Abstract
The adaptor protein SAP regulates signaling through signaling lymphocytic activation molecule (SLAM)–family receptors expressed on T and natural killer (NK) cells. In patients affected by X-linked lymphoproliferative (XLP) disease, mutations in the SH2D1A gene result in defective lytic activity. However, the mechanism by which SAP controls cytotoxic activity remains unclear. T-cell–receptor (TCR) activation of CD8+ cytotoxic T cells (CTLs) results in down-regulation of SAP, suggesting that this protein is involved in early activation events. Here, we show that SAP-deficient CTLs from patients with XLP and hemophagocytic lymphohistiocytosis (HLH) display a specific lytic defect against autologous and allogeneic Epstein-Barr virus (EBV)–positive B cells. This defect is associated with the defective polarization of 2B4, perforin, and lipid rafts at the contact area of CTLs with EBV-positive targets. Blockade of 2B4 in normal CTLs reproduces the defects in lysis and polarization observed in SAP-deficient CTLs. Expression and regulation of the SLAM-family receptors SLAM, CD84, and 2B4, as well as the lytic effectors perforin and granzyme-B are normal in SAP-deficient CTLs. In addition, TCR stimulation leads to normal proliferation and production of interleukin 2 (IL-2), IL-4, and interferon-γ (IFN-γ). These results demonstrate that the SAP/2B4 pathway plays a key role in CTL lytic activity against EBV-positive targets by promoting the polarization of the lytic machinery.
Introduction
X-linked lymphoproliferative (XLP) disease is a severe immunodeficiency characterized by variable clinical phenotypes, including fatal infectious mononucleosis, malignant B-cell lymphoma, and progressive dysgammaglobulinemia.1 The gene responsible for XLP (SH2D1A) encodes for SAP (SLAM [signaling lymphocyte-activation molecule]–associated protein), or SH2D1A, a natural killer (NK) and T-cell–specific signaling adaptor protein.2-4 Mutations in the SH2D1A gene have been detected in patients with hemophagocytic lymphohistiocytosis (HLH)5 and common variable immunodeficiency syndrome,6,7 thereby expanding the range of clinical phenotypes associated with this genetic defect. These heterogeneous clinical manifestations might be related to viral infections, which appear to act as secondary factors triggering the severe forms of SAP-deficiency syndrome. In the absence of SAP, dysregulation of T/B-cell interactions and NK-cell functions has been proposed to result in a variable ability to control Epstein-Barr virus (EBV) infection, leading to either fatal infectious mononucleosis, or infectious mononucleosis with progression toward malignant B-cell lymphoma or acquired agammaglobulinemia.8 However, in some patients with XLP, these manifestations can also occur in the absence of EBV infection.9
SAP is a protein of 128 residues with a single Src homology 2 (SH2) domain binding to a consensus T.I/V.(p)Y.x.x.V/I motif on the cytoplasmic tail of surface molecules of the SLAM family, including SLAM, 2B4, CD84, Ly-9, and NTB-A.2,10-13 These molecules form homotypic and heterotypic receptor-ligand pairs during T cell/antigen-presenting cell or NK cell/target cell contacts. SLAM is expressed on activated and memory T cells, in which it can skew cytokine production toward a T helper 1 (Th1) profile14 and can act as a costimulatory molecule for cytotoxic activity.15 SAP appears to be a natural inhibitor of the interaction of SLAM-family members with SH2-containing signal molecules such as the SH2-containing phosphatase 2 (SHP-2) phosphatase.2 In T cells, SAP recruits FynT, which is required for SLAM phosphorylation. Activation of the SLAM/SAP pathway controls the phosphorylation of dok1, dok2, and SH2-containing inositol phosphatase, thereby playing a potential role in the control of interferon-γ (IFN-γ) production.16,17 Furthermore, SAP appears to participate in the adhesion of T cells to target cells, as well as in T-cell receptor (TCR)–mediated activation.18,19 In SAP-deficient murine models,20,21 infection with lymphocytic-chorio-meningitis virus is associated with higher mortality related to increased T-cell activation and IFN-γ production, as well as to a reduction in the production of interleukin 4 (IL-4) and the generation of immunoglobulin-secreting cells. However, in this model, T- and NK-cell cytotoxic activities have not been investigated.
A number of studies in patients with XLP indicate that 2B4 stimulation on NK cells induces an abnormal dominant abrogation of NK-cell lytic activity. This defect is also present when EBV-infected cells are used as targets.22-24 Expression of CD48, the natural ligand of 2B4, is strongly increased on B cells upon EBV infection, while expression of major histocompatibility complex molecules is down-regulated.25 Thus, EBV infection may lead to a specific triggering of 2B4, thereby inducing a progressive attrition of SAP-defective NK-cell function. Initial studies on the function of T cells in patients with XLP gave controversial results that could be related to the different clinical stages and phenotypes of the patients studied.26,27 A recent report showed reduced IFN-γ production and lytic activity by EBV-specific cytotoxic T lymphocytes (CTLs) in 3 patients with XLP.28 However, the mechanism underlying this defective lytic activity of SAP-deficient CD8+ T cells was not identified. In addition, the respective regulation of expression of the SLAM-family receptors and of SAP in EBV-specific CTLs was not studied. In this study, we found that the defective lytic activity of SAP-deficient CD8+ T cells is specific to EBV-infected target cells. SLAM-family receptors are expressed normally, and perforin and granzyme-B are also produced. However, SAP-deficient CD8+ T cells fail to polarize 2B4, perforin, and the lipid raft marker GM1 at the contact with EBV-positive target cells. Our results identify the 2B4/SAP pathway as an important regulator of the assembly of the lytic synapse that forms between CD8+ CTLs and EBV-positive B-cell targets.
Patients, materials, and methods
Patients and cells
Patient 1 was diagnosed as HLH and previously described.5 His SH2D1A gene sequence carries a single nucleotide mutation in exon 3 resulting in a stop codon (Tyr76′STOP). Patients 2 and 3 were diagnosed as XLP and have been described previously.12 Patient 2 carries a single nucleotide mutation in exon 2, resulting in a Tyr54Cys amino acid substitution. Patient 3 carries a single nucleotide mutation in exon 3, resulting in a Phe87Ser amino acid substitution. Blood samples from patients and healthy donors were obtained following standard ethical procedures (Helsinki protocol) and with the approval of the concerned internal review boards.
T-cell lines
T cells from SAP-deficient patients and healthy control donors were derived from peripheral blood mononuclear cells (PBMCs) isolated with Lymphoprep (Nycomed Pharma AS, Oslo, Norway). For the generation of allogeneic T-cell lines, PBMCs were stimulated every 2 weeks at the concentration of 2 × 105 cells/mL with a feeder cell mixture which comprises irradiated allogeneic PBMCs (1 × 106/mL), irradiated EBV-transformed JY cells (1 × 105/mL), phytohemagglutinin (1 μg/mL), IL-2 (100 IU/mL), and IL-7 (10 ng/mL). For the generation of EBV-specific T-cell lines, PBMCs were stimulated every 2 weeks at the concentration of 5 × 105 cells/mL with 1 × 105/mL irradiated autologous EBV-transformed B-cell lines (B-EBV), IL-2 (100 IU/mL), and IL-7 (10 ng/mL). T-cell lines were cultured in Yssel medium (Dyaclone, Besançon, France), 10% fetal calf serum (or 5% human serum for the EBV-specific T-cell lines), and penicillin and streptomycin. CD8+ T cells were purified after 4 rounds of stimulation by depleting CD4+ T cells with anti-CD4 antibody (Abs)–coated magnetic beads (Dynal AS, Oslo, Norway).
Cytotoxic assays
Cytotoxic activity was measured in a standard 51Chromium (51Cr) release assay as described previously.29 Briefly, 1000 51Cr-labeled target cells, including JY, K562, and autologous B-EBV, were incubated for 4 hours with effector CD8+ T cells at the indicated effector-target ratios at 37°C in 5% CO2. Blocking anti-2B4 monoclonal antibodies (mAbs; Clone c1.7; Immunotech, Marseille, France) were added at a concentration of 5 μg/mL prior to adding the labeled target cells on the effector cells. The radioactivity liberated in the supernatant by the lysed targets was measured with a γ-counter. Percentage of specific lysis was calculated according to the formula: 100 × (cpm experimental release - cpm spontaneous release)/(cpm maximum release - cpm spontaneous release).
Cell proliferation and cytokine production
Resting T cells were plated at 1 × 105 cells/well in 96-well flat-bottom plates precoated with the indicated dose of anti-CD3 mAbs (OKT3; Janssen-Cilag, Milan, Italy). After 72 hours, cells were labeled with 3H-thymidine for 18 hours. Thymidine incorporation was measured in triplicates by liquid scintillation counting. IL-2, IL-4, and IFN-γ production was measured by intracytoplasmic fluorescence-activated cell sorting (FACS) analysis of cells stimulated for 3 hours with either 1 μg/mL immobilized anti-CD3 mAbs plus 10 μg/mL anti-CD28 mAbs (BD Biosciences Pharmingen, San Diego, CA) or with 10 ng/mL TPA (12-O-tetradecanoylphorbol acetate) plus 500 ng/mL ionomycin. Cells were treated with 10 μg/mL brefeldin A and were then incubated additionally for 3 hours. Cells were fixed in 2% formaldehyde, permeabilized with 0.5% Saponin, and subsequently stained with phycoerythrin (PE)–labeled anti–IL-2, PE-labeled anti–IL-4, and fluorescein isothiocyanate (FITC)–labeled anti–IFN-γ (BD Biosciences Pharmingen). After washing, cells were analyzed by using a FACScan flow cytometer (BD Biosciences, San Diego, CA) and CellQuest software (BD Biosciences).
FACS analysis of CTL activation markers
Resting CD8+ T cells were incubated for 24 hours at 1 × 106/mL in the presence of either 100 ng/mL IL-15 (R&D Systems, Abingdon, United Kingdom) or 1 μg/mL immobilized anti-CD3 mAbs. T cells were washed and resuspended in phosphate-buffered saline (PBS) containing 0.3% bovine serum albumin (BSA) and 0.1% Na-azide before incubation for 15 minutes at 4°C with one of the following mAbs: PE-conjugated anti-CD25 mAbs, FITC-conjugated anti-CD69 mAbs, PE-conjugated mouse anti-CD84 mAbs (BD Biosciences Pharmingen), phycoerythrin-cyanin 5–conjugated mouse anti-2B4 mAbs (Immunotech), and purified anti-SLAM mAbs (gift from Dr Aversa, DNAX Research Institute, Palo Alto, CA). For anti-SLAM staining, in a second step cells were incubated for 15 minutes at 4°C with 5 μg/mL PE-conjugated goat anti–mouse immunoglobulin G (IgG) Abs (Southern Biotechnology Associates, Birmingham, AL). For staining of the intracellular proteins perforin and granzyme-B, T cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences Pharmingen), blocked with 5% normal rabbit serum, and incubated with PE-conjugated anti–perforin mAbs or purified anti–granzyme-B mAbs (BD Biosciences Pharmingen) for 20 minutes at 4°C. For anti–granzyme-B staining, in a second step cells were incubated for 15 minutes at 4°C with PE-conjugated goat anti–mouse IgG Abs. Stained cells were washed and analyzed by FACS as described in “Cell proliferation and cytokine production.”
Immunofluorescence
Resting T cells were plated at 1 × 106/well on 96-well U-bottom plates, in a final volume of 100 μL. Target cells (B-EBV, JY, or K562) were added at the concentration of 2 × 106/100 μL. Plates were centrifuged for 10 seconds at 1500 rpm (300 × g) and incubated for 20 minutes at 37°C. Cell conjugates were transferred onto poly-l-lysine–coated coverslips for 20 minutes at 37°C and subsequently fixed with 4% paraformaldehyde for 30 minutes at room temperature. Staining for GM1 and perforin was performed by incubating the cells in PBS, 0.3% BSA, 0.3% saponin with 8 μg/mL FITC-conjugated Cholera toxin subunit-B (Sigma, St Louis, MO), and PE-conjugated anti-perforin mAbs (used at the dilution recommended by the manufacturer; BD Biosciences Pharmingen). Staining for 2B4 was performed by incubating the cells in PBS, 0.3% BSA, with 10 μg/mL anti-2B4 mAbs (Immunotech), and subsequently with Alexa 488–conjugated goat anti–mouse secondary Abs (Molecular Probes, Eugene, OR). Each staining step was performed at room temperature for 45 minutes. After washing, coverslips were mounted with Fluoromount-G (Southern Biotechnology Associates) and analyzed on an Olympus Provis AX70 microscope using a 100 × immersion objective lens (numerical aperture 1.30; Olympus, Melville, NY). Images were acquired with a Zeiss Axiocam camera using Zeiss Axiovision 3.1 software (Carl Zeiss, Jena, Germany).
Regulation of SAP expression in CD8+ T cells. Western blot analysis of SAP expression in CD8+ T cells from healthy donors (ND1, ND2, and ND3), and patients with HLH (SAP1) and XLP (SAP2 and SAP3) at a resting state and at 24 or 72 hours after activation with 10 μg/mL immobilized anti-CD3 mAbs.
Regulation of SAP expression in CD8+ T cells. Western blot analysis of SAP expression in CD8+ T cells from healthy donors (ND1, ND2, and ND3), and patients with HLH (SAP1) and XLP (SAP2 and SAP3) at a resting state and at 24 or 72 hours after activation with 10 μg/mL immobilized anti-CD3 mAbs.
Western blot protein analysis
Cell lysates were prepared from 1 × 106 PBS-washed cells in 20 μL lysis buffer (20 mM Tris (tris(hydroxymethyl)aminomethane) pH 7.5, 150 mM NaCl, 1% Nonidet P40 (Octylphenolpoly[ethyleneglycolether]n), 2 mM EDTA (ethylenediaminetetraacetic acid)) supplemented with 100 μg/mL phenylmethlsulfonyl fluoride and a complete set of protease inhibitors (Roche Molecular Biomedicals, Mannheim, Germany). After 30 minutes on ice, lysates were centrifuged, and supernatant was resuspended in denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. An aliquot of each lysate was used for total protein concentration determination with the bicinchoninic acid protein assay (Pierce, Rockford, IL) to normalize each sample. Following SDS-PAGE resolution, proteins were transferred onto nitrocellulose membranes, and correct loading and transfer were checked by Ponceau staining. Incubation with anti–SAP rabbit polyclonal antibody (kindly provided by Dr Nichols, Children's Hospital of Philadelphia, PA) diluted 1:500 in 5% milk in Tris-buffered saline–Tween 20 (0.05%) was performed. Horseradish peroxidase–coupled anti–rabbit Abs (Dako A/S, Glostrup, Denmark) were used at the dilution of 1:2000 as secondary Abs, and detection was performed with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) detection system.
Results
SAP deficiency in CD8+ T cells from patients with HLH and XLP
Untransformed CD8+ T-cell lines were established from 3 healthy donors (ND1, ND2, and ND3), 1 patient with HLH (SAP1), and 2 patients with XLP (SAP2 and SAP3) with SH2D1A gene point mutations, by repeated stimulation with allo-PBMCs and the EBV-positive cell line JY. CD8+ T cells from the healthy donors expressed high levels of the SAP protein at the resting stage (9-10 days after stimulation), whereas SAP expression was strongly down-regulated 24 and 72 hours after TCR stimulation with anti-CD3 mAbs (Figure 1). Resting CD8+ T cells from patients with HLH and XLP failed to show detectable expression of the SAP protein (Figure 1). Interestingly, the SAP mRNA levels detected by reverse transcription–polymerase chain reaction in normal cells, before and after TCR stimulation, were comparable to those from SAP-deficient patients were comparable (data not shown). These data demonstrate that in untransformed human CD8+ T cells, the expression of SAP protein is tightly regulated during activation and suggest that SAP plays a role relatively early during T-cell activation.
Defective lytic activity of SAP-deficient CD8+ T cells against EBV-positive targets
The lytic activity of SAP-deficient T cells was first investigated by using a system in which EBV antigens were presented by autologous B cells. To generate antigen-specific CTLs, CD8+ T cells from the patient with HLH (SAP1) were stimulated repeatedly with autologous EBV-transformed B cells. EBV-specific CD8+ T cells derived from patient SAP1 displayed a reduced lytic activity against autologous EBV-positive B-cell targets, when compared with healthy EBV-specific CD8+ T cells generated in parallel (Figure 2A). Next, we investigated the lytic activity of SAP-deficient CTLs against allogeneic EBV-infected B-cell targets. To this aim, CD8+ T cells from the 3 SAP-deficient patients were repeatedly stimulated with EBV-positive JY cells and then tested for their lytic activity against these targets. SAP-deficient CTLs had a dramatically reduced ability to lyse JY target cells. At an effector-target ratio of 90:1, SAP-deficient CD8+ T cells had a lytic activity of 8%, whereas SAP-positive control CD8+ T cells had a lytic activity of 46% (Figure 2B). The lytic activity of normal CTLs against JY cells was mediated through 2B4, since preincubation with anti-2B4 mAbs resulted in a significant inhibition, with percentages of lysis comparable to those of SAP-deficient CTLs (Figure 2C). In contrast, when the K562 cell line (EBV negative and CD48-) was used as target, the lytic activity of the SAP-deficient CD8+ T cells was not impaired. Indeed, SAP-deficient CTLs from the 3 patients were able to lyse K562 cells at even higher levels than control CD8+ T cells (Figure 2D). These results indicate that the lytic capacity of SAP-deficient CD8+ T cells is not intrinsically impaired, but that activation through 2B4, which is required for EBV-specific T-cell cytotoxic activity, is defective.
Lytic activity of SAP-deficient CD8+ T cells. (A) Lytic activity of EBV-specific CD8+ T-cell lines from a SAP-deficient HLH patient (SAP1) and from a healthy control donor. The targets are autologous EBV-transformed B-cell lines used at the indicated effector-target ratio. (B) Mean (± SD) of the lytic activity of allospecific CD8+ T-cell lines from 3 SAP-deficient patients (SAP1, SAP2, and SAP3) and from 3 healthy donors (ND1, ND2, and ND3) against the EBV-transformed B-cell line JY. (A-B) ▪ indicates ND; □, SAP deficient. (C) Mean (± SD) of the lytic activity of allospecific CD8+ T-cell lines from 3 healthy donors (ND1, ND2, and ND3) against JY cells in the presence of either blocking anti-2B4 mAbs (▨) or isotype control Abs (+IgG1; ▪). (D) Mean (± SD) of the lysis of allospecific CD8+ T-cell lines from 3 SAP-deficient patients (SAP1, SAP2, and SAP3) and from 3 healthy donors (ND1, ND2, and ND3) against the CD48- cell line K562. ▪ indicates ND; □, SAP deficient. One representative experiment of 5 performed is shown. Each mean (± SD) was obtained from 6 data points (3 cell lines studied in duplicate), and statistical analysis was performed using an unpaired t test. Significant differences of lysis between SAP and control T-cell lines are indicated by asterisks (*P < .05; **P < .01).
Lytic activity of SAP-deficient CD8+ T cells. (A) Lytic activity of EBV-specific CD8+ T-cell lines from a SAP-deficient HLH patient (SAP1) and from a healthy control donor. The targets are autologous EBV-transformed B-cell lines used at the indicated effector-target ratio. (B) Mean (± SD) of the lytic activity of allospecific CD8+ T-cell lines from 3 SAP-deficient patients (SAP1, SAP2, and SAP3) and from 3 healthy donors (ND1, ND2, and ND3) against the EBV-transformed B-cell line JY. (A-B) ▪ indicates ND; □, SAP deficient. (C) Mean (± SD) of the lytic activity of allospecific CD8+ T-cell lines from 3 healthy donors (ND1, ND2, and ND3) against JY cells in the presence of either blocking anti-2B4 mAbs (▨) or isotype control Abs (+IgG1; ▪). (D) Mean (± SD) of the lysis of allospecific CD8+ T-cell lines from 3 SAP-deficient patients (SAP1, SAP2, and SAP3) and from 3 healthy donors (ND1, ND2, and ND3) against the CD48- cell line K562. ▪ indicates ND; □, SAP deficient. One representative experiment of 5 performed is shown. Each mean (± SD) was obtained from 6 data points (3 cell lines studied in duplicate), and statistical analysis was performed using an unpaired t test. Significant differences of lysis between SAP and control T-cell lines are indicated by asterisks (*P < .05; **P < .01).
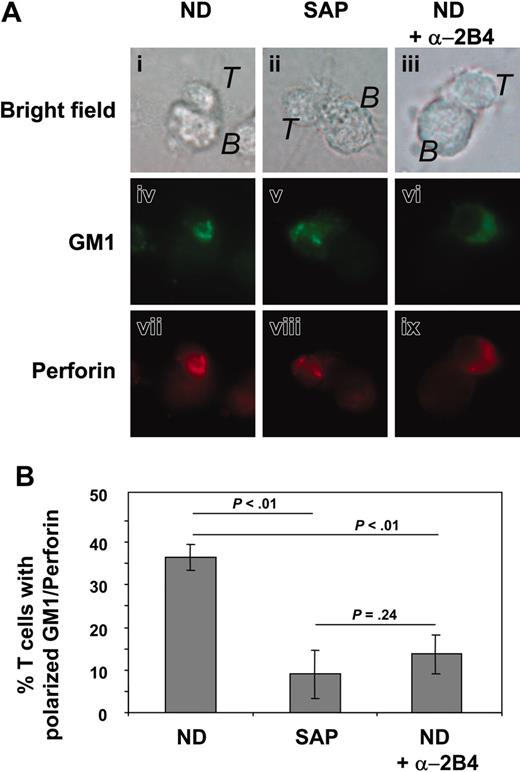
Defective polarization of perforin, GM1, and 2B4 in SAP-deficient CD8+ T cells
We next investigated the ability of SAP-deficient CTLs to polarize the lytic machinery toward the site of contact with EBV-infected target cells. The lytic activity of both NK cells and CTLs has been shown to depend on the assembly of a specialized immunologic synapse, which occurs through reorganization of lipid rafts, surface receptors, signaling molecules, cytoskeleton, and lytic granules. By immunofluorescence analysis of control and SAP-deficient CD8+ T cells conjugated to EBV-infected targets, we investigated the orientation of both perforin and ganglioside GM1 as markers for lytic granules and lipid rafts, respectively. Similarly to control CTLs, SAP-deficient CTLs were able to form conjugates with JY cells and were positive for perforin and GM1 (Figure 3A). In control CTLs, polarization of both GM1 and perforin at the contact area with JY cells was observed in 36% of the cells. In contrast, only 9% of the SAP-deficient CTLs, which formed conjugates with JY cells, polarized GM1, and perforin at the site of contact (Figure 3B). Importantly, incubation of control CTLs with blocking anti-2B4 mAbs resulted in a significative decrease in the percentage of cells with polarized GM1 and perforin (14%). A defect in GM1 and perforin polarization was also observed when autologous EBV-lymphoblastoid cells were used as targets of EBV-specific CTLs from patient SAP1, whereas normal polarization occurred when K562 cells were used as targets (data not shown). Our results indicate that in the absence of stimulation through 2B4 or SAP, the reduced lytic activity of CTLs against EBV-positive cells is associated with a defect in the orientation of both lipid rafts and lytic granules at the effector-target contact area.
Distribution of perforin and GM1 in SAP-deficient CD8+ T cells. (A) GM1 and perforin distribution in normal and SAP-deficient CD8+ T cells (T) forming conjugates with EBV-positive B-cell line JY (B). The effects of blocking anti-2B4 mAbs on normal CD8+ T cells are also shown. One representative conjugate is shown in parallel as bright field (i-iii), GM1 staining (iv-vi), and perforin staining (vii-ix). (B) Quantitative analysis of GM1 and perforin coclustering at the area of contact with B-cell targets in T cells from 3 SAP-deficient patients and T cells from 3 healthy donors, either untreated or treated with blocking anti-2B4 mAbs. Cells were considered positive for coclustered GM1 and perforin if the staining was centered at the site of contact with the B cell and occupied less than one third of the cell surface. Data are represented as mean percentages (± SDs) of 3 experiments counting T cells forming clusters with a single JY B-cell target (for each cell line a total of 250-300 cells was counted). Statistical analysis was performed using an unpaired t test, and P values corresponding to the comparison of 1 group with the other are indicated.
Distribution of perforin and GM1 in SAP-deficient CD8+ T cells. (A) GM1 and perforin distribution in normal and SAP-deficient CD8+ T cells (T) forming conjugates with EBV-positive B-cell line JY (B). The effects of blocking anti-2B4 mAbs on normal CD8+ T cells are also shown. One representative conjugate is shown in parallel as bright field (i-iii), GM1 staining (iv-vi), and perforin staining (vii-ix). (B) Quantitative analysis of GM1 and perforin coclustering at the area of contact with B-cell targets in T cells from 3 SAP-deficient patients and T cells from 3 healthy donors, either untreated or treated with blocking anti-2B4 mAbs. Cells were considered positive for coclustered GM1 and perforin if the staining was centered at the site of contact with the B cell and occupied less than one third of the cell surface. Data are represented as mean percentages (± SDs) of 3 experiments counting T cells forming clusters with a single JY B-cell target (for each cell line a total of 250-300 cells was counted). Statistical analysis was performed using an unpaired t test, and P values corresponding to the comparison of 1 group with the other are indicated.
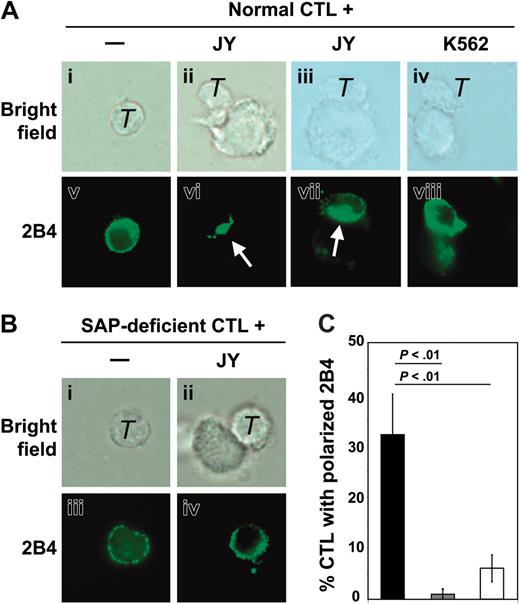
Distribution of 2B4 in normal and SAP-deficient CD8+ T cells. (A) 2B4 distribution in normal CD8+ CTLs (T) either alone or at the contact with EBV+/CD48+ JY B cells or EBV-/CD48-K562 cells. Representative cells are shown as bright field (i-iv) and 2B4 staining (v-viii). The white arrows indicate 2B4 clustering at the cell contact area. (B) 2B4 distribution in SAP-deficient CD8+ T cells either alone or at the contact with EBV+/CD48+ JY B cells. Representative cells are shown as bright field (i-ii) and 2B4 staining (iii-iv). (C) Quantitative analysis of the percentage of CTLs (normal or SAP deficient) with clustered 2B4 at the cell contact area. ▪ indicates normal CTL + JY; ▦, normal CTL + K562; and □, SAP-deficient CTL + JY cells. Mean percentages (± SDs) of 3 experiments counting T cells forming clusters with single target cells (100 conjugates were counted). Statistical analysis was performed using an unpaired t test, and P values corresponding to the comparison of 1 group with the other are indicated.
Distribution of 2B4 in normal and SAP-deficient CD8+ T cells. (A) 2B4 distribution in normal CD8+ CTLs (T) either alone or at the contact with EBV+/CD48+ JY B cells or EBV-/CD48-K562 cells. Representative cells are shown as bright field (i-iv) and 2B4 staining (v-viii). The white arrows indicate 2B4 clustering at the cell contact area. (B) 2B4 distribution in SAP-deficient CD8+ T cells either alone or at the contact with EBV+/CD48+ JY B cells. Representative cells are shown as bright field (i-ii) and 2B4 staining (iii-iv). (C) Quantitative analysis of the percentage of CTLs (normal or SAP deficient) with clustered 2B4 at the cell contact area. ▪ indicates normal CTL + JY; ▦, normal CTL + K562; and □, SAP-deficient CTL + JY cells. Mean percentages (± SDs) of 3 experiments counting T cells forming clusters with single target cells (100 conjugates were counted). Statistical analysis was performed using an unpaired t test, and P values corresponding to the comparison of 1 group with the other are indicated.
We next investigated the role of SAP in the localization of 2B4 during the lytic process. In resting normal CD8+ T cells, 2B4 was distributed all over the cell surface in small discrete clusters (Figure 4Av). The absence of SAP in CD8+ T cells from the patients had no effect on the distribution of 2B4 at the resting stage (Figure 4Biii). Upon contact with JY target cells, normal CD8+ T cells redistributed 2B4 to the site of effector-target contact area, either as a central cluster (Figure 4Avi) or as a continuous pattern covering the entire length of the contact area (Figure 4Avii). The percentage of CD8+ T cells with 2B4 clustering (Figure 4C) was very similar to the percentage of CD8+ T cells with GM1/perforin polarization. In contrast, SAP-deficient CD8+ T cells failed to cluster 2B4 at the contact area (Figure 4Bii,iv and 4C), indicating that SAP is required for the redistribution of 2B4 to the lytic immunologic synapse. Importantly, no polarization of 2B4 could be detected when K562 cells were used as targets (Figure 4Aviii and 4C), indicating that 2B4 clustering requires interaction with the CD48 ligand. Our results indicate that the 2B4/SAP pathway is crucial for the assembly of the lytic immunologic synapse at the contact of CD8+ T cells with EBV-infected target cells.
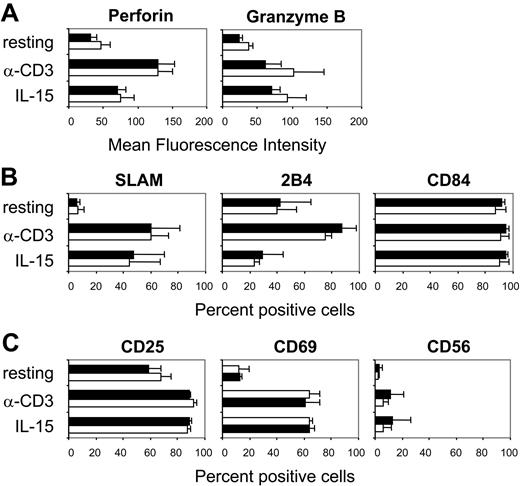
SAP-deficient CD8+ T cells express normal levels of activation markers and lytic molecules
In addition to a defect in lytic immunologic synapse assembly, the defective lytic activity of CD8+ T cells from patients with XLP and HLH could also be the result of an inappropriate activation. SAP-deficient CTLs showed normal intracytoplasmic expression of the 2 major lytic mediators, perforin and granzyme-B. The levels of perforin and granzyme-B, before and after activation with anti-CD3 mAbs or stimulation with IL-15, were comparable both in SAP-deficient and control CTLs (Figure 5A). Furthermore, the expression of SLAM, CD84, and 2B4, which are the best-characterized SAP-binding receptors, in resting and activated CTLs from SAP-deficient and control CTLs was similar. In the resting stage, CTLs expressed very low levels of SLAM, whereas most of the cells showed to be positive for CD84 and 2B4 (Figure 5B). However, TCR activation induced a rapid up-regulation of SLAM, with the majority of the cells becoming positive. Similarly, the levels of CD84 and 2B4 increased, as measured by mean fluorescence intensity, following TCR stimulation. Interestingly, stimulation with IL-15 also led to up-regulation of SLAM, but it had no effect on the expression of CD84 and 2B4 (Figure 5B). The activation markers CD25, CD56, and CD69 were expressed at similar levels in SAP-deficient and control CTLs, prior to and after stimulation through the TCR or with IL-15 (Figure 5C). Therefore, SAP-deficient CD8+ T cells acquire the typical phenotype of activated CTLs upon stimulation either through the TCR or with IL-15.
Phenotype of SAP-deficient CTLs. Phenotype of CD8+ T cells from SAP-deficient patients (SAP; □) and healthy donors (ND; ▪) in a resting state or after activation with 1 μg/mL anti-CD3 mAbs or stimulation with 100 ng/mL IL-15 (mean ± SD of 3 SAP-deficient patients and 3 healthy donors is shown). (A) Mean fluorescence intensity of the intracytoplasmic staining of the lytic molecules perforin and granzyme-B. (B) Percentage of CD8+ T cells staining positive for the CD2-superfamily receptors SLAM, 2B4, and CD84. (C) Percentage of CD8+ T cells staining positive for the T-cell activation markers CD25, CD69, and CD56. One representative experiment of 3 performed is shown.
Phenotype of SAP-deficient CTLs. Phenotype of CD8+ T cells from SAP-deficient patients (SAP; □) and healthy donors (ND; ▪) in a resting state or after activation with 1 μg/mL anti-CD3 mAbs or stimulation with 100 ng/mL IL-15 (mean ± SD of 3 SAP-deficient patients and 3 healthy donors is shown). (A) Mean fluorescence intensity of the intracytoplasmic staining of the lytic molecules perforin and granzyme-B. (B) Percentage of CD8+ T cells staining positive for the CD2-superfamily receptors SLAM, 2B4, and CD84. (C) Percentage of CD8+ T cells staining positive for the T-cell activation markers CD25, CD69, and CD56. One representative experiment of 3 performed is shown.
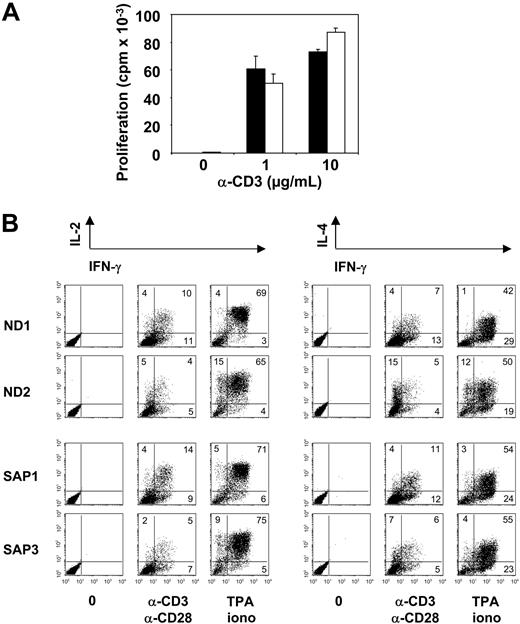
Proliferation and cytokine production of SAP-deficient CD8+ T cells. (A) Proliferation of CD8+ T-cell lines from healthy donors (mean ± SD of ND1, ND2, and ND3; ▪) and SAP-deficient patients (mean ± SD of SAP1, SAP2, and SAP3; □) after stimulation with the indicated doses of immobilized anti-CD3 mAbs. Proliferation is expressed as cpm values corresponding to 3H-thymidine uptake after a 72-hour stimulation. (B) Intracytoplasmic staining for cytokine production in T cells of healthy donors and SAP-deficient patients 6 hours after stimulation with immobilized anti-CD3 mAbs plus anti-CD28 mAbs or TPA/ionomycin. Numbers in dot plot quadrants refer to percentages of T cells positive for the indicated cytokines. One representative experiment of 3 performed is shown.
Proliferation and cytokine production of SAP-deficient CD8+ T cells. (A) Proliferation of CD8+ T-cell lines from healthy donors (mean ± SD of ND1, ND2, and ND3; ▪) and SAP-deficient patients (mean ± SD of SAP1, SAP2, and SAP3; □) after stimulation with the indicated doses of immobilized anti-CD3 mAbs. Proliferation is expressed as cpm values corresponding to 3H-thymidine uptake after a 72-hour stimulation. (B) Intracytoplasmic staining for cytokine production in T cells of healthy donors and SAP-deficient patients 6 hours after stimulation with immobilized anti-CD3 mAbs plus anti-CD28 mAbs or TPA/ionomycin. Numbers in dot plot quadrants refer to percentages of T cells positive for the indicated cytokines. One representative experiment of 3 performed is shown.
Normal proliferation and cytokine production in SAP-deficient CD8+ T cells
We then investigated whether SAP-deficient CD8+ T cells had a general defect in TCR-mediated activation, leading not only to impaired lytic activity but also to defects in proliferation and cytokine production. SAP-deficient CD8+ T cells from patients SAP1, SAP2, and SAP3 proliferated normally after TCR stimulation with immobilized anti-CD3 mAbs (Figure 6A). The production of cytokines, including IL-2, IL-4, and IFN-γ, was normal in CD8+ T cells from patients with SAP1 (HLH) and SAP3 (XLP), both after stimulation with anti-CD3/anti-CD28 mAbs or with TPA/ionomycin (Figure 6B). The normal cytokine production by SAP-deficient CD8+ T cells was confirmed by enzyme-linked immunosorbent assay (data not shown). These data indicate that, although the cytotoxic activity against B-cell targets presenting EBV antigens was impaired in the CTLs of patients, signaling through the TCR, which leads to proliferation and cytokine production, was preserved.
Discussion
The clinical onset of XLP is usually triggered by EBV infection. The defective lytic activity of NK cells has been suggested to account for the inappropriate response to EBV-infected B cells and for the development of the pathologic phenotypes of XLP.23,24 Our study, together with the recent findings by Sharifi et al28 in patients with XLP, demonstrate that the cytotoxic activity of SAP-deficient CD8+ T cells from patients with XLP and HLH toward EBV-infected B-cell targets is also impaired. Therefore, the clinical manifestations of SAP-deficiency syndromes may result from a synergistic defect in T- and NK-cell–mediated lysis of EBV-infected B cells. In the present study, we show that the absence of SAP (in CTLs from patients) or the blockade of 2B4 (in normal CTLs) results in a specific defective CTL activity toward EBV-infected targets. This lytic defect is associated with a defect in polarization of 2B4, perforin, and lipid rafts, indicating that signaling through 2B4 and SAP controls and promotes the polarization of the lytic machinery of CTLs at the site of contact with EBV-infected target cells.
Although CD8+ T cells from patients with SH2D1A gene mutations have a defective lytic activity against EBV-infected cells, they have the phenotype of effector CTLs. The activation markers CD25, CD69, CD56 are normally up-regulated in SAP-deficient CD8+ T cells following activation through the TCR or with IL-15. Furthermore, the SAP-interacting receptors SLAM, 2B4, and CD84, which costimulate lytic activity,15,30 are normally expressed in resting or activated CD8+ T cells from SAP-deficient patients. These results show that SAP deficiency does not impair the expression of these receptors. In addition, the levels of expression and regulation of granzyme-B and perforin are also normal in SAP-deficient CD8+ T cells, which can form conjugates with EBV-infected target cells, indicating that these cells are equipped to mediate lysis.
However, the polarization of the lytic mediator perforin toward the cell-cell contact area is significantly reduced in CD8+ T cells from patients with XLP and HLH, and this can be responsible for the lytic defect. Indeed, effective lysis requires the rapid polarization of perforin-containing lytic granules to the center of the lytic immunologic synapse that forms at the CTL-target cell contact.31-33 The polarization of the lipid raft marker GM1, which has been shown to cluster at the immunologic synapse,34 is also reduced in CTLs lacking SAP. In conclusion, the absence of SAP impairs the polarization of both lytic granules and lipid rafts at the contact of the CTL with the target cell, suggesting a general defect in the assembly of the lytic immunologic synapse. This defect is restricted to EBV-positive CD48+ target cells since both the polarization of perforin and GM1 and the lytic activity against the EBV-negative K562 targets are not reduced in SAP-deficient CTLs.
SAP might be specifically required for the activation of CTLs against EBV because of the sustained stimulation through 2B4 induced by EBV-infected B cells. Indeed, expression of CD48, the natural ligand of 2B4, is strongly increased on B cells upon EBV infection.25 In this context, the absence of SAP might be much more critical than during infection by other intracellular pathogens. Our data show that expression of both 2B4 and SAP are associated to a high lytic activity of normal CTLs. In contrast, SLAM is not expressed in active CTLs and requires stimulation with anti-CD3 mAbs or IL-15 to be up-regulated. Given the rapid down-regulation of SAP expression observed after TCR activation, it is likely that in human CTLs, SAP preferentially signals through 2B4, rather than through SLAM, during the first minutes of stimulation needed for the activation of the lytic machinery. In agreement with this hypothesis, experiments with blocking anti-2B4 mAbs demonstrated that the lytic activity of normal CTLs against JY cells, as well as the polarization of perforin, is mediated through 2B4. Interestingly, there is evidence that 2B4 is involved in the assembly of the immunologic synapses that form between NK cells and their targets.35,36 Recent studies show that 2B4 and SAP are rapidly recruited to the central part of the NK immunologic synapse that forms at the contact with CD48+ target cells.37,38 In addition, it has been proposed that killer cell inhibitory receptors (KIRs) negatively control NK immunologic synapse assembly and lytic activity by blocking recruitment of 2B4 into lipid rafts and by blocking clustering of lipid rafts at the synapse in a SHP-1– and SHP-2–dependent manner.34,39 Since SAP has the properties to compete with SHP-2 and to bind to 2B4, it is possible that SAP regulates the activity of both activatory (2B4) and inhibitory (KIR) receptors controlling the assembly of the lytic synapse. Similarly to the localization of 2B4 to the NK immunologic synapse, we found that 2B4 is recruited to the immunologic synapse formed between normal CD8+ CTLs and EBV-positive targets. In contrast, in the absence of SAP, 2B4 is not recruited to the site of the immunologic synapse and is confined to subtle patches distributed over the surface of the CTLs. This demonstrates that SAP is required for the recruitment of 2B4 to the site of the immunologic synapse. In addition, no 2B4 clustering was observed in normal CTLs forming conjugates with CD48- targets (K562), suggesting that the SAP-dependent 2B4 relocalization is restricted to EBV-specific CTLs. Together, our results indicate that in SAP-deficient CTLs, defective relocalization of 2B4 during encounter with a CD48+ target cell (EBV-infected) results in failure to organize the lytic synapse.
SAP expression is tightly regulated during TCR-driven activation of human untransformed CD8+ T cells. Indeed, after 24- and 72-hour stimulation, the levels of SAP protein are strongly down-regulated, confirming data obtained in murine T cells.40 Since we show that signaling through 2B4 and SAP is crucial for the assembly of the lytic immunologic synapse and for the lytic activity of EBV-specific CTLs, it is possible that the down-regulation of SAP expression decreases CTL activation after the killing of target cells has occurred. Our data point to a posttranscriptional regulation of the SAP protein since SAP mRNA is not down-regulated following TCR stimulation (data not shown). In addition, patients' T cells express normal levels of point-mutated SAP mRNA, but no protein is detected. Accordingly, we found that retroviral vector–mediated SH2D1A gene transfer into T cells of SAP-deficient patients results in very limited levels of SAP protein expression, despite the presence of SAP mRNA. This limited expression of the SAP protein after gene transfer was not sufficient to restore the ability of patients' T cells to lyse target cells and to polarize perforin and GM1 (data not shown).
Besides acting as costimulatory molecules to enhance cytotoxic activity, SLAM-family receptors, such as SLAM and CD84, are involved in the pathway regulating IFN-γ production.11,14,41 Sharifi et al28 demonstrated that SAP-deficient EBV-specific T-cell lines had a reduced frequency of IFN-γ–producing cells upon TCR or TCR/2B4 stimulation in parallel to having a defect in the lysis of autologous EBV-positive target cells. In addition, Sanzone et al18 reported a role of SAP for TCR signaling, proliferation, IL-2 production, and adhesion. However, no defects in proliferation, cytokine production, and CD25 up-regulation were observed in the SAP-deficient T-cell lines described here. This discrepancy could be due to differences in the clinical stage and history of infection of the patients investigated.
Together, our results indicate that SAP-deficient CD8+ T cells from patients with XLP and HLH have defects in the regulation of cytotoxic activity against EBV-positive targets. Although the phenotype of these CD8+ T cells is normal, they have a reduced ability to polarize key components of the lytic immunologic synapse at the contact with EBV-positive target cells. Together with the previously characterized defects in the lytic activity of NK cells, these defects could explain the clinical manifestations of XLP and related disorders resulting from SAP deficiency.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-08-3269.
Supported by a core grant from the Italian Telethon Foundation (M.-G.R.) and the New South Wales Cancer Council (S.G.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients, their families, and the medical staff that took care of them. We are grateful to Dr Nichols for the anti-SAP Abs, and to Dr Aversa for the anti-SLAM mAbs.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal