Abstract
Plasma von Willebrand factor (VWF) has been identified as an indispensable factor for platelet adhesion and thrombus formation on a collagen surface under flow conditions. VWF binds to collagen and then tethers platelets to the collagen surface through interaction with platelet glycoprotein Ib and also contributes to the thrombus formation on the collagen surface. In the present study, we demonstrated that the addition of VWF/factor VIII complex or purified VWF (> 2 ristocetin cofactor activity units/mL) increased platelet adhesion to the collagen surface in platelet-reduced blood (∼ 5 × 104 platelets/μL) to the normal level. VWF had no stimulatory effect when it was allowed to bind to the collagen surface before blood flow was initiated. Addition of an excess of FITC (fluorescein-5-isothiocyanate)–abeled VWF to platelet-reduced blood under these flow conditions demonstrated that the VWF was mainly incorporated into the platelet aggregates. These results indicated that the supplemented VWF stimulates the platelet adhesion onto the collagen surface by enhancing platelet aggregation in the platelet-reduced condition. This also suggests a possibility that supplementation of VWF to individuals with thrombocytopenia might be effective for increasing their hemostatic potential.
Introduction
Proper formation of a platelet plug at sites of vascular injury requires a spatially and temporally coordinated series of events that allow circulating platelets to adhere onto exposed collagen, become activated, and recruit additional platelets, and form multicellular aggregates, including platelets that are stabilized by fibrin. Von Willebrand factor (VWF), an adhesive plasma glycoprotein, has been indicated to play fundamental roles in the platelet plug formation under physiologic conditions.1-4 VWF is composed of identical disulfide-linked subunits, each comprising 2050 amino acid residues and up to 22 carbohydrate chains for a total mass of 250 kDa.5 VWF subunits are crosslinked at the COOH-terminal ends to form a dimer, and the dimer is further converted to a multimeric form with molecular mass from 5 × 102 kDa to 2 × 104 kDa by intersubunit disulfide linkages at the NH2-terminal ends of the dimers.6 VWF is present in the circulation at a plasma concentration of 5 to 10 μg/mL. It is also released from α-granules of megakaryocytes and platelets and Weibel-Palade bodies of endothelial cells.7,8 VWF was shown to have a critical role in platelet adhesion to a collagen surface under flow conditions, especially under high shear rate.4,9,10 Although platelets do not bind human VWF in solution under low shear rate, VWF binds reversibly with platelet glycoprotein (GP) Ib under high shear rate, and shear stress induces a permanent interaction between VWF and integrin αIIbβ3 (GPIIb/IIIa) after the initial binding of GPIb with VWF.1,11 Moroi et al12 found that the reversible binding of platelets to immobilized VWF and the tethering speed of platelets was related to the density of VWF on the surface. These and many other results suggested that the first reaction of platelet adhesion on the collagen surface is VWF binding to immobilized collagen and that circulating platelets are recruited and tethered on VWF-immobilized collagen through a weak but very fast interaction of VWF with platelet GPIb. Platelets rolling or translocating on the collagen surface are then arrested and activated on the collagen surface via the interaction with GPVI and integrin α2β1 (GPIa/IIa). Finally, the activated platelets release their granule contents, including adenosine diphosphate (ADP), and express the activated form of integrin αIIbβ3 on the cell surface, which induces thrombus formation via the interaction with fibrinogen and VWF.3,13-17 VWF also contributes to fibrin clot formation by associating with blood coagulation factor VIII (FVIII) and preventing its rapid clearance from the circulation18 and its degradation by activated protein C,19,20 thereby allowing normal thrombin generation.
It has been reported that a high plasma concentration of FVIII is a risk factor for thrombophilia in the acute phase,21 and a high plasma concentration of VWF is related to deep-vein thrombosis,22 myocardial infarction,23 and ischemic stroke.24-26 Those reports suggested that high level of FVIII or VWF in blood might have an ability to increase the hemostatic potential in patients with bleeding episodes caused by a defect in blood coagulation or thrombocytopenia. The vasopressin agonist desmopressin (DDAVP) is known to be an inducer of VWF release from endothelial cells and is used to control the bleeding of mild hemophilia and von Willebrand disease (VWD).27,28 Furthermore, it is reported that DDAVP might be applicable for thrombocytopenia.29 However, the theoretical background of DDAVP therapy has not been fully elucidated except for the increase of VWF concentration.
In the present study, we analyzed the effect of adding VWF/FVIII complex or purified VWF to human platelet-reduced blood on platelet adhesion onto a collagen surface under flow conditions. Our results indicate that the enhanced level of VWF increased platelet adhesion and aggregation onto the collagen surface under flow conditions even in platelet-reduced blood, supporting the effectiveness of the therapies of transfusing DDAVP or VWF to increase the VWF level in blood for thrombocytopenic patients.
Materials and methods
Reagents
The reagents used in the experiments were as follows: PPACK (D-Phe-Pro-Arg-Chloromethylketone; Calbiochem, San Diego, CA); mepacrine, bovine serum albumin (BSA), and busulfan (Sigma Chemical, St Louis, MO); fluorescein-5-isothiocyanate (FITC; Molecular Probes, Eugene, OR); type III bovine collagen (Koken, Tokyo, Japan); recombinant FVIII (rFVIII; Bayer, Tokyo, Japan); ristocetin (abp; American Biochemical and Pharmaceutical, Marlton, NJ); rabbit anti–human fibrinogen antibody and peroxidase-conjugated rabbit anti–human fibrinogen antibody (DAKO A/S, Glostrup, Denmark); anti-integrin α2β1 monoclonal antibody (Gi9), and anti-integrin αIIbβ3 monoclonal antibody (P2) (Immunotech S.A., Marseille, France). Anti-GPIb monoclonal antibody (NNKY5-5) was generously given by Dr S. Nomura of Kansai Medical University, Japan. The characteristics of these antibodies were reported before.30-32 All other reagents and buffer components were commercially available with the highest purity. VWF/FVIII concentrate (Confact F) was supplied by Kaketsuken (Kumamoto, Japan), and it was reconstituted by adding distilled water into the concentrate according to the manufacturer's instructions. The ratio of ristocetin cofactor activity (VWF:RCo) and FVIII clotting activity (FVIII:C) of the reconstituted VWF/FVIII solution was 2:1.
Analysis of VWF and FVIII
The multimeric structure of VWF was analyzed as previously described.33 In brief; samples containing VWF were analyzed by electrophoresis using a 2% agarose gel containing 2% sodium dodecyl sulfate (SDS). After the electrophoresis, the gel was incubated with rabbit anti-VWF antibody (Nordic Immunology, Oslo, Norway) in 100 mM phosphate, pH 7, containing 150 mM NaCl and 1% Tween 20; and after washing with the same buffer, the gel was further incubated with biotinylated anti–rabbit immunoglobulin G (IgG) antibody (Vector Laboratories, Burlingame, CA). The immunoprecipitate in the gel was stained using an avidin-biotin-peroxidase complex (ABC) kit containing avidin-conjugated horseradish peroxidase and 4-chloro-1-naphthol (Bio-Rad, Hercules, CA). VWF:RCo was determined as previously described.34 FVIII:C was measured by the activated partial thromboplastin time (APTT) method using FVIII-depleted plasma from Dade Behring (Marburg, Germany).
Preparation of VWF and FITC-labeled VWF
VWF was purified from human plasma–derived cryoprecipitate containing VWF and FVIII by gel filtration as previously described.35 In brief, VWF/FVIII concentrated fraction was dissolved in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.5, containing 100 mM NaCl (TBS) and then subjected to gel filtration with a column of Sepharose CL-4B (ϕ2.5 cm × 92 cm) equilibrated with TBS containing 350 mM CaCl2. VWF:RCo was detected at the void volume fractions in gel filtration, but no FVIII:C activity was observed in these fractions (< 0.1 U/mL). The specific activity of the purified VWF was 50 VWF:RCo U/mg protein (calculated by the extinction coefficient of VWF, E1%,1cm,280 nm = 10). Western blotting analysis of the purified VWF showed that the high-molecular-weight multimeric structure was sustained in the VWF preparation as well as the VWF/FVIII concentrate (Figure 1). Fibrinogen contents of the VWF preparation and VWF/FVIII concentrate were measured to be 0.1 and 0.5 μg/VWF:RCo U, respectively, by ELISA (enzyme-linked immunosorbent assay) method using anti–human fibrinogen antibodies. For preparation of FITC-labeled VWF, the purified VWF (1.0 mg) was incubated with FITC (6.5 μg) in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, containing 100 mM NaCl (HEPES buffer) for 15 minutes at room temperature as previously described.11 After the incubation, the reaction mixture was dialyzed against TBS. The specific activity of the FITC-labeled VWF was 37 VWF:RCo U/mg protein.
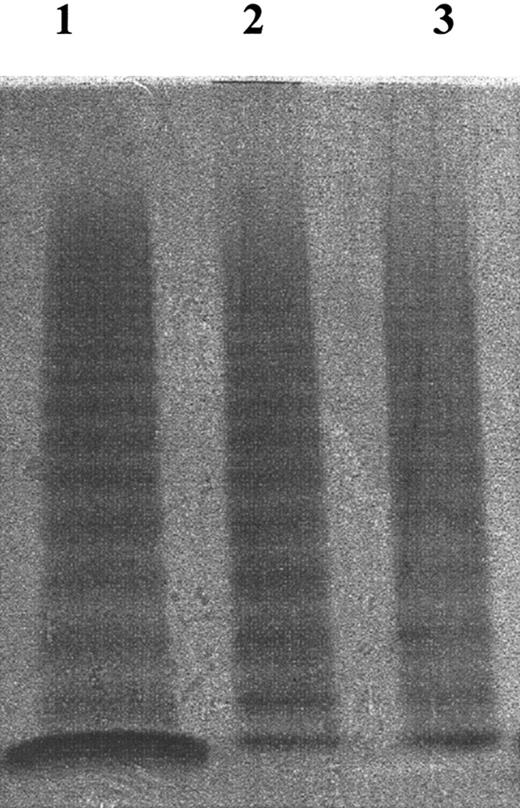
Multimeric structure analysis of VWF. VWF multimeric structure was analyzed by 2% SDS–2% agarose electrophoresis (see “Materials and methods”). Lane 1 indicates normal plasma; lane 2, purified VWF; lane 3, VWF/FVIII concentrate (supplied by Kaketsuken, Kumamoto, Japan). After adjusting the concentration of the samples to 1 VWF:RCo U/mL, they were subjected to electrophoresis. These VWF samples all contained VWF with similar multimeric structure.
Multimeric structure analysis of VWF. VWF multimeric structure was analyzed by 2% SDS–2% agarose electrophoresis (see “Materials and methods”). Lane 1 indicates normal plasma; lane 2, purified VWF; lane 3, VWF/FVIII concentrate (supplied by Kaketsuken, Kumamoto, Japan). After adjusting the concentration of the samples to 1 VWF:RCo U/mL, they were subjected to electrophoresis. These VWF samples all contained VWF with similar multimeric structure.
Preparation of platelet-reduced blood
Whole blood was drawn from healthy volunteers using PPACK (at the final concentration of 50 μM) as an anticoagulant, and platelet-rich plasma (PRP) was prepared from the whole blood by centrifugation (10 minutes at 200g at 25°C). Platelet-poor plasma (PPP) was prepared from the PRP by centrifugation (10 minutes at 500g at 25°C). Platelet-reduced blood (4-6 × 104 platelets/μL) was prepared by combining PRP, PPP, and erythrocyte fractions to obtain a proper concentration of platelets. Erythrocyte level of platelet-reduced blood was adjusted to 35% to 40% of hematocrit. The study had institutional ethics approval, and all donors provided written, informed consent at Kurume University.
Perfusion studies
Assay method. Perfusion studies were carried out in a single-pass perfusion chamber as described previously.12,32 The collagen-coated glass plate was prepared as follows: type III collagen solution (bovine) was diluted to the concentration of 200 μg/mL with 5 mM HEPES, 0.42 mM NaH2PO4, and 12 mM NaHCO3, pH 7.4, containing 136 mM NaCl, 2.7 mM KCl, and 5.5 mM glucose (HEPES-Tyrode buffer) and then layered on the glass plate. After 1 hour of incubation at room temperature in a humidified box, the collagen solution was removed, and the glass plate was incubated with HEPES-Tyrode buffer containing 2% of BSA for another 1 hour. After washing out the BSA with HEPES-Tyrode buffer, the collagen-coated glass plate was mounted in the perfusion chamber. Mepacrine-treated blood (incubated with 5 μM mepacrine for 30 minutes) was passed through the flow chamber at a controlled flow rate using a syringe pump (model STC-525; Terumo, Tokyo, Japan). Shear rate was calculated from the equation obtained by Muggli et al36 as described before.32 Platelet adhesion and aggregation in the flow chamber were monitored by a fluorescence microscope (Diaphot-TMD; Nikon, Tokyo, Japan) equipped with an objective lens (Plan Fluor, ELDW 40 ×/NA 0.60, Ph2 DM; Nikon) and a fluorescence illumination attachment (model T-FL-E; Nikon). The fluorescent images were captured by an EB-CCD camera (model 7190-21; Hamamatsu Photonics, Hamamatsu, Japan) and recorded on videotapes using an 8-mm videocassette recorder (EV-PR2 NTSC; Sony, Tokyo, Japan). The obtained photo images were analyzed by computer using Argus-50 software (Hamatsu Photonics). For analyzing platelet adhesion, the video signals were captured at 2 minutes after the start of the flow while processing background subtraction and rolling average of the captured signals using Argus-50 software. These data were further processed to obtain the area occupancy as an indicator of platelet adhesion. The percentage of area occupancy was taken at 2 minutes after starting the perfusion.
Perfusion studies using FITC-labeled VWF. To analyze the distribution of the additional VWF in the platelet deposits, FITC-labeled VWF was added to platelet-reduced blood and then the perfusion assay was performed as described in “Assay method.” After perfusion, the collagen-coated glass plates were washed briefly with HEPES-Tyrode buffer, and the deposited platelets were fixed with 4% paraformaldehyde in HEPES-Tyrode buffer for 30 minutes. Bound FITC-labeled VWF was observed with fluorescence microscopy.
Analysis of platelet adhesion on VWF-pretreated collagen surface. To prepare the VWF-pretreated collagen surface under flow conditions, 4 VWF:RCo U/mL VWF was mixed with the erythrocyte fraction (containing < 3000 platelets/μL) in HEPES-Tyrode/2% BSA buffer, and then this mixture was perfused over the collagen-coated glass plate at 1600 s–1 for 2 minutes. After perfusion of VWF, platelet-reduced blood (containing 4-6 × 104 platelets/μL) was immediately perfused over the VWF-immobilized collagen plate at 1600 s–1 for 2 minutes, and then the platelet was measured as described in “Perfusion studies using FITC-labeled VWF.”
Effects of antibodies to platelet receptors on platelet adhesion in platelet-reduced blood. To investigate the effect of platelet receptors on platelet adhesion under the present conditions, the platelet-reduced blood containing 2 VWF RCo U/mL was incubated with 20 μg/mL anti-GPIb (NNKY5-5), anti-α2β1 (Gi9), or anti-αIIbβ3 (P2) monoclonal antibodies for 30 minutes at room temperature, and then platelet adhesion under flow conditions was analyzed.
Statistical analysis
The mean surface coverage values of the flow chamber experiments were compared by the t test. P less than .05 was considered to be statistically significant.
Results
Effect of platelet counts on platelet adhesion assay
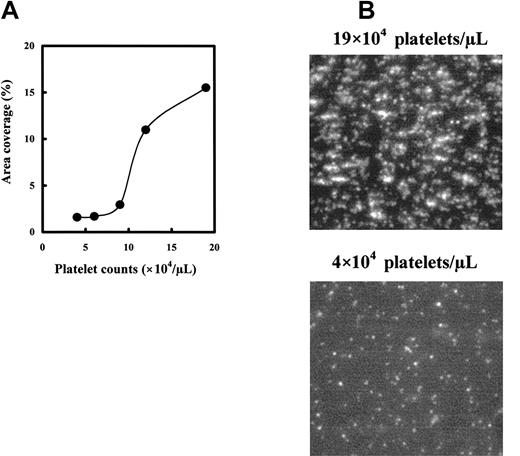
To determine the relationship between platelet counts and the area covered by adhered platelets, we measured the platelet adhesion of blood containing 4 to 19 × 104 platelets/μL under flow. The adhesion on the collagen surface increased platelet concentration dependently and markedly increased at platelet counts more than 10 × 104 platelets/μL, from 2% to 3% to more than 10% surface coverage (Figure 2A). In the normal range of platelet concentration (15-30 × 104 platelets/μL), surface coverage by adhered platelets was more than 15% (Figure 2A). Poor platelet adhesion of platelet-reduced blood and advanced platelet adhesion and aggregate formation of normal blood were also observed in fluorescent images as shown in Figure 2B. Under low platelet concentrations, platelets adhered mainly as single platelets, but at higher platelet concentrations (platelet counts in normal blood), more platelets adhered, and the adhered platelets formed aggregates.
Correlation of platelet counts to platelet adhesion on collagen. Platelet-reduced blood was prepared with PPACK-treated whole blood. Platelet-reduced blood having the appropriate platelet counts was prepared by mixing the erythrocyte fraction and diluted PRP with PPP. The mepacrine-labeled blood was perfused in the flow chamber for 2 minutes at a shear rate of 1600 s–1. (A) Relationship between platelet counts and platelet adhesion. Platelet adhesion was expressed as surface coverage at 2 minutes after the start of flow. Data are each a mean of duplicate measurement values. (B) Photo images of the platelet adhesion under normal and platelet-reduced conditions. Photo images were captured at 2 minutes after starting the perfusion using the reconstituted blood of 19 × 104 or 4 × 104 platelets/μL. Magnification, × 400.
Correlation of platelet counts to platelet adhesion on collagen. Platelet-reduced blood was prepared with PPACK-treated whole blood. Platelet-reduced blood having the appropriate platelet counts was prepared by mixing the erythrocyte fraction and diluted PRP with PPP. The mepacrine-labeled blood was perfused in the flow chamber for 2 minutes at a shear rate of 1600 s–1. (A) Relationship between platelet counts and platelet adhesion. Platelet adhesion was expressed as surface coverage at 2 minutes after the start of flow. Data are each a mean of duplicate measurement values. (B) Photo images of the platelet adhesion under normal and platelet-reduced conditions. Photo images were captured at 2 minutes after starting the perfusion using the reconstituted blood of 19 × 104 or 4 × 104 platelets/μL. Magnification, × 400.
VWF concentration–dependent platelet adherence on the collagen surface
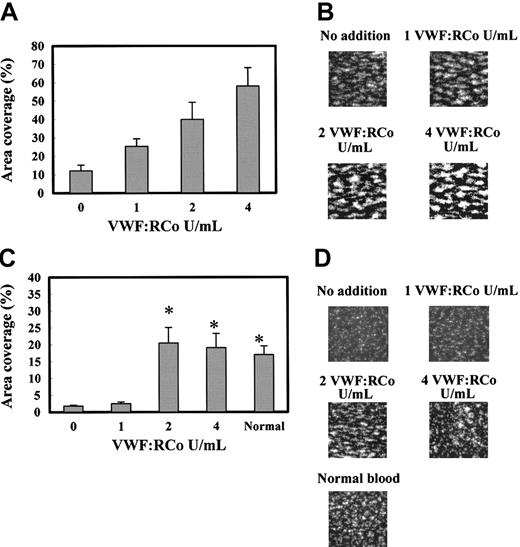
We examined the effects of VWF/FVIII complex on the platelet adhesion to the immobilized-collagen surface under flow conditions, comparing the effects in normal and platelet-reduced blood. As indicated in Figure 3A-B, the addition of VWF/FVIII complex to normal blood dose-dependently increased the platelet adhesion to the collagen surface under flow at the shear rate of 1600 s–1. For example, adding 2 VWF:RCo increased the surface coverage 3-fold. The fluorescence microscopy at 2 minutes after start of the flow showed the larger and more strongly fluorescent platelet aggregates in the normal blood with extra VWF/FVIII (Figure 3B). These results indicate that the higher level of VWF/FVIII in the normal blood promoted platelet aggregate formation on the collagen surface under flow conditions.
Effect of VWF/FVIII complex on platelet adhesion under flow conditions using normal and platelet-reduced blood. PPACK- and mepacrine-treated whole (normal) blood (19 × 104 platelets/μL) and platelet-reduced blood (4-6 × 104 platelets/μL) were added with different amounts of FVIII/VWF complex and perfused to the flow chamber for 2 minutes at a shear rate of 1600 s–1 and the platelet adhesion was analyzed. (A) Platelet adhesion of normal blood containing 0, 1, 2, and 4 VWF:RCo U/mL exogenously added VWF/FVIII complex. Platelet adhesion was expressed as surface coverage at 2 minutes after the start of flow. Data are each a mean ± SEM (n = 5-6). (B) Photo images of the platelets adhered on the collagen surface in panel A. Those images correspond to the experiments using blood containing 0 to 4 VWF:RCo U/mL of exogenously added VWF/FVIII complex shown in panel A. (C) Platelet adhesion of platelet-reduced blood exogenously added with 0 (n = 15), 1 (n = 6), 2 (n = 9), and 4 (n = 8) VWF:RCo U/mL VWF/FVIII complex. Normal blood indicates the platelet adhesion of the original blood (n = 14). Data are each a mean ± SEM. *P < .05 as compared with no addition of VWF/FVIII complex. (D) Photo images of the platelets deposited on the collagen surface, corresponding to the experiments in panel C. These photoimages were captured at 2 minutes after start of the perfusion. Magnification, × 400.
Effect of VWF/FVIII complex on platelet adhesion under flow conditions using normal and platelet-reduced blood. PPACK- and mepacrine-treated whole (normal) blood (19 × 104 platelets/μL) and platelet-reduced blood (4-6 × 104 platelets/μL) were added with different amounts of FVIII/VWF complex and perfused to the flow chamber for 2 minutes at a shear rate of 1600 s–1 and the platelet adhesion was analyzed. (A) Platelet adhesion of normal blood containing 0, 1, 2, and 4 VWF:RCo U/mL exogenously added VWF/FVIII complex. Platelet adhesion was expressed as surface coverage at 2 minutes after the start of flow. Data are each a mean ± SEM (n = 5-6). (B) Photo images of the platelets adhered on the collagen surface in panel A. Those images correspond to the experiments using blood containing 0 to 4 VWF:RCo U/mL of exogenously added VWF/FVIII complex shown in panel A. (C) Platelet adhesion of platelet-reduced blood exogenously added with 0 (n = 15), 1 (n = 6), 2 (n = 9), and 4 (n = 8) VWF:RCo U/mL VWF/FVIII complex. Normal blood indicates the platelet adhesion of the original blood (n = 14). Data are each a mean ± SEM. *P < .05 as compared with no addition of VWF/FVIII complex. (D) Photo images of the platelets deposited on the collagen surface, corresponding to the experiments in panel C. These photoimages were captured at 2 minutes after start of the perfusion. Magnification, × 400.
Next, we analyzed the effect of adding VWF/FVIII complex on platelet adhesion under platelet-reduced conditions. Using the reconstituted platelet-reduced blood containing 4 to 6 × 104 platelets/μL, VWF/FVIII addition also enhanced platelet adhesion. Although the addition of 1 VWF:RCo U/mL VWF/FVIII had no effect, addition of 2 or 4 VWF:RCo U/mL VWF/FVIII increased the platelet adhesion to the level of blood containing the normal level of platelets (Figure 3C). The fluorescent images indicate that platelet-reduced blood showing increased level of platelet adhesion (ie, added with sufficient VWF/FVIII complex) supports the formation of platelet aggregates, but platelet-reduced blood without or with 1 VWF:RCo U/mL VWF/FVIII mainly supports single platelet adhesion, with few platelet aggregates being formed (Figure 3D). These results indicate that the increased VWF/FVIII stimulated the platelet aggregate formation under the low-platelet condition.
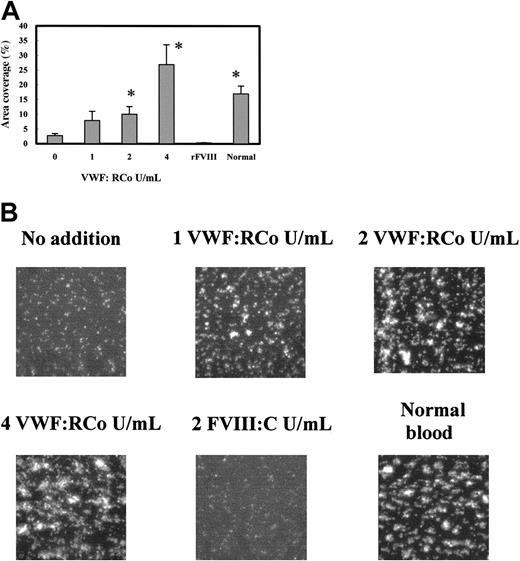
We determined which component of the VWF/FVIII complex is responsible for the platelet adhesion–stimulating effect. The addition of the purified VWF having the same VWF:RCo as in Figure 3C significantly increased the platelet adhesion to collagen (Figure 4A). Although the dose-response of the stimulating effect of VWF was different from that of the VWF/FVIII complex, the addition of more than 2 VWF:RCo U/mL VWF or VWF/FVIII had a significant stimulatory effect on platelet adhesion. However adding 2 FVIII:C U/mL rFVIII did not affect platelet adhesion (Figure 4A). The fluorescent images of the adhered platelets support this conclusion (Figure 4B). These results indicate that VWF, and not FVIII, stimulates the platelet adhesion to collagen under the platelet-reduced condition.
Effect of adding purified VWF on the platelet adhesion under flow using platelet-reduced blood. Platelet-reduced blood (4-6 × 104 platelets/μL) was prepared from PPACK-treated whole blood, added with different amounts of purified VWF or rFVIII, and perfused into the flow chamber for 2 minutes at a shear rate of 1600 s–1. (A) Platelet adhesions of platelet-reduced blood with no additions 0 (n = 11),1(n = 6),2(n = 6), or 4 (n = 5) VWF:RCo U/mL purified VWF; or 2 FVIII:C U/mL rFVIII (n = 6). Normal blood indicates the platelet adhesion of the original blood (n = 14). Data are each a mean ± SEM. *P < .05 as compared with no addition of purified VWF. (B) Photo images of the platelets adhered on collagen surface corresponding to the experiments in panel A. Those photo images were captured at 2 minutes after start of the perfusion. Magnification, × 400.
Effect of adding purified VWF on the platelet adhesion under flow using platelet-reduced blood. Platelet-reduced blood (4-6 × 104 platelets/μL) was prepared from PPACK-treated whole blood, added with different amounts of purified VWF or rFVIII, and perfused into the flow chamber for 2 minutes at a shear rate of 1600 s–1. (A) Platelet adhesions of platelet-reduced blood with no additions 0 (n = 11),1(n = 6),2(n = 6), or 4 (n = 5) VWF:RCo U/mL purified VWF; or 2 FVIII:C U/mL rFVIII (n = 6). Normal blood indicates the platelet adhesion of the original blood (n = 14). Data are each a mean ± SEM. *P < .05 as compared with no addition of purified VWF. (B) Photo images of the platelets adhered on collagen surface corresponding to the experiments in panel A. Those photo images were captured at 2 minutes after start of the perfusion. Magnification, × 400.
Analysis of incorporation of FITC-labeled VWF to the adhered platelets on the collagen surface
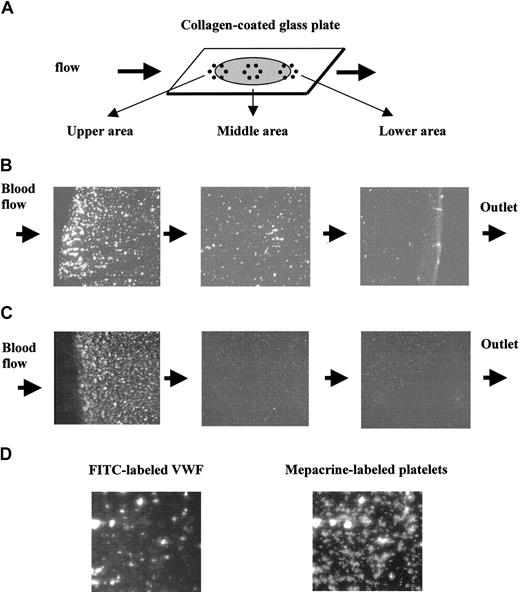
To identify which step of the platelet adhesion reaction under flow is affected by the exogenously added VWF, FITC-labeled VWF was added to platelet-reduced blood, and we analyzed its incorporation in the adherent platelets. The whole area of the collagen-coated surface showed no apparent FITC-VWF binding, which might be due to the FITC-VWF fluorescence being too weak to be detectable on the collagen-coated surface (Figure 5B). When we observed platelet adhesion using platelet-reduced platelets, platelets adhered strongly in the area close to the inlet of the blood flow and the adhesion was much less in the area around the outlet of the blood (Figure 5C). This could be attributed to the low platelet concentration because platelet adhesion was distributed evenly on the collagen-coated surface when using normal blood (data not shown). Fluorescence of the FITC-VWF appeared as spots that were stronger in fluorescence and denser near the inlet of the flow (Figure 5B). The distribution and pattern of the fluorescence of mepacrine-labeled platelets were very similar to those of FITC-VWF (Figure 5B-C). The colocalization of FITC-VWF and mepacrine-labeled platelets were further demonstrated in Figure 5D, where the same sample was observed by fluorescence of FITC and mepacrine. Fluorescence of FITC was colocalized, particularly in the large platelet aggregates (Figure 5D). These results indicate the colocalization of exogenously added VWF with the platelet aggregates and suggest that exogenously added VWF would stimulate platelet aggregate formation on the collagen surface under flow.
Colocalization of exogenous VWF with platelets adhered on collagen-immobilized surface. FITC-labeled VWF or nonlabeled VWF was added to platelet-reduced blood labeled with mepacrine (5 × 104 platelets/μL) to give a final concentration of 2.6 VWF:RCo U/mL and perfused at 1600 s–1. Blood flow was stopped at 2 minutes after start of the flow, and the cover slips were washed and fixed with paraformaldehyde solution. The fluorescent images of FITC and mepacrine were separately obtained by using B and BV filters, respectively. (A) An illustration of the collagen-coated glass plate in the flow chamber. Gray circle and bold arrows indicate the collagen-coated area and flow direction, respectively. (B) Fluorescent images of FITC-labeled VWF on the collagen surface at 3 sites: upper, middle, and lower areas of the flow, as shown in panel A. Mepacrine nonlabeled platelet reduced-blood was perfused after addition of FITC-labeled VWF. (C) Fluorescent images of mepacrine-labeled platelets on the collagen surface at 3 sites: upper, middle, and lower areas of the flow, as shown in panel A. (D) Fluorescent images of the same microscope field of the same sample using mepacrine and FITC fluorescence. Magnification, × 100 for panels B and C, and × 400 for panel D.
Colocalization of exogenous VWF with platelets adhered on collagen-immobilized surface. FITC-labeled VWF or nonlabeled VWF was added to platelet-reduced blood labeled with mepacrine (5 × 104 platelets/μL) to give a final concentration of 2.6 VWF:RCo U/mL and perfused at 1600 s–1. Blood flow was stopped at 2 minutes after start of the flow, and the cover slips were washed and fixed with paraformaldehyde solution. The fluorescent images of FITC and mepacrine were separately obtained by using B and BV filters, respectively. (A) An illustration of the collagen-coated glass plate in the flow chamber. Gray circle and bold arrows indicate the collagen-coated area and flow direction, respectively. (B) Fluorescent images of FITC-labeled VWF on the collagen surface at 3 sites: upper, middle, and lower areas of the flow, as shown in panel A. Mepacrine nonlabeled platelet reduced-blood was perfused after addition of FITC-labeled VWF. (C) Fluorescent images of mepacrine-labeled platelets on the collagen surface at 3 sites: upper, middle, and lower areas of the flow, as shown in panel A. (D) Fluorescent images of the same microscope field of the same sample using mepacrine and FITC fluorescence. Magnification, × 100 for panels B and C, and × 400 for panel D.
Analysis of the mechanism increasing the platelet adhesion by VWF
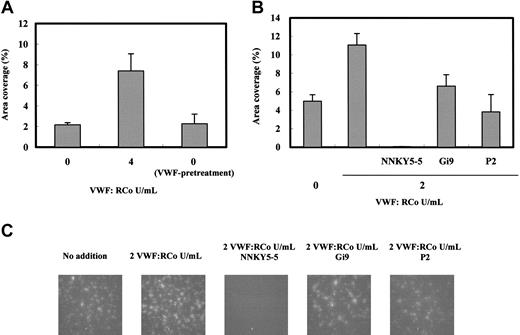
Although FITC-labeled VWF binding was not detectable on the whole area of the collagen surface (Figure 5B), it is still possible that the enhanced VWF concentration in the blood increases the amount of VWF bound to collagen, thereby increasing platelet adhesion. To test this, we compared the area coverage of platelet adhesion of the VWF-pretreated–immobilized collagen surface with that of the nontreated surface. There was no significant difference between the platelet adhesions on these 2 surfaces (Figure 6A), suggesting that increased VWF binding to the immobilized collagen does not significantly affect the platelet adhesion to the collagen under flow. Furthermore, we also could not observe any significant difference in platelet adhesion on the VWF-pretreated collagen surface in the earlier time after start of the perfusion of the platelet-reduced blood (data not shown).
Analysis of the mechanism of platelet adhesion enhancement by VWF in platelet-reduced blood under flow conditions. (A) Erythrocyte fraction (< 3000 platelets/μL) suspended in HEPES-Tyrode/2% BSA (hematocrit, 35%-40%) containing 4 RCo U/mL VWF was perfused on the collagen-coated glass plate for 2 minutes (VWF-pretreatment) and then immediately changed to flow platelet-reduced blood on the same glass plate for 2 minutes at a shear rate of 1600 s–1; the platelet adhesion was measured as indicated in “Materials and methods.” The 0 and 4 indicate the addition of 0 and 4 VWF:RCo U/mL purified VWF in the platelet-reduced blood. Data are each a mean ± SEM (n = 3). (B) Platelet-reduced blood (4-5 × 104 platelets/μL) prepared with PPACK-treated whole blood containing 2 RCo U/mL VWF/FVIII complex was perfused with anti-GPIb (NNKY5-5), anti-α2β1 (Gi9), or anti-αIIbβ3 (P2) monoclonal antibodies at the concentrations 20 μg/mL for 2 minutes at a shear rate of 1600 s–1. Platelet adhesions of platelet-reduced blood with no additions 0 (n = 11), 2 (n = 6) VWF:RCo U/mL VWF/FVIII complex, NNKY5-5 (n = 3), P2 (n = 3), and Gi9 (n = 3). Data are each a mean ± SEM.
Analysis of the mechanism of platelet adhesion enhancement by VWF in platelet-reduced blood under flow conditions. (A) Erythrocyte fraction (< 3000 platelets/μL) suspended in HEPES-Tyrode/2% BSA (hematocrit, 35%-40%) containing 4 RCo U/mL VWF was perfused on the collagen-coated glass plate for 2 minutes (VWF-pretreatment) and then immediately changed to flow platelet-reduced blood on the same glass plate for 2 minutes at a shear rate of 1600 s–1; the platelet adhesion was measured as indicated in “Materials and methods.” The 0 and 4 indicate the addition of 0 and 4 VWF:RCo U/mL purified VWF in the platelet-reduced blood. Data are each a mean ± SEM (n = 3). (B) Platelet-reduced blood (4-5 × 104 platelets/μL) prepared with PPACK-treated whole blood containing 2 RCo U/mL VWF/FVIII complex was perfused with anti-GPIb (NNKY5-5), anti-α2β1 (Gi9), or anti-αIIbβ3 (P2) monoclonal antibodies at the concentrations 20 μg/mL for 2 minutes at a shear rate of 1600 s–1. Platelet adhesions of platelet-reduced blood with no additions 0 (n = 11), 2 (n = 6) VWF:RCo U/mL VWF/FVIII complex, NNKY5-5 (n = 3), P2 (n = 3), and Gi9 (n = 3). Data are each a mean ± SEM.
To determine whether platelet membrane adhesion receptors contribute to this VWF-stimulated platelet adhesion in platelet-reduced blood, we tested the effects of the following specific antibodies for these proteins: anti-GPIb, anti-integrin α2β1, and anti-integrin αIIbβ3. As shown in Figure 6B, anti-GPIb monoclonal antibody (NNKY5-5) completely inhibited platelet deposition, indicating the major contribution of the initial GPIb-VWF interaction to platelet adhesion under flow, even in the blood containing a high level of VWF and reduced platelet concentration. The anti-αIIbβ3 monoclonal antibody (P2) reduced the area coverage to the level of blood without the addition of VWF. The obtained fluorescent image of platelet adhesion in the presence of P2 indicated that platelet aggregate formation on the collagen surface was significantly decreased (Figure 6B-C), suggesting that the interaction between integrin αIIbβ3 and VWF contributes to the enhanced platelet aggregate formation in VWF-supplemented, platelet-reduced blood. Anti-α2β1 monoclonal antibody (Gi9) partially prevented the stimulatory activity of VWF, supporting the published data indicating the weak effect of anti-integrin α2β1 antibodies on platelet adhesion to collagen under flow conditions.37
Discussion
VWF has been shown to play a key role in platelet adhesion and aggregation under flow conditions, and high plasma level of VWF is a risk factor for myocardial infarction23 or ischemic stroke.24-26 Although many reports showed decreased platelet adhesion under flow conditions when plasma VWF was genetically deficient or inhibited by antibodies,10,12,38,39 platelet adhesion and aggregation under flow conditions in the presence of higher concentration of VWF have not received much attention. In the present study, we analyzed platelet adhesion to an immobilized-collagen surface under flow using blood containing an extra amount of VWF, compared with the physiologic amount in normal blood. Addition of 1 to 4 times more VWF/FVIII to normal blood increased platelet adhesion 2- to-6 fold (Figure 3A-B), with larger and more strongly fluorescent aggregates being formed. These results support the above-described clinical observations that a high level of VWF is a risk factor for thrombosis.
The extent of platelet adhesion to the collagen surface depends on the platelet counts in the blood (Figure 2A-B). Blood containing less than 10 × 104 platelets/μL showed much less platelet adhesion than that of normal blood, with this adhesion consisting mainly of single platelets when the adhesion was analyzed at 2 minutes after initiating flow. Under such a condition, where platelet counts are 4 to 6 × 104 platelets/μL, we analyzed the effect of VWF addition on platelet adhesion to a collagen surface under flow conditions. Addition of more than 2 VWF:RCo U/mL VWF/FVIII increased the platelet adhesion of platelet-reduced blood to the level of normal blood (Figure 3-D). The addition of the same VWF:RCo of purified VWF (> 2 VWF:RCo U/mL) increased platelet adhesion, but addition of recombinant FVIII had no effect (Figure 4A-B). These results indicate that the adhesion-stimulating effect of VWF/FVIII is attributable to VWF and not to FVIII.
To determine how exogenously added VWF exerts its effect, we determined the collagen-coated surface localization of FITC-labeled VWF added into platelet (mepacrine-labeled)–reduced blood under flow conditions. The location of the FITC-labeled VWF fluorescence closely coincided with that of the mepacrine-labeled platelets. Both fluorescences appeared as spots that were densely distributed near the inlet of the blood flow (Figure 5B-C). These results indicated that exogenously added VWF binds to platelet aggregates formed on the collagen surface, suggesting that the added VWF would affect aggregate formation. This idea is also supported by our findings showing that VWF addition increased aggregate formation and anti-integrin αIIbβ3 antibody inhibited this (Figure 6B). Pretreatment of the collagen-coated surface with VWF under flow conditions showed no significant enhancing effect on platelet area coverage (Figure 6A), suggesting that even if more VWF bound to the collagen surface, such an increase in VWF density would not have any significant effects on platelet adhesion in the later stage. Since the addition of anti-GPIb antibody completely inhibited the platelet adhesion to collagen (Figure 6B) as indicated before,10,12 the initial interaction of GPIb with VWF bound to collagen is still indispensable for platelet adhesion to the collagen surface in blood containing high VWF concentration and low platelet count. These results suggested that the normal level of VWF in blood is sufficient for the initial platelet-VWF interaction on the collagen surface. Our results taken together suggest that exogenously added VWF acts by stimulating the platelet-platelet interaction to enable more efficient platelet thrombus formation on the collagen surface. In normal blood, platelets adhere as single cells on the collagen at first, becoming activated; by recruiting more platelets, these single platelet adhesion sites grow into aggregates in the later stage of adhesion.10,12,32 In our experiments, platelets adhered mainly as single cells even at 2 minutes after initiating the flow of platelet-reduced blood, compared with normal blood that showed aggregate formation by this time; then addition of VWF changed the adhesion pattern to that of normal blood (Figures 3D and 4B). Since adhesion and aggregation strongly depend on platelet concentration in the blood (Figure 2), collision of flowing platelets with activated platelets adhered on the collagen surface could be an important step for aggregate formation. Thus, after the initial contact between the platelets, VWF would “reinforce” the platelet-platelet interaction, thereby stimulating the efficiency of aggregation formation. Involvement of VWF in aggregate formation under flow conditions was indicated previously.3,17,39 In addition, shear stress–dependent binding of soluble VWF to GPIb and concomitant integrin αIIbβ3 activation11 would be another factor stimulating the platelet adhesion and aggregation.
The drug DDAVP is known to induce the release of several factors such as VWF and tissue-type plasminogen activator from the storage pool of the endothelial cell40-42 ; for many years, it has been used to treat patients with von Willebrand disease or mild hemophilia A.27 When DDAVP administrations are repeated 3 to 4 times at 24-hour intervals, the average FVIII responses of patients with hemophilia are approximately 30% less than those obtained after the first dose.43 Its efficacy against those diseases is due to the release of VWF from endothelial cells, and DDAVP has been suggested to be useful for the treatment of bleeding in patients with thrombocytopenia.29 However, it is yet unclear whether VWF released by DDAVP would really prevent the bleeding, and if it does, what level of VWF is needed to control the bleeding tendency in thrombocytopenia. As shown in Figures 3A and 4A, our present studies demonstrated that the addition of 2 VWF:RCo U/mL VWF enhances the hemostatic potential of platelet-reduced blood to the normal level. When DDAVP was administered to patients with congenital platelet thromboxane A2 abnormality, VWF concentrations in plasma were reported to increase to 3 to 5 VWF:RCo U/mL plasma.44 Since the addition of 2 VWF:RCo U/mL VWF would become 4 to 5 VWF:RCo U/mL plasma considering the 35% to 40% hematocrit of our platelet-reduced blood, this report suggested that the administration of DDAVP would increase plasma VWF concentration to the level that we found to be effective for increasing platelet deposition under the ex vivo conditions.
In this paper, we demonstrated that high levels of VWF accelerated the thrombus formation of platelet-reduced blood by enhancing the platelet aggregation under flow conditions. These data suggest that supplementation of VWF to patients with thrombocytopenia might be useful for increasing their hemostatic potential. However, for the clinical application of this therapy, more clinically oriented studies in ex vivo or animal models must be performed.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-05-1827.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Shosaku Nomura (Kansai Medical University) for providing the monoclonal antibody NNKY5-5 and Dr Takashi Okamura (Faculty of Medicine, Kurume University) for his helpful discussions. We also thank Ms Mamiko Sonoda (Institute of Life Science, Kurume University) and Ms Michiko Inaba (Blood Product Research Department, Kaketsuken) for their excellent technical assistance. We gratefully acknowledge Prof Emeritus Sadaaki Iwanaga (Kyushu University) for his valuable suggestions during the course of this research and his comments about the text.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal