Abstract
Arsenic trioxide (As2O3) is an effective agent for the treatment of relapsed and refractory acute promyelocytic leukemia by induction of partial differentiation and apoptosis. As2O3, at therapeutic concentrations (1-2 μM), induced apoptosis in Raji lymphoma cells but not in Jurkat lymphoma cells, which inversely correlated with the levels of glutathione-S-transferase π (GSTP1), but not GSTπ1 and GSTM1, expression and activity. GSTP1 mRNA, protein level, and activity were high in Jurkat cells but undetectable in Raji cells. Stable transfection of GSTP1 into Raji cells decreased the amount of As2O3-induced apoptosis. Apoptosis induced by therapeutic concentrations of As2O3 in Raji cells is related to increasing H2O2 intracellular accumulation but not to JNK activation. Forced expression of GSTP1 by transfection of Raji cells significantly decreased the basal amount of H2O2 and its levels after therapeutic concentration of As2O3 treatment. Added exogenous H2O2 was removed more rapidly, which correlated with a greater decrease in reduced glutathione level in Raji clones expressing GSTP1 than in those clones without GSTP1 expression. Overexpression of GSTP1 in transfected Raji clones was also found to decrease the retention of As2O3. These data suggest that GSTP1 blocks As2O3-induced apoptosis in lymphoma cells by decreasing intracellular amounts of H2O2 by catabolism and H2O2 production by decreasing the intracellular retention of As2O3.
Introduction
Arsenic trioxide (As2O3) is an effective agent for the treatment of relapsed and refractory acute promyelocytic leukemia (APL) and acts by inducing apoptosis and partial differentiation.1-3 Although As2O3 induces apoptosis in other forms of malignant tumors, different mechanisms of action have been identified. In some leukemia and lymphoma cells, As2O3, at therapeutic concentrations (1-2 μM), induces apoptosis through radical oxygen species–mediated pathways.4-8 Moreover, in leukemia cells, the H2O2 scavenging systems (glutathione peroxidase and catalase) and arsenic detoxification systems (glutathione-S-transferase and glutathione) are key factors in controlling cell sensitivity to As2O3-induced apoptosis.4 However, in solid tumors, As2O3, at high concentrations (greater than 10 μM), is required to induce apoptosis that involves the Jun N-terminal kinase (JNK)–mediated pathway.9-13
Glutathione-S-transferase π (GSTP1), a member of the As2O3 detoxification pathway, is increased in several arsenic-resistant cell lines.14-18 As2O3-sensitive NB4 cells have low levels of GSTP1 compared with other leukemia cell lines.4 These observations suggest that GSTP1 may be directly involved in As2O3-induced apoptosis. Jurkat cells are less sensitive to arsenic-induced apoptosis than Raji cells,19 and GSTP1 is highly expressed in Jurkat cells but undetectable in Raji cells.20 In the present report, the role of GSTP1 in As2O3-induced apoptosis was studied in these 2 lymphoma cell lines. Raji cells were stably transfected with GSTP1, and the effects of GSTP1 on apoptosis, H2O2 accumulation, intracellular accumulation of arsenic, and JNK activation were compared in GSTP1-expressing and nonexpressing cells. It was found here that the overexpression of GSTP1 in Raji cells decreased the ability of therapeutic concentrations of As2O3 to induce apoptosis. As2O3-treated GSTP1-expressing Raji cells were also found to accumulate less intracellular H2O2 and As2O3 than Raji cells not expressing GSTP1, demonstrating 2 mechanisms for GSTP1 mediation of As2O3-induced apoptosis.
Materials and methods
Reagents
Arsenic (III) oxide (As2O3, at least 99% pure) and anticatalase monoclonal antibody were purchased from Sigma (St Louis, MO); G418 sulfate was purchased from Fisher Scientific (Pittsburgh, PA); anti-GST P1-1, anti-GST M1-1, and anti-GST A1-1 polyclonal antibodies were purchased from Calbiochem (La Jolla, CA); and antiphospho-SAPK/JNK (Thr183/Tyr185) monoclonal antibody (anti–p-JNK), anti-SAPK/JNK polyclonal antibody, antiphospho-p38 mitogen-activated protein (MAP) kinase (Thr180/Thr182) polyclonal antibody, and anti-p38 MAP kinase polyclonal antibody were purchased from Cell Signaling Technology (Beverly, MA). Antiglutathione peroxidase was obtained from Abcam (Cambridge, MA). Carrier-free [73As]-arsenite (73Asv) was purchased from Los Alamos Meson Production Facility (Los Alamos, NM). [73As]-arsenate (73AsIII) was prepared from 73Asv by reduction with metabisulfite/thiosulfate reagent.34 Yields of 73AsIII in this reaction typically exceeded 95%, as determined by thin-layer chromatography (TLC).21
Cell lines and culture conditions
Raji and Jurkat human lymphoma cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and were cultured in RPMI 1640 medium adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES (N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid), 1.0 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA), and 10% fetal bovine serum (JRH BioScience, Lenexa, KS) in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Stable transfection of GSTP1
Raji cells were transfected by electroporation with a pcDNA3.1 plasmid with or without a GSTP1 expression sequence (kindly provided by Dr Ze'ev Ronai, Mount Sinai School of Medicine, New York). Briefly, 106 Raji cells in 1 mL mixed with 20 μg pcDNA3.1/GSTP1 plasmid were transferred to sterile electroporation cuvettes (Bio-Rad, Hercules, CA) and were electroporated in a GenePulser (Bio-Rad) with the voltage set at 300 V and the capacitor at 250 μF. After transfection, the cells were incubated in fresh medium containing 1 mg/mL G418 for 4 weeks. Subsequently, cell clones resistant to G418 were isolated and screened by limited dilution. Two GSTP1-expressing and 2 GSTP1-nonexpressing cell clones were selected for further study. Transfected Raji cell clones were routinely cultured in medium containing 1 mg/mL G418 and then were cultured for at least 24 hours without G418 before an experiment was initiated.
Viability assay
Cells were seeded at 1.5 to 2 × 105 cells/mL and were cultured in medium described with or without the indicated concentrations of test compounds for the times indicated. Cell viability was estimated by trypan blue dye exclusion, and cell numbers were determined by hemocytometer.
Apoptosis assay
Apoptotic cells were detected by Annexin V assay. In general, 106 cells were washed twice with phosphate-buffered saline (PBS), then labeled by Annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) in binding buffer according to the instructions in the Annexin V–FITC Apoptosis Detection Kit provided by the manufacturer (Oncogene, Cambridge, MA). Fluorescence signals of FITC and PI were detected by FL1 (FITC detector) at 518 nm and FL2 at 620 nm, respectively, on a FACScan (Becton Dickinson, San Jose, CA). The log of Annexin V–FITC fluorescence was displayed on the x-axis, and the log of PI fluorescence was displayed on the y-axis. Data were analyzed using the CELLQuest (Becton Dickinson) software. For each analysis, 10 000 events were recorded.
Intracellular H2O2 production
Intracellular H2O2 level was detected as previously reported by using 5,6-carboxy-2′,7′-dichlorofluorescein-diacetate (DCFH-DA; Molecular Probes, Eugene, OR).4 Briefly, 2 hours before ending the indicated treatment, 5 μM DCFH-DA was added to the medium and was continuously incubated for 2 hours at 37°C; then the fluorescence intensity was measured by FACScan (Becton Dickinson).
GSTP1-1 activity assay
GSTP1-1 activity was measured as previously described using 1-chloro-2, 4-dinitrobenzene (CDNB) as a high-affinity substrate and compared with that of other GST isoforms.22 Briefly, 5 × 107 cells were washed twice with cold PBS, resuspended in 300 μL of 100 mM potassium phosphate buffer (pH 6.8), and sonicated for 10 seconds at 4°C. After centrifugation at 17 000 g for 30 minutes, 50 μL cell lysate were mixed with 850 μL of 0.1 mM EDTA (ethylenediaminetetraacetic acid) (pH 6.5), 50 μL of 20 mM glutathione, and 50 μL of 20 mM CDNB. Absorbance of the mixture was continuously recorded for 2 minutes at 340 nm on a spectrophotometer (Ultrospec 2000; Pharmacia Biotec, Uppsala, Sweden).
Measurement of intracellular glutathione
Intracellular glutathione (GSH) contents were measured using the Glutathione Assay Kit (Calbiochem, San Diego, CA). In brief, 5 × 106 cells were homogenized in 5% metaphosphoric acid using a Teflon pestle (Racine, WI). Particulate matter was separated by centrifugation at 4000g. Supernatant was used for GSH measurement according to the manufacturer's instruction. The GSH content was expressed as nmol/106 cells.
Western blot analysis
Cells were centrifuged, washed with cold PBS, and lysed on ice for 30 minutes in RIPA buffer (1 × PBS, 1% nonidet P-40 [NP40], 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 5 mM NaF, 1 × protease inhibitor [Boehringer Mannheim GmbH, Mannheim, Germany]). Protein concentrations were determined with a Bio-Rad protein assay kit (Bio-Rad). Twenty-five micrograms to 70 μg total protein was electrophoresed on 8% to 12% SDS polyacrylamide gels, then transferred to a nitrocellulose membrane (Amersham, Piscataway, NJ). After incubating with 5% nonfat milk (Nestle Carnation) for an hour, the membrane was incubated with the indicated primary antibody overnight at 4°C, washed with Tris-buffered saline (pH 6.8) with Tween-20 (TBS-T) 3 times, incubated with secondary antibody for 1 hour at room temperature, and washed with TBS-T 3 times. The membrane was analyzed by autoradiography using a chemiluminescence kit (Amersham Life Science, Buckinghamshire, United Kingdom).
Northern blot analysis
Total RNA was isolated from 106 cells with an RNA isolation kit (Gentra, Minneapolis, MN). Twenty micrograms RNA was size fractionated on a 1.2% agarose–2.2 M formaldehyde gel, transferred to hybrid-N+ membrane (Amersham) in 20 × standard sodium citrate (SSC) solution, and UV cross-linked (Stratalinker; Stratagene, La Jolla, CA). Whole-length complementary DNA (cDNA) of GSTP1 was used as the probe. Probes were labeled with 32P-dCTP by random priming to a specific activity of 0.5 to approximately 1 × 109 cpm/ng. Membranes were prehybridized for 4 hours at 42°C in 50% formamide, 6 × sodium chloride, sodium phosphate, EDTA (SSPE), 5 × Denhardt reagent, and 0.2 mg/mL salmon sperm DNA and then were hybridized with a radiolabeled probe. Membranes were washed twice in 6 × SSC containing 0.1% SDS, followed by a stringent wash with 0.2 × SSC containing 0.1% SDS at 65°C.
73As retention assay
Cells (106) were incubated in complete medium with 1 μM 73AsIII–carrier at 37°C in a humidified atmosphere containing 5% CO2 for 24 hours. Cell were centrifuged at 500 rpm for 5 minutes and washed twice with PBS. Radioactivity in the sediment and in the medium plus washes was determined with the aid of a gamma counter.
Statistical analysis
Data were analyzed for statistical significance using the Student t test (Microsoft Excel; Microsoft, Seattle, WA). Differences were considered significant at P less than .05.
Results
As2O3 induces apoptosis in Raji cells, but not in Jurkat cells, at therapeutic concentrations
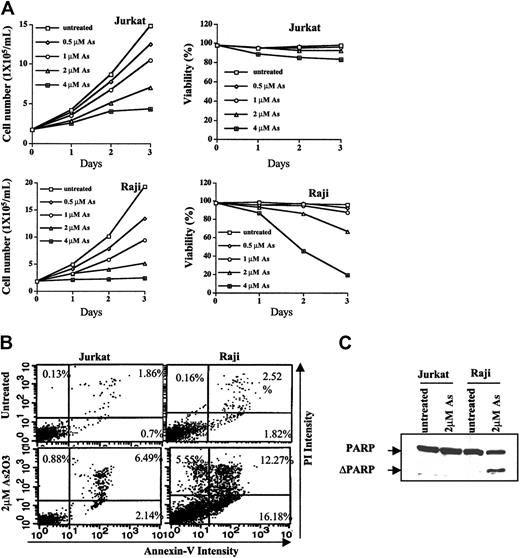
Apoptosis induction and growth inhibition abilities of As2O3 in Jurkat and Raji cells were compared after As2O3 treatment at therapeutic concentrations of 1 to 2 μM. As shown in Figure 1A, As2O3 treatment for 3 days caused more growth inhibition in Raji cells (inhibitory concentration 50% [IC50], approximately 0.9 μM) than in Jurkat cells (IC50, approximately 2 μM). In addition, the viability of Raji, but not of Jurkat, cells was decreased by As2O3 treatment in concentration- and time-dependent manners. As2O3 (2 μM) treatment for 3 days reduced the viability of Raji cells by 35%, whereas the viability of Jurkat cells was greater than 92% before and after As2O3 treatment. Percentages of apoptotic cells induced by 2 μMAs2O3 in Raji and Jurkat cells was 34% and 9%, respectively. The extent of apoptosis induction as measured by Annexin V and PI staining and by PARP cleavage was consistent with the decrease in cell viability (Figure 1B-C). PARP, a substrate of caspase protease in an apoptosis-signaling pathway, was cleaved by 2 μMAs2O3 treatment in Raji, but not in Jurkat, cells.
Growth inhibition and apoptosis induction by As2O3 in Jurkat and Raji cells. (A) Cell growth rate and viability. Jurkat and Raji cells were treated or untreated with the indicated concentrations of As2O3. Cell concentrations and percentages of viable cells after staining with trypan-blue were determined with the aid of a hematometer. Each value represents the mean ± SD of triplicates. (B) Apoptosis induction. Jurkat and Raji cells were untreated or treated with As2O3, 2 μM, for 3 days. Percentages of apoptotic cells were determined by staining with Annexin V and PI on flow cytometry, as described in “Materials and methods.” (C) Western blot analysis of PARP cleavage. Cells were untreated or were treated with As2O3, 2 μM, for 3 days. Anti-PARP antibody was used to detect the PARP cleavage product, as described in “Materials and methods.”
Growth inhibition and apoptosis induction by As2O3 in Jurkat and Raji cells. (A) Cell growth rate and viability. Jurkat and Raji cells were treated or untreated with the indicated concentrations of As2O3. Cell concentrations and percentages of viable cells after staining with trypan-blue were determined with the aid of a hematometer. Each value represents the mean ± SD of triplicates. (B) Apoptosis induction. Jurkat and Raji cells were untreated or treated with As2O3, 2 μM, for 3 days. Percentages of apoptotic cells were determined by staining with Annexin V and PI on flow cytometry, as described in “Materials and methods.” (C) Western blot analysis of PARP cleavage. Cells were untreated or were treated with As2O3, 2 μM, for 3 days. Anti-PARP antibody was used to detect the PARP cleavage product, as described in “Materials and methods.”
GSTP1 level, but not that of GSTA1 or GSTM1, is inversely correlated with As2O3-induced cell cytotoxicity
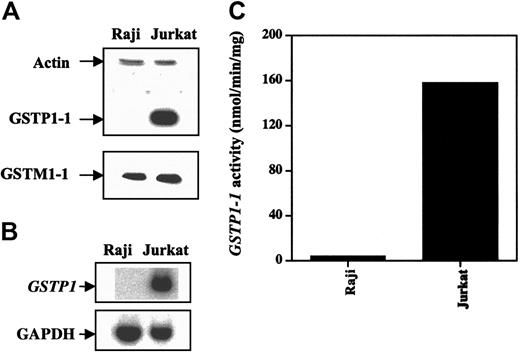
We used Western and Northern blot analyses to determine GSTP1-1, GSTA1-1, and GSTM1-1 protein levels and GSTP1 mRNA levels in Jurkat and Raji cells. Jurkat cells expressed higher levels of GSTP1-1 protein and GSTP1 mRNA than did Raji cells (Figure 2). GSTM1-1 protein expression was almost the same in both cell types and appeared not to contribute to the total GST activity using CDNB as a substrate.22 GSTA1-1 protein was not detectable in either cell line (data not shown). GSH level was the same in both cell lines (data not shown). Jurkat cells had higher levels of GSTP1-1 activity than Raji cells (Figure 2C), suggesting that the basal GSTP1 level might determine cell sensitivity to As2O3-induced apoptosis.
GSTP1 protein GSTP1 and mRNA levels and activity in Raji and Jurkat cells. (A) Western blot analysis of GSTP1-1 and GSTM1-1 proteins. (B) Northern blot analysis of GSTP1 mRNA. (C) GSTP1-1 activity.
GSTP1 protein GSTP1 and mRNA levels and activity in Raji and Jurkat cells. (A) Western blot analysis of GSTP1-1 and GSTM1-1 proteins. (B) Northern blot analysis of GSTP1 mRNA. (C) GSTP1-1 activity.
Forced expression of GSTP1 decreases cell sensitivity to As2O3-induced apoptosis
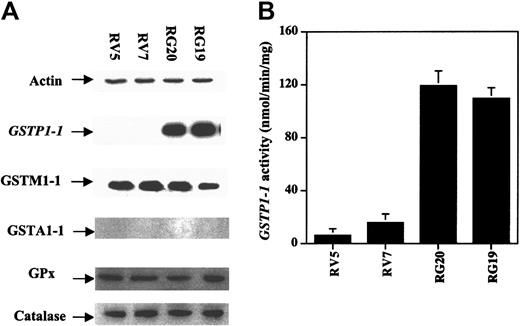
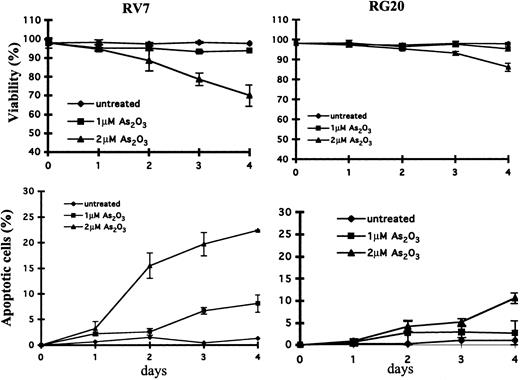
To investigate the role of GSTP1 in the regulation of cell sensitivity to As2O3, Raji cells were stably transfected with a plasmid with or without a GSTP1 expression sequence. Two Raji cell clones with GSTP1 expression (RG19 and RG20) and 2 Raji clones without GSTP1 expression (RV5 and RV7) were selected for further study. RG19 and RG20 cells had high GSTP1-1 activity—up to 120 nmol CDNB/min per milligram protein—which is similar to that in Jurkat cells, whereas RV5 and RV7 cells had low GSTP1-1 activity (less than 20 nmol CDNB/min per milligram protein) (Figure 3). Western blot analysis demonstrated that RG19 and RG20, but not RV5 and RV7, expressed GSTP1 mRNA and protein (Figure 3). Moreover, these 4 clones contained similar amounts of GSTM1-1, GSTA1-1, catalase, and glutathione peroxidase (GPx) based on Western blot analysis (Figure 3). As in parental Raji cells (Figure 1), As2O3 (2 μM) treatment induced apoptosis (23%) and reduced cell viability in RV7 cells (Figure 4). The apoptosis induction ability of As2O3 was partially blocked in GSTP1-transfected RG20 cells (Figure 4). These data suggest that GSTP1 might be one of the factors inhibiting As2O3-induced apoptosis.
Generation of GSTP1-transfected Raji cell clones. Raji cells were transfected with a PcDNA3.1 plasmid with or without an inserted GSTP1 cDNA sequence. Two clones expressing GSTP1 (RG19 and RG20) and 2 without (RV5 and RV7) were selected by Western blot analysis (A) and activity assays (B), as described in “Materials and methods.”
Generation of GSTP1-transfected Raji cell clones. Raji cells were transfected with a PcDNA3.1 plasmid with or without an inserted GSTP1 cDNA sequence. Two clones expressing GSTP1 (RG19 and RG20) and 2 without (RV5 and RV7) were selected by Western blot analysis (A) and activity assays (B), as described in “Materials and methods.”
Cytotoxicity and apoptosis induction in GSTP1-transfected Raji clones.GSTP1-transfected RG20 cells and empty vector–transfected RV7 cells were untreated or treated with As2O3 at the indicated concentrations for indicated times. Cytotoxicity (A) was determined by trypan blue staining, and relative levels of apoptotic cells (B) were determined on flow cytometry by Annexin V and PI staining, as described in “Materials and methods.”
Cytotoxicity and apoptosis induction in GSTP1-transfected Raji clones.GSTP1-transfected RG20 cells and empty vector–transfected RV7 cells were untreated or treated with As2O3 at the indicated concentrations for indicated times. Cytotoxicity (A) was determined by trypan blue staining, and relative levels of apoptotic cells (B) were determined on flow cytometry by Annexin V and PI staining, as described in “Materials and methods.”
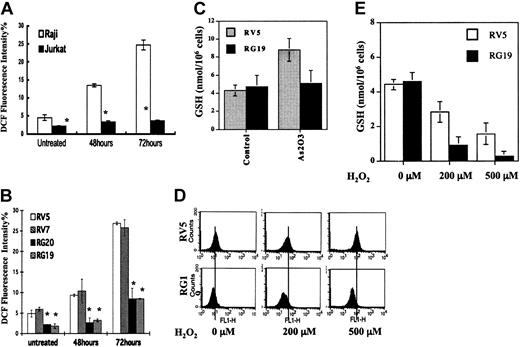
GSTP1 overexpression decreases H2O2 accumulation from As2O3 treatment in Raji cells
Amounts of intracellular H2O2 were determined in Jurkat, Raji, and GSTP1-expressing Raji cells. The basal H2O2 level in Jurkat cells was lower than it was in Raji cells. As2O3 (2 μM) treatment increased H2O2 levels in Raji cells, but not in Jurkat cells, after 3 days of treatment (Figure 5A). The effect of GSTP1 on intracellular H2O2 levels in Raji clones grown in medium with or without 2 μM As2O3 for 48 and 72 hours was tested. RG20 and RG19 cells had lower intracellular H2O2 basal levels than RV5 and RV7 cells. H2O2 levels in RV5 and RV7 cells exposed to 2 μM As2O3 obviously increased at 48 hours and then continuously accumulated, up to as much as 5-fold, at 72 hours compared with untreated cells (Figure 5B), similar to the increases in parental Raji cells. However, H2O2 levels in RG20 and RG19 cells were only slightly increased at 48 hours and were 3-fold lower than those in RV5 and RV7 cells at 72 hours after the addition of 2 μM As2O3 (Figure 5B). Intracellular GSH levels were tested before and after As2O3 treatment in RV5 and RG19 cells. As2O3 treatment increased intracellular GSH content in RV5 cells, but not in RG19 cells (Figure 5C). After exogenous H2O2 was added to the growth medium, the elimination rate of H2O2 in RV5 and RG19 cells was determined. H2O2 was eliminated more rapidly from RG19 cells than from RV5 cells (Figure 5D). Mean levels of intracellular H2O2 2 hours after the addition of 500 μMH2O2 to the growth medium were 79.6 in RV5 cells but only 39.5 in RG19 cells. Under the same conditions, the levels of GSH were much lower after H2O2 treatment in RG19 cells (Figure 5E). These data suggest that As2O3 treatment results in the accumulation of H2O2 by inhibiting GSH-involved peroxidase activity, whereas GSTP1-1 may compromise an alternative GSH-involved peroxidase pathway not inhibited by As2O3 treatment.
H2O2 and GSH levels in Raji and GSTP1-transfected Raji cells before and after As2O3 treatment. (A) Relative H2O2 levels in Jurkat and Raji cells. Growth medium was supplemented with 2 μM As2O3. After 48 and 72 hours, the intracellular level of H2O2 was measured by flow cytometry, as described in “Materials and methods.” *Statistically significant (P < .05) differences, compared with Jurkat cells. (B) Relative H2O2 amount in GSTP1-transfected Raji clones. Growth medium was supplemented with 2 μM As2O3. After 48 and 72 hours, the intracellular levels of H2O2 were measured by flow cytometry. *Statistically significant (P < .05) differences, compared with RV5 and RV7 cell clones. (C) GSH levels in GSTP1-transfected Raji clones. Growth medium was supplemented with 2 μM As2O3. After 48 hours, the intracellular level of GSH was measured, as described in “Materials and methods.” (D) H2O2 levels and (E) GSH levels after addition of exogenous H2O2 at the indicated concentrations into the growth medium of GSTP1-expressing and -nonexpressing Raji cells. After 2 hours, intracellular H2O2 and GSH levels were measured, as described in “Materials and methods.”
H2O2 and GSH levels in Raji and GSTP1-transfected Raji cells before and after As2O3 treatment. (A) Relative H2O2 levels in Jurkat and Raji cells. Growth medium was supplemented with 2 μM As2O3. After 48 and 72 hours, the intracellular level of H2O2 was measured by flow cytometry, as described in “Materials and methods.” *Statistically significant (P < .05) differences, compared with Jurkat cells. (B) Relative H2O2 amount in GSTP1-transfected Raji clones. Growth medium was supplemented with 2 μM As2O3. After 48 and 72 hours, the intracellular levels of H2O2 were measured by flow cytometry. *Statistically significant (P < .05) differences, compared with RV5 and RV7 cell clones. (C) GSH levels in GSTP1-transfected Raji clones. Growth medium was supplemented with 2 μM As2O3. After 48 hours, the intracellular level of GSH was measured, as described in “Materials and methods.” (D) H2O2 levels and (E) GSH levels after addition of exogenous H2O2 at the indicated concentrations into the growth medium of GSTP1-expressing and -nonexpressing Raji cells. After 2 hours, intracellular H2O2 and GSH levels were measured, as described in “Materials and methods.”
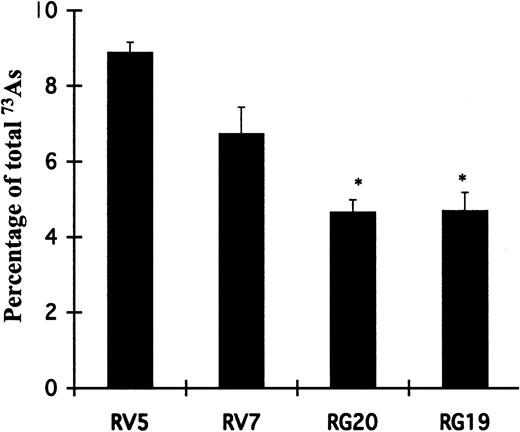
GSTP1 overexpression decreases intracellular retention of 73AsIII
To test the role of GSTP1 on the cellular accumulation of As2O3, intracellular arsenic uptake was measured using 73AsIII–arsenite. Similar 73As uptake was found in GSTP1-expressing or -nonexpressing Raji cells after incubation for 1 hour (data not shown). Percentages of intracellular 73As in RV5 and RV7 cells were 1.5- to approximately 2-fold higher than in RG20 and RG19 cells after incubation for 24 hours (Figure 6). These data indicate that overexpression of GSTP1 decreases As retention in the cells.
Expression of GSTP1 decreases As2O3 retention. Raji cells expressing GSTP1 (RG19 and RG20) and cells containing a vector not expressing GSTP1 (RV5 and RV7) were incubated in medium with 1 μM 73AsIII for 24 hours. Cell-associated radioactivity was determined as described in “Materials and methods.” Each value represents the mean ± SD of triplicates. *Statistically significant (P < .05) differences in RG20 and RG19 cells, compared with those in RV5 and RV7 cell clones.
Expression of GSTP1 decreases As2O3 retention. Raji cells expressing GSTP1 (RG19 and RG20) and cells containing a vector not expressing GSTP1 (RV5 and RV7) were incubated in medium with 1 μM 73AsIII for 24 hours. Cell-associated radioactivity was determined as described in “Materials and methods.” Each value represents the mean ± SD of triplicates. *Statistically significant (P < .05) differences in RG20 and RG19 cells, compared with those in RV5 and RV7 cell clones.
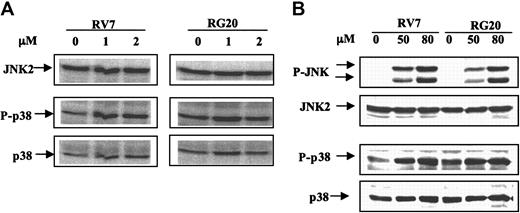
GSTP1 overexpression decreases P-JNK after treatment with high but not therapeutic concentrations of As2O3
P-JNK is not detectable by Western blot analysis in any of the untreated cells. The phosphorylated form of JNK was not detected in either RV7 or RG20 cells after treatment with 1 to approximately 10 μMAs2O3 for 72 hours, although apoptotic cells were detected (data not shown; Figure 4). However, high concentrations of As2O3 (approximately 50-80 μM) treatment for 2 hours activated the phosphorylation of JNK in RV7 cell clones and, to a lesser extent, in RG20 cells (Figure 7B). Moreover, phosphorylation of p38 was markedly increased after treatment with high concentrations of As2O3 (approximately 50-80 μM), and there was no detectable difference in p-p38 levels between GSTP1-expressing and -nonexpressing Raji cells (Figure 7). These data suggest that JNK activation represents a stress response and would not contribute to the apoptosis observed in Raji cells after treatment with As2O3 at therapeutic concentrations.
JNK was activated by higher, but not lower, therapeutic concentrations of As2O3, in transfected Raji cells with or without GSTP1 expression. RV7 and RG20 cells were untreated or treated with 1 or 2 μM As2O3 for 24 hours (A) or 50 to 80 μM As2O3 for 2 hours (B). P-JNK, JNK, P-p38, and p38 were detected by Western blot analysis.
JNK was activated by higher, but not lower, therapeutic concentrations of As2O3, in transfected Raji cells with or without GSTP1 expression. RV7 and RG20 cells were untreated or treated with 1 or 2 μM As2O3 for 24 hours (A) or 50 to 80 μM As2O3 for 2 hours (B). P-JNK, JNK, P-p38, and p38 were detected by Western blot analysis.
Discussion
Jurkat cells were less sensitive to As2O3-induced apoptosis than Raji cells, and Jurkat cells express higher levels of GSTP1 mRNA and activity than Raji cells, which are devoid of GSTP1 expression and activity (Figures 1, 2). Because other potential factors, such as GSTM1, catalase, GPx, and GSH levels, were equally expressed in both cell lines (Figure 3) and GSTA1 was absent, it appears that GSTP1 might be the factor that mediates the observed different cell sensitivities to As2O3. Stable transfection with GSTP1 in Raji cells decreased As2O3-induced apoptosis, suggesting that GSTP1 is indeed a potent inhibitor of As2O3-induced apoptosis (Figure 4).
It has been found that As2O3-induced apoptosis at therapeutic concentrations is associated with the up-regulation of H2O2.4-8 As2O3 treatment significantly increased H2O2 levels in parental Raji cells but not in Jurkat cells (Figure 5). Raji cells transfected with GSTP1 had reduced levels of H2O2 production compared with vector-transfected cells. Moreover, given that vector-transfected cells contained relatively higher levels of H2O2 than GSTP1-transfected cells and that H2O2-scavenging enzymes, such as catalase, glutathione peroxidase, and GSTA1-1 were not changed in these cells (Figure 3), it appears that GSTP1 may function as a peroxidase to diminish intracellular H2O2. GSTA1, but not GSTP1, has been reported to have selenium-independent glutathione peroxidase activity.23-26 Studies have reported that GSTP1 plays an important role in the detoxification of carcinogens and the prevention of DNA damage but not in H2O2 scavenging.27-29 However, the observations that less H2O2 accumulation (Figure 5D) and more GSH depletion (Figure 5E) in Raji cells expressing GSTP1 treated with H2O2 support the hypothesis that GSTP1 can function as a glutathione-dependent peroxidase. The nature of GSTP1 must be further studied by chemical methods.
GSTP1 has also been reported to be involved in the detoxification of arsenic by an efflux system.15,17,30 Increased GSTP1 expression levels and activity were observed in arsenic-tolerant and -resistant cells.14-18 It is possible that GSTP1 facilitates the efflux of arsenite in the cells expressing GSTP1; in turn, GPx is not inhibited, and H2O2 does not accumulate after As2O3 treatment. The retention of As2O3 in Raji clones expressing GSTP1 was less than it was in Raji clones not expressing GSTP1 (Figure 6). That H2O2 did not significantly increase in GSTP1-expressing Raji cells after As2O3 treatment supports this possibility.
It has been reported that high concentrations of As2O3 treatment activated JNK and p38, members of stress-activated signal transduction pathways, and resulted in apoptosis in several leukemia and lymphoma cell lines.11,12,31,32 Basal levels of the phosphorylated form of JNK and p38 were not detectable in Raji cell clones with or without GSTP1 expression. Although a significant apoptotic effect was observed after treatment with 2 μM As2O3 in vector-transfected Raji cells, activation of JNK and p38 was still not detectable in these cells, suggesting that JNK and p38 activation might not contribute to As2O3-induced apoptosis at therapeutic concentrations. Furthermore, as reported by other groups,10-13 the phosphorylated forms of JNK and p38 were significantly increased by As2O3 treatment at higher concentrations (Figure 7B), suggesting that JNK and p38 activation might mediate a stress response. Recently, it was found that GSTP1 is an inhibitor of JNK activity through direct protein–protein interaction.33 Forced expression of GSTP1 in Raji cells decreased JNK phosphorylation after exposure to high concentrations of As2O3, which is consistent with previous reports that GSTP1 inhibited JNK activation after stress treatments (high-dose H2O2 and As2O3). The phosphorylated form of p38 was equally increased after exposure to high concentrations of As2O3 treatment in Raji cells expressing or not expressing GSTP1 (Figure 7). These data suggest that under stress condition after high As2O3 treatments, GSTP1 may function as an inhibitor of JNK rather than of p38 kinase. However, at therapeutic As2O3 concentrations, caspase activation (Figure 1) but not JNK-mediated pathway(s) is correlated with apoptosis induction in lymphoma cells.
In conclusion, the data presented here indicate that GSTP1 might be an important factor in determining the cell sensitivity to As2O3-induced apoptosis in lymphoma. GSTP1 may inhibit arsenic-induced apoptosis through at least 2 mechanisms: the detoxification mechanism, which decreases arsenic intracellular retention, and the peroxidase mechanism, which catabolizes H2O2. Each mechanism results in a decrease in intracellular H2O2 level and in the inhibition of apoptosis observed in Raji cells transfected with GSTP1 and Jurkat cells after low-concentration As2O3 treatment.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2003-12-4299.
Supported by the National Institutes of Health (grants CA93533 and CA85478) and by the Samuel Waxman Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal