Abstract
Plasmacytoid dendritic cells (pDCs) competent to make type I interferon were rigorously defined as a Ly-6C+ and CD11cLo subset of the B220+CD19- CD43+CD24Lo bone marrow (BM) Fraction A. Otherwise similar Ly6C- cells expressed the natural killer (NK) markers DX5 and NK1.1. pDCs represented a stable, discrete, and long-lived population. Stem cells and early lymphoid progenitors (ELPs), but not prolymphocytes, were effective precursors of pDCs, and their differentiation was blocked by ligation of Notch receptors. Furthermore, pDCs were present in the BM of RAG1-/-, CD127/IL-7Ra-/-, and Pax5-/- mice. pDCs in RAG1/GFP knock-in mice could be subdivided, and immunoglobulin DH-JH rearrangements, as well as transcripts for the B-lineage–related genes Pax5, mb1/CD79a, ebf, and Bcl11a, were identified only in the green fluorescent protein–positive (GFP+) pDC1 subset. All pDCs expressed terminal deoxynucleotidyl transferase (TdT), the ETS transcription factor Spi-B, the nuclear factor-κB transcription factor RelB, toll-like receptor 9 (TLR9), and interferon consensus sequence binding protein (ICSBP)/interferon regulatory factor 8 (IRF-8) transcripts; lacked CD16 and granulocyte colony-stimulating factor receptor (G-CSFR); and were uniformly interleukin-7 receptor α (IL-7Rα-) AA4.1Lo, CD27-, Flk-2Lo, c-Kit-, DX-5-, and CD11b-, while CD4 and CD8α were variable. GFP+ pDC1 subset was less potent than GFP- pDC2s in T allostimulation and production of tumor necrosis factor α (TNFα), interferon α (IFNα), and interleukin-6 (IL-6), while only pDC2s made IFNγ and IL-12 p70. Thus, 2 functionally specialized subsets of pDCs arise in bone marrow from progenitors that diverge from B, T, and NK lineages at an early stage.
Introduction
Plasmacytoid dendritic cells (pDCs) are believed to play central roles in defense of viral infection and maintenance of T-cell tolerance. They represent a principal source of type I interferon and can produce inflammatory cytokines such as interleukin-12 (IL-12) p70, IL-6, and tumor necrosis factor α (TNFα).1,2 Furthermore, pDCs have been implicated in the pathogenesis of lupus in humans.3-5 While information is rapidly accumulating about pDCs, important questions remain about their origin, heterogeneity, and lifespan. The focus of our study was on pDCs that reside within bone marrow (BM).
CD11c-CD123HiHLA class IIHiBDCA (blood dendritic cell antigen)+ pDCs were originally defined in human blood and distinguished from conventional CD11c+CD123- dendritic cells (DCs).6-9 The murine counterparts of human pDCs express CD11c and CD45R/B220, but not CD19,10,11 while murine DCs as a whole can be divided into CD8- and CD8+ subpopulations.12 Although distinct from classical murine CD8+ DCs, pDCs express variable levels of CD8.1,13,14 They were formerly defined as CD11cLoB220+Gr1+ in spleen and as CD11c+B220+CD11b- cells when derived from BM cultured with FMS-like tyrosine kinase 3 ligand (Flt3-L).15 Expression of Ly6G/Gr1 on pDCs is controversial. A recent study demonstrated that Ly-6G/C staining is not due to Ly-6G expression on pDCs, but rather to a cross-reaction of anti-Gr1 Ab with the Ly-6C antigen (Ag), which is highly expressed on pDCs and defines their phenotype.1
It is still unclear if pDCs represent a homogenous category of cells and exactly how they arise. pDCs residing in peripheral lymphoid tissues are normally long lived, but their numbers expand when stimulated with Flt3-L.16,17 Some studies of human pDCs suggest a relationship to lymphoid cells: they express transcripts for pTα and differentiate in culture in response to IL-3, but not granulocyte-macrophage colony-stimulating factor (GM-CSF)6,18 ; and transfection of CD34+CD38- fetal liver precursors with inhibitor of differentiation 2 (Id2) or Id3 blocked their differentiation into T cells, B cells, and pDCs, but not natural killer (NK) cells or myeloid cells.19 In addition, the spleen focus-forming virus integration B (Spi-B) transcription factor is expressed by human pDCs and lymphoid cells, but not by myeloid-derived cells.20 In mice, immunoglobulin D-J gene rearrangements have been detected in pDCs and CD8+ lymphoid DCs in the thymus, but not in classical CD8- DCs.21 While these findings suggest a lymphoid origin of pDCs, this has not been shown directly. Moreover, both DC and pDC precursor activities reside in the Flt3+ fraction of common lymphoid and common myeloid progenitor fractions of BM.22
It remains to be seen if there is a single, obligate pathway for production of pDCs or if pDC progenitors have multiple differentiation options that are exercised depending on environmental cues. We now report that there are 2 stable subsets of functionally competent pDCs in bone marrow. Experiments with RAG1/GFP knock-in and gene-targeted mice suggest that progenitors for the 2 types of pDCs diverge at an early stage of differentiation.
Materials and methods
Mice
Male BALB/c, C57/BL6, 129/J, and CD127/IL-7Ra-/- mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained in the Oklahoma Medical Research Foundation Laboratory Animal Resource Center (OMRF LARC). RAG1/GFP knock-in mice have been described.23,24 Heterozygous F1 recombination activating gene 1 (RAG1)/green fluorescent protein (GFP) mice were generated at the OMRF LARC. Pax5-/- mice were generated and maintained at the Animal Facility of the Research Institute of Molecular Pathology (RIMP) in Vienna, Austria.
Cell sorting and flow cytometry
Plasmacytoid dendritic cells. BM cells from BALB/c mice were treated with Fc-receptor (FcR) block (2.4G2). After staining with allophycocyanin (APC)–anti–CD45 receptor (CD45R)/B220 (RA3/6B2), fluorescein isothiocyanate (FITC)–anti-Ly6C (6C3), phycoerythrin (PE)–anti-CD11c (HL3), and biotin–anti-CD19 (1D3), pDCs were sorted as CD19-B220+-CD11cLoLy6C+ on a MoFlo (DakoCytomation, Fort Collins, CO). For phenotyping, sorted cells were stained with the following: c-kit (2B8), CD34 (RAM34), CD27 (LG.3A10), Sca-1 (D7), interleukin-7 receptor α (IL-7Rα, SB/199), IL-3Rα (5B11), IL-2Rα (7D4), IL-2Rβ (TM-β1), Flk-2/Flt3 (A2F10.1), CD3ϵ (145-2C11), CD4 (RM4-5), CD8α (53-6.7), CD11b (M1/70), Pan-NK (DX-5), CD2 (leukocyte function antigen 2 [LFA-2]), early B-lineage C1qRp (AA4.1), BP-1 (6C3), Igβ (HM79b), CD24a (M1/69), CD43 (S7), I-A/I-E (2G9), CD40 (3/23), CD44 (IM7), CD31 (platelet-endothelial cell adhesion molecule 1 [PECAM-1], 390), or CD62 ligand (CD62-L, MEL-14) monoclonal antibodies (mAbs; BD Pharmingen, San Jose, CA). Biotinylated antibodies were revealed using R613-conjugated streptavidin. For pDC sorting from RAG1/GFP mice, magnetic beads were used to deplete CD19+ and Ter119+ cells, and an uncompensated 2-parameter procedure25 was used to discriminate and sort GFP+ and GFP- subsets of the CD19-B220+ population. After biotin-Ly6C (AL-21) plus PE-CD11c staining, the double-positive populations were resorted. Flow cytometry was performed on a FACSCalibur (BD Pharmingen), using the Cell Quest software (BD Pharmingen).
Fraction A. BALB/c BM was stained with Ab anti-CD45R/B220, -CD43, and -CD19 after FcR blocking. CD45R/B220+CD43+CD19- cells were sorted and stained with anti-CD24 before sorting again as B220+CD43+CD19-CD24-/Lo.
Precursor populations. BM cells from RAG1/GFP knock-in mice were enriched by negative selection using mAbs anti-Gr1 (RB6-8C5), anti-CD11b/Mac-1, anti-CD19, anti-CD45R/B220, and anti-Ter119, followed by the BioMag goat anti–rat IgG system (Qiagen, Valencia, CA). After staining with biotin-antilineage markers (Gr-1, Mac-1, CD19, CD45R/B220, Ter119, CD3ϵ, CD8α, and pan-NK), lineage- GFP+/- populations were sorted and stained with APC–anti–c-kit, PE–anti-Sca1, FITC–anti-CD34, and biotin-Sav-R613–anti-FcRγ. The hematopoietic stem cell (HSC)–enriched fraction was obtained as Lin-ckitHiSca1+GFP-; the myeloid progenitors, as Lin-ckit+Sca1-GFP-CD34+FcγRLo; the early lymphoid progenitors (ELPs), as Lin-ckitHiSca-1+GFP+; and prolymphocytes (Pro-Ls), as Lin-ckitLoSca-1LoGFP+.
BrdU treatment of mice and cell-cycle analyses
Mice were given an initial intraperitoneal injection of bromodeoxyuridine (BrdU; 100 μg/100 μL PBS [phosphate-buffered saline]) at zero time, and BrdU was administered continuously in drinking water for up to 7 days. The BM pDC-enriched population CD19-B220+CD11cLo was sorted, followed by intracellular staining with mAb to BrdU and 7-amino-actinomycin D (7AAD) (BrdU flow kit; BD Pharmingen).
pDC stimulation and cytokine production
Freshly isolated pDCs were cultured for 12 or 18 hours in RPMI-1640 with phosphorothiolated cytosine-phosphate-guanosine A (CpG A)–containing oligonucleotide (CpG-ODN) 1826 (TCCTTCCATGACGTTCCTGACGTTTCCT, 1 μM; Qiagen). Costimulatory molecules were stained with anti-H2Db (KH95), –I-Ad/I-Ed (2G9), -CD80 (16-10A1), -CD86 (GL1), and -CD40 (3/23) antibodies, while supernatants were assayed using enzyme-linked immunosorbent assay (ELISA) kits for interferon α (IFNα; PBL, Piscataway, NJ), IL-6 (eBioscience, San Diego, CA), IFNγ, TNFα, and IL-12p70 (R & D Systems, Minneapolis, MN).
Cell morphology
Cytospins of freshly isolated or 12-hour CpG-stimulated pDCs were stained with Giemsa-May-Grünwald (Sigma Diagnostics, St. Louis, MO), embedded in Immersol (Zeiss, Thornwood, NY), and analyzed on a Zeiss Axioplan 2i microscope with a 100×/1.4 NA Plan Achromat objective, using an AxioCam MRc color camera (Zeiss), and AxioVision 3.1 software (Zeiss).
Reverse-transcriptase–polymerase chain reaction (RT-PCR) analysis of gene expression
mRNAs were isolated from sorted cells using MicroPoly (A) pure (Ambion, Austin, TX) and converted to cDNA with Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen, Carlsbad, CA). The PCR was conducted using ampli-Taq DNA polymerase (TaKaRa, Shiga, Japan) and TaqStart antibody (Clontech, Palo Alto, CA). Anti-Taq Ab was inactivated by heating at 95°C for 7 minutes prior to amplification of 30 seconds at 94°C, 30 seconds at 56°C, and 45 seconds at 72°C. Gene-specific primers were as follows: PU.1: forward, 5′CGGATGACTTGGTTACTTACG and reverse, 5′GTAGGAAACCTGGTGACTGAG; Ikaros: forward, 5′AGCATAAAGAGCGATGCCACAAC and reverse, 5′ATGACACAGACTTGGCCTTGGAC; E47: forward, 5′CGCACTGACCACGAGCTTCAC and reverse, 5′TCCAGGGACAGCACCTCATCTG; B29: forward, 5′GGTGAGCCGGTACCAGCAATG and reverse, 5′AGTTCCGTGCCACAGCTGTCG; GATA-3: forward, 5′GGCCAGGCAAGATGAGAAAGA and reverse, 5′TCTGACAGTTCGCGCAGGATG; Notch 1: forward, 5′CGGTGTGAGGGTGATGTCAATG and reverse, 5′GAATGTCCGGGCCAGCGCCACC; terminal deoxynucleotidyl transferase (TdT): forward, 5′GTCTGAATTCCTCCAGAGCCTAAG and reverse, 5′CAGGTCTAGAGCCATGGCCTGGAC; RAG1: forward, 5′TGCAGACATTCTAGCACTCTGG and reverse, 5′ACATCTGCCTTCACGTCGAT; Bcl11a: forward, 5′CCATGACGGCTCTCCCACAAT and reverse, 5′GCGAGAATTCCCGTTTGCTT; ebf (early B cell factor): forward, 5′GAGATTTTTCCACAAGAAAAGGTTG and reverse, 5′GGAAGAACCTGTCAATTATCACTGG; Mb1: forward, 5′GCCAGGGGGTCTAGAAGC and reverse, 5′TCACTTGGCACCCAGTACAA; Pax5: forward, 5′CTACAGGCTCCGTGACGCAG and reverse, 5′GTCTCGGCCTGTGACAATAGG; FcγRIII: forward, 5′ATGTTTCAGAATGCACACTCT, and reverse, 5′CAGAAATCACTCCCAGATCTA; macrophage colony-stimulating factor receptor (M-CSFR): forward, 5′GAGCATCTTTGACTGCGTCTAC and reverse, 5′TTAGCATAGTCCTGGTCTCTCCTC; granulocyte colony-stimulating factor receptor (G-CSFR): forward, 5′CTCAAACCTATCCTGCCTCATG and reverse, 5′TCCAGGCAGAGATGAGCGAATG; interferon consensus sequence binding protein (ICSBP): forward, 5′ATGTGTGACCGGAACGGCGG and reverse, 5′CAGAAGGTTCCTTGATCAGC; RelB: forward, 5′CTTTGCCTATGATCCTTCTG and reverse, 5′ATTCTGTAAAATGGGCTCAA; Spi-B: forward, 5′CCCAGAAGGAGTCTTCTACGACCTG and reverse, 5′ATAAGCCAAGGAGCCCAGGGTCTG; toll-like receptor 9 (TLR9): forward, 5′CCGCAAGACTCTATTTGT and reverse, 5′TGTCCCTAGTCAGGGCTG; TLR4: forward, 5′AGTGGGTCAAGGAACAGAAGCA and reverse, 5′CTTTACCAGCTCATTTCTCACC; and β-actin: forward, 5′CCTAAGGCCAACCGTGAAAAG and reverse, 5′TCTTCATGGTGCTAGGAGCCA.
Ig gene rearrangement assay
Genomic DNA was prepared from sorted GFP+ and GFP- pDCs and used for PCR reaction as described.26 The primers were α-actin,27 DHL(5′), and J3(3′) to detect DH-JH rearrangement28 ; Q52(5′)29 and J3(3′) to detect VH-DJH rearrangements; and Mu0(5′) and J1(3′) to detect germ-line alleles.28
B220+CD19-Ly-6C+CD11cLo cells in bone marrow are functional pDCs. (A, left) Whole bone marrow (wBM) from BALB/c mice was stained for CD45R/B220, Ly-6C, CD19, and CD11c. The B220+CD19- population, gated with an open box, was further resolved and sorted according to CD11c and Ly-6C expression (middle). (Right) Purity of sorted cells. (B) The sorted B220+CD19-Ly-6C+CD11cLo cells were stimulated with CpG-ODNs for 12 hours, and their morphology was assessed by May-Grünwald-Giemsa staining. (Top) Unstimulated cells; (bottom) stimulated cells. (C) Culture supernatants were analyzed for cytokine levels by ELISA after stimulation with CpG-ODNs (▪) or not (▦). These results are representative of those obtained in 4 independent experiments.
B220+CD19-Ly-6C+CD11cLo cells in bone marrow are functional pDCs. (A, left) Whole bone marrow (wBM) from BALB/c mice was stained for CD45R/B220, Ly-6C, CD19, and CD11c. The B220+CD19- population, gated with an open box, was further resolved and sorted according to CD11c and Ly-6C expression (middle). (Right) Purity of sorted cells. (B) The sorted B220+CD19-Ly-6C+CD11cLo cells were stimulated with CpG-ODNs for 12 hours, and their morphology was assessed by May-Grünwald-Giemsa staining. (Top) Unstimulated cells; (bottom) stimulated cells. (C) Culture supernatants were analyzed for cytokine levels by ELISA after stimulation with CpG-ODNs (▪) or not (▦). These results are representative of those obtained in 4 independent experiments.
Stromal-cell cocultures
Sorted cells were cocultured for 8 days with OP-9 and/or MS-5 stromal cells. Delta-like-1 and GFP retrovirally transduced OP9 stromal cells (OP9-DL1 and OP9-GFP, respectively) were kindly provided by Dr J. C. Zúñiga-Pflücker, University of Toronto, ON. The α–modified essential medium (α-MEM) 20% (for OP-9) or 10% (for MS-5 and transduced cells) fetal calf serum (FCS) contained 100 ng/mL Flt3-L, 2 mM l-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin.
T-cell stimulation
Allogeneic splenic T cells (1 × 105) were cocultured for 6 days with CpG-preactivated pDC1s or pDC2s, or with GM-CSF–preactivated DCs. BrdU 10 μM was added continuously during culture, and T proliferation was measured by intracellular staining with mAb to BrdU of T-cell receptor β–positive (TCRβ+) cells. To test their capacity to produce cytokines, T cells were restimulated with 50 ng/mL phorbol myristate acetate (PMA) plus 500 ng/mL ionomycin. After one hour, 10 μg/mL Brefeldin A (eBioscience) was added and the cells were cultured for an additional 4 hours. Harvested cells were stained with anti-TCRβ Ab, followed by intracellular staining with PE–anti-IFNγ and PE–anti–IL-4 Ab (eBioscience).
The pDCs in bone marrow are homogeneous with respect to most lympho-hematopoietic–cell antigens and represent a conspicuous subset of Fraction A. (A) Purified B220+CD19-Ly-6C+CD11cLo BM pDCs were labeled with the indicated cell surface markers and analyzed by flow cytometry. Dashed lines indicate isotype controls. (B) BALB/c wBM was stained for CD45R/B220, CD43, and CD19. CD45R/B220+CD43+CD19- cells were gated and sorted as indicated with boxes (i). The recovered cells (middle panel) were then stained with CD24 and resorted as B220+CD43+CD19-CD24-/Lo (Fraction A; ii). Sorted Fraction A cells were then stained and analyzed with respect to CD11c and Ly6C to discriminate B220+CD19-Ly-6C+CD11cLo pDCs among B220+CD43+CD19-CD24-/Lo Fraction A cells. (iii) Total numbers of pDCs (▪) and total Fraction A cells (▦) recovered from 4 harvested bones from 3 strains of mice are shown in the bar graph. Results are presented as the mean ± SD of 5 different experiments.
The pDCs in bone marrow are homogeneous with respect to most lympho-hematopoietic–cell antigens and represent a conspicuous subset of Fraction A. (A) Purified B220+CD19-Ly-6C+CD11cLo BM pDCs were labeled with the indicated cell surface markers and analyzed by flow cytometry. Dashed lines indicate isotype controls. (B) BALB/c wBM was stained for CD45R/B220, CD43, and CD19. CD45R/B220+CD43+CD19- cells were gated and sorted as indicated with boxes (i). The recovered cells (middle panel) were then stained with CD24 and resorted as B220+CD43+CD19-CD24-/Lo (Fraction A; ii). Sorted Fraction A cells were then stained and analyzed with respect to CD11c and Ly6C to discriminate B220+CD19-Ly-6C+CD11cLo pDCs among B220+CD43+CD19-CD24-/Lo Fraction A cells. (iii) Total numbers of pDCs (▪) and total Fraction A cells (▦) recovered from 4 harvested bones from 3 strains of mice are shown in the bar graph. Results are presented as the mean ± SD of 5 different experiments.
Results
Identification of functional pDCs in murine bone marrow
While pDCs have been intensively studied, most of the information pertains to ones present in peripheral tissues.1,13,14,16 Therefore, we began our study with an extensive analysis of pDCs resident in BM. The CD45R/B220+CD19-CD11cLo subset could be further resolved according to Ly-6C (Figure 1A). We found that the Ly-6C+ subset responded to CpG-ODNs and differentiated to TNFα–, IL-12–, IL-6–, and IFNα-producing pDCs (Figure 1B-C). The nature of the Ly-6C- population is unclear because it did not produce type I interferon efficiently, nor did it give rise to Ly-6C+ pDCs when placed in culture (“Developmental origins of pDCs”). Since they express NK1.1 and DX-5, we assume that they are related to the NK lineage (Supplemental Figure 1; see the Supplemental Figures link at the top of the online article on the Blood website). Thus, CD11c may not represent a defining characteristic of dendritic lineages, and we will refer to CD45R/B220+CD19-CD11cLoLy-6C+ cells throughout this paper as functional pDCs.
In an attempt to find evidence for maturational stages and relationships to other hematopoietic lineages, pDCs were sorted from BALB/c marrow to high purity and screened with antibodies (Figure 2A). The pDCs were surprisingly homogeneous and lacked markers associated with early progenitors; that is, they were c-kit-CD34-/LoCD27-Sca-1Lo/-. Cytokine receptors were very low to absent (Flk-2LoIL-7Rα-IL-3R-IL-2Rα-IL-2Rβ-), and the same was true for several lympho-myeloid lineage markers (CD11b/Mac-1-DX5-CD2-CD3-BP-1-Igβ-). Approximately 40% of pDCs expressed CD4, and most variably displayed CD8α. Interestingly, pDCs became uniformly CD8α+ after stimulation with CpG-ODNs (R.P., unpublished data, March 2004). Several cell interaction/adhesion molecules were present (CD44HiCD31+CD40LoI-A/I-ELo).
The pDC population was homogeneous with respect to CD43 and BP-1 (CD43+BP-1-) and displayed low levels of CD24 (Figure 2A). This suggested that pDCs might reside within the CD45R/B220+CD19-CD24-/LoCD43+ Fraction A subset of BM described by Hardy et al.27 They obtained additional resolution with the AA4.1 antibody,30,31 and we found that it weakly recognized pDCs (Figure 2A). Furthermore, we previously reported that many cells in Fraction A express Ly-6C.32 We now show that CD45R+CD43+Ly-6C+CD19-CD11cLo pDCs comprise approximately one fourth of the total Fraction A category in BALB/c, C57BL/6, or 129/J mice (Figure 2B).
These observations suggest that resident marrow pDCs are highly differentiated and distinct from B220-CD11b+CD11c+ classical dendritic, CD11b/Mac-1+ myeloid, CD19+ or CD3+ lymphoid, or DX-5+ NK cells. Their characteristics do not evoke obvious relationships to other lympho-hematopoietic lineages.
Developmental origins of pDCs
Unstimulated pDCs in the spleen are long lived relative to classical DCs.16 It seemed possible that pDCs within BM would be rapidly replaced; however, DNA histograms for the pDC-enriched CD45R/B220+CD19-CD11cLo subset showed a much lower mitotic index than for CD19+ B-lineage cells sorted from the same tissue (Figure 3A). For additional information about population dynamics, mice were injected and fed BrdU for various intervals before flow cytometry comparison of the same 2 cell types (Figure 3B). Labeling of pDCs was much slower than B lymphocytes, and only 25% of the population incorporated BrdU during 7 days of treatment. These findings suggest that most pDCs in BM are relatively long-lived, but it is possible that a small subset is rapidly replenished.
Preliminary experiments established that pDCs could be made in stromal cell–free, serum-free cultures containing the cytokine Flt3-L (or Flt3-L + stem cell factor [SCF] + IL-7 + IL-15), but yields of recovered cells were very low (usually 3 per input progenitor). Subsequent cultures used OP9 stromal cells, 20% FCS, and Flt3-L, and we routinely recovered substantial numbers of CD45R/B220+CD19-CD11cLoLy-6C+ pDCs under these conditions. While pDCs could also be produced on the nonosteopetrotic MS5 stromal cell line, the cultures contained large numbers of classical DCs (R.P., unpublished data, March 2004). A strain of heterozygous RAG1/GFP knock-in mice has recently been used to identify the earliest known lymphoid progenitors in adult BM and fetal liver.25,33 These Lin-c-KitHiSca-1+CD27+Flk-2+ ELPs have greatly reduced potential for generating nonlymphoid cells and likely give rise to Lin-c-KitLoSca-1+/-CD27+Flk-2+ Pro-Ls. Both of these subsets, as well as fractions enriched for stem cells (Lin-c-KitHiSca-1+RAG1-) or myeloid progenitors (Lin-c-KitHiSca-1+RAG1-CD34+FcγRLo), were tested for their potential to generate pDCs. Approximately 20 pDCs were produced per input stem cell within 8 days of culture, and the yield was almost as high when cultures were initiated with ELPs (Figure 4A, bottom). In contrast, neither myeloid progenitors nor prolymphocytes tested at the same time were efficient progenitors of pDCs.
The pDCs in bone marrow are slowly replenished. (A) Purified B220+CD19-CD11cLo pDCs were stained with 7AAD for analysis of DNA content. (B) Mice were injected and then fed BrdU in their drinking water for the indicated intervals when cells were isolated and evaluated by flow cytometry. Enriched B220+CD19-CD11cLo pDCs (□) were compared with B220+CD19+ B-lineage lymphocytes (○).
The pDCs in bone marrow are slowly replenished. (A) Purified B220+CD19-CD11cLo pDCs were stained with 7AAD for analysis of DNA content. (B) Mice were injected and then fed BrdU in their drinking water for the indicated intervals when cells were isolated and evaluated by flow cytometry. Enriched B220+CD19-CD11cLo pDCs (□) were compared with B220+CD19+ B-lineage lymphocytes (○).
Many recent studies have demonstrated that strong signals delivered to the Notch family of receptors suppress B lymphopoiesis and initiate T-cell formation.34-36 Therefore, it was of interest to learn if coculture on Delta-like-1–expressing OP9 stromal cells (OP9-DL1) would support pDC differentiation. Lin-c-KitHiSca-1+CD27+ ELPs were sorted from C57BL/6 mouse BM and placed on either OP9-GFP as control, or OP9-DL1 plus Flt3-L for 8 days. While approximately 15 pDCs were produced per input progenitor in the vector control cultures, essentially none (0.28 per input cell) recovered when the progenitors were exposed to the Delta-like-1 Notch ligand (Figure 4C). Similar experiments were done using primitive cells from BM of BALB/c or RAG1/GFP mice. It is perhaps noteworthy that some CD45R/B220-CD11cLoLy-6C- cells were recovered in these cultures, but their identity was not further investigated. Accordingly, progenitors of pDCs and B cells are negatively regulated by Notch receptor ligation, and differ in this respect from progenitors that support T lymphopoiesis.
These experiments defined stem/progenitor cells that can produce pDCs, but did not suggest a sequence of differentiation. In contrast to freshly isolated BM cells, discrete subsets were not resolved among cells recovered from culture and there was a continuous range of Ly-6C (compare Figure 1A with Figure 4A). It appeared possible that CD45R/B220+CD19-CD11c+Ly-6C- cells represent immature pDCs, which might acquire this marker and attain functional competence. Cultured cells were then resorted according to these parameters and returned to culture with CpG-ODNs. Only the Ly-6C+ subset produced high amounts of IFNα (R.P., unpublished data, March 2004). CD45R/B220+CD19-CD11cLoLy-6C- cells were then freshly harvested from bone marrow and placed in OP9 stromal cell cocultures. While this culture condition supports production of pDCs from ELPs and stem cells, none were generated from the CD45R/B220+CD19-CD11cLoLy-6C- cohort (Figure 4A). Furthermore, few cells in this subset expressed RAG1 either before or after culture (R.P., unpublished data, March 2004). In addition, the Ly6C- cohort displayed NK-lineage markers (Supplemental Figure 1). We conclude that stem cells and very primitive lymphoid progenitors in bone marrow can efficiently replenish pDCs. pDCs may also be produced from prolymphocytes and myeloid progenitors, but the yield is very low.
Developmental independence of pDCs on RAG1, RAG2, IL-7Rα, or Pax5
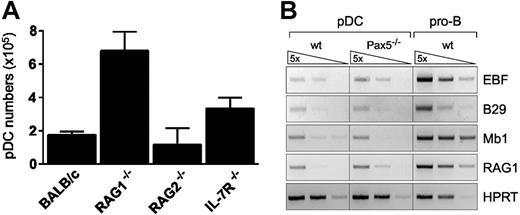
Additional information was obtained by examination of knock-out mice. Total numbers of nucleated cells were reduced in RAG1-/- or RAG2-/- BM relative to littermate controls, but the incidences of pDCs were within the normal range (Figure 5A). The IL-7 and thymic stromal lymphopoietin (TSLP) cytokines, which are known to use the IL-7Rα receptor component,37,38 appear to be dispensable for pDC formation, because pDC numbers were normal in CD127/IL-7Ra-/- mice (Figure 5A).
The progenitors of pDCs are within stem cell–containing fraction and early lymphoid progenitors, and are negatively regulated by Notch receptor ligation. (A) Lin-ckitHiSca1+GFP- (stem cell enriched), Lin-ckit+Sca1- GFP-CD34+FcγRLo (myeloid progenitors), Lin-ckitHi Sca-1+GFP+ (early lymphoid progenitors), Lin-ckitLo Sca-1LoGFP+ (prolymphocytes), and B220+CD19- CD11cLoLy6C- (Ly6C- cohort) were isolated from RAG1/GFP knock-in mice. Cells (10 000) of each kind were cultured for 8 days on OP9 stromal cells with Flt3-L. Cells were harvested and stained with CD45R/B220 and CD19. The B220+CD19- population was sorted and restained with CD11c and Ly-6C (i). The yield of CD11cLoLy-6C+ pDCs recovered per input precursor is shown in the bar graph (ii). CMP indicates common myeloid progenitors. (B) GFP expression was analyzed in B220+CD19-CD11cLoLy6C+ pDCs recovered from HSCs, ELPs, and Pro-L cultures. (C) Lin-ckitHi Sca-1+CD27+ ELPs (10 000) from C57BL/6 BM were cultured for 8 days on OP9-GFP or OP9-DL1 stromal cells. The yield of pDCs per input precursor is shown in the bar graph.
The progenitors of pDCs are within stem cell–containing fraction and early lymphoid progenitors, and are negatively regulated by Notch receptor ligation. (A) Lin-ckitHiSca1+GFP- (stem cell enriched), Lin-ckit+Sca1- GFP-CD34+FcγRLo (myeloid progenitors), Lin-ckitHi Sca-1+GFP+ (early lymphoid progenitors), Lin-ckitLo Sca-1LoGFP+ (prolymphocytes), and B220+CD19- CD11cLoLy6C- (Ly6C- cohort) were isolated from RAG1/GFP knock-in mice. Cells (10 000) of each kind were cultured for 8 days on OP9 stromal cells with Flt3-L. Cells were harvested and stained with CD45R/B220 and CD19. The B220+CD19- population was sorted and restained with CD11c and Ly-6C (i). The yield of CD11cLoLy-6C+ pDCs recovered per input precursor is shown in the bar graph (ii). CMP indicates common myeloid progenitors. (B) GFP expression was analyzed in B220+CD19-CD11cLoLy6C+ pDCs recovered from HSCs, ELPs, and Pro-L cultures. (C) Lin-ckitHi Sca-1+CD27+ ELPs (10 000) from C57BL/6 BM were cultured for 8 days on OP9-GFP or OP9-DL1 stromal cells. The yield of pDCs per input precursor is shown in the bar graph.
While E2A and EBF transcription factors are essential for lymphoid lineage specification,39-42 Pax5 induces B-cell commitment and controls subsequent events in B-lymphocyte formation.39-42 The finding of Pax5 transcripts in pDCs suggested that this factor might also be important for their formation. However, the incidences of pDCs were increased in BM of 12-day-old gene-targeted Pax5-/- mice (A.D., unpublished data, January 2004). Older mice could not be studied because disruption of the Pax5 gene results in early postnatal death. Nonetheless, transcripts for mb1/Igα and RAG1 were detectable in pDCs sorted from both knock-out and wild-type control littermates (Figure 5B). These findings strongly suggest that pDC progenitors diverge before and independent of RAG1-, RAG2-, IL-7R–, or Pax5-dependent events.
Two subsets of pDCs distinguished according to RAG1 expression
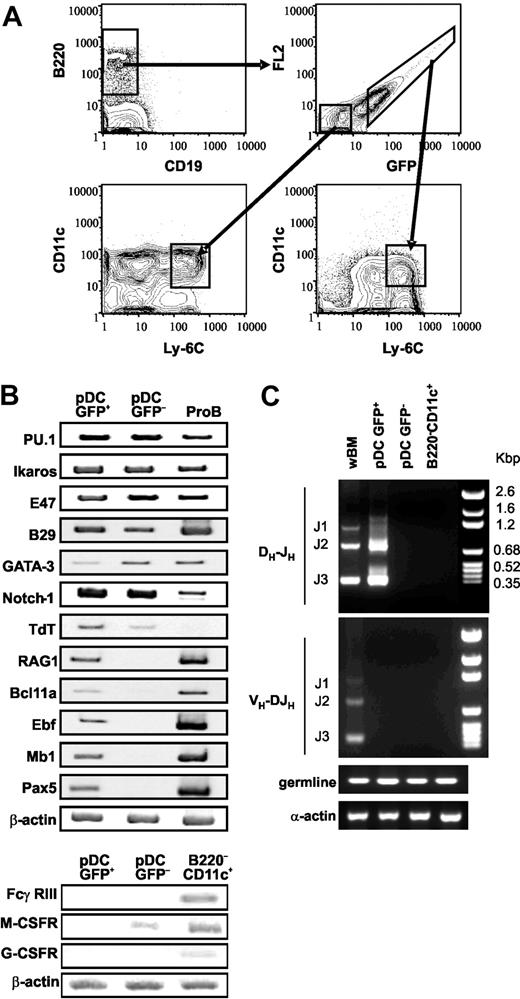
Cultures established with RAG1/GFP+ ELPs gave rise to GFPLo pDCs. In contrast, GFP+ and GFP- pDCs were produced from the stem cell–enriched fraction (Figure 4B). Persisting GFP protein need not correspond to active transcription of RAG1,23 but this finding might be informative with respect to precursor-product relationships. Therefore, we used a sensitive GFP gating procedure to sort CD45R/B220+CD19- cells directly from BM (Figure 6A). Both GFP+ and GFP- subsets contained conspicuous populations of CD45R/B220+CD19-CD11cLoLy-6C+ pDCs.
These 2 categories of pDCs were then sorted to high purity and evaluated by RT-PCR with respect to lymphoid-related genes (Figure 6B). Transcripts corresponding to early development were indistinguishable and both types of pDCs were PU.1+ Ikaros+ E47+ B29/Igβ+ GATA-3+ Notch1+ and TdT+. They both lacked transcripts for FcγRIII/CD16 and G-CSF receptors that would indicate relatedness to myeloid cells, but GFP- pDCs had trace amounts of mRNA corresponding to the M-CSFR (Figure 6B).
However, only the GFP+ subset of pDCs contained transcripts for RAG1 and 4 B-lymphoid lineage–related genes: Bcl11a, EBF, mb1/CD79, and Pax5. The GFP+ pDCs were distinguishable from pro-B cells only in terms of the strength of these signals. Genomic DNA from the same cells was then evaluated with respect to immunoglobulin gene rearrangement status (Figure 6C), and DH-JH, but not VH-DJH rearrangement products were exclusively present in the RAG1/GFP+ subset of pDCs. Like the GFP- pDCs, CD45R/B220-CD11c+ classical DCs lacked evidence of Ig gene rearrangement.
The finding of early lymphoid transcription factors and Ig rearrangement products in one pDC subset suggested that these might have differentiation potential. Therefore both types of pDCs were isolated and placed in a series of standard cultures. Myeloid and erythroid colonies were efficiently produced in growth factors (SCF, IL-3, IL-6, and erythropoietin [Epo]) containing methylcellulose cultures initiated with Lin-c-kit+ marrow cells, but none were produced from RAG1/GFP+ or RAG1/GFP- pDCs (Supplemental Figure 2A). The same was true when the 3 populations were placed in serum-free, stromal cell–free cultures containing recombinant factors (SCF, Flt3-L, IL-7, and IL-15) and able to support production of CD19+ B- or DX-5+ NK-lineage cells (Supplemental Figure 2B). Furthermore, no T-lineage differentiation potential was demonstrable when the 2 pDC populations were placed in fetal thymus organ cultures (Supplemental Figure 2C).
Developmental independence of pDCs on RAG1, RAG2, IL-7Rα, or Pax5. (A) pDC incidence in marrow of RAG1, RAG2, CD127/IL-7Ra, and Pax5 knock-out mice. Results are presented as the mean ± SD of 3 different experiments. (B) pDCs were sorted from BM of 12-day-old Pax5-/- and littermate control mice. RT-PCR analysis for expression of the indicated genes was performed with 5-fold serial dilutions of cDNA prepared from these cells. The cDNA input was normalized according to the expression of the control HPRT gene.
Developmental independence of pDCs on RAG1, RAG2, IL-7Rα, or Pax5. (A) pDC incidence in marrow of RAG1, RAG2, CD127/IL-7Ra, and Pax5 knock-out mice. Results are presented as the mean ± SD of 3 different experiments. (B) pDCs were sorted from BM of 12-day-old Pax5-/- and littermate control mice. RT-PCR analysis for expression of the indicated genes was performed with 5-fold serial dilutions of cDNA prepared from these cells. The cDNA input was normalized according to the expression of the control HPRT gene.
A category of pDCs residing in bone marrow expresses low levels of RAG1, as well as B-lineage–associated genes, and undergoes DH-JH rearrangements. (A) An uncompensated 2-parameter procedure was used to discriminate and sort GFP+ and GFP- subsets of CD45R/B220+CD19--enriched cells from BM of RAG1/GFP knock-in mice. The purified populations were then stained with Ly-6C and CD11c. The boxes in the bottom 2 histograms indicate gates used for an additional sorting of B220+CD19-Ly-6C+CD11cLoGFP+ and B220+CD19-Ly-6C+CD11cLoGFP- pDC subsets. (B) RT-PCR analyses of the indicated genes were performed with cDNA prepared from these 2 sorted subsets and compared with B220+CD19+ CD43+CD24+ pro-B/large pre-B cells or to B220-CD11c+ DCs, while β-actin was used as a loading control. (C) Genomic DNA from these same fractions was evaluated for Ig DHJH and VH-DJH rearrangements and germ-line loci by PCR. Specific products with the expected sizes are labeled with respect to rearrangement to J1, J2, or J3. Alpha actin was used as a control for genome representation.
A category of pDCs residing in bone marrow expresses low levels of RAG1, as well as B-lineage–associated genes, and undergoes DH-JH rearrangements. (A) An uncompensated 2-parameter procedure was used to discriminate and sort GFP+ and GFP- subsets of CD45R/B220+CD19--enriched cells from BM of RAG1/GFP knock-in mice. The purified populations were then stained with Ly-6C and CD11c. The boxes in the bottom 2 histograms indicate gates used for an additional sorting of B220+CD19-Ly-6C+CD11cLoGFP+ and B220+CD19-Ly-6C+CD11cLoGFP- pDC subsets. (B) RT-PCR analyses of the indicated genes were performed with cDNA prepared from these 2 sorted subsets and compared with B220+CD19+ CD43+CD24+ pro-B/large pre-B cells or to B220-CD11c+ DCs, while β-actin was used as a loading control. (C) Genomic DNA from these same fractions was evaluated for Ig DHJH and VH-DJH rearrangements and germ-line loci by PCR. Specific products with the expected sizes are labeled with respect to rearrangement to J1, J2, or J3. Alpha actin was used as a control for genome representation.
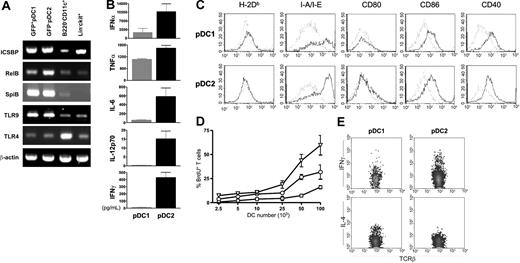
A number of functional parameters have been used to distinguish plasmacytoid and classical DCs obtained from peripheral tissues.12,43,44 We extended our analysis to marrow cells and sought properties that might be unique to one of the pDC subsets. The IFN consensus sequence binding protein (ICSBP) is essential for type I interferon–producing cells, and corresponding transcripts were high in both RAG1/GFP+ and RAG1/GFP- categories of marrow pDCs (Figure 7A). Both expressed more RelB than DCs, and levels were consistently highest in the RAG1/GFP- subset. The Spi-B transcription factor has been found in human pDCs,20 and we found this to be the case for all pDCs (Figure 7A). Similarly, toll-like receptors (TLRs) play unique functions in innate immunity,45-48 and transcripts for TLR9 and TLR4 appear to distinguish pDCs from classical DCs. Of note, TLR9 mediates responses to microbial DNA, is critically involved in the recognition of CpG motifs, and was highly expressed in marrow-derived pDCs.
Since our study focused on pDCs resident in bone marrow, it was important to learn if the same discrete subsets were present elsewhere. As was the case for bone marrow, CD45R+ CD19-CD11cLo Ly-6C+ pDCs in the spleens of lymphocyte-deficient homozygous RAG1/GFP knock-in mice could be clearly resolved into GFP- and GFP+ populations (Supplemental Figure 3). The latter had fluorescence intensity similar to their counter-parts in BM, and RT-PCR analysis of heterozygous mice revealed that they contained transcripts for RAG1. These findings suggest that RAG1-expressing pDCs produced within BM give rise to stable populations with similar characteristics in peripheral lymphoid tissues.
Plasmacytoid dendritic-cell subsets differ with respect to cytokine production and T-cell allostimulation
We stimulated the 2 categories of pDCs with CpG-ODNs in culture to evaluate their potential for cytokine production (Figure 7B). The RAG1/GFP- subset consistently produced higher levels of IFNα, IL-6, and TNFα than did their RAG1/GFP+ counterparts. Remarkably, only GFP- pDCs produced IFNγ and IL-12 p70. The cells were then characterized with respect to costimulatory molecules before and after 18 hours of exposure to CpG-ODNs (Figure 7C). There was up-regulation of class II, CD86, and CD40 on both, but the degree of change in class II and CD86 densities on GFP- pDCs was consistently higher. We then compared the 2 types of pDCs with CD45R/B220-CD11c+ DCs with respect to T-allostimulatory capability (Figure 7D). While both categories of pDCs elicited T-cell proliferation measurable by BrdU incorporation, it was less than that stimulated by classical DCs, and GFP- pDCs were the more active of the 2. Finally, we determined that only T cells stimulated by the GFP+ category of pDCs synthesized IL-4, while IFNγ production was most remarkable when GFP- pDCs stimulated the response (Figure 7E).
We conclude that the RAG1 locus becomes active in a discrete and functionally specialized category of pDCs that we now designate pDC1s. Neither subset is a progenitor for other types of blood cells. On the contrary, both categories are stable and present in lymphoid tissues. While pDC1s, but not GFP- pDC2s, share properties with B-lineage lymphoid progenitors, they are unlike T-lineage cells with respect to Notch receptor ligation sensitivity. Both may function as antigen-presenting cells, but pDC1s may elicit different cytokine responses in T cells and pDC2s more efficiently produce inflammatory cytokines in response to CpG DNA stimulation.
Discussion
Recent studies have revealed a surprising degree of diversity in dendritic cells and suggested that they might be resolved into functionally specialized subsets with distinct developmental origins. Our analysis of murine bone marrow indicates that there are 2 stable categories of plasmacytoid dendritic cells that arise and diverge from each other at a very early stage of lympho-hematopoietic differentiation. Furthermore, patterns of cytokine production, T-cell allostimulation, and gene expression suggest that the 2 types of pDCs may have unique roles in immune responses.
RAG1+ pDC1 and RAG1- pDC2 subsets express pDC-related genes but differ with respect to cytokine production and T-cell allostimulation. (A) RT-PCR analyses were performed with cDNA from freshly purified RAG1+ pDC1s, RAG1- pDC2s, B220-CD11c+ DCs, and Lin-ckit+ cells. Primer pairs were used to detect transcripts for ICSBP, TLR9, TLR4, RelB, and SpiB along with β-actin as a control. (B) The 2 categories of pDCs (▦, pDC1; ▪, pDC2) were stimulated for 12 hours with CpG-ODNs. Supernatants were analyzed for IFNα, TNFα, IL-6, IL-12p70, and IFNγ levels by ELISA. Results are presented as the mean ± SD of 3 different experiments. (C) Expression of costimulatory molecules was assessed on pDC1s and pDC2s after 18-hour stimulation with CpG-ODNs. Dotted lines indicate unstimulated cells. (D) Preactivated RAG1+ pDC1s (□), RAG1- pDC2s (○), and B220-CD11c+ DCs (▵) were cultured for 6 days with 1 × 105 allogeneic T cells. Proliferation of TCRβ+ cells was measured by BrdU incorporation as described in “Material and methods.” Of 3 experiments, 1 representative is shown. Results are presented as the mean ± SD of 3 different culture wells; one representative experiment of 3 is shown. (E) Intracellular cytokine accumulation was determined in T cells 6 days after priming with preactivated pDC1s or pDC2s, and 5-hour restimulation with PMA and ionomycin.
RAG1+ pDC1 and RAG1- pDC2 subsets express pDC-related genes but differ with respect to cytokine production and T-cell allostimulation. (A) RT-PCR analyses were performed with cDNA from freshly purified RAG1+ pDC1s, RAG1- pDC2s, B220-CD11c+ DCs, and Lin-ckit+ cells. Primer pairs were used to detect transcripts for ICSBP, TLR9, TLR4, RelB, and SpiB along with β-actin as a control. (B) The 2 categories of pDCs (▦, pDC1; ▪, pDC2) were stimulated for 12 hours with CpG-ODNs. Supernatants were analyzed for IFNα, TNFα, IL-6, IL-12p70, and IFNγ levels by ELISA. Results are presented as the mean ± SD of 3 different experiments. (C) Expression of costimulatory molecules was assessed on pDC1s and pDC2s after 18-hour stimulation with CpG-ODNs. Dotted lines indicate unstimulated cells. (D) Preactivated RAG1+ pDC1s (□), RAG1- pDC2s (○), and B220-CD11c+ DCs (▵) were cultured for 6 days with 1 × 105 allogeneic T cells. Proliferation of TCRβ+ cells was measured by BrdU incorporation as described in “Material and methods.” Of 3 experiments, 1 representative is shown. Results are presented as the mean ± SD of 3 different culture wells; one representative experiment of 3 is shown. (E) Intracellular cytokine accumulation was determined in T cells 6 days after priming with preactivated pDC1s or pDC2s, and 5-hour restimulation with PMA and ionomycin.
The CD45R/B220+CD24-/LoCD43+CD19- Fraction A of murine BM has been extensively studied and shown to contain multiple subsets.27,30,31,49 Fraction A includes some progenitors destined to follow the B-lymphoid lineage, although it is not particularly rich in this activity.32 While many NK-lineage cells never express the CD45R/B220 antigen, DX-5+ AA4.1-Ly-6C- NK cells represent a conspicuous component of Fraction A.50 An additional part of Fraction A (A1), defined by expression of sterile immunoglobulin heavy chain (IgH) locus transcripts and the AA4.1+ CD4+ surface phenotype, rapidly differentiated into classical CD11b+CD8LoCD11c+ major histocompatibility complex class II–positive (MHC-II+) dendritic cells in stromal cell cultures.51 Some Fraction A cells display the Ly-6C antigen, as well as low levels of CD11c, a marker thought to be definitive for DCs.11,32,52 We now formally demonstrate that the CD11cLoLy-6C+ cells in Fraction A are functional pDCs. That is, they differentiated in response to CpG-ODNs and produced large amounts of type I interferon. Their independence from myeloid-related DCs was further indicated by their lack of CD11b. Furthermore, they were unable to give rise to B-, T-, NK-, or myeloid-lineage cells in culture.
BrdU labeling experiments suggested that pDCs in BM have a very low turnover, and they were not efficiently produced in culture from rapidly dividing prolymphocytes. This is compatible with the prolonged lifespan in the spleen reported for pDCs.16 While rapidly replaced lymphoid progenitors are estrogen sensitive, short-term treatment with this hormone does not deplete pDC numbers in BM (Medina et al53 and R.P., unpublished data, December 2003). These observations make it difficult to conclude that pDCs are actively made in either central or peripheral lymphoid tissues, and it remains to be seen if their turnover is substantially increased under conditions of inflammation or disease. In that context, it is important to note that the Flt3-L cytokine alone did not efficiently drive pDC formation in culture, and stromal cells may provide additional cues needed for their development. The factors that support this process are not fully understood, but it has been reported that G/M-CSF or TNF, cytokines that drive the generation of myeloid DCs, block the development of pDCs.15
D'Amico and Wu demonstrated that thymic CD4Lo progenitors gave rise to very small numbers of pDCs following transplantation.22 It was also found by them22 and by Shigematsu et al54 that both common myeloid (Lin-CD16/32LoCD34+c-kit+Sca-1-) and common lymphoid (Lin-IL-7Rα+Sca-1Loc-kitLoThy-1.1-) progenitors produced low pDC yields per input when engrafted in irradiated recipients. However, a relevant comparison, that is, to c-kitHi stem cells and ELPs, was not performed. Furthermore, a rigorous Ly6C+ definition of pDCs was not used. Thus, it has been extremely difficult to ascribe a particular developmental pathway and sequence of differentiation events to pDC formation. We now show that substantial numbers of pDCs were produced when BM fractions enriched for stem cells or ELPs were cultured with stromal cells and Flt3-L. In contrast, the yields of pDCs from myeloid progenitors or prolymphocytes under the same culture conditions were very low. The inefficient production of pDCs from cells dedicated to those lineages may suggest that a small degree of reprogramming is possible.55 We conclude from these and observations discussed at a later point that pDCs normally derive from very primitive hematopoietic cells in BM and subsequently persist in various tissues.
RAG1/GFP mice permit very rare lymphoid progenitors to be identified and sorted.25,33 This is because fluorescence corresponds to the presence of RAG1 transcripts in cells that are primitive in terms of transcription factors, surface markers, and time required to differentiate. We originally intended to exploit these mice to probe the origins of pDCs. While that was highly successful, we also learned that approximately 60% of the pDCs in bone marrow were GFP+. That subset, that we now designate pDC1, was unique in having immunoglobulin D-J rearrangement products, a fact that suggests they or their progenitors also expressed RAG2. Transcripts for Pax5, Bcl11a, EBF, and mb1/Igα were exclusively present in pDC1s. These findings extend previous reports of D-J rearrangements in spleen or thymic pDCs21 by demonstrating that the phenomenon pertains to a particular subset. Furthermore, we found RAG1-expressing cells in the spleen and lymph nodes that had characteristics indistinguishable from pDCs present in BM.
Signals that control cell-fate decisions of lympho-hematopoietic progenitors are poorly understood but likely reflect the interplay of cytokines and transcription factors. It has been reported that Flt3-L is indispensable for commitment and differentiation of hematopoietic progenitors to all DC lineages,17,22,56,57 and signal transducer and activator of transcription 3 (STAT3) is the intracellular mediator of Flt3-L signaling required for DC development.58 A general depletion of both CD8α+ and CD8α- DCs is in lymphoid organs in Ikaros-/- mice,59 and CD11c+CD8α+DEC205+ but not CD11c+DEC205-CD8α- DCs are present in PU.1-/- mice.60 Moreover, the development of myeloid-related CD8α- but not lymphoid-related CD8α+ DCs is dependent on RelB.61 We showed that both categories of pDCs express PU.1, Ikaros, E47, and GATA-3 transcription factors, and both are ICSBPHiTLR4LoTLR9Hi. This latter profile distinguishes them from ICSBPLoTLR4HiTLR9Lo myeloid DCs.47 Interestingly, both subsets of pDCs expressed CD8α following stimulation with CpG-ODNs.
While the importance of E2A, EBF, or Pax5 transcription factors to pDC development has not been explored, loss of any one blocks B-cell development.39-41 Pax5 was of particular interest because it was selectively expressed by pDC1s and is known to control production of mb1/Igα. Pax5 controls the commitment and thus entry into the B-lymphocyte lineage, and its removal confers a remarkable degree of lineage plasticity on lymphopoietic cells.42 Therefore, it seemed possible that this transcription factor accounted largely or in part for the distinctive properties of this subset and we examined pDCs in Pax5 gene–targeted mice. Pax5-/- mice have a short lifespan, but pDCs defined according to expression of RAG1 and mb1/Igα were clearly present in BM at 12 days of age. Thus, the differentiation lineage that produces pDC1s must diverge from that corresponding to B cells at a Pax5-independent stage. The involvement of Id2 or Id3 and Spi-B in the differentiation of human pDCs19,20 may indicate that these factors control the T-B-NK-pDC lineage decision at an extremely early stage in differentiation. In further support of this concept, ligation of Notch receptors on stem cells and ELPs by coculture on Delta-1–transduced stromal cells blocked formation of pDCs (Figure 4C and data not shown). In this respect, their progenitors in BM are B-lineage related and dissimilar from those that produce T and NK cells.
Additional information is needed about developmental relationships between pDC1s and pDC2s. RAG1+ ELPs were exclusively progenitors of pDC1s, while both subsets could be generated from stem cells (Figure 4). Similarly, many NK-lineage cells derive from RAG1/GFP+ progenitors, but DX-5+ NK cells are GFP-.33 It could be significant that even pDC1s in the spleen contain RAG1 transcripts as well as GFP protein, and thus probably have an active RAG1 locus. CD45R/B220+CD19-CD11cLoLy-6C+ pDCs were present in bone marrow of RAG1, RAG2, and CD127/IL-7Ra knock-out mice, and subsequent study may reveal if proportions of pDC subsets are normal. The presence of Ig gene rearrangements only in pDC1s precludes it giving rise to pDC2s, but the reverse relationship remains a possibility.
It was important to investigate the nature and fate of CD45R/B220+CD19-CD11cLoLy-6C- cells in BM. With the exception of Ly-6C, they are similar to and potentially represented an immature form of pDCs. However, freshly isolated CD45R/B220+CD19-CD11cLoLy-6C- cells did not produce type I interferon efficiently when cultured with CpG-ODNs and did not give rise to Ly-6C+ pDCs when held on stromal cells with Flt3-L. On the contrary, the NK1.1 and DX-5 NK-lineage markers were expressed and substantial numbers of DX-5+ cells were produced when they were held in serum-free, stromal cell–free cultures containing SCF, Flt3-L, IL-7, and IL-15 (R.P., unpublished data, 2004). As Ly-6C is an interferon-inducible gene that can be expressed on some lymphocyte subsets and monocytes,62-64 it remains possible that some environmental condition or inflammatory circumstance can convert CD45R/B220+CD19-CD11cLoLy-6C- cells into functional pDCs.
This study provides an initial description of 2 plasmacytoid dendritic cell categories and demonstrates that they likely arise from very primitive progenitors in bone marrow. Although the 2 differ with respect to cytokine synthesis and allostimulatory behavior, further investigation is needed to attribute them to distinct biologic processes. Of particular interest is whether both differ from classical dendritic cells with respect to antigen presentation and T-cell priming capability and if they are similarly biased toward tolerogenic or stimulatory signals.43,44,65 It also seems possible that their cytokine profiles will correspond to differential contributions to the pathogenesis of autoimmune disease. Indeed, the discovery of a new cell type may launch an investigation of its role in tumor surveillance and immunity.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-07-2529.
Supported by grants AI 20069 and AI 058162 from the National Institutes of Health. M.B.'s research was supported by Boehringer Ingelheim. P.W.K. holds the William H. and Rita Bell Chair in biomedical science.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Dr J. C. Zúñiga-Pflücker for Delta-like-1 and GFP-expressing OP9 cell lines. We thank Ms Viji Dandapani and Mr Jacob Bass for expert cell sorting, and Ms Shelli Wasson for professional editorial assistance.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal