Abstract
CpG oligodeoxynucleotides (CpG-ODNs) affect innate and adaptive immune responses, including antigen presentation, costimulatory molecule expression, dendritic cell maturation, and induction of cytokines enhancing antibody-dependent cell-mediated cytotoxicity (ADCC). We conducted a phase 1 study evaluating 4 dose levels of a CpG-ODN (1018 ISS) with rituximab in 20 patients with relapsed non-Hodgkin lymphoma (NHL). Patients received CpG once a week for 4 weeks beginning after the second of 4 rituximab infusions. Adverse events were minimal. Quantitative polymerase chain reaction (PCR) measurements of a panel of genes inducible by CpG-ODN and interferons were performed on blood samples collected before and 24 hours after CpG. A dose-related increase was measured in the expression of several interferon–inducible genes after CpG and correlated with serum levels of 2′-5′ oligoadenylate synthetase (OAS), a validated interferon response marker. Genes induced selectively by interferon-γ (IFN-γ) were not significantly induced by CpG. In conclusion, we have defined a set of gene expression markers that provide a sensitive measure of biologic responses of patients to CpG therapy in a dose-related manner. Moreover, all the genes significantly induced by this CpG are regulated by type 1 interferons, providing insight into the dominant immune mechanisms in humans. CpG treatment resulted in no significant toxicity, providing rationale for further testing of this exciting combination immunotherapy approach to NHL.
Introduction
Few advances in the treatment of non-Hodgkin lymphoma (NHL) have had such dramatic impact as the anti-CD20 chimeric monoclonal antibody rituximab (Rituxan; BiogenIdec, Cambridge, MA, and Genentech, South San Francisco, CA).1 Rituximab compares favorably with single-agent chemotherapy, and has a significantly better toxicity profile, when used in patients with relapsed indolent NHL.2 However, indolent NHL remains an incurable disease with standard therapy.3 At least 50% of patients with recurrent indolent NHL do not respond to rituximab therapy, and all patients will experience disease progression at some point after rituximab monotherapy.
The mechanism of cytotoxicity induced by rituximab therapy in humans is not completely understood,4 and probably includes several interactive mechanisms.5 Direct apoptosis from cross-linking of CD20 has been observed in some malignant B-cell lines.6-8 Complement-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) have been demonstrated in vitro.9,10 Rituximab in vitro has been shown to translocate CD20 into lipid rafts and to activate complement-mediated lysis;11 however, there is little convincing evidence that this finding is important therapeutically.12 In studies of normal and malignant human B cells in vitro, B-cell depletion was observed with rituximab therapy in the presence of mononuclear cells, but not in the presence of complement,13 suggesting the importance of cell-mediated mechanisms (ADCC). Moreover, the expression of complement inhibitors (CD55 and CD59) on tumor cells and their susceptibility to in vitro complement-mediated killing did not predict clinical outcome after in vivo treatment with rituximab in a recent clinical trial.14 ADCC, therefore, appears to be a major in vivo mechanism of rituximab.15 A contribution of ADCC is supported by a clear role for the FcγR-bearing effectors in mediating response to rituximab in certain lymphoma histologies.16,17
Immunostimulatory sequences (ISSs) are short sequences of synthetic DNA containing unmethylated CpG dimers that have multiple effects on the human immune system.18 ISSs signal through the Toll-like receptor 9, which is expressed on only a few specific subsets of immune cells.19 Immunostimulatory effects of CpG-ODN include the induction of proliferation and immunoglobulin production by B cells and the induction of interferon-α (IFN-α), IFN-β, interleukin-12 (IL-12), and tumor necrosis factor α (TNF-α) by plasmacytoid dendritic cells (DCs). These cytokines, in turn, produce potent responses in many other immune cell types not directly responsive to CpG-ODN. IL-12, TNF-α, and, especially, IFN-α activate cytotoxicity by natural killer (NK) cells, and IL-12 induces strong NK production of IFN-γ.20 This cytokine milieu induces the differentiation of naive CD4+ T cells into T-helper 1 (TH1) cells on encountering specific antigens. Moreover, CpG-ODN increases antigen presentation and costimulatory molecule expression.21 CpG-ODN specifically affects the maturation of DCs, which are recognized as distinct antigen-presenting cells (APCs) largely responsible for the initiation of T-cell and NK-cell responses to tumor antigens.22-25 Synthetic CpG-ODN sequences (ISSs) are under investigation for a number of therapeutic indications—including protection from infectious pathogens, adjuvant for vaccines, and treatment of allergy—by encouraging TH1 rather than TH2 immunity.26
We hypothesized that an ISS could have significant synergistic effects with antitumor monoclonal antibodies such as rituximab through the augmentation of ADCC and the enhancement of presentation of antigens released by killed tumor cells. Therefore, we designed a phase 1 study testing 4 doses of a CpG-ODN (1018 ISS; Dynavax Technologies, Berkeley, CA) with rituximab in patients with relapsed or refractory advanced-stage NHL. In the results reported in this article, the combination was well tolerated and demonstrated significant biologic activity, measured through a dose-related increase in type 1 interferon–inducible genes. Unlike therapy with exogenous interferon or interleukins, the 1018 ISS and rituximab combination resulted in no significant toxicity, providing rationale for further testing of this exciting combination immunotherapy approach to NHL.
Patients, materials, and methods
Informed consent was obtained in writing from all patients before enrollment. This study was approved by the Institutional Review Board at Dana-Farber/Partners Cancer Care and abides by the tenets of the Declaration of Helsinki.
Patients
Eligible patients had histologically documented CD20+, B-cell NHL as defined by the World Health Organization (WHO) classification, and received at least 1 previous treatment regimen for lymphoma. All patients were 18 years of age or older and had a performance status using the Eastern Cooperative Oncology Group (ECOG) score of 2. Eligible patients were required to have white blood cell (WBC) counts greater than 2000 (1000/μL), absolute neutrophil counts (ANCs) greater than 1000 (1000/μL), platelet counts greater than 75 000/mm3, and expected survival times greater than 4 months.
Patients not eligible to be enrolled in the study included those who were pregnant or lactating or who used chemotherapy, systemic steroids, or radiation within 30 days before study enrollment. Additionally, patients treated with radioimmunotherapy (including iodine I 131 tositumomab or ibritumomab tiuxetan), autologous transplantation, or fludarabine chemotherapy within the previous 6 months were excluded because of the immunosuppressive nature of these therapies. Patients experiencing disease progression, as defined by the National Cancer Institute (NCI)–Sponsored International Working Group criteria27 within 6 months of any previous rituximab-containing therapy were also excluded. Other exclusion criteria included history of allogeneic (bone marrow or stem cell) transplantation, including nonmyeloablative transplantation; history of unstable angina, symptomatic cardiac arrhythmia, or clinical heart failure; severe pulmonary disease, symptomatic pleural effusions, or clinically significant pulmonary symptoms; uncontrolled bacterial, viral, or fungal infection; clinically apparent central nervous system (CNS) lymphoma; major surgery within 2 weeks before enrollment; known presence of human antimurine antibodies (HAMAs) or antichimeric antibodies (HACAs), or history of any clinically significant autoimmune disorder. Because other phosphorothioate compounds interfere with the interpretation of coagulation assays in vitro,28 an additional exclusion criterion was current therapeutic use of anticoagulants or history of symptomatic coagulopathy.
Treatment
Rituximab was given on days 1, 8, 15, and 22 (weekly × 4 doses) at a dose of 375 mg/m2, as previously described.29 Between 30 and 60 minutes before the start of the rituximab infusion, the patient was given oral acetaminophen (650 mg) and diphenhydramine hydrochloride (50 mg). Corticosteroids were avoided.
The CpG oligonucleotide 1018 ISS (provided by Dynavax Technologies) is a single-stranded, 22–base pair (bp) immunostimulatory phosphorothioate oligonucleotide prepared by standard solid-phase chemistry techniques (sequence 5′-TGACTGTGAACGTTCGAGATGA-3′) with a molecular mass of approximately 7150 Da.30 Patients were assigned to 1 of 4 doses of 1018 ISS: 0.01 mg/kg, 0.05 mg/kg, 0.2 mg/kg, or 0.5 mg/kg. Dose assignments were sequential and were based on the order in which patients consented and completed the screening process. Dose assignments began with the lowest dose, and the next cohort at the next higher dose began treatment only after investigators reviewed the safety parameters of the preceding dose cohort.
The first injection of 1018 ISS was administered subcutaneously 30 to 60 minutes after the second dose of rituximab (on day 8). The second and third doses of 1018 ISS were administered 30 to 60 minutes after the third and fourth infusions of rituximab. The fourth (last) dose of 1018 ISS was administered 1 week after the fourth (last) dose of rituximab, on day 29.
Dose-limiting toxicity and rules for stopping
A classical phase 1 design was used for this study. Dose-limiting toxicities (DLTs) (defined by the NCI Common Toxicity Criteria, version 2, http://ctep.cancer.gov/forms/CTCv2.0_4-30-992.pdf) were grade 2 or higher allergic reaction to 1018 ISS and any nonhematologic toxicity grade 3 or higher that occurred during the 1018 ISS treatment period, and that was deemed possibly or probably related to 1018 ISS treatment. Hematologic toxicities were defined in this study as any episode of febrile neutropenia, platelet count below 20 000, and platelet transfusion for bleeding.
Each dose cohort consisted of 3 patients who completed both rituximab and 1018 ISS treatments. Patients withdrawn because of toxicity counted as having had a DLT. Patients who withdrew for any reason other than toxicity were replaced. If no patient had a DLT after all 3 patients in a cohort completed therapy, enrollment of the next cohort commenced. If 1 patient had a DLT, 3 additional patients were enrolled at that dose level; if none of these 3 additional patients had a DLT, then enrollment at the next dose level commenced. If 2 or more patients had DLTs, then the previous dose level was declared the maximum tolerated dose (MTD). At the conclusion of dose escalation, 6 additional patients were entered in the candidate MTD tier; if 1 or fewer DLTs were observed in these 6 additional patients, the candidate MTD was declared the MTD; otherwise, de-escalation to the next lower dose occurred, and 6 additional patients were added at that dose.
Response criteria and follow-up
Clinical responses were as previously defined by the International Workshop for NHL response criteria.27 Patients underwent comprehensive restaging, including physical examination and anatomic imaging, on day 56 of therapy, then every 3 months for the first year, then every 6 months, until evidence of disease progression.
RNA preparation and quantitative gene expression analysis of PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated using Vacutainer CPT tubes (BD Biosciences, San Jose, CA), and duplicate samples of 1 × 106 cells were resuspended in RLT-buffer (Qiagen, Valencia, CA) and subsequently were stored at –80°C. From each sample, RNA was purified using RNeasy mini kits (Qiagen). The RNA was DNase treated and reverse transcribed, and subsequently cDNA levels were quantitated by real-time polymerase chain reaction (PCR) using a Perkin-Elmer 5700 Sequence Detection System (all methods as previously described).31 The intercalating dye SYBR green (Qiagen) or a specific probe labeled with a fluorescent dye (Perkin-Elmer) was used to detect the PCR product at each cycle. Specific PCR primer/probe pairs were obtained from Perkin-Elmer or were designed and validated by us. When using SYBR green, the specificity of each reaction was confirmed by a melting temperature curve at the end of each run. Results were calculated as a ratio of the test gene to the internal control gene ubiquitin. This gene/ubiquitin ratio is directly proportional to the fraction of total mRNA represented by the test gene and normalizes for experimental variations in RNA recovery, purification, and quality. We have confirmed that the expression of ubiquitin is linear over a broad range of RNA concentrations by processing, under identical conditions, human PBMC samples ranging from 5 × 103-107 cells. The standard deviation of the gene/ubiquitin ratio in replicate samples is generally less than 50% of the mean of the replicates.
Serum markers of ISS activity
Because the induction of interferon is an expected effect of ISS, we incorporated the evaluation of validated interferon-induced markers that are dose-dependent indicators of serum interferon levels.32 These included evaluation serum 2′5′ oligoadenylate synthetase (OAS), which is inducible in monocytes and lymphocytes by IFN-α and IFN-β and has been widely used to monitor pharmacokinetics in phase 1 trials of type 1 interferon.33 In addition, serum levels of neopterin, which is produced by the interferon-inducible enzyme GTP cyclohydrolase and is secreted by macrophages in response to interferons, were determined. Both 2′5′ OAS and neopterin are at least as sensitive as enzyme-linked immunosorbent assay (ELISA) detection of serum interferon.34
Biostatistical analysis
A standard phase 1 design was selected for this study. The primary end point was to determine safety and tolerability of 1018 ISS in conjunction with rituximab, as determined by type and severity of adverse events and by clinically significant changes in laboratory results, and to determine the MTD for 1018 ISS given after rituximab infusions. Secondary exploratory variables included clinical response rate and biologic activity of 1018 ISS in conjunction with rituximab. Gene expression data were evaluated by Kruskal-Wallis nonparametric analysis of variance (ANOVA) with Dunn posttest to compare differences between groups.
Results
Patients
Twenty patients (11 women, 9 men) were enrolled; the median age was 59 (range, 40-73 years). Histology included follicular NHL in 17 patients, diffuse large B-cell NHL in 2 patients, and small lymphocytic lymphoma in 1 patient. Patients had received a median of 3 previous therapies for NHL, detailed in Table 1. Of note, 4 patients were treated with aggressive salvage chemotherapy, 2 underwent autologous stem cell transplantation, 7 received rituximab, 1 received previous radioimmunotherapy, and 4 underwent previous external beam radiation therapy.
Patient characteristics
Characteristics . | No. . |
|---|---|
| Total enrolled | 20 |
| Women/men | 11/9 |
| Mean age, y (range) | 59 (40-73) |
| Histology | |
| Follicular NHL grade 1* | 15 |
| Follicular NHL grade 2* | 2 |
| Diffuse large B cell NHL* | 2 |
| Small lymphocytic NHL* | 1 |
| Previous therapy | |
| CHOP | 9 |
| CVP | 9 |
| Rituximab | 7 |
| Oral alkylating agents | 4 |
| Purine analog chemotherapy | 3 |
| Other combination chemotherapy | 4 |
| Autologous stem cell transplantation | 2 |
| Ibritumomab tiuxetan | 1 |
Characteristics . | No. . |
|---|---|
| Total enrolled | 20 |
| Women/men | 11/9 |
| Mean age, y (range) | 59 (40-73) |
| Histology | |
| Follicular NHL grade 1* | 15 |
| Follicular NHL grade 2* | 2 |
| Diffuse large B cell NHL* | 2 |
| Small lymphocytic NHL* | 1 |
| Previous therapy | |
| CHOP | 9 |
| CVP | 9 |
| Rituximab | 7 |
| Oral alkylating agents | 4 |
| Purine analog chemotherapy | 3 |
| Other combination chemotherapy | 4 |
| Autologous stem cell transplantation | 2 |
| Ibritumomab tiuxetan | 1 |
CHOP indicates cyclophosphamide, hydroxydaunomycin/doxorubicin, Oncovin, prednisone; CVP, cyclophosphamide, vincristine, prednisone.
World Health Organization lymphoma classification.
Median time from diagnosis to enrollment was 36 months (range, 12 to 145 months). Bone marrow involvement was present histologically at enrollment in 5 of 20 patients who underwent evaluable bone marrow biopsy. Two patients had B symptoms. One patient did not achieve remission. The remainder of the patients had disease progression after response to previous therapy.
Treatment
Eighteen patients completed 4 infusions of rituximab and the 4 prescribed doses of 1018 ISS. One patient discontinued protocol participation after the third infusion of rituximab because of the exacerbation of underlying chronic back pain, unrelated to disease status or protocol therapy. One additional patient discontinued protocol participation before receiving any ISS therapy.
Adverse events and laboratory changes
Fifty percent of patients had expected infusion reactions associated with the initial dose of rituximab; there was no exacerbation of rituximab toxicity after the institution of ISS therapy. Common adverse events observed in this study are detailed in Table 2. Injection site reactions occurred in a dose-dependent fashion. The only grade 3 adverse events included the aforementioned back pain, an episode of sepsis thought unrelated to therapy, an episode of atypical pneumonia thought possibly related to therapy, and an episode of confusion caused by narcotic use for pain secondary to NHL. No patient developed HAMA after combination therapy. Antinuclear antibody (ANA) levels did not significantly change compared with baseline levels.
Frequent and serious adverse events reported during treatment period
Event . | Grade 1 . | Grade 2 . | Grades 3-4 . | Total, % . |
|---|---|---|---|---|
| Allergic reaction* | 5 | 5 | 0 | 50 |
| Injection site reaction† | 6 | 3 | 0 | 45 |
| Upper respiratory‡ | 7 | 1 | 0 | 40 |
| Headache | 6 | 2 | 0 | 40 |
| Fatigue | 2 | 4 | 0 | 30 |
| Pneumonia | 0 | 0 | 2 | 10 |
| Confusion | 0 | 0 | 1 | 5 |
| Back pain | 0 | 0 | 1 | 5 |
| Sepsis | 0 | 0 | 1 | 5 |
Event . | Grade 1 . | Grade 2 . | Grades 3-4 . | Total, % . |
|---|---|---|---|---|
| Allergic reaction* | 5 | 5 | 0 | 50 |
| Injection site reaction† | 6 | 3 | 0 | 45 |
| Upper respiratory‡ | 7 | 1 | 0 | 40 |
| Headache | 6 | 2 | 0 | 40 |
| Fatigue | 2 | 4 | 0 | 30 |
| Pneumonia | 0 | 0 | 2 | 10 |
| Confusion | 0 | 0 | 1 | 5 |
| Back pain | 0 | 0 | 1 | 5 |
| Sepsis | 0 | 0 | 1 | 5 |
Values reflect events that occurred in more than 20% of patients and all grades 3-4 events. No grade 5 events were reported.
Includes hives, rhinitis, rigors, chills, and dyspnea.
Injection site reactions were dose-dependent.
Includes cough, congestion, infection.
Response to 1018 ISS measured by interferon-induced gene expression
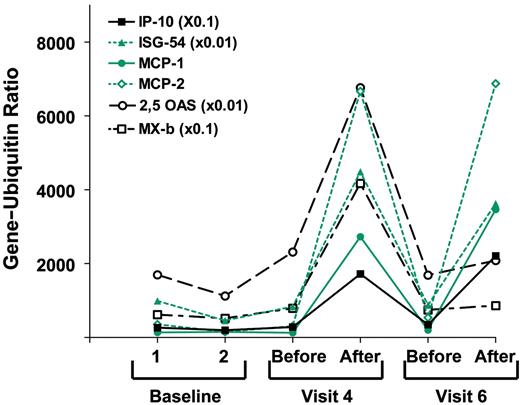
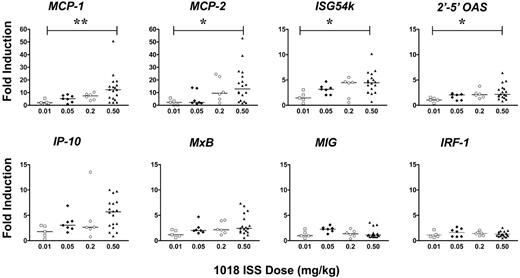
We used quantitative PCR analysis to evaluate changes in mRNA expression in a panel of interferon-inducible genes between PBMCs isolated before and 24 hours after the second and fourth doses of 1018 ISS. These genes are detailed in Table 3. Gene expression levels in baseline PBMC samples, collected on 2 visits before the start of treatment, varied 10- to 100-fold among patients (n = 19) but were relatively stable within individual patients (r = 0.978; P < .001). There was no evidence of gene induction in vivo by the 0.01 mg/kg dose, but a dose-related increase was observed in the induction of several IFN-α/β–inducible genes 24 hours after the injection of 1018 ISS in the 3 higher-dose groups. Gene expression data from a representative patient in the second cohort is depicted in Figure 1. Specifically, 2 of 4 patients in cohort 2 (0.05 mg/kg) showed greater 3-fold induction of 2 or more IFN-inducible genes. In cohorts 3 and 4 (0.20 mg/kg and 0.50 mg/kg), all patients showed greater than 3-fold induction in at least 2 of these genes. Moreover, the mean induction levels of most of these genes tended to increase with increasing doses. There was no significant difference in biologic response between cohorts 3 and 4, suggesting we were reaching a plateau in biological activity. This dose-related gene induction was only observed, however, in genes inducible with type 1 interferons or inducible equally well with types 1 and 2 interferons. The 2 genes induced selectively by IFN-γ (MIG, IRF-1) were not significantly increased by 1018 ISS at any dose level. Figure 2 summarizes gene-induction data for all patients, demonstrating this selective type-1 interferon effect.
Biologic response marker genes for IFN and ISS activity
. | . | Induced in vitro . | . | . | |
|---|---|---|---|---|---|
| Symbol . | Name . | IFN-α . | IFN-γ . | Induced in patients ISS . | |
| 2′-5′ OAS | 2′-5-Oligoadenylate synthetase | + | - | + | |
| ISG-54 | Interferon-stimulated gene, 54 kDa | + | - | + | |
| MxB | Myxoma resistance-B | + | - | + | |
| MCP-1 | Monocyte chemotactic protein | + | + | + | |
| MCP-2 | Monocyte chemotactic protein | + | + | + | |
| IP-10 | IFN-γ—inducible protein 10 | + | + | + | |
| IRF-1 | Interferon-responsive factor-1 | - | + | - | |
| MIG | Monokine induced by IFN-γ | - | + | - | |
. | . | Induced in vitro . | . | . | |
|---|---|---|---|---|---|
| Symbol . | Name . | IFN-α . | IFN-γ . | Induced in patients ISS . | |
| 2′-5′ OAS | 2′-5-Oligoadenylate synthetase | + | - | + | |
| ISG-54 | Interferon-stimulated gene, 54 kDa | + | - | + | |
| MxB | Myxoma resistance-B | + | - | + | |
| MCP-1 | Monocyte chemotactic protein | + | + | + | |
| MCP-2 | Monocyte chemotactic protein | + | + | + | |
| IP-10 | IFN-γ—inducible protein 10 | + | + | + | |
| IRF-1 | Interferon-responsive factor-1 | - | + | - | |
| MIG | Monokine induced by IFN-γ | - | + | - | |
+ Indicates that gene was induced.
- Indicates that gene was not induced.
Gene induction by 1018 ISS in a representative patient. Gene/ubiquitin ratios for 6 interferon-inducible genes in PBMCs from patient 113, treated with the highest dose of 1018 ISS (0.5 mg/kg). Values for the more highly expressed genes were multiplied as indicated. Samples were taken before and 24 hours after 1018 ISS injection. Visit 4 included the second and visit 6 included the last of the 4 injections of 1018 ISS.
Gene induction by 1018 ISS in a representative patient. Gene/ubiquitin ratios for 6 interferon-inducible genes in PBMCs from patient 113, treated with the highest dose of 1018 ISS (0.5 mg/kg). Values for the more highly expressed genes were multiplied as indicated. Samples were taken before and 24 hours after 1018 ISS injection. Visit 4 included the second and visit 6 included the last of the 4 injections of 1018 ISS.
Summary of interferon-inducible gene response to injection of 1018 ISS. Data are expressed as fold induction after 1018 ISS compared with the matching pre–1018 ISS value. Significance of the differences among groups was analyzed by the Kruskal-Wallis test, with the following values: MCP-1, P = .005; MCP-2, P = .016; ISG54k, P = .024; 2'-5' OAS, P = .033; all other genes, P > .05. Comparisons of the 0.01 and the 0.5 mg/kg groups by Dunn posttest are indicated, if significant, on the figure. **P < .01; *P < .05.
Summary of interferon-inducible gene response to injection of 1018 ISS. Data are expressed as fold induction after 1018 ISS compared with the matching pre–1018 ISS value. Significance of the differences among groups was analyzed by the Kruskal-Wallis test, with the following values: MCP-1, P = .005; MCP-2, P = .016; ISG54k, P = .024; 2'-5' OAS, P = .033; all other genes, P > .05. Comparisons of the 0.01 and the 0.5 mg/kg groups by Dunn posttest are indicated, if significant, on the figure. **P < .01; *P < .05.
Interferon-induced serum markers
There was no significant increase in serum levels of neopterin after therapy with ISS (data not shown). In cohorts 1 and 2, there were no significant increases in serum OAS concentration. However, 6 of 9 patients with adequate samples in cohorts 3 and 4 demonstrated an increase in OAS after ISS therapy compared with baseline. Five of these 6 patients also demonstrated increases in OAS gene expression as measured by quantitative PCR.
Clinical response and patient outcome
Nineteen patients were evaluable for response. Six patients showed response (1 unconfirmed complete remission [CRu], 5 partial remission [PR]) for an overall response rate of 32% (90% confidence interval, 17%-64%; computed using logarithmic transformation). Additionally, 13 patients had stable disease after therapy. Median progression-free survival in responding patients is 12 months (range, 5-23.5 months). Four patients remain alive without progression at a median of 10 months follow-up (range, 3.2 to 23.4 months). Four patients have died: 1 of progressive disease, 1 of complications of chronic obstructive pulmonary disease (COPD) with lymphoma present at death, 1 of sepsis after additional chemotherapy, and 1 of a pulmonary embolism without evidence of lymphoma at the time of death.
Discussion
Our study represents the first published experience of a CpG ODN in combination with an antitumor monoclonal antibody. In our study, 1018 ISS treatment was extremely well tolerated, at weekly doses of up to 0.5 mg/kg, without the toxicities usually associated with pharmacologic doses of cytokines. This dose is approximately 10-fold higher than that used in previous studies of injected 1018 ISS as a vaccine adjuvant.35 Moreover, the demonstration of significant dose-related increases in ISS-inducible genes demonstrates that this study encompassed a range of 1018 ISS doses with substantial pharmacologic activity in humans.
Many of the therapeutic actions of ISS are likely mediated through the induction of IFN-α and IFN-β from ISS-responsive plasmacytoid dendritic cells and IFN-γ indirectly from NK cells.26 Genes prominently induced by interferons have been extensively characterized, and several gene products have been used to monitor clinical trials of interferons. Interferon-inducible mRNAs, however, have not been widely used in the same manner, though a recent study in patients with hepatitis C virus (HCV) used microarrays to measure changes in the mRNA of PBMCs 3 and 6 hours after IFN-α therapy.36 For our study, we selected 8 genes with known induction patterns and, in some cases, well-studied regulatory regions. In preliminary experiments with cultured human PBMCs (data not shown), these genes were shown to be strongly induced by 1018 ISS and IFN-α or IFN-γ, or both. Although the kinetics of expression of these genes varied, mRNA levels remained substantially elevated after 24 hours for all genes. In contrast, IFN-α and IFN-γ mRNAs were transient and variable, and mRNA levels were relatively low. Thus, the 24-hour time point was chosen for evaluation of posttreatment blood samples to assess ISS activity.
Genes regulated by IFN-α, or by either type of interferon, were induced in a dose-dependent fashion. The 2 genes regulated primarily by IFN-γ, however, showed no induction, suggesting that much of the biologic activity of 1018 ISS in vivo is mediated by type-1 interferons. This was surprising because 1018 ISS is representative of the CpG-B class of ISS and induces low, often undetectable levels of IFN-α in cultured PBMCs in vitro.37,38 Other CpG-ODN sequences, including those under clinical evaluation, may have different effects.39 Clearly, though, our studies provide evidence that 1018 ISS administered subcutaneously has a biologically relevant, systemic effect in patients with lymphoma treated with an ISS/antibody combination.
CpG-ODNs have been shown in animal models to significantly enhance monoclonal antibody therapy against malignancy in vivo. In one study of anti-idiotype monoclonal antibody treatment of murine B-cell lymphoma, ISS alone had no effect on the survival of mice inoculated with 38C13 murine lymphoma cells. However, a single injection of an ISS significantly enhanced the antitumor response to monoclonal antibody therapy: 90% of mice treated with monoclonal antibody alone developed lymphoma compared with only 20% of mice treated with antibody and ISS.40 This combination was as effective as multiple doses of IL-2 at inhibiting tumor growth when combined with monoclonal antibody therapy, with significantly less toxicity. Further studies using the 38C13 tumor model suggest that efficacy of monoclonal antibodies is most enhanced when CpG-ODN is given within 2 days of antibody therapy.41 Furthermore, in indolent lymphoma cell lines, CD20 expression, in particular, has been observed to increase in response to CpG-ODN.42,43 Interestingly, an inverse correlation was detected between baseline expression of CD20 and expression after exposure to CpG-ODN; the most significant increases in CD20 expression were found in samples that had the lowest baseline levels, suggesting another possible mechanism through which 1018 ISS could augment responses to anti-CD20 therapy with rituximab.
A murine study provides further insight into the mechanism of possible antitumor effects of CpG-ODN in NHL.44 Mice receiving CpG-ODN had significant suppression of tumor growth after subcutaneous injection with EL4 cells, with the greatest effect seen in mice given CpG DNA near the tumor inoculation site. NK cells, monocytes, and macrophages were necessary for this antitumor effect. Mice that received CpG-ODN rejected subsequent tumor rechallenge, and further studies support a T-cell memory response. Another murine study demonstrated significant enhancement of TH1 immunity through IL-12 production and HLA class 1 and 2 molecules when CpG-ODN was combined with an antigen/granulocyte macrophage–colony-stimulating factor (GM-CSF) fusion protein.45 Finally, a recent study reveals that daily injection of CpG-ODN dramatically alters the morphology and functionality of mouse lymphoid organs, potentially altering the lymphoma microenvironment.46
Because ADCC appears to play an important role in the response to rituximab and other monoclonal antibodies, several alternative approaches to stimulate effector-cell function in vivo have been used to augment the clinical response. Several years ago, Vlasveld et al47 treated 7 patients with indolent B-cell lymphoma with a combination of a murine anti-CD19 antibody and continuous infusion low-dose IL-2, a lymphokine that enhances ADCC in vitro. A gradual and sustained increase in CD8+ and CD56+ cells occurred, and 2 responses were observed. Lymphocytes from involved lymph nodes also showed enhanced ADCC, which provided proof-of-principle. Others48 and we29 have published results of clinical trials evaluating systemic IL-2 combined with rituximab in patients with NHL, demonstrating relative safety and effector-cell enhancement and suggesting a prolonged time to progression in a subset of patients with follicular NHL. Rituximab has also been safely combined with IL-12,49 another cytokine that mediates effector cell number and function; G-CSF,50 which greatly enhances the cytotoxicity of neutrophils in ADCC; and IFN-α,51 an immunomodulatory cytokine that induces antigen expression, enhances cytotoxicity of immunotoxins, and has limited clinical activity as a single agent for NHL.52 Additional clinical trials of these combinations are ongoing;53 however, the doses of cytokines required to achieve adequate effector-cell responses are associated with greater toxicity than those of rituximab alone.54
Recently, a phase 1 study of a similar ISS (CpG 7909; Coley Pharmaceutical Group, Wellesley, MA) was completed in patients with previously treated NHL.55 In 15 patients evaluable for effector cell changes, dose-related increased ADCC activity and increased NK-cell activity were observed at day 21 compared with baseline levels. The regimen was easily tolerated, and, despite no clinical responses, the significant immunomodulatory activity suggested potential for benefit in combination with standard treatment approaches. One potential concern in using CpG-ODN for lymphoma is that CpG-containing sequences, including 1018 ISS, have been observed to induce proliferation of primary human B cells in vitro.56-59 Lymphoma proliferation was not observed in vivo in the single-agent study by Link et al55 in patients with relapsed NHL or in our study. Moreover, in the EG7/EL4 murine lymphoma model, CpG-ODN treatment resulted in a significant suppression of tumor growth.44
Given the favorable safety and tolerability profile to date in humans, including our current study, 1018 ISS appears to be an ideal agent for combination immunotherapy with rituximab. The use of 1018 ISS and rituximab clearly has the potential to enhance ADCC and to induce the antigen-presenting function of DCs, macrophages (MFs), and B cells. Subsequently, activated APC may induce TH1 and cytolytic T-cell expansion, thereby generating tumor-specific immune responses. We have developed a phase 2 study to evaluate the efficacy of 1018 ISS combined with rituximab in patients with relapsed follicular NHL, using the 0.2 mg/kg dose, the lowest dose observed that provided the maximum biologic effects. In this trial, we plan to further explore the effects of this rational combination on the tumor microenvironment and systemic immune function through surrogate markers of biologic activity. Additionally, we will correlate these findings, including the gene expression data, with clinical responses. Through detailed analysis of these surrogate markers, we hope to better define the optimal schedule of this combination, which may require additional phase 2 studies. Other combination immunotherapy trials, using different ISSs, are also ongoing.60 We anticipate that these CpG-ODN combinations with rituximab will have very low toxicity in this patient population, which is a critical part of developing novel therapeutics for patients with indolent NHL.
Prepublished online as Blood First Edition Paper, September 9, 2004; DOI 10.1182/blood-2004-06-2156.
Supported by the National Institutes of Health (grant CA102216) (J.W.F.), the Leukemia and Lymphoma Society (A.S.F.), and the Norman Hirschfield Foundation and by research support from Dynavax Technologies (J.W.F., A.S.F.).
Three of the authors (E.M.H., P.S., R.L.C.) are employed by a company (Dynavax Technologies) whose potential product was studied in the present work.
Presented in part at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal