Abstract
Jab1 is a multifunctional protein associated with the signaling pathway, cell-cycle regulation, and development, and acts as a key subunit of COP9 signalosome (CSN). Jab1 promotes degradation of the cyclin-dependent kinase inhibitor p27Kip1 by transportation from the nucleus to the cytoplasm. However, there has been no clear evidence for whether and how Jab1 contributes to malignant transformation in human cancers. Here we show that Bcr-Abl tyrosine kinase facilitates the down-regulation of p27 by modulating complex formation of Jab1/CSN through the mitogen-activated protein (MAP) kinase and phosphatidylinositol 3 (PI3) kinase signaling pathways. Nearly half of the chronic myelogenous leukemia cell lines and the murine hematopoietic precursor cells expressing Bcr-Abl exhibited a marked increase in the small loose Jab1 complex located in the cytoplasm. Inhibition of Bcr-Abl kinase by STI571 induced G1 arrest and caused a recovery of the p27 level with reduction of the small Jab1 complex from the cytoplasm. Either blockade of the MAP kinase and PI3 kinase pathways by specific inhibitors or Jab1 knockdown by small interfering RNA (siRNA) prevented p27 down-regulation as well as formation of the small complex. Thus, regulation of p27 via modulation of the Jab1 subcomplex is a novel mechanism whereby Bcr-Abl oncogenic signals accelerate abnormal cell proliferation.
Introduction
The signals triggered by oncogenic protein kinases ultimately control the cell-cycle machinery to promote cell proliferation via constitutive activation of downstream pathways. However, little is known about how oncogenic signals reach cell-cycle regulators and override the restrictions that suppress undesirable cell proliferation. Bcr-Abl is a cytoplasmic chimeric oncoprotein produced by Philadelphia chromosome translocation and found in more than 90% of patients with chronic myelogenous leukemia (CML). The constitutive tyrosine kinase activity of Bcr-Abl confers growth factor independency and transforming activity to hematopoietic cells in vitro.1,2 Bcr-Abl activates multiple signaling cascades including the Ras/mitogen-activated protein kinase (MAPK), phosphatidylinositol 3 kinase (PI3K)/Akt, Janus kinase (Jak)/signal transducer and activator of transcription (Stat), and Myc pathways, which are required for mitogen-activated cell proliferation, cell survival, and stress responses. Clinical studies and animal models prove that Bcr-Abl expression serves as the initiating event in the transformation by activating these pathways.3-5 However, down-stream elements of the signaling pathways leading to the cell-cycle machinery are poorly understood.
The proliferation of mammalian cells is strictly controlled by both negative and positive regulators that specifically function during the G1 phase of the cell-cycle.6 Among G1 regulators, the cyclin-dependent kinase inhibitor p27Kip1 is a key component in growth control in response to extracellular signals.7 Although mutations in the p27 gene are rare in human tumors, reduced expression of the p27 protein correlates well with both histologic aggressiveness and poor prognosis in approximately half of all human cancers.8-10 These low levels of p27 result from increased 26S proteasome-mediated proteolysis. In fact, overexpression of Skp2, a Skp1-Cullin-F-box (SCF) ubiquitin ligase required for degradation of p27, inversely correlates with the reduced level of p27 in several human cancers.11,12 Recent studies have shown that inhibition of the nuclear import of p27 by protein kinase B (PKB)/Akt-mediated phosphorylation is linked to a poor prognosis in breast cancer.13-15 These results indicate that both accelerated degradation of p27 and the segregation of p27 from the nucleus to the cytoplasm are essential in tumorigenesis. In the case of CML, Bcr-Abl is reported to down-regulate the intracellular level of p27 or to inactivate p27 by cytoplasmic segregation.16-19 It is possible that other signaling pathways involved in oncogenesis target the machinery that controls the stability and localization of p27.
We previously found that Jab1 promotes p27 degradation by transporting it from the nucleus to the cytoplasm in a CRM1-dependent manner.20 Jab1 is also known as the fifth component of the Constitutive Photomorphogenesis 9 (COP9) signalosome complex (CSN).21,22 In mammals, CSN appears to be involved in important signal transduction pathways because of its ability to regulate protein kinases that phosphorylate c-Jun, p53, IκBα and ICSBP/IRF-8.21,23-25 The phosphorylation of c-Jun and p53 requires direct binding to Jab1.26 In addition, CSN cleaves the ubiquitinlike protein Nedd8 from the Cul1 subunit of the SCF ubiquitin ligase.27 This “deneddylation” activity of CSN depends on the JAMM motif within the MPN domain of the Jab1/CSN5 subunit.28 Thus, Jab1 and CSN seem to play an important role in various physiologic processes of signal transduction and proteolysis. At least 2 different Jab1-containing complexes are found in proliferating cells.29-32 One is the 450-kDa CSN complex exhibiting a nuclear localization, and the other is the approximately 100-kDa form located in the cytoplasm. Leptomycin B, a chemical inhibitor of CRM1-dependent nuclear export, reduces the amount of the 100-kDa Jab1 complex and prevents p27 down-regulation, indicating that the small complex, rather than CSN, is a direct player in nuclear export–dependent p27 degradation.32 Recently, in contrast to the small complex, CSN was reported to inhibit the p27 degradation by promoting deneddylation of Cul1.33
These recent findings raise several critical questions: is Jab1 involved in malignant transformation in human cancer, and do the 2 Jab1 complexes contribute to the regulation of p27 independently or together via cross talk? In this study, we show that an oncogenic signal initiated by Bcr-Abl tyrosine kinase modulates the Jab1-containing subcomplexes of CSN through both Ras/MAPK and PI3K pathways, and an increase in the cytoplasmic small Jab1 complex results in a decrease in p27 level. The resultant down-regulation of p27 is one of the key events by which Bcr-Abl accelerates abnormal cell proliferation leading to leukemogenesis. Jab1/CSN is a critical mediator of the oncogenic signaling, which enforces cell-cycle progression. In this oncogenic process, Jab1 has an essential function because Jab1 knockdown using the small interfering RNA (siRNA) technique abolished both the formation of the small complex and down-regulation of p27.
Materials and methods
Cell culture, transfection, and cell-cycle analysis
Hematopoietic cell lines used in this study were maintained in RPMI-1640 medium containing 2 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS). Cells were treated for 8 hours with 5 μM STI571 (kindly provided by Novartis, Basel, Switzerland), 5 μM MG132 (Biomol, Plymouth Meeting, PA), 20 μM LLM (Biomol), 2 ng/mL leptomycin B (LMB; kindly provided by Minoru Yoshida, RIKEN), 60 μM curcumin (Sigma, St Louis, MO), 1 μM H89 (SEIKAGAKU, Tokyo, Japan), 10 nM staurosporine (Sigma), 100 μM PD-98059 (Biomol), 10 μM wortmannin (Sigma), and 10 μM LY-294002 (Sigma) before harvest. In a chase experiment, cells were treated with STI571 as described above, followed by additional 30-minute incubation in 2 μg/mL cycloheximide together with STI571, and released in an STI571-free medium with and without cycloheximide. In a murine BaF3–derived cell line (TonB cells34 ; kindly provided by George Q. Daley, Whitehead Institute), Bcr-Abl was induced to express a tetracycline derivative by incubation in 1 μg/mL doxycycline (DOX) for 16 to 24 hours. A dominant-negative mutant of Ras was induced to express in K562-derived cell lines35 (kindly provided by Yuzuru Kanakura, Osaka University) by incubation in 10 μg/mL IPTG for 24 hours. K562 cells were transfected by electroporation with an expression vector containing hemagglutinin protein (HA)–tagged Jab1 cDNA together with a neo marker–containing plasmid, and selected in 1 mg/mL G418 (Geneticin; GIBCO, Carlsbad, CA) for 2 weeks.
The vectors for RNA interference (RNAi) specific to mouse and human Jab1 were constructed based upon the pSilencer expression vector system (Ambion, Austin, TX) according to the manufacturer's instructions using the following oligonucleotides: human Jab1 (hJab1) sense (5′-GCTCAGAGTATCGATGAAACGAGTCTCATAGCTACTTTTTTTTT-3′), hJab1 antisense (5′-AATTAAAAAAGCTCAGAGTATCGATGAAATCTCTTGAACGAGTCTCATAGCTACTTTGGCC-3′), murine Jab1 (mJab1) sense (5′-CAACAACAAGAAATCCTGGTTCAAGAGACCAGGATTTCTTGTTGTTGTTTTTT-3′), and mJab1 antisense (5′-AATTAAAAAACAACAACAAGAAATCCTGGTCTCTTGAACCAGGATTTCTTGTTGTTGGGCC-3′). Vectors were transfected together with the green fluorescent protein (GFP) expression vector as a marker into cells using the Lipofectamine 2000 kit (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions. After 12 hours, the same transfection procedures were repeated and cells were harvested 48 and 72 hours after initial transfection. Transfection efficiency was constantly 50% to 60%, as determined by the GFP expression under the fluorescent microscope.
For flow cytometric analysis of DNA content, cells were suspended in a 1 mL solution of 0.1% sodium citrate and 0.1% Triton X-100 containing 50 g/mL propidium iodide and treated with 1 g/mL RNase for 30 minutes at room temperature. Fluorescence from the propidium iodide–DNA complex was measured with a FACScan flow cytometer (Becton Dickinson, San Jose, CA). For cell-cycle analysis of GFP-expressing cells, cells were incubated in 10 μg/mL Hoechst 33342 for 90 minutes at 37°C, and GFP-positive fractions were gated and analyzed using a FACS Vantage flow cytometer (Becton Dickinson). The percentages of cells in G1, S, and G2/M phases of the cell-cycle were determined using the Cell Fit cell-cycle software, Becton Dickinson.
Clinical samples and cell preparation
All patient samples and normal peripheral blood cells were obtained with informed consent. Mononuclear cells from heparinized bone marrows and peripheral blood of patients with Bcr-Abl–positive CML in chronic and accelerated phases and negative acute leukemia were separated by lymphoprep (Nycomed, Oslo, Norway) density gradient centrifugation and frozen as pellets or cultured for further study.
Immunofluorescent staining
K562 cells were cytocentrifuged onto glass slides, fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, stained with affinity-purified anti-Jab1 rabbit polyclonal antibody, and incubated with fluorescein-linked antirabbit immunoglobulin G (IgG; Hoechst Aktiengesellschaft, Frankfurt, Germany). Chromosomal DNA was stained in 1 μg/mL Hoechst 33342 for 2 minutes. The samples were viewed by phase-contrast or fluorescence microscopy.
Antibodies
Rabbit polyclonal antibodies specifically recognizing mouse Jab1 and Skp2 were produced against 6xHis-tagged full-length polypeptides expressed in bacteria, and were tested for cross-reactivity with mouse and human proteins. Antibodies were affinity-purified using purified glutathione s-transferase (GST)–tagged recombinant proteins immobilized onto CNBr-activated Sepharose beads. Elution of bound IgG was performed at pH 2.5 and 3.5.
Rabbit polyclonal antibodies to p27 (C-19), mouse monoclonal antibody to phosphotyrosine (PY-20), and rat monoclonal antibody to cyclin D3 (18B6-10) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies to retinoblastoma protein (XZ91, G3-245) and Abl (8E9) were obtained from PharMingen (San Diego, CA). Mouse monoclonal antibodies to γ-tubulin (GTU-88) and an HA peptide epitope (12CA5) were from Sigma. Mouse monoclonal antibodies to p27 (clone 57) and β-actin (clone C4), and rabbit polyclonal antibody to Cul1 (ZL18) were from Transduction Laboratories (Lexington, KY), Boehringer Mannheim (Mannheim, Germany), and Zymed (South San Francisco, CA), respectively.
Protein analyses
Gel electrophoresis, immunoblotting, and glycerol gradient centrifugation were performed as described.20,32 For nondenaturing gel electrophoresis (native-PAGE), cells were lysed in a digitonin-lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane, pH 8.0], 120 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], and 0.1% digitonin) at 1 × 105 cells/μL (more than 1 × 107 cells were required for quantitative, reproducible native-PAGE analysis). Equal amounts (∼ 100 μg) of total protein were separated on precast native gradient gels (5%-15%; Bio-Craft, Tokyo, Japan) without sodium dodecyl sulfate (SDS) at 5 mA for 16 hours followed by 10 mA for 6 hours, and analyzed by anti-Jab1 immunoblotting. To prepare direct lysates for immunoblotting, cell lysates in a digitonin-lysis buffer were mixed with an equal volume of a sample buffer (80 mM Tris-HCl, pH 6.8, 0.2 M dithiothreitol [DTT], 2% SDS, 20% glycerol, and 0.1% Bromophenol Blue) and boiled for 4 minutes. Developed films were quantitatively analyzed with a densitograph (ATTO, Tokyo, Japan).
Retroviral transfection and transformation assay with primary bone marrow cells
The bone marrow assay was performed according to methods described previously.1,36 Bone marrow cells were collected from the femurs and tibias of 6- to 8-week-old Balb/c mice (Clea Japan, Tokyo, Japan) and incubated overnight in bone marrow medium (Dulbecco modified Eagle medium [DMEM], 15% FBS, 5% WEHI3B-conditioned medium, 2 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 6 ng/mL mouse interleukin-3 [IL-3], 10 ng/mL human IL-6, and 50 ng/mL mouse stem cell factor (SCF); recombinant cytokines were from Genzyme, Minneapolis, MN). Nucleated bone marrow cells plated at approximately 107 cells per well in 6-well plates were infected with 5 mL MSCV-p210Bcr-Abl-IRES-GFP retroviral supernatant by centrifugation at 1000g for 90 minutes. The spin infection was repeated the next day and cells were cultured in bone marrow medium for 72 hours. The cells were washed and cultured in selection medium (RPMI-1640, 10% FCS, 100 μM 2-mercaptoethanol, 2 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin) for several days.
Results
Small complex of the Jab1/COP9 signalosome is up-regulated in CML cells
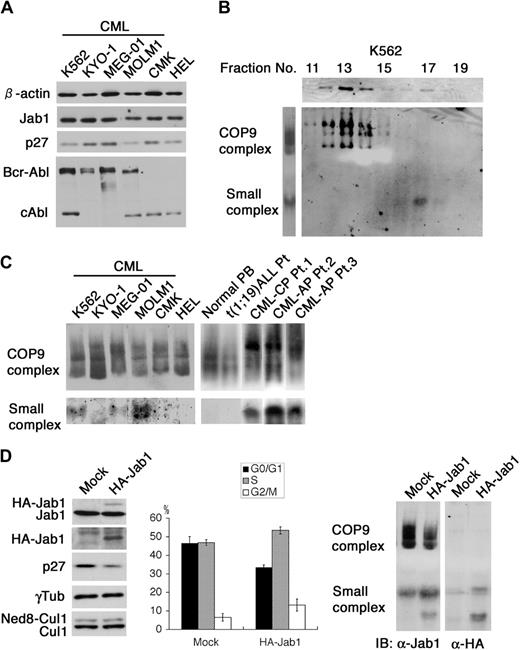
We evaluated endogenous levels of p27 protein and the status of Jab1/CSN in various human hematopoietic cell lines derived from patients with leukemia. Of 4 CML cell lines carrying Bcr-Abl, 2 showed low expression of p27 without any change in the total level of Jab1 (Figure 1A). Since Jab1 is suggested to act as a small complex in the cytoplasm when promoting p27 degradation,32 it is more important to know the distribution of the 100-kDa small complex and the conventional 450-kDa complex than the total protein expression level. We chose K562 cells as a representative CML cell line expressing a low level of p27 to analyze endogenous Jab1 complexes by glycerol gradient fractionation analysis. Jab1 in K562 cells was found in 2 different fractions corresponding to molecular masses of 100 and 450 kDa (Figure 1B, upper panel). As a more convenient and quantitative way to analyze the Jab1 complex, we optimized the nondenaturing polyacrylamide gel electrophoresis (native-PAGE) method, which had previously proved useful in analyses of large complexes such as the 26S proteosome and cytochrome bc/bf complexes. On separating cell lysate by native-PAGE and immunoblotting with antibody to Jab1, 2 major bands were detected (Figure 1B, left panel). To determine what fractions correspond to these bands, cell lysate was fractionated by glycerol gradient centrifugation and each fraction was sequentially separated by native-PAGE. Immunoblotting with antibody to Jab1 showed that the multiple upper bands obtained using whole lysate were identical to CSN and the lower band corresponded to the small complex (Figure 1B, lower panels). These results indicate that this method is a powerful and convenient way to analyze the Jab1-containing complexes.
Characterization of the Jab1-containing complexes in hematopoietic cell lines. (A) Equal amounts of cell lysate from CML cell lines (K562, KYO-1, MEG-01, and MOLM1) and non-CML cell lines (CMK and HEL) were analyzed by immunoblotting using antibodies against Jab1, p27, Abl, and β-actin. (B) Lysate from K562 was fractionated through a 10% to 40% glycerol gradient by centrifugation at 27 000 rpm for 24 hours at 4°C. Each fraction was analyzed by immunoblotting using antibody against Jab1 (first row). Direct lysates (second row, left) or the same fractions used in the upper panels (second row, right) were separated by nondenaturing gel electrophoresis (native-PAGE) and analyzed by immunoblotting using antibody against Jab1. (C) Cell lysates extracted from several CML cell lines and primary cells from patients with CML in chronic phase (CML-CP) and accelerated phase (CML-AP) as well as non-CML cell lines and other primary cells were separated by native-PAGE and analyzed by immunoblotting using antibody against Jab1. (D) K562 cells were electroporated with a mock vector and an HA-tagged Jab1 expression vector together with a neo marker–containing plasmid. After G418 selection, cell lysates were separated by SDS-PAGE (left panels) and native-PAGE (right panels), and analyzed by immunoblotting using antibodies against Jab1, HA-epitope, p27, γ-tubulin, and Cul1. The cell-cycle distributions of the GFP-positive cells were analyzed using a flow cytometer after staining with Hoechst 33342. The averages of 3 independent experiments and standard deviations are shown.
Characterization of the Jab1-containing complexes in hematopoietic cell lines. (A) Equal amounts of cell lysate from CML cell lines (K562, KYO-1, MEG-01, and MOLM1) and non-CML cell lines (CMK and HEL) were analyzed by immunoblotting using antibodies against Jab1, p27, Abl, and β-actin. (B) Lysate from K562 was fractionated through a 10% to 40% glycerol gradient by centrifugation at 27 000 rpm for 24 hours at 4°C. Each fraction was analyzed by immunoblotting using antibody against Jab1 (first row). Direct lysates (second row, left) or the same fractions used in the upper panels (second row, right) were separated by nondenaturing gel electrophoresis (native-PAGE) and analyzed by immunoblotting using antibody against Jab1. (C) Cell lysates extracted from several CML cell lines and primary cells from patients with CML in chronic phase (CML-CP) and accelerated phase (CML-AP) as well as non-CML cell lines and other primary cells were separated by native-PAGE and analyzed by immunoblotting using antibody against Jab1. (D) K562 cells were electroporated with a mock vector and an HA-tagged Jab1 expression vector together with a neo marker–containing plasmid. After G418 selection, cell lysates were separated by SDS-PAGE (left panels) and native-PAGE (right panels), and analyzed by immunoblotting using antibodies against Jab1, HA-epitope, p27, γ-tubulin, and Cul1. The cell-cycle distributions of the GFP-positive cells were analyzed using a flow cytometer after staining with Hoechst 33342. The averages of 3 independent experiments and standard deviations are shown.
We subjected cell lysates extracted from several CML cell lines and primary CML cells as well as non-CML myeloid cell lines and primary cells to this native-PAGE (Figure 1C). Notably, 3 of 4 CML cell lines, but none of the non-CML myeloid cells, exhibited clear lower bands identical to the small complex. In addition, all primary CML cells (3 of 3) obtained from patients, but no other primary cells, contained a substantial amount of the small complex, and the levels were even higher than those in cell lines, suggesting that prolonged tissue culture reduced dependency of the small Jab1 complex presumably due to additional genetic alterations. CML cells expressing low levels of p27 (K562 and MOLM1) had markedly increased amounts of the small complex with a slightly decreased level of CSN, while cells with a lower level of the small complex (MEG-01) contained a relatively higher level of p27. These findings lead to the idea that signals triggered by Bcr-Abl promote p27 degradation by inducing formation of the small Jab1 complex and accelerate cell proliferation.
Since we previously showed that the ectopic overexpression of Jab1 induces down-regulation of p27 in mouse fibroblasts,20 we investigated the effect of Jab1 overexpression on the distribution of Jab1 complexes in CML cells. We introduced an HA-tagged Jab1 expression vector into K562 cells followed by selection in G418 and performed native-PAGE and immunoblotting. Ectopic expression of HA-Jab1 induced p27 down-regulation with an obvious decrease and an increase in G1 and S phase populations, respectively, in K562 cells (Figure 1D, left and middle panels). Most HA-Jab1 was in a free form with approximately 20% incorporated into the small complex. No HA-Jab1 was detected in CSN (Figure 1D, right panel). In addition, the proportion of neddylated Cul1 subunit remained unchanged, supporting the notion that the neddylation activity associated with CSN was unaffected. These results suggest that it is the small Jab1 complex, not CSN, that controls the intracellular abundance of p27.
Bcr-Abl kinase induces the small Jab1 complex and p27 down-regulation
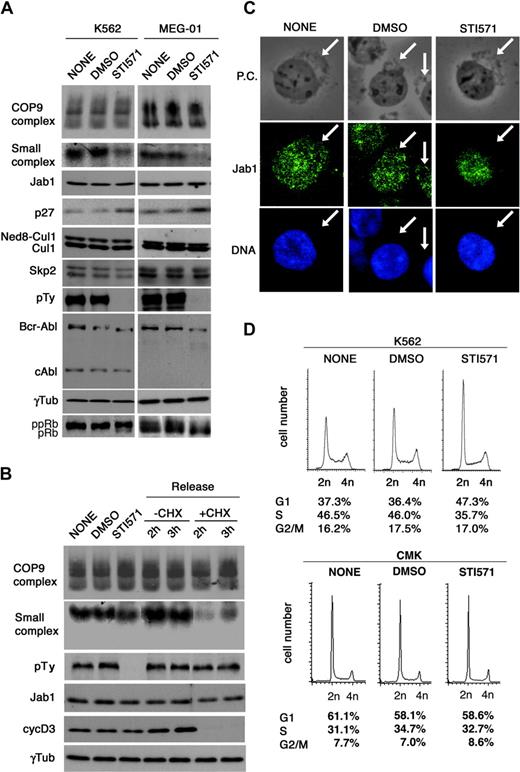
To determine whether Bcr-Abl tyrosine kinase affects the amount of small complex and resultant p27 degradation, we examined the effect of an Abl kinase–specific inhibitor, STI571, which demonstrated striking efficacy on clinical application in patients with CML.37 There were 2 CML cell lines (K562 and MEG-01 cells) expressing different levels of p27 treated with STI571, and the Jab1 complex formation was examined. Under the conditions, the inhibitor completely suppressed the Bcr-Abl kinase activity, as shown by an absence of autophosphorylation of Bcr-Abl (Figure 2A, antiphosphotyrosine blot in the seventh panel, and mobility shift in the eighth panel). After treatment with STI571, a drastic down-regulation of the small complex and marked accumulation of p27 were observed in K562 cells and MEG-01 cells (Figure 2A, second and fourth panels). The level of neddylated Cul1 remained the same (Figure 2A, fifth panel). These results indicate the specific link of the small complex to p27 degradation.
Bcr-Abl kinase regulates the small Jab1 complex in CML cells. (A) K562 cells and MEG-01 cells were treated with DMSO and STI571 (5 μM) for 8 hours. After 8 hours, cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to bottom rows), and analyzed by immunoblotting using antibodies against Jab1, p27, Cul1, Skp2, phosphotyrosine (pTy), Abl, γ-tubulin, and pRb. (B) K562 cells were treated with DMSO and STI571 for 8 hours (second and third lanes). After 8 hours, STI571 was washed out and cells were resuspended in culture medium with and without cycloheximide. Lysates from cells harvested at the indicated times (fourth to seventh lanes) were separated by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows), and analyzed by immunoblotting using antibodies against Jab1, phosphotyrosine, cyclin D3, and γ-tubulin. (C) K562 cells were treated with DMSO and STI571 for 8 hours, cytocentrifuged, and fixed with 4% paraformaldehyde for 10 minutes. The fixed cells were immunostained using antibody to Jab1 (second row) and DNA was visualized with Hoechst 33342 (third row). The image was acquired using an Olympus IX 71 microscope (Olympus, Tokyo, Japan) equipped with a Cool SNAP color digital camera (Roper Scientific, Tucson, AZ). Fluorescence images were electronically recorded with Meta Com software (Universal Imaging, Downingtown, PA). Original magnification × 400. The cytoplasmic boundaries are shown with arrows. P.C. indicates phase-contrast. (D) K562 and CMK cells were treated with DMSO and STI571 for 8 hours. Cells were harvested and stained with PI, and the cell-cycle profile was analyzed using a flow cytometer. Representative histograms for each cell and the distribution of the cells are shown.
Bcr-Abl kinase regulates the small Jab1 complex in CML cells. (A) K562 cells and MEG-01 cells were treated with DMSO and STI571 (5 μM) for 8 hours. After 8 hours, cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to bottom rows), and analyzed by immunoblotting using antibodies against Jab1, p27, Cul1, Skp2, phosphotyrosine (pTy), Abl, γ-tubulin, and pRb. (B) K562 cells were treated with DMSO and STI571 for 8 hours (second and third lanes). After 8 hours, STI571 was washed out and cells were resuspended in culture medium with and without cycloheximide. Lysates from cells harvested at the indicated times (fourth to seventh lanes) were separated by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows), and analyzed by immunoblotting using antibodies against Jab1, phosphotyrosine, cyclin D3, and γ-tubulin. (C) K562 cells were treated with DMSO and STI571 for 8 hours, cytocentrifuged, and fixed with 4% paraformaldehyde for 10 minutes. The fixed cells were immunostained using antibody to Jab1 (second row) and DNA was visualized with Hoechst 33342 (third row). The image was acquired using an Olympus IX 71 microscope (Olympus, Tokyo, Japan) equipped with a Cool SNAP color digital camera (Roper Scientific, Tucson, AZ). Fluorescence images were electronically recorded with Meta Com software (Universal Imaging, Downingtown, PA). Original magnification × 400. The cytoplasmic boundaries are shown with arrows. P.C. indicates phase-contrast. (D) K562 and CMK cells were treated with DMSO and STI571 for 8 hours. Cells were harvested and stained with PI, and the cell-cycle profile was analyzed using a flow cytometer. Representative histograms for each cell and the distribution of the cells are shown.
In K562 cells, withdrawal of the inhibitor from the culture allowed a prompt re-elevation of the tyrosine kinase activity and an increase in the formation of the small complex within 2 hours (Figure 2B) and induced a relatively slow reduction of p27 within 24 hours (data not shown). The reversible inhibitory effects of STI571 imply that Bcr-Abl kinase activity promotes the formation of the small complex and down-regulation of p27. The treatment with cycloheximide after withdrawal of STI571 blocked protein synthesis by reactivating Bcr-Abl signaling. In the presence of cycloheximide, the amount of small complex decreased transiently 2 hours after STI571 withdrawal and partially recovered 3 hours after withdrawal. Because transcriptionally regulated cyclin D3 protein synthesis is completely suppressed under the conditions, the small complex appears to derive from CSN, independent of transcription (Figure 2B, right).
Immunofluorescent staining in K562 cells revealed that Jab1 is present both in the nucleus and in the cytoplasm, but more abundant in the nucleus as in murine fibroblasts. STI571 treatment significantly attenuated the cytoplasmic but not the nuclear signal (Figure 2C), which coincides with the disappearance of the small but not the large complex from the cells (Figure 2A). These results are consistent with the findings that the small complex is located in the cytoplasm and Bcr-Abl kinase regulates the subcellular localization as well as the complex formation of Jab1.
Since these inhibitory effects by STI571 were accompanied by the expression of dephosphorylated Rb protein in K562 cells or the reduction of phosphorylated Rb protein in MEG-01 cells (Figure 2A, bottom panel), the cell-cycle profile was examined. Mock- and dimethyl sulfoxide (DMSO)–treated K562 cells contained 37% G1 phase, 46% S phase, and 17% G2/M phase cells, whereas STI571 treatment increased the G1 phase population by 10% at the expense of the S phase population (Figure 2D). By contrast, CMK cells, a non-CML cell line, did not change the cell-cycle distribution with and without STI571 (Figure 2D).
To clarify whether K562 and MEG-01 are dominant in CML cell lines, we tested 9 CML cell lines with p210 Bcr-Abl as well as 6 non-CML cell lines. An obvious increase in the small complex was detected in 5 of 9 CML cell lines, but in none of the non-CML cell lines (Table 1). STI571 treatment of these cells induced an increase in the p27 level following a decrease in the amount of small complex in all the 5 CML cell lines (Table 1). These results suggest that the response to STI571 observed in K562 cells is a common feature of CML.
Relative protein levels of small Jab1 complex and p27 on STI571 treatment
. | . | . | STI571 treatment . | . | |
|---|---|---|---|---|---|
| Cell line . | Origin . | Small Jab1 complex* . | Small Jab1 complex† . | p27‡ . | |
| K562 | CML | + + + | ↓ ↓ | ↑ ↑ | |
| KYO-1 | CML | - | → | → | |
| MEG-01 | CML | +/+ | ↓ ↓ | ↑ ↑ | |
| MOLM1 | CML | + + + | ↓ ↓ | ↑ ↑ | |
| KCL-22§ | CML | + + + | ↓ | ↑ | |
| YS9;22 | CML | + | → | → | |
| MOLM7 | CML | + + + | ↓ ↓ | ↑ | |
| JURL-MK | CML | + | → | → | |
| NALM1 | CML¶ | - | → | → | |
| CMK | MegL | + | → | → | |
| HEL | EL | + | NT | NT | |
| HL-60 | APL | - | NT | NT | |
| THP-1 | AMoL | - | NT | NT | |
| KY821 | AML | - | NT | NT | |
| NALM16 | ALL | + | NT | NT | |
. | . | . | STI571 treatment . | . | |
|---|---|---|---|---|---|
| Cell line . | Origin . | Small Jab1 complex* . | Small Jab1 complex† . | p27‡ . | |
| K562 | CML | + + + | ↓ ↓ | ↑ ↑ | |
| KYO-1 | CML | - | → | → | |
| MEG-01 | CML | +/+ | ↓ ↓ | ↑ ↑ | |
| MOLM1 | CML | + + + | ↓ ↓ | ↑ ↑ | |
| KCL-22§ | CML | + + + | ↓ | ↑ | |
| YS9;22 | CML | + | → | → | |
| MOLM7 | CML | + + + | ↓ ↓ | ↑ | |
| JURL-MK | CML | + | → | → | |
| NALM1 | CML¶ | - | → | → | |
| CMK | MegL | + | → | → | |
| HEL | EL | + | NT | NT | |
| HL-60 | APL | - | NT | NT | |
| THP-1 | AMoL | - | NT | NT | |
| KY821 | AML | - | NT | NT | |
| NALM16 | ALL | + | NT | NT | |
NT indicates not tested; CML, chronic myeloid leukemia; MegL, megakaryoblastic leukemia; EL, erythroleukemia; APL, acute promyelocytic leukemia; AMoL, acute monocytic leukemia; AML, acute myeloblastic leukemia; and ALL, acute lymphoblastic leukemia.
Protein levels of the small Jab1 complex were assessed by immunoblotting: -, no detectable signal even after a long exposure; +, detection of a very weak signal (less than one fifth that in MEG-01 cells); + +, detection of a strong signal (equal to that in MEG-01 cells; + + +, detection of a very strong signal (more than twice that in MEG-01 cells). The intensity of the immunoblotting signals was analyzed with a densitograph and the average of 2 experiments used
Relative decreased levels of the small Jab1 complex on STI571 treatment: →, no change; ↓, decrease in more than 40% and up to 80% of pretreated cells; ↓ ↓, decrease in more than 80% of pretreated cells
Relative increased levels of p27 on STI571 treatment: →, no change; ↑, increase in more than 30% and up to 70% of pretreated cells; ↑ ↑, increase in more than 70% of pretreated cells
KCL-22 cells are known to be relatively resistant to STI571
NALM1 cells are derived from lymphoid crisis of CML
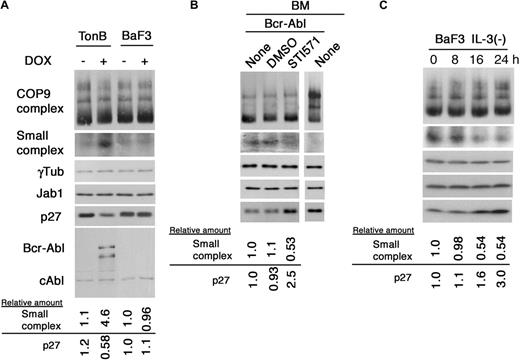
Ectopic expression of p210Bcr-Abl induces down-regulation of p27 protein in myelogenous and B-cell hematopoietic cell lines.16-18 To assess whether Bcr-Abl stimulates the production of the small Jab1 complex in these cells, we used the murine pro-B-cell line TonB, in which Bcr-Abl expression is induced by a doxycyclin (DOX)–responsive promoter. Untreated TonB cells in the absence of IL-3 contained a low level of the small complex; however, DOX-treated TonB cells, in which Bcr-Abl was induced to express, exhibited a 4-fold increase in the small complex and reduction of p27 to 60%, whereas parental BaF3 cells without IL-3 contained the same low level of small complex as in untreated TonB cells and DOX treatment did not alter the expression level (Figure 3A). To verify ex vivo the results obtained with TonB cells, we infected murine primary bone marrow cells with retroviruses encoding p210 Bcr-Abl and green fluorescent protein (GFP). After selection of Bcr-Abl–transformed cells in growth factor–depleted medium, the cells were treated with STI571. The bone marrow cells carrying Bcr-Abl exhibited a marked increase in the small complex and reduction of p27, and treatment of STI571 inhibited the formation of small complex (∼ 50%) and p27 degradation (2.5-fold). As a control, normal bone marrow cells expressed an undetectable level of small complex (Figure 3B). Taken together, the cytoplasmic Jab1 complex appears to be one of the direct targets of Bcr-Abl kinase to disturb normal cell-cycle regulation.
Ectopic expression of Bcr-Abl induced the small Jab1 complex. (A) TonB cells, able to express Bcr-Abl on addition of tetracycline or its analog doxycycline, and parental cells, BaF3, were cultured with and without doxycycline in the absence of IL-3 for 24 hours. After 24 hours of incubation, cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows), and analyzed by immunoblotting using antibodies against Jab1, γ-tubulin, p27, and Abl. The relative amounts of the small Jab1 complex and p27 are presented as the ratio of the small Jab1 complex–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated BaF3 cells as 1.0. (B) Murine bone marrow cells were infected with the MSCV-p210Bcr-Abl-IRES-GFP retrovirus and plated in liquid culture. Transformed bone marrow cells were cultured with and without STI571 for 8 hours. After 8 hours of treatment, cell lysate was separated by native-PAGE (first and second rows) and SDS-PAGE (third to fifth rows), and analyzed by immunoblotting using antibodies against Jab1, γ-tubulin, and p27. The relative amounts of the small Jab1 complex and p27 are presented as the ratio of the small Jab1 complex–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated bone marrow cells expressing Bcr-Abl as 1.0. BM indicates bone marrow cells. (C) BaF3 cells, which were routinely maintained in IL-3, were cultured in the absence of IL-3. At the indicated times, cells were harvested and the cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to fifth rows), and analyzed by immunoblotting using antibodies against Jab1, γ-tubulin, and p27. The relative amounts of the small Jab1 complex and p27 are presented as the ratio of the small Jab1 complex–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated BaF3 cells as 1.0.
Ectopic expression of Bcr-Abl induced the small Jab1 complex. (A) TonB cells, able to express Bcr-Abl on addition of tetracycline or its analog doxycycline, and parental cells, BaF3, were cultured with and without doxycycline in the absence of IL-3 for 24 hours. After 24 hours of incubation, cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows), and analyzed by immunoblotting using antibodies against Jab1, γ-tubulin, p27, and Abl. The relative amounts of the small Jab1 complex and p27 are presented as the ratio of the small Jab1 complex–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated BaF3 cells as 1.0. (B) Murine bone marrow cells were infected with the MSCV-p210Bcr-Abl-IRES-GFP retrovirus and plated in liquid culture. Transformed bone marrow cells were cultured with and without STI571 for 8 hours. After 8 hours of treatment, cell lysate was separated by native-PAGE (first and second rows) and SDS-PAGE (third to fifth rows), and analyzed by immunoblotting using antibodies against Jab1, γ-tubulin, and p27. The relative amounts of the small Jab1 complex and p27 are presented as the ratio of the small Jab1 complex–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated bone marrow cells expressing Bcr-Abl as 1.0. BM indicates bone marrow cells. (C) BaF3 cells, which were routinely maintained in IL-3, were cultured in the absence of IL-3. At the indicated times, cells were harvested and the cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to fifth rows), and analyzed by immunoblotting using antibodies against Jab1, γ-tubulin, and p27. The relative amounts of the small Jab1 complex and p27 are presented as the ratio of the small Jab1 complex–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated BaF3 cells as 1.0.
To examine whether the Jab1-p27 pathway is part of the malignant transformation mediated by Bcr-Abl or normal growth factor (IL-3) signaling, we analyzed the effect of IL-3 depletion on BaF3 cells that had been maintained in IL-3. Figure 3C shows that, in the presence of IL-3, BaF3 cells contained a significant amount of the small complex and depletion of IL-3 reduced its level within 24 hours. During this period, the expression of p27 was induced. Thus, the small complex and p27 are involved in the IL-3–mediated signaling pathway, which is shared with Bcr-Abl.
Bcr-Abl signaling pathways located upstream of the Jab1/CSN complex
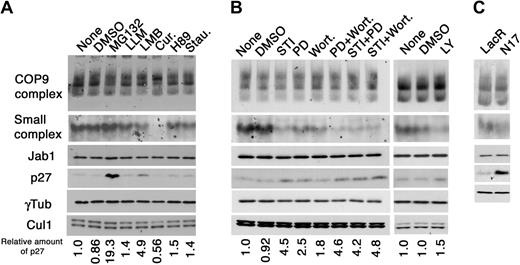
Bcr-Abl activates multiple signaling cascades including the Ras/MAPK, PI3K/Akt, and Jak/Stat pathways. We investigated which pathways activated by Bcr-Abl are involved in modulation of Jab1 complexes and subsequent down-regulation of p27. For this purpose, we used chemicals, which specifically inhibit signaling pathways functioning downstream of Bcr-Abl (PD-98059 for MAPK pathway; wortmannin and LY-294002 for PI3K; H89 and staurosporin for protein kinases A and C, respectively, and used as negative controls) and CSN (curcumin for CSN-associated kinases), or upstream of p27 (MG132 for proteasome; LMB for CRM1). Lysates prepared from K562 cells treated with chemical inhibitors shown at the top of Figure 4A were analyzed by native/SDS-PAGE and immunoblotting. MG132, a specific inhibitor of the 26S proteasome, but not a control calpine inhibitor, LLM, induced a significant accumulation of p27 protein with little effect on the Jab1 complex, indicating that p27 is regulated in a 26S proteasome-dependent manner in K562 cells and the ubiquitin/proteasome pathway is located downstream of Jab1. Treatment of the cells with LMB, an inhibitor of CRM1-dependent nuclear export, reduced the amount of the small complex with accumulation of p27 as we previously showed in murine fibroblasts.32 Treatment of the cells with curcumin completely abolished the small complex and rearranged the multiple band pattern in the CSN region. The molecular targets of curcumin in vivo are largely unknown, but curcumin is reported to be a potent inhibitor of CSN-associated kinases,38 which phosphorylate c-Jun, nuclear factor κB (NFκB), and p53. It is, therefore, possible that the CSN-associated kinases phosphorylate the CSN subunits and play an important role in the CSN complex organization. Alternatively, curcumin may target other biochemical reactions (eg, ubiquitination or proteolysis), thereby regulating the CSN dynamics. Neddylation of Cul1 was largely unaffected in response to a variety of chemicals except for curcumin, which somehow accelerated deneddylation by an unknown mechanism.
Bcr-Abl induces the small Jab1 complex through MAPK and PI3K pathways. (A) K562 cells were treated with the chemicals indicated at the top of panel A for 8 hours. Cell lysates were analyzed by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows), followed by immunoblotting using antibodies against Jab1, p27, γ-tubulin, and Cul1. The relative amounts of p27 are presented as the ratio of p27–γ-tubulin and calculated with the level of untreated K562 cells as 1.0. Cur. indicates curcumin; Stau., staurosporine. (B) K562 cells were treated with DMSO, STI571 (STI), PD-98059 (PD), Wortmannin (Wort.), and the mixture of chemicals indicated for 8 hours. Cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows) and analyzed by immunoblotting using antibodies against Jab1, p27, γ-tubulin, and Cul1. The relative amounts of p27 are presented as the ratio of p27–γ-tubulin and calculated with the level of untreated K562 cells as 1.0. (C) K562 cells, able to produce a dominant-negative form of Ras (N17) on addition of IPTG, were treated with IPTG for 24 hours. Cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to fifth rows), and analyzed by immunoblotting using antibodies against Jab1, p27, and γ-tubulin.
Bcr-Abl induces the small Jab1 complex through MAPK and PI3K pathways. (A) K562 cells were treated with the chemicals indicated at the top of panel A for 8 hours. Cell lysates were analyzed by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows), followed by immunoblotting using antibodies against Jab1, p27, γ-tubulin, and Cul1. The relative amounts of p27 are presented as the ratio of p27–γ-tubulin and calculated with the level of untreated K562 cells as 1.0. Cur. indicates curcumin; Stau., staurosporine. (B) K562 cells were treated with DMSO, STI571 (STI), PD-98059 (PD), Wortmannin (Wort.), and the mixture of chemicals indicated for 8 hours. Cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to sixth rows) and analyzed by immunoblotting using antibodies against Jab1, p27, γ-tubulin, and Cul1. The relative amounts of p27 are presented as the ratio of p27–γ-tubulin and calculated with the level of untreated K562 cells as 1.0. (C) K562 cells, able to produce a dominant-negative form of Ras (N17) on addition of IPTG, were treated with IPTG for 24 hours. Cell lysates were separated by native-PAGE (first and second rows) and SDS-PAGE (third to fifth rows), and analyzed by immunoblotting using antibodies against Jab1, p27, and γ-tubulin.
Next, we examined the effect of PD-98059 and wortmannin, specific inhibitors of MAPK and PI3K, respectively, on the small complex and p27 expression, because the role of MAPK and PI3K signaling pathways is well established and essential for Bcr-Abl–mediated leukemogenesis. A moderate reduction of the small complex with an increase in the p27 level was detected in K562 cells treated with PD-98059 and wortmannin, and wortmannin was less effective for the accumulation of p27 than PD-98059 (Figure 4B). The same results were obtained using another chemical inhibitor, LY294002, which is more specific to PI3K than wortmannin. Interestingly, coordinated treatment with both PD-98059 and wortmannin completely suppressed the formation of the small complex and induced marked accumulation of p27, which is equivalent to the effect of STI571. Furthermore, treatment with STI571 and either PD-98059 or wortmannin was as effective as that with STI571 alone. These results indicate that Jab1 is a downstream target of both MAPK and PI3K pathways, which collaboratively promote the small Jab1 complex formation and resultant p27 down-regulation.
To confirm the above findings, we used an inducible dominant-negative (DN) mutant of Ras (designated as N17) in K562 cells.35 A significant decrease of the small complex with highly accumulated p27 was induced by enforced expression of N17, which blocks the Ras activity, functioning upstream of MAPK and PI3K pathways (Figure 4C). These results are consistent with those obtained in the chemical inhibitor experiments.
Small Jab1 complex is essential for Bcr-Abl–mediated p27 down-regulation
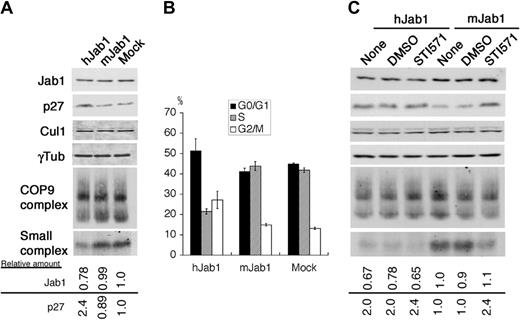
To assess whether the small Jab1 complex is a direct and critical mediator of the Bcr-Abl oncogenic signal to the p27 cell-cycle regulator, we used an RNA interference (RNAi) technique to reduce the amount of endogenous protein in mammalian cells. A GFP expression vector was transfected together with small interfering RNA (siRNA) vectors specific for human Jab1 (hJab1) and mouse Jab1 (mJab1, as a negative control). After careful optimization, more than 60% of K562 cells were successfully transfected under the conditions used, as judged by GFP expression. At 60 hours after transfection, the amount of endogenous Jab1 protein was reduced by 20% to 30% in hJab1 siRNA-transfected cells, but not in mJab1 siRNA-transfected cells (Figure 5A,C), implying that the effect of Jab1 siRNA is highly specific. A small reduction of total Jab1 protein resulted in a preferential decrease of the small complex, but not CSN, with accumulation of p27 and an increase in G1 population of the cells (Figure 5B). Transfection with Jab1 siRNA did not alter the level of neddylated Cul1.
The small Jab1 complex is required for down-regulation of p27 in K562 cells. (A) K562 cells were transfected with the siRNA expression vectors indicated at the top of the panel together with the GFP expression vector. After 60 hours of transfection, cell lysates were separated by SDS-PAGE (first to fourth rows) and native-PAGE (fifth and sixth rows), and analyzed by immunoblotting using antibodies against Jab1, p27, Cul1, and γ-tubulin. The relative amounts of Jab1 and p27 are presented as the ratio of Jab1–γ-tubulin and p27–γ-tubulin and calculated with the level of K562 cells transfected with the empty siRNA expression vector as 1.0. (B) The cell-cycle distributions of the GFP-positive cells in panel A were analyzed using a flow cytometer after staining with Hoechst 33342. The averages of 3 independent experiments are shown. (C) K562 cells transfected with the siRNA expression vectors were treated with DMSO and STI571 for 8 hours. Cell lysates were separated by SDS-PAGE (first to fourth rows) and native-PAGE (fifth and sixth rows), and analyzed by immunoblotting using antibodies to Jab1, p27, Cul1, and γ-tubulin. The relative amounts of Jab1 and p27 are presented as the ratio of Jab1–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated K562 cells transfected with the mJab1 siRNA expression vector as 1.0.
The small Jab1 complex is required for down-regulation of p27 in K562 cells. (A) K562 cells were transfected with the siRNA expression vectors indicated at the top of the panel together with the GFP expression vector. After 60 hours of transfection, cell lysates were separated by SDS-PAGE (first to fourth rows) and native-PAGE (fifth and sixth rows), and analyzed by immunoblotting using antibodies against Jab1, p27, Cul1, and γ-tubulin. The relative amounts of Jab1 and p27 are presented as the ratio of Jab1–γ-tubulin and p27–γ-tubulin and calculated with the level of K562 cells transfected with the empty siRNA expression vector as 1.0. (B) The cell-cycle distributions of the GFP-positive cells in panel A were analyzed using a flow cytometer after staining with Hoechst 33342. The averages of 3 independent experiments are shown. (C) K562 cells transfected with the siRNA expression vectors were treated with DMSO and STI571 for 8 hours. Cell lysates were separated by SDS-PAGE (first to fourth rows) and native-PAGE (fifth and sixth rows), and analyzed by immunoblotting using antibodies to Jab1, p27, Cul1, and γ-tubulin. The relative amounts of Jab1 and p27 are presented as the ratio of Jab1–γ-tubulin and p27–γ-tubulin and calculated with the level of untreated K562 cells transfected with the mJab1 siRNA expression vector as 1.0.
In control K562 cells (mJab1 siRNA–transfected cells), STI571 treatment reduced the amount of small complex and increased the level of p27. However, hJab1-siRNA transfected K562 cells already contained reduced levels of the small complex and STI571 treatment did not further lower levels of the complex and, importantly, did not alter the level of p27 (Figure 5C). These results clearly demonstrate that the small Jab1 complex is an essential downstream transmitter functioning between Bcr-Abl oncogenic kinase and the cell-cycle machinery.
Discussion
Low-level expression of the Cdk inhibitor p27 is well correlated with a poor prognosis in human cancer.6,39,40 Although substrate-specific and cell-cycle–dependent proteolysis plays a pivotal role in regulating the intracellular abundance of p27,41 the signaling pathways leading to the down-regulation of p27 remain to be fully clarified. While analyzing the relevance of p27 expression to the Jab1 complex formation in various human hematopoietic cell lines, we found that Jab1 is a novel target of Bcr-Abl oncogenic signals through both the Ras/MAPK and the PI3K/Akt pathways, and modulation of the complex formation is linked to the regulatory mechanism for p27.
Ras/MAPK and PI3K/Akt pathways are well-characterized signaling cascades that transduce numerous extracellular mitogenic stimuli and are deregulated in a variety of human cancers.42,43 Both not only are major pathways required for primary transformation and cell survival in CML cells, but also affect the stability of p27 in mammalian cell lines. In fact, Bcr-Abl has been reported to down-regulate p27 through the PI3K/Akt pathway in a proteasome-dependent manner.17 However, the direct mediators of p27 down-regulation activated by these oncogenic factors have not been identified. In breast cancer, recent studies showed that an oncogenically activated kinase Akt mediates cell-cycle progression by phosphorylating p27 at a specific threonine residue located within a nuclear localization signal sequence, which results in impairment of p27 nuclear import.13-15 Therefore, the factors regulating the degradation and cytoplasmic segregation of p27 are likely to participate in tumorigenesis. Our findings suggest that Jab1/CSN is a common downstream target of oncogenically activated Ras/MAPK and PI3K/Akt pathways, which lead to p27 down-regulation in many cancers. The finding that on overexpression of proto-oncogene HER2/neu, Jab1 is translocated to the cytoplasm and p27 degradation is promoted through the Ras/MAPK pathway in a proteasome-dependent manner supports this view.44
Jab1 interacts with a variety of factors involved in tumorigenesis, including p27, c-Jun, p53, Bcl3, and hypoxia-inducible factor 1 α (HIF1α).20,26,45-47 In some human tumors, Jab1 expression is inversely correlated with p27.48-50 However, there is not enough evidence yet that Jab1 contributes to malignant transformation in human cancers, because the total amount of Jab1 protein is relatively constant among tumor cells and not necessarily correlated with low levels of p27 protein and its cytoplasmic localization. In this study, we showed that the distribution of Jab1 between different complexes rather than its total expression level is critical to mediate oncogenic signals to a downstream effector, p27, in CML. It is largely unknown how the small Jab1 complex is regulated and what the functional relevance is to CSN. The small complex promotes p27 degradation partly by nuclear export,32 and CSN inhibits p27 degradation via Cul1 deneddylation of the SCF ubiquitin ligase,33 which seems to be contradictory to p27 regulation. However, our results from experiments using chemicals, a dominant-negative mutant, and siRNA clearly prove that the small Jab1 complex–mediated p27 down-regulation is independent of proteolysis of nedd8-Cul1 conjugation. Therefore, it seems that other components in the small complex play an important role in p27 regulation. We have little information as to the identity of this mysterious subunit at the moment. Because the total amount of Jab1 is limited, CSN, but not the small complex, is dominant in mammalian cells. CSN seems to be more essential for early development31,51 and maintenance of cells, while subcomplexes are recent products of evolutionary necessity. It has been suggested that each subunit or subcomplex has a unique function when separated from CSN. Therefore, CSN itself may serve as a docking station for assembly of the CSN subcomplexes or recruit proteins such as kinases, ligases, proteases, and their substrates in response to various intracellular stimuli by a currently undefined mechanism.
We showed here that the Jab1 complex is a novel mediator of Bcr-Abl oncogenic signaling. The question is what molecule directly transduces the Ras/MAPK and PI3K/Akt signaling to Jab1/CSN. Although a number of proteins interacting with each CSN subunit have been isolated by 2-hybrid screenings and pull-down assays,25,52 identification of a direct mediator will be critical for future studies. Moreover, the native-PAGE method provided us an easier, more precise, and more convenient way to analyze the quantitative complex formation. Detection of the small Jab1 complex may help in predicting the efficacy of STI571/imatinib administration in individual patients suffering from CML. As numerous signal transduction inhibitors that target Ras/MAPK and PI3K/Akt pathways have now entered clinical trials,53 Jab1/CSN-mediated regulation of cell proliferation will increase in clinical importance for future cancer therapy.
Prepublished online as Blood First Edition Paper, September 7, 2004; DOI 10.1182/blood-2004-04-1242.
Supported by Grants-in-Aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, and Culture of Japan and by Yamazaki Spice Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank George Q. Daley and Yuzuru Kanakura for cell lines, Owen N. Witte for a plasmid, Elisabeth Buchdunger for STI571, and Minoru Yoshida for LMB.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal