Comment on Hammerman et al, page 4477
Hammerman and colleagues have shown that the Pim-2 kinase is required to confer rapamycin resistance in hematopoietic cells, thus providing a strong rationale for the development of Pim kinase inhibitors as potential therapeutic agents in hematologic malignancies.
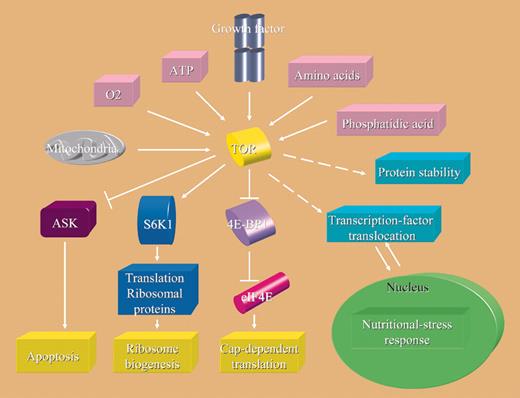
Clinical exploration of the phosphatidyl-inositol 3-kinase (PI3K)/Akt/mTOR (mammalian target of rapamycin) pathway as a therapeutic target in leukemia is in its infancy. Myriad preclinical data support this endeavor, ranging from demonstrable constitutive up-regulation of these kinases in leukemia to the in vitro cytotoxicity of rapamycin against a broad spectrum of hematologic malignancies. Rapamycin first binds to a cyclophilin FKBP12 in mammalian cells, and this complex binds to, and inhibits the function of, mTOR. As mTOR is the only identified target, rapamycin and its analogs are the most selective kinase inhibitors currently in the clinic. mTOR acts as a central controller sensing cellular environment (nutritional status or mitogenic stimulation) and regulating translation initiation through both the eukaryotic initiation factor 4E and ribosomal p70 S6 kinase pathways. Rapamycin's adverse pharmacokinetic properties have encouraged the development of analogs, three of which, RAD001, CCI-779, and AP23573, are in early studies in patients with leukemia.1 In general these drugs are well tolerated with mucositis, or easily manageable metabolic adverse effects being the dominant observations. Initial data indicate that single-agent activity of the rapamycin analogs is limited, with the possible exception of advanced mantle-cell lymphoma. With increasing evidence that many oncogenic kinases, including vascular endothelial growth factor receptor (VEGFR), FMS-like tyrosine kinase 3 (FLT3), platelet-derived growth factor receptor β (PDGFRβ), Raf, and break-point-cluster region/Abelson leukemia (BCR-ABL), mediate critical activities via the mTOR pathway, the therapeutic focus on mTOR also increases. It thus becomes vital to understand what mediates rapamycin resistance. Previous postulates to explain such resistance (in various in vitro systems) have included mutations in mTOR, FKBP12, ATM, S6K1, PP2A-related phosphatases, or 14-3-3 scaffold proteins and/or deregulation of eIF4E or p27kip1.2 The relative contribution of these mechanisms to mTOR inhibitor resistance in humans requires further study. A clear advance has been taken in this area, with the elegant demonstration by Hammerman and colleagues that Pim-2 is required to confer rapamycin resistance in primary hematopoietic cells. Noting the absence of significant hematopoietic toxicity in humans receiving rapamycin, the authors investigated pathways that could maintain hematopoietic cell growth independently of Akt and mTOR. This led them to focus on Pim-2, an oncogene that promotes apoptosis resistance in hematopoietic stem cells that is not inhibited by mTOR inhibitors.3 The authors show that cells from Pim-2– and Pim-1/2– deficient animals failed to accumulate and underwent apoptosis in the presence of rapamycin. Primary hematopoietic cells from Akt-1/Pim-1/2– deficient animals had marked impairments in cell growth and survival while ectopic expression of either Pim-2 or Akt-1 induced increased cell size and apoptotic resistance. The effects of ectopic Akt-1 were reversed by rapamycin or nonphosphorylatable form of 4EBP-1, while those of Pim-2 were not. The authors also demonstrated that coexpression of Akt-1 and Pim-2 in transgenic mice cooperatively induced lymphoma in all progeny. These data indicate that Pim-2 and Akt-1 are important components of overlapping, independent pathways, either of which is sufficient to promote the growth and survival of nontransformed hematopoietic cells. Cohen and colleagues4 have reported evidence for a linkage of Pim-2 up-regulation to the development ofhumanlymphomaandchroniclymphocytic leukemia (CLL). Based on these data, Pim-2 is an important new target kinase. An important next step will be to assay baseline levels and to monitor sequential changes in Pim-1 and/or Pim-2 in patients who are receiving mTOR inhibitor therapy. Demonstrating a relationship between Pim up-regulation and mTOR inhibitor resistance would significantly encourage the development of Pim kinase inhibitors, which may be active alone, or could be combined with mTOR inhibitors, to potentially maximize the latter's antileukemic activity.5 ▪


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal