Abstract
The value of administering sequential courses of chemotherapy containing high-dose cytarabine in both induction and consolidation therapy for acute myeloid leukemia (AML) has not been assessed in a prospective randomized trial. Two hundred ninety-two AML patients aged 15 to 60 years were enrolled in the Australasian Leukaemia and Lymphoma Group (ALLG) AML trial number 7 (M7) protocol to evaluate this question. All received induction therapy with the ICE protocol (idarubicin 9 mg/m2 × 3; cytarabine 3 g/m2 twice a day on days 1, 3, 5, 7; etoposide 75 mg/m2 × 7). Complete remission was achieved in 234 (80%) patients. Two hundred two patients in remission were then randomized to either a further identical cycle of ICE or 2 attenuated courses (cytarabine 100 mg/m2 daily × 5, idarubicin × 2, etoposide × 5 [IcE]). ICE consolidation therapy was more toxic than IcE, however, the treatment-related death rate was not significantly different. There was no difference between the 2 consolidation arms for relapse-free survival at 3 years (49% for ICE vs 46% for IcE; P = .66), survival following randomization (61% vs 62%; P = .91), or the cumulative incidence of relapse (43% vs 51%; P = .31), and there was no difference within cytogenetic risk groups. Intensive induction chemotherapy incorporating high-dose cytarabine results in high complete remission rates, but further intensive consolidation treatment does not appear to confer additional benefit.
Introduction
Despite more than 30 years of clinical investigation, the optimal treatment of acute myeloid leukemia (AML) in adults remains to be defined. The pyrimidine analog cytarabine and the anthracycline antibiotics, such as daunorubicin and idarubicin, remain the mainstay of current clinical practice but their optimal dose and scheduling remain uncertain. AML is usually sensitive to both of these classes of agents, and there is accumulating evidence that a steep dose-effect relationship exists in this disease, suggesting that dose escalation of cytarabine and/or anthracyclines could produce higher response and cure rates. However, the most appropriate scheduling of dose-intensified therapy for AML remains controversial.
A considerable amount of clinical research has been conducted over the past 15 years to evaluate the effects of cytarabine dose escalation in the initial treatment of AML. Earlier studies had established that a dose of cytarabine of 100 mg/m2 per day by continuous intravenous infusion was effective in inducing remissions in the majority of untreated adults with AML, when used in combination with an anthracycline.1,2 Considerably higher doses of cytarabine, up to 6 g/m2 per day given in divided boluses, could however be given safely and induce remissions in patients with AML in relapse after previous conventional-dose therapy.3 The Cancer and Leukemia Group B (CALGB) subsequently conducted a study to evaluate the role of high-dose cytarabine as consolidation treatment after successful induction treatment with daunorubicin and conventional-dose cytarabine.4 Three cytarabine dose levels were compared: 3 g/m2 versus 400 mg/m2 versus 100 mg/m2. This study demonstrated a statistically significant prolongation of relapse-free survival in the 2 higher-dose arms, indicating a benefit for cytarabine dose escalation in consolidation.4
Two studies have explored the use of high-dose cytarabine during induction treatment for AML. The Australian Leukaemia Study Group (ALSG) compared the effects of increasing the dose of cytarabine to 3 g/m2 every 12 hours on days 1, 3, 5, and 7 (total of 8 doses) from the conventional 100 mg/m2 daily for 7 days by continuous intravenous infusion, with all patients also receiving daunorubicin 50 mg/m2 for 3 doses and etoposide 75 mg/m2 daily for 7 doses.5 All responding patients subsequently received 2 identical attenuated consolidation courses with conventional dose cytarabine, daunorubicin, and etoposide. Although there was no improvement in complete remission rate, the high-dose cytarabine arm was associated with a significant improvement in relapse-free survival.5 Interestingly, the magnitude of this improvement closely approximated the benefit seen in the CALGB consolidation study.6
The South West Oncology Group (SWOG) conducted a more complex study, which included an initial randomization to high- or conventional-dose cytarabine.7 Again, as in the ALSG study, there was no difference in complete remission rates between the 2 arms, but the high-dose arm was associated with improved relapse-free survival, suggesting that increased dose intensity of cytarabine in the initial treatment course resulted in greater kill of leukemic cells with improved long-term cure rates.7
The SWOG study included a second randomization for patients who responded to conventional-dose cytarabine induction therapy to then receive either 2 cycles of conventional-dose consolidation treatment or a single cycle of high-dose cytarabine. Patients who responded to high-dose cytarabine induction received a second cycle as consolidation treatment. This study was unable to confirm the improved relapse-free survival results of the CALGB study for the high-dose cytarabine consolidation arm. However, the small group of patients who received high-dose cytarabine both in induction and consolidation phases of treatment had the highest rate of relapse-free survival, although this did not reach statistical significance.7
These studies raise a number of important questions about the use of high-dose cytarabine during the early phases of treatment of adult AML. One issue is the best timing of dose escalation, bearing in mind that the ALSG and CALGB studies appeared to show equivalent long-term results for using high-dose cytarabine in either induction or consolidation phases, respectively. A second issue is whether successive cycles of treatment containing high-dose cytarabine delivered in both induction and consolidation would be more effective than dose escalation during either phase alone, as suggested by the SWOG study. In order to clarify this question, the Australasian Leukaemia and Lymphoma Group conducted a study in which all newly diagnosed adult AML patients received identical induction therapy consisting of high-dose cytarabine, idarubicin, and etoposide. Patients achieving a complete remission were then randomized to receive an identical course as consolidation therapy or to be treated with 2 attenuated courses of chemotherapy with conventional-dose cytarabine, as well as idarubicin and etoposide.
Patients, materials, and methods
Patients
The Australasian Leukaemia and Lymphoma Group (ALLG) AML trial number 7 (M7) study was conducted in 26 centers affiliated with the ALLG between November 1995 and May 2000. Eligibility criteria for the study included a diagnosis of de novo AML, except for cases of acute promyelocytic leukemia (who were treated on a different protocol), aged between 15 and 60 years inclusive, and absence of irreversible major organ failure. Diagnostic slides were reviewed centrally to confirm the diagnosis of AML. Where the material sent was inadequate, the referring institution was contacted to review the evidence available. In 2 cases, the diagnosis of AML could not be confirmed and the patients were correspondingly excluded from the primary analyses. The study protocol was approved by the Human Ethics Committee, or equivalent body, of each participating institution prior to the start of enrollment at each center. All patients gave written informed consent prior to registration on the study. On-study patient details are shown in Table 1.
Patient characteristics at registration
. | No. of patients (% of 292) . |
|---|---|
| Sex | |
| Male | 160 (55) |
| Female | 132 (45) |
| Age, y | |
| Median | 43 |
| Range | 15-60 |
| Younger than 20 | 18 (6) |
| 20-29 | 52 (18) |
| 30-39 | 59 (20) |
| 40-49 | 70 (24) |
| 50-60 | 93 (32) |
| ECOG performance status | |
| 0 | 147 (50) |
| 1 | 113 (39) |
| 2 | 23 (8) |
| 3 | 9 (3) |
| FAB classification, institutional | |
| M0 | 18 (6) |
| M1 | 41 (14) |
| M2 | 97 (33) |
| M4 | 75 (26) |
| M5 | 40 (14) |
| M6 | 6 (2) |
| M7 | 9 (3) |
| Biphenotypic M4, M6 | 1 (0) |
| Unknown | 5 (2) |
| WCC at diagnosis | |
| At least 100 × 109/L | 29 (10) |
| Clinical bleeding | |
| None | 226 (77) |
| Petechiae | 25 (9) |
| Mild blood loss | 39 (13) |
| Gross | 2 (1) |
| Febrile at time of registration | |
| Yes | 84 (29) |
| Cytogenetic risk group | |
| Favorable | 42 (14) |
| Intermediate | 211 (72) |
| Adverse | 21 (7) |
| Unknown | 18 (6) |
. | No. of patients (% of 292) . |
|---|---|
| Sex | |
| Male | 160 (55) |
| Female | 132 (45) |
| Age, y | |
| Median | 43 |
| Range | 15-60 |
| Younger than 20 | 18 (6) |
| 20-29 | 52 (18) |
| 30-39 | 59 (20) |
| 40-49 | 70 (24) |
| 50-60 | 93 (32) |
| ECOG performance status | |
| 0 | 147 (50) |
| 1 | 113 (39) |
| 2 | 23 (8) |
| 3 | 9 (3) |
| FAB classification, institutional | |
| M0 | 18 (6) |
| M1 | 41 (14) |
| M2 | 97 (33) |
| M4 | 75 (26) |
| M5 | 40 (14) |
| M6 | 6 (2) |
| M7 | 9 (3) |
| Biphenotypic M4, M6 | 1 (0) |
| Unknown | 5 (2) |
| WCC at diagnosis | |
| At least 100 × 109/L | 29 (10) |
| Clinical bleeding | |
| None | 226 (77) |
| Petechiae | 25 (9) |
| Mild blood loss | 39 (13) |
| Gross | 2 (1) |
| Febrile at time of registration | |
| Yes | 84 (29) |
| Cytogenetic risk group | |
| Favorable | 42 (14) |
| Intermediate | 211 (72) |
| Adverse | 21 (7) |
| Unknown | 18 (6) |
ECOG indicates Eastern Cooperative Oncology Group; FAB, French-American-British; M, FAB classification code denoting myeloid leukemia; and WCC, white cell count.
Treatment protocol
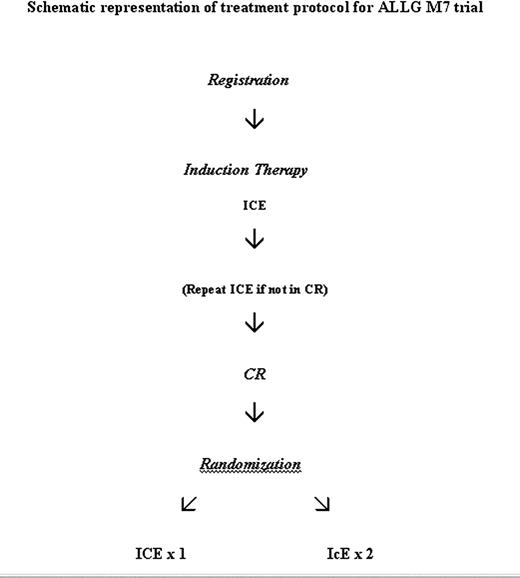
Following registration, all patients were treated with identical induction chemotherapy, as detailed in Figure 1. This treatment course, abbreviated as ICE, consisted of high-dose cytarabine (3 g/m2 by 3 hours intravenous infusion at 12-hour intervals on days 1, 3, 5, and 7); idarubicin 12 mg/m2 by intravenous bolus daily on days 1, 2, and 3; and etoposide 75 mg/m2 intravenous daily infused over 1 hour on days 1 to 7 inclusive. The dose of idarubicin was reduced to 9 mg/m2 daily after patient 44 had been registered because of toxicity concerns (see “Results”). The initial 114 patients registered on the study were randomized at registration to receive lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor [G-CSF]) 5 μg per kg subcutaneously daily from day 8 after the start of chemotherapy until granulocyte recovery (defined as a neutrophil count of > 1.0 × 109/L on 3 successive days or a count of > 5.0 × 109/L on any day) or no growth factor in induction as previously reported.8 All subsequent patients received lenograstim as described in the previous sentence. All patients received fluconazole 200 mg daily as prophylaxis against fungal infection.
A mandatory bone marrow biopsy was performed at day 28 after the start of chemotherapy to assess response. Complete remission was defined as previously reported: absence of symptoms or signs relating to leukemia, neutrophil count higher than 1.0 × 109/L, platelet count higher than 100 × 109/L, and normocellular bone marrow aspirate with less than 5% blast cells.5 If residual leukemia was present, a second cycle of ICE was permitted, depending on the medical condition of the patient. Patients failing to achieve a complete remission after 2 cycles of ICE were taken off study. Patients in complete remission were randomized to receive a second course of ICE as consolidation therapy or to be treated with 2 cycles of attenuated consolidation chemotherapy, abbreviated as IcE: idarubicin 9 or 12 mg/m2 intravenously daily on days 1 and 2, cytarabine 100 mg/m2 daily by continuous intravenous infusion on days 1 to 5 inclusive, and etoposide 75 mg/m2 intravenous daily on days 1 to 5 inclusive. The second cycle of IcE was begun as soon as possible after recovery from the first cycle. Lenograstim was to be given in all consolidation courses. Bone marrow biopsies were performed after each consolidation course to confirm remission status. Following the completion of all consolidation courses, no further therapy was given, however, allogeneic hematopoietic stem cell transplantation was permitted for those patients with histocompatible donors, at the discretion of the treating physician. Autologous stem cell transplantation was not a treatment protocol option, however, a small number of patients underwent this procedure following completion of protocol therapy.
Statistical analysis
Patients were randomized at the time of consolidation by telephoning the Trial Centre. Patients were assigned to 1 of 3 risk groups based on their karyotype and the marrow status after the first induction course or were assigned to an unknown risk group if the karyotype was unknown at the time of randomization. Approximately equal numbers of patients were assigned to each of the randomization arms, using an adaptive biased coin design.
The trial had a target sample size of 200 eligible patients randomized in consolidation. This sample size was chosen essentially on pragmatic grounds but had an 80% power to detect an increase in the 3-year relapse-free survival rate from an anticipated 47% to 71%, using a 2-sided test of significance at significance level .05.
The duration of neutropenia following a given course of treatment was measured from the first day in the cycle with neutrophil count lower than 0.5 × 109/L until the first day with neutrophil count of at least 0.5 × 109/L, provided this was sustained. The duration of thrombocytopenia following a given course of treatment was measured from the first day in the cycle with platelet count lower than 50 × 109/L until the first day with platelet count of at least 50 × 109/L, provided this was sustained and was transfusion independent.
Patients with AML characterized by t(8;21) or inv16 were considered to have favorable cytogenetics. Patients with –5, –7, del(5q), abnormal 3q, or complex cytogenetics, defined as the presence of at least 5 unrelated cytogenetic abnormalities, were considered to have adverse cytogenetics. The remaining patients were considered to have intermediate cytogenetics.9
The data were analyzed with a closeout (study censor) date of March 1, 2002. The status of all but 9 (3%) of the 298 registered patients was known at this date, including 7 patients randomized in consolidation. Relapse-free survival (RFS) was measured from the date of (consolidation) randomization to the date of relapse or the date of death in remission from any cause. Survival following randomization was measured from the date of randomization to the date of death from any cause. The data were censored at the earlier of the date of last contact or the closeout date where applicable. Survival estimates and estimated durations of neutropenia and thrombocytopenia were obtained using the Kaplan-Meier method. The cumulative incidence of relapse (CIR) and the cumulative incidence of death in complete remission (CR) were estimated using a competing risks analysis.10,11 The median duration of follow-up was estimated using the reverse Kaplan-Meier method.12
The primary comparisons of randomization arms were made on an intention-to-treat basis, analyzing all eligible patients according to their randomization arm, no matter whether they received treatment according to protocol or not, and ignoring any subsequent therapies received.
The primary end point of the study was the 3-year relapse-free survival rate. Differences in 3-year rates between arms were calculated by obtaining the difference in the Kaplan-Meier estimates of the rate for each arm and estimating the standard error of the differences from the estimated standard errors of the individual estimates. The differences were assumed to have normal distributions for calculating the significance of the differences and obtaining 95% confidence intervals (CIs). Hazard ratios (HRs) for the survival comparisons were estimated using the Cox proportional hazards model, stratified by cytogenetic group, idarubicin dose (12 mg/m2 or 9 mg/m2), and whether prophylactic G-CSF was given during induction for overall comparisons, and by idarubicin dose and induction G-CSF for comparisons within cytogenetic groups. These hazard ratios and their corresponding P values have been illustrated in the relevant figures and represent the risk of failure (or relapse or death as appropriate) for ICE patients relative to IcE patients. For the overall comparisons, the proportional hazards model was tested using a time-dependent covariate, allowing the hazard ratios to differ in the first and subsequent years. There were no significant departures from the proportional hazards model (P = .85, .94, and .78 for RFS, survival following randomization, and remission duration, respectively).
Comparisons of nonhematologic toxicities were made using the exact Cochran-Armitage test for trend for comparing the individual toxicity grades and the exact common odds-ratio test for comparing the incidences of grades 3 and 4 toxicities. Comparisons of days febrile and other uncensored durations were made using the Wilcoxon Mann-Whitney test. In the comparison of induction toxicities for 9 mg/m2 versus 12 mg/m2 of idarubicin, these tests were stratified by the lenograstim or no-cytokine induction randomization arm. These comparisons were based on the first induction course only. In the comparison of consolidation toxicities for the ICE versus IcE arms, the tests were stratified by the idarubicin dosage (9 mg/m2 or 12 mg/m2). For patients who had 2 courses of consolidation, the worst toxicity was calculated over the 2 courses, and durations of different events were summed over the courses.
One-sided P values were calculated for all toxicity comparisons anticipating greater toxicities with the higher idarubicin doses and greater toxicities with ICE compared with IcE. Two-sided P values were calculated for survival comparisons. No adjustment was made for multiple comparisons. Statistical analyses were carried out using SPSS (Chicago, IL), StatXact (CYTEL Software, Cambridge, MA), and S-Plus (MathSoft, Seattle, WA) statistical software.
Results
Outcome of induction therapy
A total of 298 patients were registered on the study between September 1995 and May 2000. Six patients were subsequently considered ineligible (final diagnosis of acute lymphoblastic leukemia [ALL] in 2 patients, primary myelodysplastic syndrome [MDS] in 2 patients, and the diagnosis of AML was unable to be confirmed in the remaining 2 patients). Complete remission was achieved in 234 of the 292 eligible patients (80%; 95% CI, 75%-85%), with 225 receiving 1 induction course and 9 requiring 2 courses of ICE. Sixteen patients (5%) had persistent leukemia, 9 of whom were taken off study after 1 course of induction and 7 after 2 courses. Nine patients (3%) had prolonged aplasia or thrombocytopenia. Of the remaining 33 patients (11%), 32 did not have the day-28 bone marrow biopsy performed (30 dying within 29 days of commencing induction, 1 taken off study on day 8 due to toxicity, and 1 admitted to the intensive care ward after 2 days of ICE). An additional patient did not have remission documented and was taken off protocol treatment due to infection. These 33 patients were included in the denominator when calculating the response rate.
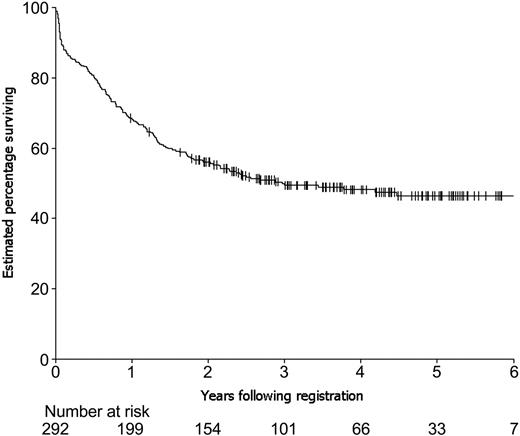
An estimated 69% of the 292 eligible patients survived 1 year following registration on study, 56% survived 2 years, 49% survived 3 years, 48% survived 4 years, and 47% survived 5 years (Figure 2). A reduction in the idarubicin dose in ICE induction therapy, from the initial 12 mg/m2 daily for 3-day schedule to 9 mg/m2 per dose, was made by the ALLG Safety Monitoring Committee after the first 44 patients had been entered on the study. This decision was made because of concerns about the nonhematologic toxicity of ICE, particularly gastrointestinal (see first paragraph of “Toxicity of induction therapy”). There were no significant differences in complete remission rates (81% vs 80%) or overall survival rates (3-year rates of 52% vs 49%) for the 42 eligible patients on the higher-dosage schedule versus the 250 eligible patients on the lower-dosage schedule, respectively.
Toxicity of induction therapy
Detailed information on the toxicity of the first course of induction therapy for the first 112 randomized patients enrolled on the cytokine trial has been published previously.8 In brief, the median duration of neutropenia less than 0.5 × 109/L was 18 days for those patients receiving lenograstim and 22 for those not receiving cytokine therapy (P = .0005). The median duration of thrombocytopenia less than 50 × 109/L was 22 days in both arms. Clinically or microbiologically confirmed infections were observed in 68% of patients, while fever higher than 38°C occurred for a median of 10.5 days. There was no difference between the 2 arms with respect to nonhematologic toxicities, summarized in Table 2 for the 112 evaluable patients.
Toxicity of induction therapy
Toxicity . | % grade 3 or 4 events . |
|---|---|
| CNS | 7 |
| Cerebellar | 2 |
| Cardiac | 3 |
| Cutaneous | 4 |
| Ocular | 6 |
| Stomatitis | 22 |
| Nausea and vomiting | 32 |
| Diarrhea | 34 |
| GIT* | 11 |
| Renal | 4 |
| Hepatic-bilirubin | 11 |
Toxicity . | % grade 3 or 4 events . |
|---|---|
| CNS | 7 |
| Cerebellar | 2 |
| Cardiac | 3 |
| Cutaneous | 4 |
| Ocular | 6 |
| Stomatitis | 22 |
| Nausea and vomiting | 32 |
| Diarrhea | 34 |
| GIT* | 11 |
| Renal | 4 |
| Hepatic-bilirubin | 11 |
Toxicities are serious (grades 3 and 4) nonhematologic toxicities as defined by World Health Organization (WHO) criteria.
CNS indicates central nervous system.
Additional toxicity criterion created for this study to define serious gastrointestinal toxicities of therapy: grade 3 defined as severe pain, ileus; grade 4, life-threatening GIT bleeding, diarrhea, perforation.
Comparison of the 43 evaluable patients in the induction trial treated on the initial 12 mg/m2 idarubicin dose level with the subsequent 69 evaluable patients treated on the 9 mg/m2 dose level showed a significant increase in grades 3 and 4 stomatitis (33% vs 16% for the higher and lower dosages, respectively; P = .035) but no significant differences in the remaining nonhematologic toxicities. There were, however, statistically significant increases in the duration of neutropenia less than 0.5 × 109/L (median 21 vs 19 days; P = .0125) and a possible increase in the duration of thrombocytopenia less than 50 × 109/L (median 24 vs 22 days; P = .074).
Outcome of consolidation therapy
Of the 298 patients registered for induction, 206 patients (69%) were randomized to consolidation between October 19, 1995 and June 27, 2000: 103 to receive a single cycle of ICE consolidation therapy and 103 to receive 2 cycles of IcE. This included 1 patient who was excluded from analysis following morphology review, as the diagnosis of AML could not be confirmed, and 3 patients who were randomized in error as they were not in remission at the time of randomization, although 1 subsequently achieved remission. All 4 patients had been randomized to receive ICE. There were thus 202 eligible patients randomized on the consolidation trial, of whom 99 (49%) were randomized to receive ICE and 103 (51%) to receive IcE. These 202 patients represented 86% of the 234 eligible induction patients who achieved CR. One patient who achieved CR after 2 courses of induction was randomized in error after the first course before achieving CR and was thus ineligible at the time of randomization. Reasons for not being randomized for the remaining 31 potentially eligible patients who achieved CR consisted of the following: toxicity (20), infection (8), transplantation (1), relapse (1), and investigator decision (1). The remainder of the report will be based on the 202 eligible randomized patients.
Details of the 2 patient randomization groups are described in Table 3. The median time to commencement of consolidation therapy was 40 days following commencement of induction therapy (39 days for ICE and 40 days for IcE). Four patients randomized to ICE did not receive ICE, 3 because of infections and 1 because of patient refusal. Two further patients did not complete consolidation, both due to cardiac toxicity (1 acute myocardial infarction, 1 cardiac tamponade), and 1 further patient received only 4 cytarabine doses due to cerebellar toxicity. All patients randomized to IcE commenced consolidation but 7 received only 1 course: 2 due to early deaths (multiorgan failure in 1 patient; hemorrhage, infection, and cerebrovascular accident secondary to neutropenic fever in the other patient), 2 taken off study to receive transplants, 1 relapse, 1 toxicity (pancytopenia), and 1 refused to continue. One patient received etoposide on 2 days only instead of 5 in both consolidation courses (investigator error).
Characteristics of consolidation trial patients
. | ICE . | IcE . |
|---|---|---|
| No. patients | 99 | 103 |
| Sex, % | ||
| Male | 53 | 50 |
| Female | 47 | 50 |
| Age, y | ||
| Median | 37 | 42 |
| Mean | 38 | 40 |
| Range | 16-59 | 15-60 |
| ECOG performance status, % | ||
| 0 | 58 | 56 |
| 1 | 39 | 39 |
| 2 | 3 | 4 |
| 3 | 0 | 1 |
| FAB classification, institutional, % | ||
| M0 | 7 | 2 |
| M1 | 17 | 12 |
| M2 | 31 | 38 |
| M4 | 27 | 31 |
| M5 | 13 | 11 |
| M6 | 1 | 2 |
| M7 | 1 | 2 |
| Biphenotypic M4, M6 | 0 | 1 |
| Unknown | 2 | 2 |
| WCC at diagnosis, % | ||
| Greater than 100 × 109/L | 8 | 5 |
| Cytogenetic risk group, % | ||
| Favorable | 17 | 16 |
| Intermediate | 74 | 69 |
| Adverse | 4 | 10 |
| Unknown | 6 | 6 |
| No. of induction courses, % | ||
| 1 | 96 | 97 |
| 2 | 4 | 3 |
. | ICE . | IcE . |
|---|---|---|
| No. patients | 99 | 103 |
| Sex, % | ||
| Male | 53 | 50 |
| Female | 47 | 50 |
| Age, y | ||
| Median | 37 | 42 |
| Mean | 38 | 40 |
| Range | 16-59 | 15-60 |
| ECOG performance status, % | ||
| 0 | 58 | 56 |
| 1 | 39 | 39 |
| 2 | 3 | 4 |
| 3 | 0 | 1 |
| FAB classification, institutional, % | ||
| M0 | 7 | 2 |
| M1 | 17 | 12 |
| M2 | 31 | 38 |
| M4 | 27 | 31 |
| M5 | 13 | 11 |
| M6 | 1 | 2 |
| M7 | 1 | 2 |
| Biphenotypic M4, M6 | 0 | 1 |
| Unknown | 2 | 2 |
| WCC at diagnosis, % | ||
| Greater than 100 × 109/L | 8 | 5 |
| Cytogenetic risk group, % | ||
| Favorable | 17 | 16 |
| Intermediate | 74 | 69 |
| Adverse | 4 | 10 |
| Unknown | 6 | 6 |
| No. of induction courses, % | ||
| 1 | 96 | 97 |
| 2 | 4 | 3 |
For the eligible patients who commenced consolidation, the total duration of therapy from the commencement of induction to the delivery of the last of the consolidation therapy drugs had a mean of 48 days for ICE patients (median, 46 days; range, 35-122 days) compared with a mean of 85 days for IcE patients (median, 82 days; range, 38-171 days). Ninety-five percent of patients had completed therapy within 63 days for ICE patients and 122 days for IcE patients.
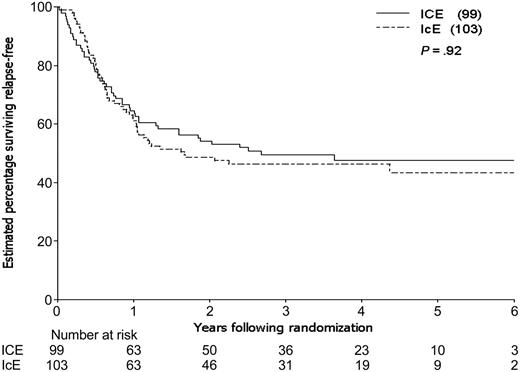
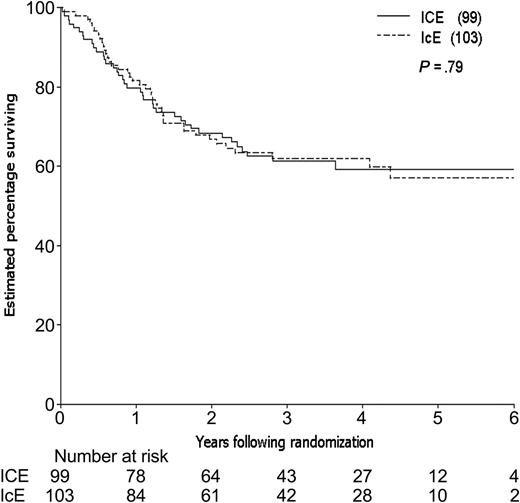
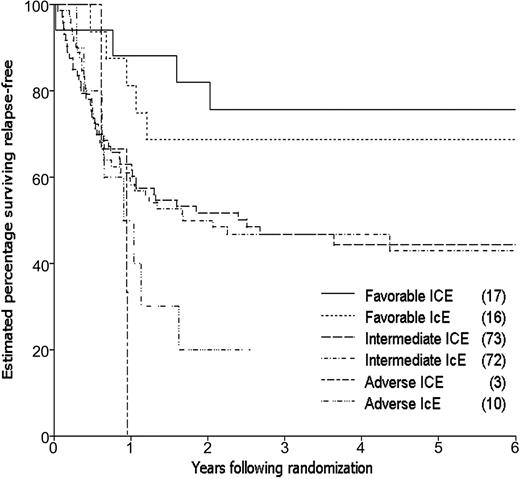
By the time of study closeout, with a median follow-up of 45 months (3.8 years) from consolidation randomization, 94 patients had relapsed and 12 patients had died in CR, one (in an ICE patient) due to graft-versus-host disease (GVHD) following an allogeneic transplantation. The estimated 3-year relapse-free survival (RFS) for the 202 patients randomized to receive consolidation therapy was 48%, 49% for the ICE arm and 46% for IcE (Table 4). The estimated difference in 3-year RFS between the 2 arms (the primary end point of the study) was 3% (95% CI, –11% to 17%; P = .66). The estimated rate of failure (relapse or death) for ICE patients relative to IcE patients was 0.98 adjusting for idarubicin dose, cytogenetic group, and prophylactic G-CSF in induction (95% CI, 0.66 to 1.46; P = .92; Figure 3). Estimated survival following randomization was 61% at 3 years, 61% for the ICE arm and 62% for IcE (P = .91; Table 4; Figure 4). The similarity in outcomes for the 2 randomization arms appeared to hold within each of the cytogenetic risk categories (Table 4; Figure 5).
Outcome of consolidation trial
. | ICE . | . | IcE . | . | Total . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | % . | 95% CI . | % . | 95% CI . | % . | 95% CI . | |||
| RFS at 3 years | |||||||||
| All cases | 49 | 40-59 | 46 | 37-56 | 48 | 41-55 | |||
| Favorable cytogenetics | 76 | 50-91 | 69 | 43-86 | 72 | 55-85 | |||
| Intermediate cytogenetics | 47 | 35-58 | 47 | 36-58 | 47 | 39-55 | |||
| Adverse cytogenetics | 0 | — | 20* | 5-54 | 15* | 4-45 | |||
| Unknown cytogenetics | 33 | 8-73 | 20† | 3-69 | 27 | 9-59 | |||
| Survival at 3 years | |||||||||
| All cases | 61 | 51-71 | 62 | 52-71 | 62 | 55-68 | |||
| Favorable cytogenetics | 88 | 63-97 | 79 | 50-93 | 84 | 66-93 | |||
| Intermediate cytogenetics | 59 | 47-70 | 65 | 53-75 | 62 | 53-69 | |||
| Adverse cytogenetics | 33 | 4-85 | 33* | 10-70 | 35 | 13-65 | |||
| Unknown cytogenetics | 33 | 8-73 | 0 | — | 23 | 6-57 | |||
. | ICE . | . | IcE . | . | Total . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | % . | 95% CI . | % . | 95% CI . | % . | 95% CI . | |||
| RFS at 3 years | |||||||||
| All cases | 49 | 40-59 | 46 | 37-56 | 48 | 41-55 | |||
| Favorable cytogenetics | 76 | 50-91 | 69 | 43-86 | 72 | 55-85 | |||
| Intermediate cytogenetics | 47 | 35-58 | 47 | 36-58 | 47 | 39-55 | |||
| Adverse cytogenetics | 0 | — | 20* | 5-54 | 15* | 4-45 | |||
| Unknown cytogenetics | 33 | 8-73 | 20† | 3-69 | 27 | 9-59 | |||
| Survival at 3 years | |||||||||
| All cases | 61 | 51-71 | 62 | 52-71 | 62 | 55-68 | |||
| Favorable cytogenetics | 88 | 63-97 | 79 | 50-93 | 84 | 66-93 | |||
| Intermediate cytogenetics | 59 | 47-70 | 65 | 53-75 | 62 | 53-69 | |||
| Adverse cytogenetics | 33 | 4-85 | 33* | 10-70 | 35 | 13-65 | |||
| Unknown cytogenetics | 33 | 8-73 | 0 | — | 23 | 6-57 | |||
There were 99 patients in the ICE group, 103 in the IcE group, and 202 patients total. RFS indicates relapse-free survival; —, not applicable; and survival, measured from the time of consolidation randomization.
At 2.6 years, longest surviving patient not followed beyond this time.
At 1.9 years, longest surviving patient not followed beyond this time.
Relapse-free survival for eligible patients entered on the 2 consolidation arms.
Relapse-free survival for eligible patients entered on the 2 consolidation arms.
Using a competing risk analysis, the estimated cumulative incidence of relapse (CIR) was 43% at both 3 and 5 years for ICE patients and 51% at 3 and 5 years for IcE patients. The estimated relapse rate for ICE patients relative to IcE patients was 0.92 adjusting for idarubicin dose, cytogenetic group, and G-CSF during induction (95% CI, 0.60 to 1.40; P = .69). The estimated cumulative incidence of death in CR was 7% and 9% at 3 and 5 years, respectively, for ICE patients and 3% and 6% for IcE patients.
A total of 35 randomized patients received stem cell transplantation in first remission, 16 in the ICE arm (1 autologous and 15 allogeneic) and 19 in the IcE arm (10 autologous and 9 allogeneic). The median time to transplantation was 129 days (range, 73-508 days), with a median of 126 for ICE patients and 139 for IcE patients. Two IcE patients were taken off protocol to receive their transplant after 1 consolidation course. The remaining 33 patients received their transplants after they had completed all protocol treatment. Estimated survival rates were similar when the data were censored at the time of transplantation: at 3 years estimated RFS was 49% for ICE and 42% for IcE, and survival following randomization was 61% for ICE and 61% for IcE.
Seven of the randomized patients had 2 courses of induction, 4 in the ICE arm and 3 in the IcE arm. Six of the 7 patients relapsed within 13 months of randomization and all 7 patients died within 3 years of randomization. RFS and survival following randomization were significantly shorter for these patients compared with patients who only required 1 induction course to achieve remission (P = .030 and .037, respectively), after adjusting for cytogenetics, prophylactic G-CSF in induction, idarubicin dose, and consolidation randomization arm. Remission duration was also shorter but not statistically significantly (P = .055).
Toxicity of consolidation therapy
Treatment-related death occurred in 5 patients randomized to the ICE arm (4 due to infection, 1 adult respiratory distress syndrome) and 2 patients randomized to IcE (l infection, 1 bleeding and infection). The treatment-related death rate was not statistically significant between the 2 arms (P = .26). ICE was more toxic than IcE in consolidation therapy (Table 5). ICE was associated with a significant increase in the overall duration of neutropenia. This was reflected in the significant increase in the total number of days febrile and the overall duration of therapeutic intravenous antibiotics. There was no significant difference between ICE and IcE with respect to the overall duration of thrombocytopenia. ICE was associated with significant increases in diarrhea, nausea and vomiting, stomatitis, and gastrointestinal (GIT), cerebellar, renal, and ocular toxicities (Table 5).
Toxicity of consolidation therapy
. | ICE . | IcE consolidation course 1 . | IcE consolidation course 2 . | IcE . | P . | P for trend* . |
|---|---|---|---|---|---|---|
| No. patients | 95 | 103 | 96 | 103 | NA | NA |
| Neutropenia less than 0.5 × 109/L, d | 17 | 5 | 8 | 13 | .0005 | — |
| Thrombocytopenia less than 50 × 109/L, d | 27 | 13 | 16 | 29 | .50 | — |
| Infections, %† | 79 | 50 | 59 | 75 | .30 | — |
| Fever lower than 38.0°C, d | 8 | 2 | 2 | 4 | < .0001 | — |
| Duration of therapeutic IV antibiotics, d | 19 | 7 | 9 | 15 | .0020 | — |
| Clinical bleeding, %‡ | 5 | 1 | 0 | 1 | .092 | .16 |
| CNS, % | 6 | 1 | 0 | 1 | .050 | .056 |
| Cerebellar, % | 3 | 0 | 0 | 0 | .11 | .0036 |
| Cardiac, % | 1 | 2 | 0 | 2 | .53 | .079 |
| Cutaneous, % | 3 | 1 | 0 | 1 | .28 | .26 |
| Ocular, % | 5 | 2 | 1 | 3 | .32 | .024 |
| Stomatitis, % | 15 | 6 | 3 | 7 | .059 | .0031 |
| Nausea and vomiting, % | 20 | 8 | 3 | 8 | .010 | .0013 |
| Diarrhea, % | 4 | 1 | 2 | 3 | .47 | .0001 |
| GIT, % | 5 | 2 | 1 | 3 | .31 | .0096 |
| Renal, % | 1 | 0 | 0 | 0 | .48 | .023 |
| Hepatic, bilirubin, % | 3 | 1 | 1 | 2 | .46 | .20 |
| Other, % | 5 | 2 | 1 | 3 | .32 | .81 |
. | ICE . | IcE consolidation course 1 . | IcE consolidation course 2 . | IcE . | P . | P for trend* . |
|---|---|---|---|---|---|---|
| No. patients | 95 | 103 | 96 | 103 | NA | NA |
| Neutropenia less than 0.5 × 109/L, d | 17 | 5 | 8 | 13 | .0005 | — |
| Thrombocytopenia less than 50 × 109/L, d | 27 | 13 | 16 | 29 | .50 | — |
| Infections, %† | 79 | 50 | 59 | 75 | .30 | — |
| Fever lower than 38.0°C, d | 8 | 2 | 2 | 4 | < .0001 | — |
| Duration of therapeutic IV antibiotics, d | 19 | 7 | 9 | 15 | .0020 | — |
| Clinical bleeding, %‡ | 5 | 1 | 0 | 1 | .092 | .16 |
| CNS, % | 6 | 1 | 0 | 1 | .050 | .056 |
| Cerebellar, % | 3 | 0 | 0 | 0 | .11 | .0036 |
| Cardiac, % | 1 | 2 | 0 | 2 | .53 | .079 |
| Cutaneous, % | 3 | 1 | 0 | 1 | .28 | .26 |
| Ocular, % | 5 | 2 | 1 | 3 | .32 | .024 |
| Stomatitis, % | 15 | 6 | 3 | 7 | .059 | .0031 |
| Nausea and vomiting, % | 20 | 8 | 3 | 8 | .010 | .0013 |
| Diarrhea, % | 4 | 1 | 2 | 3 | .47 | .0001 |
| GIT, % | 5 | 2 | 1 | 3 | .31 | .0096 |
| Renal, % | 1 | 0 | 0 | 0 | .48 | .023 |
| Hepatic, bilirubin, % | 3 | 1 | 1 | 2 | .46 | .20 |
| Other, % | 5 | 2 | 1 | 3 | .32 | .81 |
Percentage of patients in each consolidation arm experiencing WHO grades 3 and 4 nonhematologic toxicities, or median durations of hematologic toxicities as defined. NA indicates not applicable; —, trend not assessed; and IV, intravenous.
Significance of trend based on individual toxicity grades rather than grade 3 or 4 toxicities.
Percentage of patients with clinically or microbiologically proven infections.
Coded as none, petechiae, mild blood loss, gross blood loss, debilitating blood loss. Percentages reported correspond to gross or debilitating blood loss
ICE was also associated with a significant increase in the total number of days in intensive care: mean 2.7 days for ICE and 0.6 days for IcE (P = .045), with 15% and 8% of patients, respectively, requiring at least 1 admission. However there was a similar overall duration of the total days in hospital, including 1-day admissions and outpatient visits, with a mean of 38 days for ICE and 40 days for IcE (medians, 34 and 40 days, respectively).
Discussion
This randomized study was designed to test the hypothesis that sequential cycles of combination chemotherapy based on high-dose cytarabine could produce greater leukemic cytoreduction, and therefore ultimately superior long-term relapse-free survival, than chemotherapy based on high-dose cytarabine in induction and conventional-dose cytarabine in consolidation. The rationale for the study was based on a number of previously published observations. First, the Australian Leukaemia Study Group described superior relapse-free survival for patients induced with high-dose cytarabine plus daunorubicin and etoposide, followed by conventional-dose cytarabine in consolidation therapy compared with induction treatment with standard doses of cytarabine.5 The South West Oncology demonstrated similar findings in the SWOG 8601 study.7 Secondly, CALGB showed a significant benefit for consolidation therapy with high-dose cytarabine compared with conventional-dose treatment.4 Finally, the SWOG 8601 study suggested that inclusion of high-dose cytarabine in both induction and consolidation phases gave the best long-term relapse-free survival, although this was not a randomized comparison.7 We therefore assessed whether sequential courses of chemotherapy containing high-dose cytarabine, given in both induction and consolidation phases of initial treatment for newly diagnosed AML, would improve the long-term cure rate compared with high-dose induction therapy followed by conventional cytarabine doses in consolidation, as employed in our previous clinical trial. The major finding of the present study is that there was no significant difference in outcome, as measured by survival following consolidation randomization, relapse-free survival, or remission duration, between the 2 groups receiving either 2 cycles of treatment containing high-dose cytarabine or high-dose induction followed by conventional consolidation. Thus, the benefits of high-dose cytarabine in this strategy appear to be conferred by the induction cycle, and there appeared to be no additional effect of administering a second identical cycle compared with giving conventional consolidation treatment.
The major potential disadvantage to the upfront use of high-dose cytarabine in induction therapy is the potential for increased treatment-related toxicity. This study, however, demonstrates that the ICE protocol could be delivered to adults aged 60 years or younger with newly diagnosed AML, following a 25% reduction in idarubicin dose from the initial total induction dose of 36 mg/m2 over 3 days. The early death rate of 10% in induction was comparable to that reported in other studies of younger AML patients using protocols using conventional doses of cytarabine and substantially lower than previously reported studies using high-dose cytarabine in induction therapy.5,7,13 The latter observation may reflect improvements in supportive care over the last decade. The major toxicity encountered in the present study was prolonged neutropenia, shortened by G-CSF, and gastrointestinal mucosal toxicity, including neutropenic enterocolitis which, interestingly, was also observed in a small minority of patients receiving conventional-dose cytarabine in consolidation. Although the ICE combination frequently was complicated by GIT toxicities, the precise role of high-dose cytarabine in the etiology of these problems remains unclear since both idarubicin and etoposide are known to cause mucosal toxicity. We have previously reported that the duration of neutropenia occurring after ICE induction therapy is shortened by G-CSF but that the use of this cytokine did not result in a detectable improvement in either complete remission rate or overall survival.8
The ICE regimen proved to be effective in inducing complete remission in this younger patient population with AML, with a complete remission rate of 80%, and 7% of patients having evidence of drug-resistant leukemia. These results compare favorably with those obtained in patients of comparable age treated on standard-dose induction regimens.5,7,13,14 One major difference however was that 95% of complete remissions were observed with a single induction cycle, indirectly implying more leukemic cytoreduction with the ICE protocol compared with regimens containing standard-dose cytarabine. It can be argued that any increase in hematologic and nonhematologic toxicity from the ICE protocol is offset by a higher frequency of complete remission after the first induction cycle and a corresponding reduction in the proportion of patients requiring a second attempt at induction of remission. It is also noteworthy that, despite intensified induction chemotherapy, we observed a small proportion of patients with residual leukemia after 1 or 2 induction cycles; clearly, other forms of therapy are required for this subset of patients with highly chemorefractory disease, for whom even upfront high-dose cytarabine was insufficient to produce remission.
The ICE protocol also showed acceptable toxicity when used for consolidation therapy, with no prolongation of myelosuppression compared with induction treatment, no evidence of cumulative nonhematologic toxicity (particularly mucosal), and a treatment-related death rate of 5%, not significantly different from that observed on the conventional cytarabine dose arm. These results are comparable with the reported risks associated with autologous stem cell transplantation for AML in first remission and for consolidation therapy with multiple cycles of high-dose cytarabine.4,15-17 All patients receiving ICE in this study received the prescribed high-dose consolidation therapy promptly after completing induction treatment. Thus both dose escalation and increased dose intensity of therapy were achieved. There was however, no demonstrable benefit from the use of a second cycle of ICE, as no significant differences were detected in any of the clinical outcomes between the 2 randomization groups. Thus, it would appear that, at least in the context of the 3-drug program used in ICE, that the long-term effects of the early use of high-dose cytarabine were largely conferred by the first induction course and that further intensive cycles did not provide additional clinical benefit. Indeed, it remains uncertain as to whether any further chemotherapy after an induction cycle of ICE is necessary. A conservative interpretation of the data from this study, however, would be that induction with ICE, followed by 2 cycles of standard-dose consolidation, provides relapse-free survival comparable to that reported for alternative strategies using multiple courses of intensified consolidation treatment, with or without stem cell transplantation, but with the advantage of considerably shorter total duration of treatment and overall drug exposure. For younger patients judged sufficiently fit to tolerate 2 intensive ICE cycles, the total duration of therapy could be reduced to an average of 48 days, with equal efficacy to 3 cycles of treatment.
Cytogenetic and molecular changes in leukemic cells at diagnosis remains one of the most powerful pretreatment prognostic factors for predicting outcome in AML.9,18 Analysis of data from this study taking into account cytogenetic risk groups failed to detect a difference between the randomization arms. Notably, there was no significant difference in outcome between high- and conventional-cytarabine dose arms for the 144 patients with intermediate-risk chromosome changes. We did not examine for expression of mutations in the Fms-like tyrosine kinase 3 (Flt-3) receptor (which has been shown to identify a subgroup of intermediate risk cytogenetics with adverse outcome19 ) in this group. It was also notable that the small group of patients with adverse risk cytogenetics fared equally badly with 2 cycles of ICE compared with 2 cycles of IcE, indicating that radically different therapeutic approaches will be necessary to improve the outcome of these patients. The group of patients with favorable cytogenetics (core binding factor–positive cases) had a relatively good outcome, with no statistically significant benefit from 2 cycles of high-dose cytarabine compared with only 1 cycle in the small number of patients studied; this is of relevance in view of suggestions that multiple cycles of high-dose cytarabine are the most important component of curative therapy for this subgroup of AML.20,21
In conclusion, this study demonstrates that high-dose cytarabine, as used in the ICE protocol for induction therapy, results in a high rate of remission for younger adults with de novo AML, with acceptable toxicity. Although administration of a second cycle of ICE as consolidation therapy proved feasible, we were unable to demonstrate a clinical benefit over 2 cycles of attenuated consolidation therapy with conventional-dose cytarabine. Given the equivalent antileukemic efficacy and overall toxicity of 2 cycles of ICE, 1 in induction followed by 1 as consolidation therapy, this treatment strategy may be considered as a realistic alternative to other current forms of treatment for AML, with the advantage of being delivered within 2 months for the majority of patients.
Appendix: contributors to the ALLG M7 Trial
Westmead Hospital, Westmead, Sydney, NSW, Australia: W. Benson, D. Gottlieb, P. Castaldi, M. Hertzberg; Royal Prince Alfred Hospital, Camperdown, Sydney, NSW, Australia: H. Kronenberg, J. Gibson, H. Iland; Mater Hospital, Newcastle, NSW, Australia: M. Seldon, S. Deveridge, A. Spencer, I. Kerridge; Royal North Shore Hospital, St Leonards, Sydney, NSW, Australia: D. Ma, K. Fay, C. Ward, R. Mckinley; St George Hospital, Kogarah, Sydney, NSW, Australia: Y. Kwan; Prince of Wales Hospital, Randwick, Sydney, NSW, Australia: R. Lindeman, P. Rowlings; Liverpool Hospital, Liverpool, Sydney, NSW, Australia: J. Gallo, P. Motum, D. Rosenfeld; Concord Hospital, Concord, Sydney, NSW, Australia: I. Cunningham; Royal Melbourne Hospital, North Melbourne, VIC, Australia: A. Grigg, A. Roberts, P. Bardy; Alfred Hospital, Melbourne, Prahran, VIC, Australia: P. Elliott, A. Spencer; Peter MacCallum Cancer Institute, East Melbourne, VIC, Australia: H. Januszewicz, M. Wolf; Monash Medical Centre, Clayton, Melbourne, VIC, Australia: E. Gan; Austin Hospital, Heidelberg, Melbourne, VIC, Australia: C. Smith; Royal Brisbane Hospital, Herston, Brisbane, QLD, Australia: A. Nicol, I. Bunce; Mater Hospital, South Brisbane, QLD, Australia: S. Wright; Princess Alexandra Hospital, Wollongabba, Brisbane, QLD, Australia: P. Marlton, G. Cull; Wesley Medical Centre, Brisbane, QLD, Australia: I. Bunce, S. Fanning, J. Morton; Royal Adelaide Hospital, Adelaide, SA, Australia: N. Horvath, J. Dart, J. Ho, T. Hughes; Queen Elizabeth Hospital, Woodville Park, Adelaide, SA, Australia: J. Norman; Royal Perth Hospital, Perth, WA, Australia: R. Hermann, R. Baker; Fremantle Hospital, Fremantle, WA, Australia: F. Cordingley, M. Webb; Sir Charles Gardner Hospital, Perth, WA, Australia: S. Rule, D. Joske; Canberra Hospital, Canberra, ACT, Australia: I. Prosser, M. Pidcock, M. Webb; Royal Hobart Hospital, Hobart, Tasmania, Australia: R. Kimber, D. Jupe; Pretoria Academic Hospital, Pretoria, South Africa: C. Falkson; Medical Oncology Clinic of Rosebank, Johannesburg, South Africa: B. Rapoport.
Prepublished online as Blood First Edition Paper, June 22, 2004; DOI 10.1182/blood-2004-01-0326.
A complete list of the members of the Australasian Leukaemia and Lymphoma Group appears in the “Appendix.”
Supported by research grants from the National Health and Medical Research Council of Australia (grants 950971 and 9937673) and by financial support from Amrad Pharmaceuticals Australia and Pharmacia Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The Australasian Leukaemia and Lymphoma Group is the successor organization to the Australian Leukaemia Study Group (ALSG).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal