Abstract
The incidence of treatment-related myelodysplastic syndromes and acute myeloid leukemia (tMDSs/tAML) after tositumomab and iodine I131 tositumomab administration to previously treated and untreated patients with non-Hodgkin lymphoma (NHL) was evaluated. A total of 1071 patients were enrolled in 7 studies: 995 with relapsed/refractory low-grade NHL, ± transformation (median, 3 prior regimens [range, 1-13 regimens]) and 76 patients with previously untreated low-grade follicular NHL. A single dose of iodine tositumomab and I131 tositumomab was administered. For tMDS/tAML patients, baseline and posttherapy peripheral blood and marrow specimens were reviewed in a blinded fashion. Median follow-up was 6 years from diagnosis and 2 years from radioimmunotherapy (RIT) for previously treated patients, and 4.6 years from radioimmunotherapy for previously untreated patients. tMDS/tAML was reported in 35 (3.5%) of 995 patients (annualized incidence, 1.6%/y [95% confidence interval, 1.0%-2.0%/y]), and 52% of the tMDS/tAML diagnoses of tMDS/tAML were confirmed in a blinded review (annualized incidence of 1.1%/y [95% confidence interval, 0.7%-1.6%/y]). Of the 25 cases, 10 patients (40%) were diagnosed with tMDS/tAML prior to receiving radioimmunotherapy; 2 (8%) had no pathologic or clinical evidence to support such a diagnosis; and 13 (52%) were confirmed to have developed tMDS/tAML following RIT. This incidence is consistent with that expected on the basis of patients' prior chemotherapy for NHL. With a median follow-up approaching 5 years, no case of tMDS/tAML has been reported in any of the 76 patients receiving iodine I131 tositumomab as their initial therapy (P = .011 compared with previously treated patients). (Blood. 2005;105:4576-4582)
Introduction
Patients with the low-grade non-Hodgkin lymphomas (LG-NHLs) characteristically achieve multiple remissions and sustain multiple relapses during their long (median, 10 to 12 years) disease course. With each successive relapse, patients tend to be treated with more intense cytotoxic therapies to which the disease eventually becomes refractory.1-4 There is a need for new, non-cross-resistant therapies. B-lymphoid-specific CD20 monoclonal antibodies (MAbs) such as rituximab have partially fulfilled this need. However, despite the fact that more than 90% of the malignant lymphocytes express CD20, responses are achieved in only about 50% of the patients and the responses generally last less than a year.5
Given the exquisite radiosensitivity of LG-NHL, radioimmunotherapy (RIT; ie, the conjugation of a CD20 MAb with a radioisotope) offers an alternative treatment for patients with refractory LG-NHL.6-12 With RIT, the monoclonal antibody induces antibody-dependent and complement-mediated cytotoxicity13,14 and apoptosis15 and also enables targeted radiation-induced cytotoxicity. The radiation is delivered not only to the antibody-bound tumor cell, but also to neighboring, non-antibody-bound tumor cells through a “cross-fire” effect consistent with the path length of the radioactive isotope.16 The cross-fire effect also has the potential to damage normal cells within the path length of the isotope. Since cytopenias have been the prominent dose-limiting toxicities for RIT, treatment programs have limited RIT to patients whose bone marrow contains 25% or less tumor infiltration.6-12
Since its introduction in 1990, considerable experience has been obtained with the CD20 RIT regimen, tositumomab and iodine I131 tositumomab (BEXXAR therapeutic regimen; Corixa, Seattle, WA, and GlaxoSmithKline, Philadelphia, PA), a murine immunoglobulin G2a (IgG2a) MAb conjugated to iodine 131.17 Tositumomab and iodine I131 tositumomab are approved by the US Food and Drug Administration for the treatment of patients with CD20+ follicular NHL with or without transformation whose disease is refractory to rituximab and has relapsed following chemotherapy. Of significant clinical importance, long-term durable remissions extending to more than 10 years after a single treatment have been achieved in a heavily pretreated patient population that includes patients refractory to chemotherapy and/or rituximab.18 Additionally, durable remissions extending to more than 7 years have been achieved in newly diagnosed patients with follicular NHL who had received tositumomab and iodine I131 tositumomab as their initial therapy.19
Treatment-related myelodysplastic syndromes and acute myeloid leukemia (tMDSs/tAML) have become a well-recognized delayed complication in patients with LG-NHL.20 This has been attributed to repetitive exposure to cytotoxic drugs over the relatively long natural history of the disease. The success of RIT in evoking durable remissions in patients with relapsed and refractory NHL further extends the period of observation for delayed effects such as tMDS/tAML. In this context, the question as to whether the systemic radiation associated with RIT adds to the expected incidence of tMDS/tAML in these heavily treated patients is important. The present report evaluates the incidence of tMDS/tAML in 2 patient populations: one with relapsed and refractory LG-NHL that had received extensive cytotoxic therapies prior to RIT, and another population that received RIT as the initial therapy for follicular LG-NHL. Some of the cases included in this series have been previously reported.21
Patients, materials, and methods
Patients
Since 1990, 1071 patients with LG- and transformed LG-NHL have been treated in 5 clinical trials and an Expanded Access Program (EAP) for patients with relapsed and refractory NHL (n = 995), and a clinical trial in which RIT was used as initial therapy for patients with follicular NHL (n = 76) (Table 1). For the previously treated patients, the median follow-up time from diagnosis was 6.0 years (range, 0.6 to 29 years) and from therapy with RIT, 2.0 years (range, < 0.1 to 9.8 years). Patients had received a median of 3 prior cytotoxic therapies (range, 1 to 13 therapies) before the RIT. For the previously untreated patients, the median follow-up times from diagnosis and RIT therapy were 5.6 years (range, 1.1 to 14.2 years) and 4.6 years (range, 0.6 to 6.7 years), respectively. For the purposes of performing the analyses, the database was locked as of May 1, 2003. All patients provided informed consent according to the institutional review board guidelines from the centers participating in the 5 clinical trials and the Expanded Access Program. Informed consent was provided according to the Declaration of Helsinki. Copies were sent to Corixa for permanent storage.
Study population: clinical trials with tositumomab and iodine I131 tositumomab in patients with low-grade and transformed low-grade NHL and their respective follow-up times
Study . | No. patients . | No. prior therapies, median (range) . | Median follow-up from diagnosis, y (range) . | Median follow-up from RIT, y (range) . |
|---|---|---|---|---|
| Previously treated | ||||
| RIT-I-000 | 22 | 5 (1-13) | 10.1 (1.0-25.5) | 3.8 (0.2-9.8) |
| RIT-II-001 | 47 | 4 (1-13) | 7.5 (1.8-27.7) | 2.8 (<0.1-7.2) |
| RIT-II-002 | 61 | 2 (1-8) | 5.8 (1.1-23.3) | 3.2 (0.2-6.0) |
| RIT-II-004 | 60 | 4 (2-13) | 7.7 (2.4-29.0) | 2.5 (<0.1-5.9) |
| CP-97-012 | 40 | 4 (2-13) | 7.2 (1.8-17.7) | 3.2 (0.1-4.6) |
| CP-98-020 | 765 | 2 (1-12) | 5.8 (0.6-27.1) | 1.9 (<0.1-4.5) |
| Total | 995 | 3 (1-13) | 6.1 (0.6-29.0) | 2.0 (<0.1-9.8) |
| Previously untreated | ||||
| RIT-II-003 | 76 | 0 (0-0) | 5.6 (1.1-14.2) | 4.6 (0.6-6.7) |
Study . | No. patients . | No. prior therapies, median (range) . | Median follow-up from diagnosis, y (range) . | Median follow-up from RIT, y (range) . |
|---|---|---|---|---|
| Previously treated | ||||
| RIT-I-000 | 22 | 5 (1-13) | 10.1 (1.0-25.5) | 3.8 (0.2-9.8) |
| RIT-II-001 | 47 | 4 (1-13) | 7.5 (1.8-27.7) | 2.8 (<0.1-7.2) |
| RIT-II-002 | 61 | 2 (1-8) | 5.8 (1.1-23.3) | 3.2 (0.2-6.0) |
| RIT-II-004 | 60 | 4 (2-13) | 7.7 (2.4-29.0) | 2.5 (<0.1-5.9) |
| CP-97-012 | 40 | 4 (2-13) | 7.2 (1.8-17.7) | 3.2 (0.1-4.6) |
| CP-98-020 | 765 | 2 (1-12) | 5.8 (0.6-27.1) | 1.9 (<0.1-4.5) |
| Total | 995 | 3 (1-13) | 6.1 (0.6-29.0) | 2.0 (<0.1-9.8) |
| Previously untreated | ||||
| RIT-II-003 | 76 | 0 (0-0) | 5.6 (1.1-14.2) | 4.6 (0.6-6.7) |
Treatment
Drug preparation, dosimetry, and administration of tositumomab and iodine I131 tositumomab have been previously described.7,9,22
The total body dose (TBD) of radiation was standardized to 75 cGy and was attenuated to 65 cGy for patients with a baseline platelet count of 100 to 149 × 109/L. Obese patients had their dose adjusted downward based on 137% of their lean body weight.22 To block uptake of iodine I131 by the thyroid gland, patients were treated with saturated solution of potassium iodide, Lugol solution, or potassium iodide tablets beginning 24 hours before the dosimetric dose and continuing for 14 days after the therapeutic dose.
MDS/AML reporting and analyses
The occurrence of tMDS/tAML was a serious adverse event requiring a report to the study sponsor within 48 hours of diagnosis. Following the diagnosis of tMDS/tAML, baseline peripheral blood (Wright-Giemsa stain) and marrow specimens (aspirate [Wright-Giemsa stain], biopsy [hematoxylin and eosin stain], and Prussian blue iron stains), and similar post-RIT specimens were requested. Cytogenetic analyses of these same specimens were also requested.
Independent review of tMDS/tAML
Specimens were masked to all identifying characteristics, then randomly coded with new numbers to enable a blinded morphologic review. The baseline evaluation specimen (pre-RIT) and the post-RIT specimen received separate, nonrelated coded numbers. An independent, experienced hematomorphologist (J.M.B.), who was not a participant in any of the studies, provided a morphologic diagnosis of each individual specimen without the benefit of any clinical history or other identifying characteristics. This review included cell lineage dysplasia and a differential count, including the percentage of blasts, percentage of ringed sideroblasts, bone marrow cellularity, and the presence of myelofibrosis. An interpretation of the specimens was made as one of the following categories: normal marrow, inadequate material to render a diagnosis, tMDS, tAML, myeloproliferative disorder, or aplastic anemia.
Another independent hematologist/oncologist reviewed each patient and summarized the clinical history, hemogram, marrow reports, and cytogenetics and composed a clinical narrative. Following the submission of the reports of the blinded morphologic review, the individual clinical narratives were made available to the hematomorphologist who then provided a final diagnosis by synthesizing the morphologic review with the clinical narrative for each patient. This final review is noted as the “blinded independent review” of the reported cases of tMDS/tAML.
Statistical methods
A number of statistics are used to summarize the frequency and timing of events that require prolonged observations such as the diagnosis of tMDS/tAML following NHL therapy.20 There were 3 statistical summaries of tMDS/tAML performed: crude incidence, annualized incidence, and cumulative incidence accounting for competing risks. Crude incidence, the proportion of patients diagnosed with tMDS/tAML, was calculated along with exact 95% binomial confidence limits. Annualized incidence, the number of cases of tMDS/tAML per person-year of follow-up, was calculated along with likelihood-based 95% confidence limits.23 Cumulative incidence, the proportion of patients estimated to be diagnosed with tMDS/tAML as a function of time following therapy, was calculated using nonparametric methods along with associated standard errors.23 The cumulative incidence estimate incorporated the presence of competing risks such as death from progressive NHL or other causes. In the long run, the cumulative incidence will estimate the actual proportion of patients that were diagnosed with tMDS/tAML.
Exploratory univariate analyses that compared the crude incidence across patient subgroups were performed using the chi-square test with Yates' correction24 for characteristics with 2 subgroups. Comparisons of the time to tMDS/tAML were performed using the log-rank test.25 The following factors were included in the univariate analyses: sex; age (≤ 60 versus > 60 years); ethnicity (white versus other); years from NHL diagnosis (< 4 versus ≥ 4 years); histology (follicular versus other); baseline lactate dehydrogenase (LDH, elevated versus low/normal); bulky disease (≤ 5 cm versus > 5 cm); bone marrow involvement (yes versus no); number of prior chemotherapies (1 to 3 versus > 3); number of prior therapies (1 to 3 versus > 3); prior rituximab; prior fludarabine-containing regimen; prior anthracycline/anthracenedione-containing regimen; prior alkylating agent-containing regimen; prior radiotherapy; baseline platelet count (< 150 × 109/L versus ≥ 150 × 109/L); and baseline absolute neutrophil count (ANC, < 3 × 109/L versus ≥ 3 × 109/L). Multivariate analyses of the time to tMDS/tAML were performed using the Cox model.26 Factors for age, bone marrow involvement, time from diagnosis, type of prior therapy, number of prior therapies, and baseline blood count were included in the stepwise model. There were 2 separate multivariate analyses conducted. Data on the type of prior chemotherapy were not collected for the 765 patients in the Expanded Access Program, Study CP-98-020. Thus, the analyses excluded the type of prior chemotherapy in the integrated population. Separate multivariate analyses including type of prior chemotherapy were run with all 230 patients from the integrated patient population who were not enrolled in Study CP-98-020.
The rates of tMDS/tAML were compared between patients in the integrated relapsed and refractory NHL population and patients from Study RIT-II-003 who received tositumomab and iodine I131 tositumomab as their initial therapy for follicular NHL. The differences in the rates of tMDS/tAML were compared using the log-rank test.25
Separate analyses for each of the end points were performed based on the investigator-reported cases of tMDS/tAML and the blinded independent assessment of tMDS/tAML. Although it is likely that some of the nonreviewed cases would have been found to have preexisting tMDS/tAML, we elected to adopt a conservative approach and therefore classified these as having a confirmed case following therapy.
Results
Baseline patient demographics for the patient populations are reported in Table 2. Besides the significant differences in age, grade, and prior therapy, the previously treated and untreated populations had significantly different baseline characteristics in bone marrow involvement, 42% and 64% (P < .001) and platelets less than 150 × 109/L, 25% and 11% (P = .005), respectively. There was a trend for a difference in ANC less than 3 × 109/L, 30% in previously treated versus 21% in untreated patients (P = .090). The previously treated patients had received a median of 3 prior therapies (range, 1 to 13 therapies) and 35% had received 4 or more prior therapies. Nearly half of the patients had received a rituximab-containing regimen. For the 230 patients with detailed prior treatment history, 99% had received a regimen that contained an alkylating agent, 45% had received a fludarabine-containing regimen, 80% had received at least one topoisomerase II inhibitor, and 25% had received at least one course of radiation treatment. For the 995 previously treated patients, the median follow-up time from the diagnosis of NHL and RIT was 6 years (range, 0.6 to 29 years) and 2 years (range, < 0.1 to 9.8 years), respectively. For the 76 patients who received RIT as their initial therapy for follicular NHL, the corresponding figures were 5.6 years (range, 1.1 to 14.2 years) and 4.6 years (range, 0.6 to 6.7 years), respectively.
Patient demographics
Demographic characteristic . | Relapsed/refractory NHL . | First-line therapy . |
|---|---|---|
| No. patients | 995 | 76 |
| Sex | ||
| Male, no. (%) | 586 (59) | 41 (54) |
| Female, no. (%) | 409 (41) | 35 (46) |
| Median age, y (range) | 58 (21-88) | 49 (23-69) |
| Median time from diagnosis to protocol entry, mo (range) | 45 (2-334) | 8 (1-105) |
| Grade at protocol entry, no. (%) | ||
| Low grade | 766 (77) | 76 (100) |
| Transformed low grade | 218 (22) | 0 (0) |
| Intermediate/high grade | 11 (1) | 0 (0) |
| Ann Arbor stage III/IV at enrollment, no. (%) | 883/994 (89) | 76 (100) |
| Bone marrow involvement, no. (%) | 414/990 (42) | 48/75 (64) |
| Elevated LDH, no. (%) | 90/224 (40) | 23/75 (31) |
| Decreased platelets,* no. (%) | 245 (25) | 8 (11) |
| Decreased ANC,† no. (%) | 301 (30) | 16 (21) |
| Median number of prior therapies (range) | 3 (1-13) | 0 (0-0) |
| Patients with 4 or more prior therapies, no. (%) | 351 (35) | 0 (0) |
| Previously received rituximab, no. (%) | 470 (47) | 0 (0) |
| Previously received fludarabine, no. (%) | 103/230 (45) | 0 (0) |
| Previously received anthracycline or anthracenedione, no. (%) | 185/230 (80) | 0 (0) |
| Previously received alkylator, no. (%) | 227/230 (99) | 0 (0) |
| Previously received radiotherapy, no. (%) | 244 (25) | 0 (0) |
Demographic characteristic . | Relapsed/refractory NHL . | First-line therapy . |
|---|---|---|
| No. patients | 995 | 76 |
| Sex | ||
| Male, no. (%) | 586 (59) | 41 (54) |
| Female, no. (%) | 409 (41) | 35 (46) |
| Median age, y (range) | 58 (21-88) | 49 (23-69) |
| Median time from diagnosis to protocol entry, mo (range) | 45 (2-334) | 8 (1-105) |
| Grade at protocol entry, no. (%) | ||
| Low grade | 766 (77) | 76 (100) |
| Transformed low grade | 218 (22) | 0 (0) |
| Intermediate/high grade | 11 (1) | 0 (0) |
| Ann Arbor stage III/IV at enrollment, no. (%) | 883/994 (89) | 76 (100) |
| Bone marrow involvement, no. (%) | 414/990 (42) | 48/75 (64) |
| Elevated LDH, no. (%) | 90/224 (40) | 23/75 (31) |
| Decreased platelets,* no. (%) | 245 (25) | 8 (11) |
| Decreased ANC,† no. (%) | 301 (30) | 16 (21) |
| Median number of prior therapies (range) | 3 (1-13) | 0 (0-0) |
| Patients with 4 or more prior therapies, no. (%) | 351 (35) | 0 (0) |
| Previously received rituximab, no. (%) | 470 (47) | 0 (0) |
| Previously received fludarabine, no. (%) | 103/230 (45) | 0 (0) |
| Previously received anthracycline or anthracenedione, no. (%) | 185/230 (80) | 0 (0) |
| Previously received alkylator, no. (%) | 227/230 (99) | 0 (0) |
| Previously received radiotherapy, no. (%) | 244 (25) | 0 (0) |
See “Results” for P values for differences between the 2 patients populations.
Defined as having a platelet count < 150 × 109/L.
Defined as having an ANC < 3 × 109/L.
Of the patients, 35 were reported by the investigators to have developed tMDS/tAML (Table 3). The crude and annualized incidence of tMDS/tAML for each of the studies and the person-years of follow-up are noted in Table 3. The variable crude incidence rates for each of the studies may be a reflection not only of the number of prior therapies but also the nature and intensity of drug usage. For example, study RIT-I-000 was a phase 1 trial that enrolled not only patients in whom multiple regimens containing conventional-dose chemotherapy had failed, but also patients in whom high-dose chemotherapy and stem cell transplantations had failed.6
Investigator-reported and independent review-reported cases of MDS/AML after tositumomab and iodine I131 tositumomab
Study . | No. . | No. MDS/AML . | Crude incidence, % (95% CI) . | After tositumomab and iodine I131 tositumomab, person-years . | Median follow-up, y . | Annualized incidence, %, y (95% CI) . |
|---|---|---|---|---|---|---|
| Investigator-reported | ||||||
| RIT-I-000 | 22 | 5 | 22.7 (7.8-45.3) | 104 | 3.8 | 4.8 (2.0-11.5) |
| RIT-II-001 | 47 | 5 | 10.6 (3.5-23.1) | 167 | 2.8 | 3.0 (1.2-7.2) |
| RIT-II-002 | 61 | 4 | 6.6 (1.8-15.9) | 177 | 3.2 | 2.3 (0.8-6.0) |
| RIT-II-004 | 60 | 4 | 6.7 (1.8-16.2) | 152 | 2.5 | 2.6 (1.0-7.0) |
| CP-97-012 | 40 | 2 | 5.0 (0.6-16.9) | 103 | 3.2 | 1.9 (0.5-7.7) |
| Expanded access | 765 | 15 | 2.0 (1.1-3.2) | 1,440 | 1.9 | 1.0 (0.6-1.7) |
| Subtotal | 995 | 35 | 3.5 (2.5-4.9) | 2144 | 2.0 | 1.6 (1.2-2.3) |
| RIT-II-003 | 76 | 0 | 0.0 (0.0-4.7) | 346 | 4.6 | 0.0 (0.0-0.9) |
| Total | 1071 | 35 | 3.3 (2.3-4.5) | 2490 | 2.1 | 1.4 (1.0-2.0) |
| Independent review-reported | ||||||
| RIT-I-000 | 20 | 3 | 15.0 (3.2-37.9) | 96 | 3.8 | 3.1 (1.0-9.6) |
| RIT-II-001 | 46 | 4 | 8.7 (2.4-20.8) | 166 | 2.9 | 2.4 (0.9-6.4) |
| RIT-II-002 | 60 | 3 | 5.0 (1.0-13.9) | 176 | 3.2 | 1.7 (0.5-5.3) |
| RIT-II-004 | 59 | 2 | 3.4 (0.4-11.7) | 154 | 2.4 | 1.3 (0.3-5.2) |
| CP-97-012 | 40 | 2 | 5.0 (0.6-16.9) | 103 | 3.2 | 1.9 (0.5-7.7) |
| Expanded access | 760 | 9 | 1.2 (0.5-2.2) | 1436 | 1.9 | 0.6 (0.3-1.2) |
| Subtotal | 985 | 23 | 2.3 (1.5-3.5) | 2132 | 2.0 | 1.1 (0.7-1.6) |
| RIT-II-003 | 76 | 0 | 0.0 (0.0-4.7) | 346 | 4.6 | 0.0 (0.0-0.9) |
| Total | 1061 | 23 | 2.2 (1.4-3.2) | 2479 | 2.1 | 0.9 (0.6-1.4) |
Study . | No. . | No. MDS/AML . | Crude incidence, % (95% CI) . | After tositumomab and iodine I131 tositumomab, person-years . | Median follow-up, y . | Annualized incidence, %, y (95% CI) . |
|---|---|---|---|---|---|---|
| Investigator-reported | ||||||
| RIT-I-000 | 22 | 5 | 22.7 (7.8-45.3) | 104 | 3.8 | 4.8 (2.0-11.5) |
| RIT-II-001 | 47 | 5 | 10.6 (3.5-23.1) | 167 | 2.8 | 3.0 (1.2-7.2) |
| RIT-II-002 | 61 | 4 | 6.6 (1.8-15.9) | 177 | 3.2 | 2.3 (0.8-6.0) |
| RIT-II-004 | 60 | 4 | 6.7 (1.8-16.2) | 152 | 2.5 | 2.6 (1.0-7.0) |
| CP-97-012 | 40 | 2 | 5.0 (0.6-16.9) | 103 | 3.2 | 1.9 (0.5-7.7) |
| Expanded access | 765 | 15 | 2.0 (1.1-3.2) | 1,440 | 1.9 | 1.0 (0.6-1.7) |
| Subtotal | 995 | 35 | 3.5 (2.5-4.9) | 2144 | 2.0 | 1.6 (1.2-2.3) |
| RIT-II-003 | 76 | 0 | 0.0 (0.0-4.7) | 346 | 4.6 | 0.0 (0.0-0.9) |
| Total | 1071 | 35 | 3.3 (2.3-4.5) | 2490 | 2.1 | 1.4 (1.0-2.0) |
| Independent review-reported | ||||||
| RIT-I-000 | 20 | 3 | 15.0 (3.2-37.9) | 96 | 3.8 | 3.1 (1.0-9.6) |
| RIT-II-001 | 46 | 4 | 8.7 (2.4-20.8) | 166 | 2.9 | 2.4 (0.9-6.4) |
| RIT-II-002 | 60 | 3 | 5.0 (1.0-13.9) | 176 | 3.2 | 1.7 (0.5-5.3) |
| RIT-II-004 | 59 | 2 | 3.4 (0.4-11.7) | 154 | 2.4 | 1.3 (0.3-5.2) |
| CP-97-012 | 40 | 2 | 5.0 (0.6-16.9) | 103 | 3.2 | 1.9 (0.5-7.7) |
| Expanded access | 760 | 9 | 1.2 (0.5-2.2) | 1436 | 1.9 | 0.6 (0.3-1.2) |
| Subtotal | 985 | 23 | 2.3 (1.5-3.5) | 2132 | 2.0 | 1.1 (0.7-1.6) |
| RIT-II-003 | 76 | 0 | 0.0 (0.0-4.7) | 346 | 4.6 | 0.0 (0.0-0.9) |
| Total | 1061 | 23 | 2.2 (1.4-3.2) | 2479 | 2.1 | 0.9 (0.6-1.4) |
The annualized incidence and 95% CI for the investigator-reported cases of tMDS/tAML for the previously treated patients, initial RIT patients, and total patient populations were 1.6%/y (95% CI, 1.2%-2.3%/y), 0%/y (95% CI, 0%-0.9%/y), and 1.4%/y 95% CI, 1.0%-2.0%/y), respectively.
An independent, blinded review of specimens from 25 patients with investigator-reported tMDS/tAML was conducted. Specimens could not be located for 10 patients; these 10 patients were classified as confirmed cases of tMDS/tAML. Following the independent review of the 25 cases, 10 (40%) were diagnosed as having tMDS prior to receiving RIT, 2 (8%) had no evidence of tMDS (ie, the site diagnosis of tMDS was not confirmed), and 13 (52%) were confirmed to have developed tMDS/tAML after treatment with tositumomab and iodine I131 tositumomab (ie, the independent review of the peripheral blood and marrow prior to treatment with tositumomab and iodine I131 tositumomab had no evidence of MDS, and the hematologic specimens following RIT had confirmed documentation of tMDS/tAML). Thus, on the basis of the masked independent review, 23 of 985 patients were confirmed to have developed tMDS/tAML after tositumomab and iodine I131 tositumomab. This provided an overall crude incidence rate of 2.3%/y (95% CI, 1.5%/y-3.5%/y) and an annualized incidence of 1.1%/y (95% CI, 0.7%/y-1.6%/y) (Table 3).
Results from study RIT-II-003 are useful in interpreting the possible risk of tMDS/tAML following RIT. Study RIT-II-003 was a phase 2, single-arm, open-label study of tositumomab and iodine I131 tositumomab for patients with previously untreated, advanced-stage (stage III/IV) follicular LG-NHL. With a median follow-up of 4.6 years after RIT (range, 0.7 to 6.7 years), none of the 76 patients in this study has developed tMDS/tAML. The upper bound of the 95% CI for this observation is 0.86%/y. The incidence of tMDS/tAML for patients without prior therapy was significantly less (P = .011) than that for the patients who had received prior cytotoxic therapies.
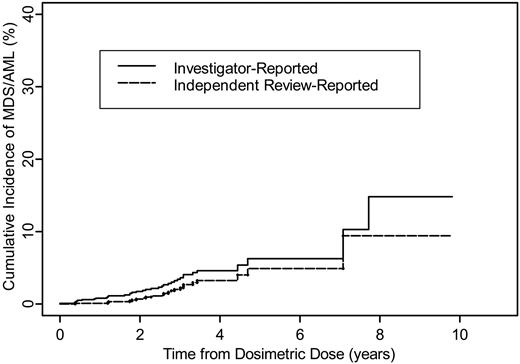
The cumulative incidence of investigator-reported tMDS/tAML after tositumomab and iodine I131 tositumomab is provided in Figure 1 for the 995 patients with relapsed/refractory NHL. The 2-year and 5-year cumulative incidences were 1.7% and 6.3%, respectively. The cumulative incidence for those patients confirmed by the independent reviewer is also provided in Figure 1 for the 985 evaluable patients with relapsed/refractory NHL. The 2-year and 5-year cumulative incidences were 0.8% and 5.0%, respectively.
Table 4 compares the demographics for the 35 patients who developed tMDS/tAML with the 960 who did not. Most of the parameters were comparable. Patients who developed tMDS/tAML tended to have lower baseline platelet and neutrophil counts and a larger number were treated with fludarabine-containing combinations. The median time to tMDS/tAML was 5.6 years (range, 3.1 to 25.4 years) from initial therapy and 2.1 years (range, 0.1 to 7.7 years) from RIT.
Demographics for patients with and without investigator-reported tMDS/tAML
Demographic characteristic . | Investigator-reported tMDS/tAML . | No investigator-reported tMDS/tAML . |
|---|---|---|
| No. patients | 35 | 960 |
| Sex | ||
| Male, no. (%) | 21 (60) | 565 (59) |
| Female, no. (%) | 14 (40) | 395 (41) |
| Median age, y (range) | 59 (41-81) | 58 (21-88) |
| Median time from diagnosis to protocol entry, mo (range) | 43 (8-214) | 45 (2-334) |
| Grade at protocol entry, no. (%) | ||
| Low grade | 26 (74) | 740 (77) |
| Transformed low grade | 8 (23) | 210 (22) |
| Intermediate/high grade | 1 (3) | 10 (1) |
| Ann Arbor stage III/IV at enrollment, no. (%) | 31 (89) | 852 (89) |
| Bone marrow involvement, no. (%) | 15/34 (44) | 399/956 (42) |
| Elevated LDH, no. (%) | 4/20 (20) | 86/204 (42) |
| Decreased platelets,* no. (%) | 17 (49) | 228 (24) |
| Decreased ANC,† no. (%) | 16 (46) | 285 (30) |
| Median number of prior therapies (range) | 3 (1-13) | 3 (1-13) |
| Patients with 4 or more prior therapies, no. (%) | 16 (46) | 335 (35) |
| Previously received rituximab, no. (%) | 10 (29) | 460 (48) |
| Previously received fludarabine, no. (%) | 13/20 (65) | 90/210 (43) |
| Previously received anthracycline or anthracenedione, no. (%) | 18/20 (90) | 167/210 (80) |
| Previously received alkylator, no. (%) | 19/20 (95) | 208/210 (99) |
| Previously received radiotherapy, no. (%) | 11 (31) | 233 (24) |
Demographic characteristic . | Investigator-reported tMDS/tAML . | No investigator-reported tMDS/tAML . |
|---|---|---|
| No. patients | 35 | 960 |
| Sex | ||
| Male, no. (%) | 21 (60) | 565 (59) |
| Female, no. (%) | 14 (40) | 395 (41) |
| Median age, y (range) | 59 (41-81) | 58 (21-88) |
| Median time from diagnosis to protocol entry, mo (range) | 43 (8-214) | 45 (2-334) |
| Grade at protocol entry, no. (%) | ||
| Low grade | 26 (74) | 740 (77) |
| Transformed low grade | 8 (23) | 210 (22) |
| Intermediate/high grade | 1 (3) | 10 (1) |
| Ann Arbor stage III/IV at enrollment, no. (%) | 31 (89) | 852 (89) |
| Bone marrow involvement, no. (%) | 15/34 (44) | 399/956 (42) |
| Elevated LDH, no. (%) | 4/20 (20) | 86/204 (42) |
| Decreased platelets,* no. (%) | 17 (49) | 228 (24) |
| Decreased ANC,† no. (%) | 16 (46) | 285 (30) |
| Median number of prior therapies (range) | 3 (1-13) | 3 (1-13) |
| Patients with 4 or more prior therapies, no. (%) | 16 (46) | 335 (35) |
| Previously received rituximab, no. (%) | 10 (29) | 460 (48) |
| Previously received fludarabine, no. (%) | 13/20 (65) | 90/210 (43) |
| Previously received anthracycline or anthracenedione, no. (%) | 18/20 (90) | 167/210 (80) |
| Previously received alkylator, no. (%) | 19/20 (95) | 208/210 (99) |
| Previously received radiotherapy, no. (%) | 11 (31) | 233 (24) |
The denominator is used when the number of patients is smaller than the total population.
Defined as having a platelet count < 150 × 109/L.
Defined as having an ANC < 3 × 109/L.
Cumulative incidence of tMDS/tAML following tositumomab and iodine I131 tositumomab for patients with relapsed/refractory NHL.
Cumulative incidence of tMDS/tAML following tositumomab and iodine I131 tositumomab for patients with relapsed/refractory NHL.
Patients who had received a fludarabine-containing regimen (relative risk, 3.08 [P = .011]) and patients with a platelet count less than 150 000/mm3 (relative risk, 2.93 [P = .004]) had significantly higher rates of tMDS/tAML in univariate and multivariate analyses. A reduced platelet count (ie, < 150 × 109/L) was associated with preexisting tMDS/tAML. Of the 10 cases with preexisting tMDS/tAML, 7 (70%) had a reduced platelet count. None of the disease-related demographics, such as bone marrow involvement with lymphoma, were prognostic for tMDS/tAML.
Almost all of the 35 patients were previously treated with repetitive cycles of mustard-type alkylating agents, nitrosoureas, procarbazine, inhibitors of topoisomerase II, and fludarabine. In addition, some of the same patients received external beam radiation to marrow-bearing areas.
Cytogenetic profiles were available for 26 of the 35 patients. All demonstrated chromosomal abnormalities. These included structural alterations or deletions of chromosomes 5 and/or 7 in 26.9% of cases and alterations in chromosomes 5 and/or 7 and other complex aberrations in an additional 38.5% of cases, for a total of 65.5%, and other deletions, rearrangements, or translocations in 34.6% of cases. Nonhematologic neoplasms were reported in 43 patients (51 different cancers in these 43 patients) or less than 5% of the integrated safety population. These included skin cancers (20); breast cancer (5); head and neck cancer (5); bladder cancer (4); lung cancer (3); colorectal cancer (3); and miscellaneous cancers (6). These chromosomal abnormalities are widely recognized as associated with alkylating agent-induced tMDS/tAML. Of the patients, 35% had complex and/or other chromosomal abnormalities.
Discussion
RIT with the anti-CD20 MAb tositumomab conjugated to 131-iodine enables the targeted delivery of radiation to B-cell lymphomas, offering a renewed opportunity for response in patients with chemotherapy- and rituximab-refractory LG- and transformed LG-NHL. Multiple studies in these heavily pretreated patients have yielded overall response rates of 47% to 68% and complete responses (CRs) in 20% to 33% of patients.7-10 Of clinical significance, this therapy has consistently resulted in durable responses in a sizable proportion of patients following a single therapy delivered over 7 to 14 days. Remissions extending out to 10 years have been reported.18,19 Given the systemic nature of the radiation, the overall assessment of safety from this RIT has included the continued follow-up of patients for the occurrence of tMDS/tAML to determine whether the RIT would add to the reported incidence of chemotherapy-related, and in some cases, stem cell transplant-related tMDS/tAML.
Therapy-related MDS/AML is a well-recognized delayed complication following cytotoxic drug and radiation therapy.27 Indeed, for curable malignancies such as Hodgkin disease and childhood malignancies, tMDS/tAML has been the price of therapeutic success. Similar instances of tMDS/tAML have been noted in patients with non-Hodgkin lymphomas20 and other neoplastic28-31 and nonneoplastic diseases.32-34 The common denominator for these complications has been the use of alkylating agents and/or inhibitors of topoisomerase II. The antimetabolite, fludarabine, has also been implicated as an etiologic agent, especially when combined with an alkylating agent.35 Reports have related the cumulative dose of alkylating agents and their use in combination with more aggressive radiation regimens such as extended field, hemibody, and total body radiation.36 Restriction of the radiation ports to localized areas of lymphoma does not appear to add to the risk associated with the drugs alone.37-39
The common link in the occurrence of tMDS/tAML irrespective of the primary disease is clonal chromosomal aberrations somewhat unique to the therapy. Rowley et al40 noted that more than 90% of patients developing tMDS/tAML subsequent to treatment for lymphoma had a chromosomally abnormal clone, with the most common abnormalities being loss of either part or all of chromosomes 5 and/or 7.41,42 Similar cytogenetic observations have been noted for the patients in the present series.
The high-energy (1.71 MeV) beta radiation emitted by radioactive phosphorous (32P) has been associated with an increased occurrence of tAML. In a randomized trial conducted by the Polycythemia Rubra Vera Study Group, within 6 years after treatment, tAML developed in 1% of patients treated with phlebotomy alone, in 6% of patients treated with 32P32, and in 11% of patients treated with chlorambucil.43 The lower-energy (0.606 MeV) beta emitter, 131-iodine, has been in clinical use for more than 50 years for the treatment of benign and malignant diseases of the thyroid. With long-term follow-up, there has been no consistent pattern of increased cancer risk. In a report of a 25-year follow-up of 47 712 patients administered therapeutic or diagnostic doses of 131-iodine, there has been no increased incidence of acute leukemia.44 In most instances, the treatment of thyroid disease uses doses of 100 to 150 mCi (37 to 55 × 108 Bq) 131-iodine, similar to the median doses delivered from tositumomab and iodine I131 tositumomab (median, 85 mCi [31.45 × 108 Bq]; range, 40 to 239 mCi [14.8 to 88.43 × 108 Bq]). However, rare instances of acute leukemia have been reported after cumulative doses of more than 800 mCi (296 × 108 Bq) 131-iodine delivered over relatively short periods of time.45
In patients with NHL, the time to occurrence of tMDS/tAML after the initiation of therapy is typically a median of 6 years (range, 2 to 10 years).36,42,46-48 The long-term and repetitive treatment with alkylating agents as occurs following the multiple relapses and remissions during the natural history of LG-NHL results in cumulative chromosomal damage to marrow stem cells and a consequent dose-dependent increase in the risk of tMDS/tAML.36,48-50 The annualized risk of tMDS/tAML after the long-term use of alkylating agents ± external beam radiation therapy has been estimated to be 1%/y to 1.5%/y from 2 to 10 years after the start of therapy.20,46 It is expected that up to 10% of the patients with NHL treated with either conventional or high-dose therapy would develop tMDS/tAML within 10 years of initiation of therapy.20
The present series included 995 patients with LG- and transformed LG-NHL who had been treated with a median of 3 prior therapies (range, 1 to 13 therapies) prior to RIT. This series also included 76 patients who received RIT as their initial therapy for follicular NHL. These 2 patient populations enable a reasonable estimate of the relative contribution of the systemic radiation as delivered by RIT to the incidence of tMDS/tAML over and above that associated with the multiple regimens of cytotoxic therapy typically offered to patients with LG-NHL. For the previously treated patients, the median follow-up from the diagnosis of NHL and RIT was 6 years and 2 years, respectively; for the patients who received RIT as their initial therapy, the corresponding median follow-up times were 5.6 years and 4.6 years, respectively.
Of the 995 previously treated patients, investigators reported 35 cases of tMDS/tAML. Of these cases, blinded independent review of peripheral blood and bone marrow specimens were available for 25 patients; 52% were confirmed to have occurred after the RIT treatment. The confirmed cases represent a crude incidence of 2.3% and an annualized incidence of 1.1%/y (95% CI, 0.7%-1.6%/y). The cumulative incidence at 2 and 5 years is 0.8% and 5.0%, respectively. These statistics for this heavily pretreated patient population compare favorably with the reported rates following multiple regimens used in the treatment of LG-NHL.20
However, because of the retrospective nature of the study design that included 6 studies of previously treated patients and one untreated population, it is not possible to determine the extent to which iodine I131 tositumomab may have contributed to the incidence of tMDS/tAML. Moreover the EAP study for relapsed and/or refractory patients accrued 765 patients, by far the largest number of any of the programs. Although the annualized incidence was lower than the other treated groups, the confidence interval was broad. The requirements for reporting of serious adverse events was identical for all 7 studies, with all sites queried every 6 months to year 5 and then yearly thereafter with a specific question regarding the development of tMDS/tAML.
In LG-NHL, as in Hodgkin disease, the search continues for effective therapies with diminished delayed toxicity. RIT with tositumomab and iodine I131 tositumomab appears to provide durable responses in a significant proportion of patients with relapsed/refractory LG-NHL. Therefore, this regimen offers renewed hope for durable response in these patients, and a highly effective therapy for patients with newly diagnosed disease. The present series includes patients who received RIT as a single course of tositumomab and iodine I131 tositumomab delivered over 7 to 14 days as their initial and only therapy for follicular NHL. In addition to the high overall and CR rates, 95% and 75%, respectively, and the relatively long disease-free survival (DFS) extending to 6.7 years, there have been no reported cases of tMDS/tAML.19 These results are encouraging. While longer follow-up is needed, these results suggest that RIT will, with acceptable toxicity, take its place among the more effective therapies for LG-NHL.
Prepublished online as Blood First Edition Paper, February 24, 2005; DOI 10.1182/blood-2004-12-4690.
One of the authors (S.M.K.) is employed by Corixa Corporation and has a financial interest in the company, whose product was studied in the present work. In addition, the following authors have received consultant fees in conjunction with the conduct of the studies: J.M.B., M.S.K., A.D.Z., and R.L.C.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Ms Phoebe Downing for administrative support and to Dr Richard Schwartz for preparing the clinical cases summaries.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal