been demonstrated to require the Pbx HD60 ; therefore, we wanted to test whether deleting the Meis1 HD would prevent it from inducing apoptosis. We used an isoform of Meis1, Meis1d, which lacks exon 8; this resulted in a truncated protein lacking an HD. Unlike Meis1a and Meis1b, Meis1d failed to induce apoptosis (Figure 3A), and stable transfectants of Meis1d could be produced. Therefore, we concluded that the apoptosis-inducing ability of Meis1 required a functional HD, possibly indicating that Meis1 induces apoptosis through transcriptional activation. Interestingly, the cotransfection of Meis1d with Meis1a or Pbx1b suppressed Meis- or Pbx-mediated apoptosis in a dominant-negative fashion (Figure 3A).
Ability of Meis1 to induce apoptosis is dependent on the presence of the HD and of the Pbx-interaction motif. (A) Reh cells were transfected with murine Meis1a, murine Meis1d (lacking the HD), or murine Pbx1b alone or in all possible cotransfection combinations. Total amounts of DNA were kept constant by transfecting empty vectors with single transfections. Results are pooled means and standard deviations of 3 separate experiments consisting of 3 replicate samples. Transfection efficiency, 38%. (B) Reh cells were transfected with murine Meis1b, murine Meis1bΔM2 (lacking the Pbx-interaction motif), or murine Pbx1b alone or in all possible cotransfection combinations. Transfection efficiency, 36%. (C) Nuclear extracts from transiently transfected Reh cells were separated on a 10% SDS-PAGE gel, blotted onto PVDF membrane, and probed with a polyclonal Meis1 antibody (78-5) directed at an epitope at the Meis1 N-terminus. THP-1 nuclear extract was used as a positive control for Meis1 expression, whereas mock-transfected Reh nuclear lysate was used to indicate baseline expression in Reh cells. Cytoplasmic extracts were used for cultures transfected with Meis1bΔM2 because this protein was expected to remain in the cytoplasm. (D) Murine Meis1a was transfected into human Reh lymphoblastoma cells, human HL-60 promyelomonocytic cells, murine 32Dcl3 pre–B-cell leukemia, and murine NIH3T3 fibroblasts. Apoptosis was scored as previously described with the exception of NIH3T3 cells, for which detached cells in the media were collected and added to trypsinized cells for the purpose of trypan blue counting. (E) Murine Pbx1b was transfected into the 4 cell types described in panel D. (D-E) Transfection efficiencies: Reh, 39%; HL-60, 36%; 32D, 37%; NIH3T3, 32%
Ability of Meis1 to induce apoptosis is dependent on the presence of the HD and of the Pbx-interaction motif. (A) Reh cells were transfected with murine Meis1a, murine Meis1d (lacking the HD), or murine Pbx1b alone or in all possible cotransfection combinations. Total amounts of DNA were kept constant by transfecting empty vectors with single transfections. Results are pooled means and standard deviations of 3 separate experiments consisting of 3 replicate samples. Transfection efficiency, 38%. (B) Reh cells were transfected with murine Meis1b, murine Meis1bΔM2 (lacking the Pbx-interaction motif), or murine Pbx1b alone or in all possible cotransfection combinations. Transfection efficiency, 36%. (C) Nuclear extracts from transiently transfected Reh cells were separated on a 10% SDS-PAGE gel, blotted onto PVDF membrane, and probed with a polyclonal Meis1 antibody (78-5) directed at an epitope at the Meis1 N-terminus. THP-1 nuclear extract was used as a positive control for Meis1 expression, whereas mock-transfected Reh nuclear lysate was used to indicate baseline expression in Reh cells. Cytoplasmic extracts were used for cultures transfected with Meis1bΔM2 because this protein was expected to remain in the cytoplasm. (D) Murine Meis1a was transfected into human Reh lymphoblastoma cells, human HL-60 promyelomonocytic cells, murine 32Dcl3 pre–B-cell leukemia, and murine NIH3T3 fibroblasts. Apoptosis was scored as previously described with the exception of NIH3T3 cells, for which detached cells in the media were collected and added to trypsinized cells for the purpose of trypan blue counting. (E) Murine Pbx1b was transfected into the 4 cell types described in panel D. (D-E) Transfection efficiencies: Reh, 39%; HL-60, 36%; 32D, 37%; NIH3T3, 32%
Pbx-interaction motif of Meis1 is necessary for induction of apoptosis
Meis1 is known to interact with Pbx using motifs in the conserved N-terminal Meinox domain resulting in nuclear localization of the Meis/Pbx complex.15,20 The Meis1bΔM2 construct deletes the M2 motif required for Meis1 to interact with Pbx. Meis1bΔM2 fails to induce apoptosis after transfection into Reh cells (Figure 3B) and stable Meis1bΔM2 transfectants can be selected. As with Meis1d, cotransfecting Meis1bΔM2 with either Meis1a or Pbx1b suppresses their ability to induce apoptosis (Figure 3B). Expression of Meis1 constructs was verified by Western blot (Figure 3C).
Meis and Pbx can induce apoptosis in a variety of cell types of murine and human origin
We next wanted to determine whether the ability of Meis and Pbx to induce apoptosis was restricted to lymphoid cells or if it was a more general capability. Therefore, we repeated our experiments in HL-60 human promyelomonocytic cells, 32Dcl3 murine lymphoblastic cell line, and murine NIH-3T3 fibroblast cells. Meis1a (Figure 3C) or Pbx1b (Figure 3D) induced apoptosis in all the cell lines tested and at comparable levels to apoptosis induced in Reh cells. Results demonstrated that the ability of Meis1 and Pbx1 to induce apoptosis is not restricted to lymphoid cells but includes myeloid and nonhematopoietic cells. Because HL60 cells lack functional p53,66 Meis1 and Pbx1 induce apoptosis through a p53-independent pathway.
Meis1b expression affects cellular levels of multiple apoptosis pathway proteins
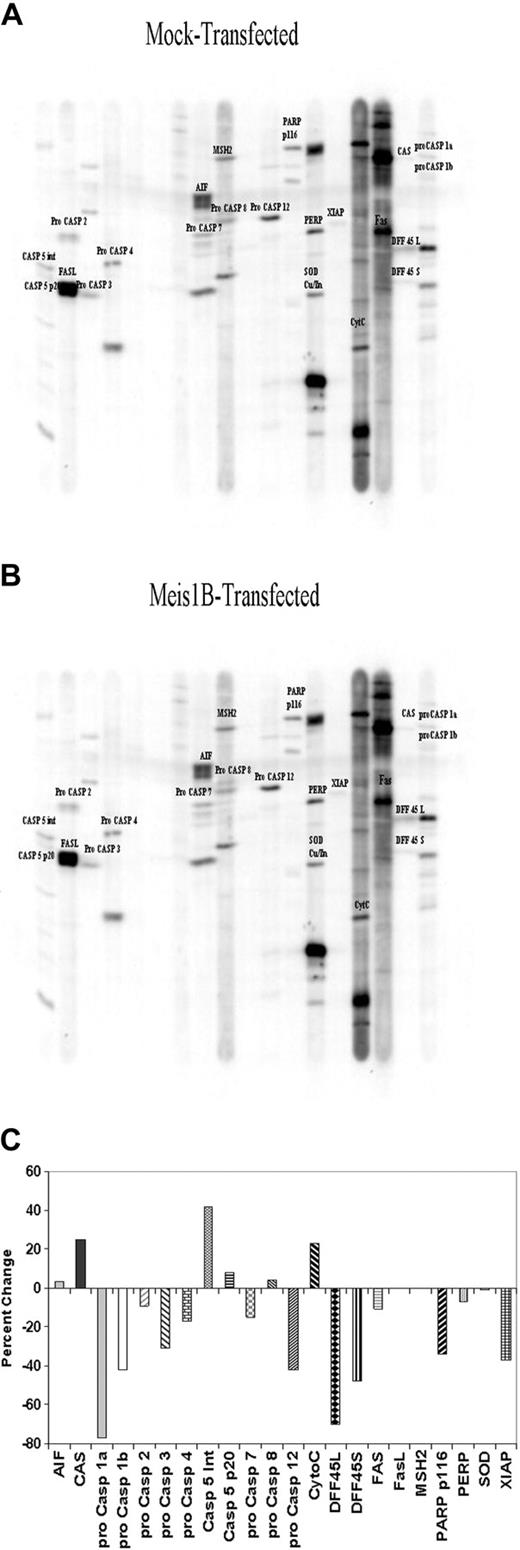
Because apoptosis can be induced through a variety of intrinsic and extrinsic pathways, we examined the effects of Meis1b expression on levels of a variety of 25 proteins relevant to apoptosis using the Kinexus Apoptosis Protein Screen.63 Protein lysates from mock-transfected and Meis1b-transfected cells were loaded onto separate SDS-PAGE gels, electrophoresed, and probed using a panel of specific antibodies (Figure 4A-B). Protein expression levels were estimated by measuring the area of the relevant band's intensity profile. Levels of each protein in the mock-transfected sample were considered baseline expression levels and were used to calculate percentage differences in the Meis1b-transfected lysates (Figure 4C). Results indicated that Meis1b expression reduced the amount of 2 inhibitors of apoptosis: X-linked inhibitor of apoptosis (XIAP), which decreased 57%, and the long and short forms of DFF45, which decreased 70% and 48%, respectively. Levels of full-length poly-(ADP-ribosylation) protein (PARP) decreased 34%, and the amount of PERP decreased 7%. Several procaspases also decreased in Meis1b-transfected lysates, indicating cleavage into activated caspases. Specifically, decreases were seen in procaspase 1α (77%), procaspase 1β (42%), procaspase 2 (9%), procaspase 3 (17%), procaspase 4 (17%), procaspase 7 (15%), and procaspase 12 (42%) levels. Two processed forms of caspase 5 increased in the Meis1b-expressing sample: the 35-kDa intermediate form increased 42%, and the activated 22-kDa form increased 8%. Cellular apoptosis susceptibility (CAS) protein levels increased 25%, and, finally, cytochrome c levels increased 23%.
Kinexus apoptosis screen shows multiple changes in protein levels. Western blots of mock-transfected (A) and Meis1b-transfected (B) protein lysates. Each lane was probed with up to 3 different specific primary antibodies followed by incubation with the appropriate secondary antibody and was visualized using enhanced luminescence. (C) Protein expression levels from lysates derived from mock-transfected and murine Meis1b-transfected cells were analyzed from SDS-PAGE gels probed with multiple apoptosis protein-specific antibodies. Band intensities were measured for each protein. The analysis algorithm used is based on the assumption that equal levels of loaded protein will have equal intensities. In generating the final report, the algorithm determines the total counts on each blot and corrects each band's count such that the resultant total counts on the blots equal the total average counts among all blots. Levels of mock-transfected samples were arbitrarily set to 0 for each protein and were compared with Meis1b-transfected samples. Graph shows the percentage difference from the mock sample. Transfection efficiency, 36%.
Kinexus apoptosis screen shows multiple changes in protein levels. Western blots of mock-transfected (A) and Meis1b-transfected (B) protein lysates. Each lane was probed with up to 3 different specific primary antibodies followed by incubation with the appropriate secondary antibody and was visualized using enhanced luminescence. (C) Protein expression levels from lysates derived from mock-transfected and murine Meis1b-transfected cells were analyzed from SDS-PAGE gels probed with multiple apoptosis protein-specific antibodies. Band intensities were measured for each protein. The analysis algorithm used is based on the assumption that equal levels of loaded protein will have equal intensities. In generating the final report, the algorithm determines the total counts on each blot and corrects each band's count such that the resultant total counts on the blots equal the total average counts among all blots. Levels of mock-transfected samples were arbitrarily set to 0 for each protein and were compared with Meis1b-transfected samples. Graph shows the percentage difference from the mock sample. Transfection efficiency, 36%.
Meis- and Pbx-mediated apoptosis are caspase 8 and caspase 3 dependent
Given these results demonstrating changes in many procaspase protein levels, we investigated whether Meis- and Pbx-mediated apoptosis were caspase-dependent. To examine this, we incubated transiently transfected Reh cells with a variety of peptide caspase inhibitors67 and measured their effect on the apoptosis-inducing ability of Meis or Pbx. Meis1b-transfected cells were incubated with varying concentrations of the pan-caspase inhibitor z-VAD-fmk. Dose-dependent inhibition of apoptosis was observed, with 5 μM concentrations producing levels of apoptosis equivalent to levels in vector-transfected cells (Figure 5A). A similar effect was seen in Pbx1b-transfected cultures (Figure 5B), with apoptosis levels decreasing in a concentration-dependent fashion.
Meis1- and Pbx-mediated apoptosis is caspase dependent. Increasing concentrations of the pan-caspase inhibitor z-VAD-fmk were added to cells transfected with either murine Meis1b (A) or murine Pbx1b (B) and were assayed for cell death. Apoptosis was assayed as previously described. (C-D) Caspase-specific inhibitors were added to cells transfected with murine Meis1b or murine Pbx1b and were assayed for cell death. Results displayed are from the addition of 5 μM of all inhibitors used. (A-D) Transfection efficiency, 36%.
Meis1- and Pbx-mediated apoptosis is caspase dependent. Increasing concentrations of the pan-caspase inhibitor z-VAD-fmk were added to cells transfected with either murine Meis1b (A) or murine Pbx1b (B) and were assayed for cell death. Apoptosis was assayed as previously described. (C-D) Caspase-specific inhibitors were added to cells transfected with murine Meis1b or murine Pbx1b and were assayed for cell death. Results displayed are from the addition of 5 μM of all inhibitors used. (A-D) Transfection efficiency, 36%.
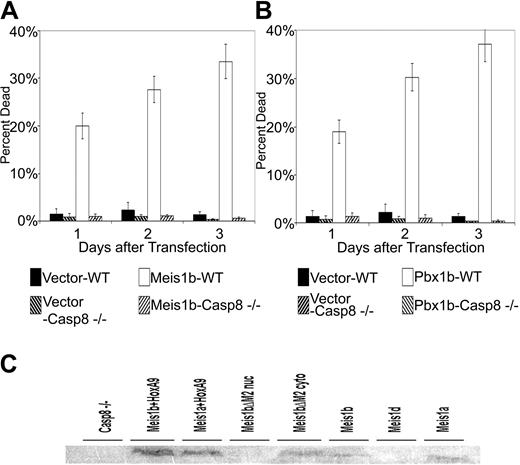
We next incubated transfected cultures with the following inhibitors: z-DEVD-fmk (caspase 3), z-VEID-fmk (caspase 6), z-IETD-fmk (caspase 8), and z-LEHD-fmk (caspase 9). The z-VEID and z-LEHD inhibitors showed no effect on either Meis1-mediated apoptosis (Figure 5C) or Pbx1b-mediated apoptosis (Figure 5D) at all concentrations used (data not shown). However, the z-DEVD and z-IETD inhibitors reduced apoptosis levels to baseline for both proteins (Figure 5A-B) in a concentration-dependent manner (data not shown). As a final test of the dependence of Meis1b- and Pbx1b-mediated apoptosis on caspase 8, we transiently transfected our constructs into wild-type Jurkat T cells and mutant Jurkat T cells functionally null for caspase 8 expression.68 Transient transfection of wild-type Jurkat T cells with Meis1b produced apoptosis levels closely resembling those observed in Reh lymphoblastoma cells, approaching 40% by day 3 after transfection (Figure 6A). However, transfection of Meis1b into caspase 8–null Jurkat T cells produced only baseline apoptosis levels. Finally, similar results were seen in transfections using Pbx1b. Again, expected levels of Pbx1b-induced apoptosis were observed in wild-type Jurkat T cells (Figure 6B) but not in the caspase 8–null cells. Stable cell lines were easily generated using Meis1 constructs, even in the absence of HoxA9 coexpression. Meis1 expression in these cell lines was examined by Western blot (Figure 6C). The observed lack of apoptosis was not caused by the resistance of caspase 8-/- cells to apoptosis induction because exposure of these cells to apoptosis inducers, such as etoposide and staurosporine, produced 90% apoptosis within 24 hours of exposure to these agents (data not shown).
Meis1b and Pbx1b cannot induce apoptosis in caspase 8–null Jurkat T cells. Wild-type and caspase 8–null Jurkat T cells were transiently transfected with murine Meis1b (A) or murine Pbx1b (B) and were assayed for cells undergoing apoptosis at 1-day intervals after transfection. (A-B) Transfection efficiency, 38%. (C) Nuclear lysates from stably transfected Jurkat caspase 8–null cells were separated on a 10% SDS page gel, blotted onto PVDF membrane, and probed with Meis1 antibody 78-5. Nontransfected Jurkat caspase 8–null nuclear extracts were included to assess baseline expression of Meis1 in these cells. Cytoplasmic extract was included for the cell line transfected with Meis1bΔM2.
Meis1b and Pbx1b cannot induce apoptosis in caspase 8–null Jurkat T cells. Wild-type and caspase 8–null Jurkat T cells were transiently transfected with murine Meis1b (A) or murine Pbx1b (B) and were assayed for cells undergoing apoptosis at 1-day intervals after transfection. (A-B) Transfection efficiency, 38%. (C) Nuclear lysates from stably transfected Jurkat caspase 8–null cells were separated on a 10% SDS page gel, blotted onto PVDF membrane, and probed with Meis1 antibody 78-5. Nontransfected Jurkat caspase 8–null nuclear extracts were included to assess baseline expression of Meis1 in these cells. Cytoplasmic extract was included for the cell line transfected with Meis1bΔM2.
Discussion
The studies presented here demonstrate that the HD proteins Meis1a and Meis1b potently induce apoptosis in a variety of human and murine cell types. This adds a new level of complexity to the web of interactions between the Meis, Hox, and Pbx protein families. On transient transfection of Meis1a or Meis1b, Reh lymphoblastoma cells undergo apoptosis, with nearly 40% of the total culture dying within 3 days of transfection. Based on control staining of LacZ-transfected cells, we estimate that nearly 100% of transfected cells undergo apoptosis within 3 days. Affected cells exhibited characteristic apoptotic morphology, such as membrane blebbing, and DAPI staining of transfected cells confirmed the presence of nuclear condensation in apoptotic cells. Selection of parallel cultures transfected with Meis1a or Meis1b produced no surviving cells, again indicating that effectively 100% of Meis1-positive cells had undergone apoptosis.
Pbx1b, Pbx3a, and Pbx3b yielded the same results on transfection into Reh cells. This was not unexpected because E2A-Pbx1 transgenic mice, in addition to demonstrating increased proliferation, sustain increased apoptosis of lymphoid cells.59 Additionally, E2A-Pbx1 induces apoptosis in Reh and HL-60 cells on induction of expression.60 This apoptosis was p53 independent and was suppressed by coexpressing Bcl2. Because Meis and Pbx induce apoptosis in myeloid, lymphoid, and fibroblast cells of human and murine origin, this ability is not cell type or species specific and may reflect a general consequence of Meis or Pbx dysregulation.
Of great interest to us was the effect coexpressing HoxA9 had on Meis1- and Pbx1-mediated apoptosis. HoxA9 completely prevents apoptosis in cells overexpressing Meis1. Cell death levels observed were indistinguishable from vector-transfected cultures. Apoptosis levels were inversely proportional to the concentration of HoxA9, indicating that the ectopic HoxA9 protein mediated this suppression, possibly because of increased HoxA9 levels quenching Meis-induced apoptosis. Interestingly, HoxA9 coexpression did not affect Pbx-induced apoptosis. Taken in conjunction with extensive evidence in the literature demonstrating a strong bias for Meis-HoxA7 or Meis-HoxA9 coexpression in myeloid leukemia,21-24,48 this argues that coexpression confers a powerful selective advantage not present for singly expressing cells. Given that Pbx does not interact with HoxA9, it is perhaps not surprising that Pbx-mediated apoptosis is not affected by HoxA9 expression, raising the possibility that other Hox proteins capable of interaction with Pbx may provide a similar protective function.
Of particular significance are our data demonstrating that coexpressing Meis1 and HoxA9 not only protect against Meis-mediated apoptosis, they provide more generalized protection against apoptosis inducers, such as etoposide, staurosporine, and valinomycin; such protection is not conferred by the expression of HoxA9 alone. Each of these inducers operates differently to induce apoptosis. Etoposide inhibits DNA synthesis, producing double- and single-stranded breaks.69 Staurosporine operates as a phospholipid/calcium-dependent protein kinase inhibitor, preventing adenosine triphosphate (ATP) binding.70 Valinomycin induces mitochondrial potential (ΔΨM) disruption.71 Immunity from multiple apoptosis–inducting pathways would provide strong selective pressure for coexpression of Meis1 and HoxA9 not offered by the transformation potential of HoxA9 alone. This could conceivably allow cells to survive multiple subsequent mutations without triggering apoptosis, thereby decreasing the latent period for oncogenesis in vivo.
Previous studies have demonstrated that Meis1 can be expressed in murine bone marrow, seemingly in contradiction to the results presented here.48-50 The obvious difference is that Meis1 overexpression was achieved in long-term repopulating–enriched populations in those studies. Hox gene expression is elevated in this population compared with total bone marrow.72 Thus, it may be possible for these cells to tolerate higher Meis1 expression because of higher endogenous levels of potentially protective Hox proteins. Additionally, Meis1-transduced bone marrow cells were selected on the basis of antibiotic resistance.48-50 We isolated antibiotic-resistant clones, but they did not sustain elevated expressions of Meis1. Low proviral signals were obtained in Southern blot analysis of marrow isolated from chimeric mice produced with Meis1 or Pbx singly transduced cells. We speculated that this might be caused by low-level repopulation by these transduced cells, suggesting a proliferative or survival disadvantage to these cells.
Data obtained from the transfection of Meis1d into cells indicate that the presence of Meis1HD is necessary for Meis1 to induce apoptosis. Meis1d is generated by alternative splicing, producing a Meis1 cDNA lacking exon 8. The resultant protein is identical to Meis1a and Meis1b through the first 228 amino acids, followed by 5 unique amino acids and premature termination. This results in a protein completely lacking an HD. We also found that cotransfection of Meis1d with apoptosis-inducing forms of Meis1 or Pbx results in the suppression of apoptosis in a dominant-negative fashion. Expression of this isoform could conceivably have wide-ranging effects in vivo. It has recently been reported that a Meis1 knockout mouse exhibits a variety of developmental effects resulting in lethality by 14.5 days after conception.73 The abnormalities in hematopoiesis observed include a complete lack of megakaryocytes, a dramatic decrease in the total number of colony-forming units of the myeloerythroid lineages, and a decreased ability to compete in the repopulation all hematopoietic lineages. Interestingly, it is possible that this mouse, instead of representing a true Meis1 knockout, instead expresses a truncated HD-less form of Meis1 and that the defects seen are the result of the dominant-negative properties of this isoform.
The functions of Meis and Pbx are often difficult to separate because of their mutual requirement for nuclear translocation. Our results show that the overexpression of Meis1a, Meis1b, or any of the Pbx isoforms induces comparable levels of apoptosis with similar kinetics through a p53-independent, caspase 8–dependent pathway. It appears that Meis1 and Pbx can activate the same apoptosis pathway, but it is unclear whether they act in concert to produce this effect. Our data demonstrate that the Meis1bΔM2 mutant lacking the M2 motif in the Meinox domain is incapable of inducing apoptosis. This, however, may be solely attributed to the need for Meis1 to interact with Pbx to access the nucleus.54-56 Pbx1 also uses this interaction to enter the nucleus, but it can enter the nucleus independently of Meis1.74 E2A-Pbx1, which lacks the Pbx N-terminus that interacts with Meis1, can enter the nucleus and induce apoptosis independently of Meis1.60 An experiment in which Meis1bΔM2 is linked to a Pbx-independent nuclear localization signal will allow us to discern whether the seeming requirement for Pbx is due solely the need for Meis1 to enter the nucleus.
Meis1d and Meis1bΔM2 may exert their dominant-negative effects by different mechanisms. Meis1d can complex with Pbx and is capable of entering the nucleus. Its failure to induce apoptosis possibly results from its inability to activate downstream target gene transcription, irrespective of the amount of Pbx present. Meis1bΔM2, however, does not interact with Pbx and cannot enter the nucleus. Therefore, it cannot function as a transcription factor. Coexpression of Pbx with Meis1bΔM2 may have no proapoptotic effect simply because Meis1bΔM2 can form dimers with endogenous Meis1b, preventing importation into the nucleus with Pbx.
Protein expression data of cells undergoing Meis1b-induced apoptosis will allow us to begin elucidating mechanisms behind this apoptotic effect. Multiple procaspase protein levels decrease on activation of Meis1b-induced apoptosis, arguing that procaspases are processed into active forms. Additionally, we show that the pan-caspase inhibitor zVAD-fmk totally inhibits Meis1b- and Pbx1b-mediated apoptosis, as do the caspase 8–specific (Z-IETD-fmk) and caspase 3–specific (Z-DEVD-fmk) inhibitors. Taken together, these results support the contention that Meis1b- and Pbx1b-mediated apoptosis occurs through a caspase 8–dependent pathway because caspase 3 is a major effector of caspase 8–mediated apoptosis. The observation that Meis1b and Pbx1b cannot induce apoptosis in caspase 8–null Jurkat T cells supports this result.
Our data support the hypothesis that Meis1 overexpression may trigger the apoptotic program to defend against uncontrolled cellular proliferation. Meis1-overexpressing cells exhibit perturbations in several genes regulating apoptosis. Anti-apoptotic proteins such as XIAP,75 DFF45,76,77 and PARP78 decrease on Meis1b expression, whereas proapoptotic proteins cytochome c79-81 and CAS82-84 increase. Additionally, multiple procaspase protein levels decrease, indicating processing to active forms. Inhibition of caspase 8 and caspase 3 suppress Meis1b- and Pbx1b-mediated apoptosis, and these proteins cannot induce apoptosis in caspase-null Jurkat T cells. Intriguingly, the Drosophila homolog of Meis1, homothorax, induces apoptosis through a JNK-dependent pathway.85 Meis1b overexpression results in increased levels of several proteins involved in tumor necrosis factor (TNF)–mediated apoptosis that controls JNK activation.
Given these data, it is possible to envision a model (Figure 7) in which Meis/Pbx complexes form in the cytoplasm, allowing entry into the nucleus. Then Meis1, either alone or cooperating with Pbx, modulates target gene transcription activating apoptosis in a caspase 8–dependent manner. However, if sufficient quantities of HoxA9 are present, Meis/Hox complexes form and modulate the transcription of other target genes, which act to suppress Meis-induced, but not Pbx-induced, apoptosis. This suppression could act at any of several points, allowing suppression of both caspase 8–dependent and caspase 8–independent pathways. HoxA9 may fail to suppress Pbx-induced apoptosis because of its inability to directly interact with Pbx. Alternatively, it could leave at least one of the caspase 8–dependent apoptosis pathways intact.
Generalized pathway showing various possible interactions of Meis1, Pbx, and HoxA9 in the induction and suppression of apoptosi
Generalized pathway showing various possible interactions of Meis1, Pbx, and HoxA9 in the induction and suppression of apoptosi
In summary, our most significant findings are that coexpressing HoxA9 suppresses Meis1-but not Pbx1b-mediated apoptosis and that this suppression is not limited to Meis-induced apoptosis but is more generalized. This suppression of Meis-mediated apoptosis may provide an explanation for observations that in many human and murine tumors, Meis1 up-regulation is concurrent with the up-regulation of HoxA7 or HoxA9, which greatly reduces the latency of tumors transformed by HoxA9 in murine bone marrow. Meis and Hox coexpression, by conferring protection against several apoptotic stimuli, may provide a selective advantage during oncogenesis, permitting cells to accumulate additional growth stimulatory mutations without incurring the risk for triggering the cell's default apoptotic machinery. This presents a potential therapeutic target for treating tumors exhibiting up-regulated Meis1/HoxA9 expression. Targeting HoxA9 expression while leaving Meis1 expression intact may eliminate the advantage of HoxA9 coexpression, leaving only Meis1 apoptotic effects.
Prepublished online as Blood First Edition Paper, October 12, 2004; DOI 10.1182/blood-2004-03-0802.
Supported by National Institutes of Health research grant CA-81358 (A.M.B., P.J.W.) and training grant T32-HL07780 (P.J.W.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jeffrey Montgomery for the pspFlag-Meis1bΔM2 construct. We thank Drs Linda D. Siracusa, Jianke Zhang, and Jay L. Rothstein for stimulating discussions and constructive critical reading of the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal