Abstract
T-cell dysfunction after human hematopoietic stem-cell transplantation (HSCT) is generally attributed to intrinsic T-cell defects. Here we show that the characteristic impaired proliferative responses to polyclonal stimulation of post-HSCT peripheral blood mononuclear cells (PB-MCs) were markedly (4-fold) improved by T-cell enrichment. Conversely, addback of post-HSCT monocytes to these enriched T cells dampened their proliferative responses, suggesting that post-HSCT monocytes effectively mediate T-cell suppression. As a mechanism possibly contributing to monocyte-mediated T-cell suppression, we investigated monocyte tryptophan catabolism by indoleamine 2,3-dioxygenase into kynurenine, which has been implicated in regulating T-cell responses. Compared with controls, all post-HSCT monocyte-containing cell cultures (total PBMCs, monocytes, and monocyte/T-cell cocultures), but not monocyte-depleted populations, secreted elevated amounts of kynurenine. Blockade of tryptophan catabolism improved the proliferative responses. The slightly increased kynurenine release and substantial release of neopterin by unstimulated post-HSCT monocytes suggests that they were in a state of continuous activation. Superimposed on this state, stimulation of these cells caused a striking, additional increase (10-fold) in kynurenine release, and they triggered marked apoptosis of autologous post-HSCT T cells. We conclude that the amplified kynurenine release by post-HSCT monocytes, particularly induced upon stimulation, may underlie their suppressor activity, which in turn may contribute to the depressed T-cell immune responses after HSCT.
Introduction
Hematopoietic stem-cell transplantation (HSCT) profoundly alters host immunity by several mechanisms. First, a state of transient immunodeficiency is induced by the lymphocytotoxic effects of myeloablative and even nonmyeloablative preparative regimens.1-3 Second, the transfusion of hematopoietic stem cells (HSCs) will modulate the recipient immune system, by eliciting host-versus-graft or graft-versus-host reactions in the allogeneic setting4-6 and through immunomodulatory effects of the cytokines used to prepare HSCs in the autologous setting.7 Third, allogeneic HSCT requires post-HSCT pharmacologic immunosuppression. The ensuing immunodeficient state renders HSCT recipients particularly susceptible to opportunistic infections that require T-cell control, such as caused by viruses8-11 and fungi.12
The profound alteration of T-cell immunity thus represents a still-unsolved core problem of HSCT (reviewed in Storek and Witherspoon13 ). It occurs on the basis of discordant regeneration kinetics, a skewed T-cell receptor (TCR) repertoire,14,15 and unbalanced function of individual T-cell subpopulations.16-18 Dysfunction of T cells, frequently observed as a decreased lymphoproliferative response to activation,16 has been attributed to numerous defects intrinsic to T cells. These include inappropriate cellular activation19,20 and disturbed cytokine production,21,22 especially of interleukin 2 (IL-2),23,24 and increased susceptibility to apoptosis.25,26
Here, we show that an impaired T-cell proliferative capacity early after HSCT may be mediated by potent suppressor activity of monocytes. Monocytes obtained from HSCT recipients (autologous and allogeneic) 1 to 6 months after HSCT, when cocultured with highly enriched T cells, had a profound inhibitory effect on the T-cell proliferative response to polyclonal stimulation in contrast to the effect of monocytes from healthy controls. In efforts to trace the mechanism, we discovered enhanced kynurenine production and release into cell culture supernatants by post-HSCT monocytes, particularly upon stimulation. Kynurenine, the first stable down-stream metabolite of tryptophan, is generated through the enzymatic activity of indoleamine 2,3-dioxygenase (IDO; reviewed in Moffett and Namboodiri27 ). Its release correlates to the rate of tryptophan catabolism,28 which has been recently described to represent a key mechanism regulating T-cell responses in vitro and in vivo (reviewed in Mellor and Munn29 ). The studies reported here suggest that after HSCT monocytes are dysfunctional, generating enhanced amounts of kynurenine and thereby contributing to the suppressed T-cell responses frequently observed after HSCT.
Patients, materials, and methods
Patients
Peripheral blood samples of 31 recipients of HSCT were included in the study, which was approved by the institutional review boards of the participating institutions. Informed consent was obtained from all patients and/or their parents. Blood sampling was performed upon routine examinations to minimize harm to the patients. There were 21 pediatric and 10 adult patients; 16 males and 15 females. The median age of the pediatric patients was 10 years (range 2-17 years) and of the adults was 33 years (range 18-62 years). The donors for transplantation were matched unrelated donors (n = 17), matched sibling donors (n = 7), autologous bone marrow (n = 6), and 1 haplomatched donor. Twenty-five patients had either leukemia or lymphoma as their primary diagnosis; 6 patients had other diagnoses including hematologic or immunologic diseases or solid tumors. All HSCT recipients received a myeloablative preparative regimen, including high-dose chemotherapy with or without total body irradiation. After allogeneic HSCT, graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine A (CSA) alone in 20 patients, CSA combined with mycophenolate mofetil in 1 patient, and CSA combined with steroids in 4 patients. Examination of lymphocyte function was performed between 1 and 6 months after HSCT (median 3 months). Patients were studied when they were in complete remission and had a good performance status and were free of infectious complications. GVHD was absent in 22 patients and grade II in 3 (< 50% skin involvement, bilirubin < 3 mg/dL, diarrhea < 500 mL/d).30
Cell isolation and culture
Heparinized peripheral blood from HSCT recipients and from healthy volunteers was, in parallel, subjected to the same procedures in each experiment to control for inter-experimental variations. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway) and resuspended in complete medium, consisting of RPMI 1640 (PAA Laboratories, Linz, Austria) supplemented with 10% fetal calf serum (FCS; PAA Laboratories), 2 mM l-glutamine (PAA Laboratories), and 100 μg/mL penicillin/streptomycin (PAA Laboratories). PBMCs were separated into a T-cell population (CD3+) or a monocyte population (CD14+) by magnetic cell sorting using the T-cell and monocyte isolation kits (MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany). This routinely yielded a 95% CD3+ and a 94% CD14+ population, respectively. Control experiments to test functional effectiveness of enrichment procedures20 showed that separate T-cell and monocyte populations obtained from controls had only minimal proliferative responses upon stimulation with phytohemagglutinin (PHA; see “Lymphocyte proliferation assay”), but full proliferative responses when recombined (not shown).
To induce the expression of IDO, PBMCs or monocytes were cultured in medium supplemented with 20 ng/mL macrophage-colony-stimulating factor (M-CSF; Chemicon International, Temecula, CA) for 4 days, and graded doses of interferon γ (IFN-γ; Boehringer Ingelheim, Ingelheim, Germany) were added for the last 18 hours of culture, as indicated.31 The competitive inhibitor of IDO, 1-methyl-dl-tryptophan (MDLT; Sigma, St Louis, MO),32 was dissolved in 1 N NaOH, adjusted to pH 7.4, and added to PBMC cultures at 0.05 M to 0.5 M concentrations.
Quantification of mononuclear-cell subpopulations by flow cytometry
The proportions of total T cells (CD3+) and of monocytes (CD14+) contained in PBMCs and in the enriched cell populations were assessed by flow cytometry prior to and subsequent to enrichment procedures. Monoclonal antibodies (mabs) were obtained from BD Biosciences (San Jose, CA). Cells were analyzed on a FACScan flow cytometer (BD Biosciences) and the list mode data were analyzed using the Cell Quest software (BD Biosciences). To detect T-cell apoptosis, anti-CD3 mab was used to gate for the T-cell population, and apoptotic cells were defined as the annexin V-positive (fluorescein isothiocyanate [FITC]) and propidium iodide-negative population. Absolute cell counts (cells/μL) of CD3+ and CD14+ cells were calculated from the differential count of total white blood cells.
Lymphocyte proliferation assay
Cells were stimulated with optimal concentrations of PHA (1 μg/mL; Sigma) or of anti-CD3 (250 ng/mL; Sigma). Anti-CD3 was immobilized by precoating 48-well multiplates (Falcon; BD Labware, Franklin Lakes, NJ) with goat anti-mouse immunoglobulin G (IgG; Sigma). Routinely, 6 × 105 PBMCs/well in 1 mL complete medium were cultured in humidified air containing 5% CO2 at 37°C. In experiments assessing the effect of monocytes on the T-cell proliferative response, 4.8 × 105 T cells/well were stimulated with immobilized anti-CD3 and cocultured with monocytes as indicated (10%-40%). PBMCs or T cells alone or T cells cocultured with monocytes, maintained in medium only (“resting conditions”), served as negative controls. After 48 hours, 400 μL of the cell culture supernatant was carefully removed and immediately frozen at -20°C. For measuring the proliferative capacity, cell populations were collected by gentle pipetting and transferred to 96-well flat-bottom tissue-culture plates (Falcon; BD Biosciences). 1 μCi (3.7 × 104 Bq) [3H]thymidine (ICN Biomedicals, Costa Mesa, CA) per well was added and 18 hours later the cells were harvested (Skatron, Lier, Norway) and [3H]thymidine incorporation was assessed by β-scintillation counting. Net counts per minute (cpm) were calculated by subtracting the cpm of resting cultures from those of stimulated cultures. In selected experiments the T-cell proliferative response (1 × 105) to allogeneic monocytes (0.3 × 104) was measured in 5-day cultures (mixed lymphocyte reaction [MLR]).
Quantification of kynurenine, tryptophan, and neopterin
Free tryptophan and kynurenine concentrations were determined by high-perfomance liquid chromatography (HPLC) as previously described.33,34 Briefly, tryptophan was detected by its natural fluorescence at 286 nm excitation and 366 nm emission wavelengths. 3-nitro-l-tyrosine, used as an internal standard, and kynurenine were determined by ultraviolet absorption at 360 nm. An albumin-based external standard mix was prepared and included 50 μM tryptophan (Serva, Heidelberg, Germany), 10 μM kynurenine (Sigma), and a frozen serum pool. Upon the addition of 25 μL2 M trichloroacetic acid (Merck, Darmstadt, Germany) the reaction vials were immediately vortexed and centrifuged at 12 000g (13 000 rpm) for 6 minutes at room temperature to precipitate protein. The concentration of the components was calculated according to peak heights and was compared with 3-nitro-l-tyrosine as a reference standard. The amount of neopterin released was measured by enzyme-linked immunosorbent assay (ELISA; BRAHMS Diagnostica, Berlin, Germany)
Detection of IDO protein expression
IDO expression was examined by flow cytometry.35 PBMCs (5 × 105) were stained with CD14 mab and then subjected to fixation and permeabilization with Cytofix/Cytoperm (BD Pharmingen, San Jose, CA) according to the manufacturer's instructions. Cells were incubated with rabbit anti-IDO antibody (Chemicon International) followed by Cy5-conjugated antirabbit secondary antibody (Chemicon International).
Statistics
Statistical analyses (Wilcoxon signed rank test, Wilcoxon 2-sample test, 2-tailed) were used where appropriate to detect differences between findings in control and post-HSCT samples. A P value of less than .05 was considered to indicate statistical significance.
Results
Monocytes mediate inhibition of the T-cell proliferative capacity in the post-HSCT period
In our initial experiments, we compared the proliferative response of PBMCs obtained from 15 HSCT recipients (GVHD less than or equal to grade II, no apparent infection), 1 to 6 months after HSCT, to that of 15 controls. In agreement with current knowledge,16,36 the post-HSCT PBMCs showed an impaired in vitro proliferative response to polyclonal stimulation by immobilized anti-CD3 (Figure 1A), with a median reduction of 89%, compared with the control (P < .001).
The reduction of proliferative responses of total PBMCs after HSCT has been shown to occur on the basis of both a reduced T-cell content and a reduced T-cell responsiveness.20 Both were found in this study cohort. PBMCs of 15 HSCT recipients contained reduced proportions and numbers of T cells (median 40%, or 0.32 × 109 cells/L [range 0.06 to 1.06] whole blood), compared with healthy controls older than 10 years of age (normal range 55%-82%, or 1.0 × 109-3.9 × 109 cells/L).37,38 In contrast, monocytes were present in increased proportions (median 14%) as compared with healthy controls (normal range 3%-8%) and were within the normal range by absolute counts (median, 0.39 × 109 cells/L whole blood; range 0.13 to 0.83 × 109 cells/L).39 Second, to probe for the possibility of a reduced T-cell responsiveness in our current studies of mechanisms of inhibition, we highly purified the T cells (95% CD3+ cells) from the PBMC populations and stimulated equal numbers of these highly enriched T cells (1 × 105/well) with immobilized anti-CD3. In this case, T-cell stimulation through the T-cell receptor (TCR) is largely independent of monocytes40 and allows examination of T-cell proliferative responses independent of interactions with non-T cells. Strikingly, when T cells from the poorly responding post-HSCT PBMC populations were highly purified, the proliferative responses to immobilized anti-CD3 were significantly increased (Figure 1A, right; 4.1-fold median increase; P < .001) and were almost equivalent to those of control T cells, which in themselves showed no increase over the response of control PBMCs (Figure 1A, left). These improved proliferative responses by T-cell enrichment were detectable despite the heterogeneity of the transplant conditions in this cohort of patients (eg, different types of HSCT, various underlying diseases, different conditioning regimens, and different treatments for GVHD prophylaxis). The findings suggested that the inhibition of proliferative responses to TCR-mediated T-cell stimulation, after HSCT, was caused by a cell population contained in the non-T-cell fraction of PBMCs.
Monocytes mediate inhibition of T-cell proliferative responses after HSCT. (A) Total PBMCs or T cells highly enriched from total PBMCs obtained from healthy controls (□) or HSCT recipients (▪) were stimulated by immobilized anti-CD3 (250 ng/mL) for 72 hours and the proliferative response was quantified by net [3H]thymidine incorporation. Each bar depicts the median proliferative response of 15 experiments. Proliferation of post-HSCT PBMCs was significantly lower than that of control PBMCs (P < .001). The asterisk indicates that in HSCT recipients proliferation of enriched T cells was significantly increased over that of total PBMCs (P < .001). (B) T cells were cocultured with graded proportions of autologous monocytes as indicated and stimulated as in panel A. The bars depict net [3H]thymidine incorporation of control cultures (□) and cultures obtained from HSCT recipients (▪) as percent of the response of the respective purified T cells alone (horizontal line = 100%). *Significant (P < .05) differences of post-HSCT and control cultures. Error bars indicate standard error of the mean (SEM).
Monocytes mediate inhibition of T-cell proliferative responses after HSCT. (A) Total PBMCs or T cells highly enriched from total PBMCs obtained from healthy controls (□) or HSCT recipients (▪) were stimulated by immobilized anti-CD3 (250 ng/mL) for 72 hours and the proliferative response was quantified by net [3H]thymidine incorporation. Each bar depicts the median proliferative response of 15 experiments. Proliferation of post-HSCT PBMCs was significantly lower than that of control PBMCs (P < .001). The asterisk indicates that in HSCT recipients proliferation of enriched T cells was significantly increased over that of total PBMCs (P < .001). (B) T cells were cocultured with graded proportions of autologous monocytes as indicated and stimulated as in panel A. The bars depict net [3H]thymidine incorporation of control cultures (□) and cultures obtained from HSCT recipients (▪) as percent of the response of the respective purified T cells alone (horizontal line = 100%). *Significant (P < .05) differences of post-HSCT and control cultures. Error bars indicate standard error of the mean (SEM).
Several cell populations can potentially inhibit T-cell responses after transplantation.4,41 Because monocytes may have such a suppressor effect on T cells,42-44 here we have studied the role of the monocyte population in suppressing T-cell proliferation after HSCT. Constant numbers of highly enriched T cells (1 × 105/well) were cocultured with increasing proportions of highly enriched autologous monocytes (94% CD14+ cells). These coculture experiments (Figure 1B) showed that in controls the addback of 10% and 25% monocytes slightly increased the T-cell proliferative response (145% and 115%, respectively), and that even the addback of a high (40%) proportion of monocytes resulted in a proliferative response equivalent to that of T cells alone. In contrast, the proliferation of post-HSCT T cells was inhibited at all 3 levels of monocyte addback, and this inhibition was quantitatively related to the number of monocytes added. The median inhibition of [3H]thymidine uptake was 20%, 28%, and 59%, by addition of 10%, 25% and 40% monocytes, respectively, suggesting monocyte-mediated impairment of T-cell proliferation on a per cell basis.
Enhanced kynurenine accumulation in post-HSCT cell cultures
One effective pathway by which monocytes acquire the capacity to suppress T-cell responses in vitro and in vivo is through the induction of monocyte tryptophan catabolism, by the enzymatic activity of IDO. Tryptophan catabolism is induced upon monocyte activation, for example, by in vitro exposure to lipopolysaccharide (LPS), M-CSF or IFN-γ.31,45 As established in previous studies, this eventually results in tryptophan depletion31 and in the accumulation of tryptophan catabolic products such as the kynurenines. To assess tryptophan catabolism as a mechanism of monocyte-mediated T-cell inhibition after HSCT, we compared kynurenine release in the same sets of cell populations studied in Figure 1. As kynurenine is the first stable molecular species of tryptophan catabolism, its release was used as a measure of tryptophan catabolism. We reasoned that if elevated kynurenine release was related to the proliferative defect in PBMCs, this should be detectable in the unstimulated state and/or after stimulation with immobilized anti-CD3.
The first set of analyses evaluated kynurenine content of the culture supernatants of resting and activated PBMCs and T-cell populations (Figure 2A). Several important observations were made. First, the median level of kynurenine accumulation of unstimulated PBMCs was somewhat elevated in post-HSCT PB-MCs, compared with control PBMCs (0.81 μM versus 0.41 μM kynurenine, P < .001). Second, there was no difference in kynurenine release of unstimulated highly enriched control versus post-HSCT T cells. Third, activated PBMC cultures of post-HSCT samples accumulated significantly greater quantities of kynurenine than did activated control PBMC cultures (3.4 μM versus 1.2 μM, median, P < .001), whereas, finally, activation of either control or post-HSCT highly enriched T-cell populations did not enhance their kynurenine accumulation. These findings clearly implicate the non-T cell-containing population as the source of the kynurenine. Furthermore, what these experiments in their composite suggest, is that in addition to a state of enhanced baseline kynurenine release (which could be associated with chronic activation), post-HSCT cells appear highly sensitive to activation, evidenced as exceptionally high kynurenine production and release in stimulated cultures.
Monocyte-containing cell cultures after HSCT contain elevated amounts of kynurenine. (A) The culture supernatants of cell populations assessed for their proliferative response to immobilized anti-CD3 in Figure 1 were examined for kynurenine content by HPLC. Each point represents the μM kynurenine released per single experiment in cultures of total PBMCs or of T cells, maintained in resting conditions (top row) or stimulated with anti-CD3 (bottom row). ♦ indicates controls; ▪, HSCT. The median of 15 experiments is shown by the horizontal line. (B) T cells were cocultured with increasing proportions of autologous monocytes (as in Figure 1B). The bars indicate the median fold increase of kynurenine release over that of T cells alone. □ indicates controls; ▪, HSCT. - indicates not done. *Significant differences of post-HSCT and control cultures (P < .05). Error bars indicate SEM.
Monocyte-containing cell cultures after HSCT contain elevated amounts of kynurenine. (A) The culture supernatants of cell populations assessed for their proliferative response to immobilized anti-CD3 in Figure 1 were examined for kynurenine content by HPLC. Each point represents the μM kynurenine released per single experiment in cultures of total PBMCs or of T cells, maintained in resting conditions (top row) or stimulated with anti-CD3 (bottom row). ♦ indicates controls; ▪, HSCT. The median of 15 experiments is shown by the horizontal line. (B) T cells were cocultured with increasing proportions of autologous monocytes (as in Figure 1B). The bars indicate the median fold increase of kynurenine release over that of T cells alone. □ indicates controls; ▪, HSCT. - indicates not done. *Significant differences of post-HSCT and control cultures (P < .05). Error bars indicate SEM.
These observations were pursued by the experiments shown in Figure 2B, in which we assessed the effect on kynurenine release of adding graded quantities of either control or post-HSCT monocytes to constant numbers of autologous purified T cells. This addback controlled for possible variations in monocyte number as a cause for altered kynurenine release in PBMC cultures. In resting cultures, the addition of 25% monocytes to autologous control T cells had essentially no effect on kynurenine release, whereas the same proportion of post-HSCT monocytes added to post-HSCT T cells resulted in a significant (1.7-fold) increase in kynurenine release. Even more striking was the effect observed in cultures activated by exposure to immobilized anti-CD3. In this case, while the addition of 10% to 40% control monocytes to purified control T cells increased kynurenine release only marginally (up to 1.4-fold), a striking, up to 4.3-fold (P < .025) monocyte-related increase was observed in the post-HSCT samples. These data provide further evidence that post-HSCT monocytes are highly sensitive to activation by the release of kynurenine.
Evidence that post-HSCT monocyte kynurenine release reflects a state of enhanced activation
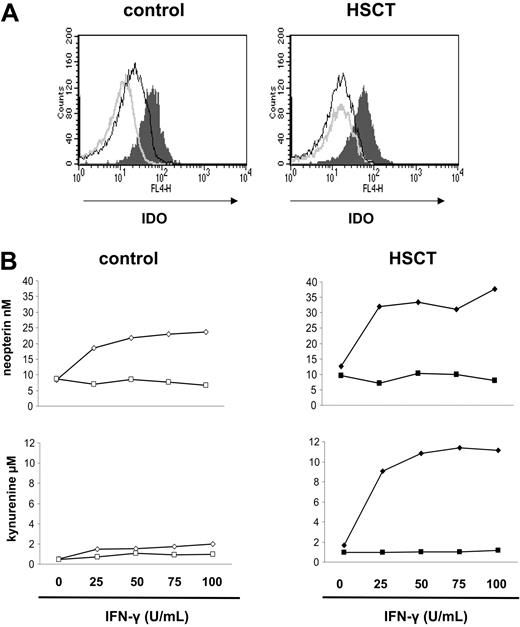
Since kynurenine is the first stable catabolic product of tryptophan, and tryptophan catabolism is generally understood to be initiated upon cellular activation, the enhanced generation of kynurenine by post-HSCT monocytes could indicate a state of increased monocyte activation.31 To test the relationship between enhanced kynurenine production and the state of cellular activation, we correlated the release of kynurenine by monocytes to that of neopterin. Neopterin is a guanosine triphosphate (GTP) cleavage product. Although its precise function remains unknown, its release has been understood as a highly sensitive indicator of monocyte activation.46,47 Enriched monocytes (5 × 105/0.5 mL) were cultured for 4 days with or without the IDO-inducing stimulants M-CSF and IFN-γ. We first ascertained by flow cytometry that, as in control cells, IDO protein expression in post-HSCT PBMCs induced by M-CSF and IFN-γ (100 U/mL) segregated in the CD14+ population31 (Figure 3A) and was barely detectable in the CD14- populations.
Then, total PBMCs were separated by MACS into a CD14+ population (monocytes) and a CD14- population and exposed to 20 ng/mL M-CSF for 4 days and graded amounts of IFN-γ during the last 18 hours of culture. Kynurenine and neopterin release into the cell culture supernatants was quantified. Several interesting observations were made (Figure 3B). First, in the absence of in vitro activation, supernatants of the unstimulated CD14+ cultures from post-HSCT patients had a higher kynurenine content (3-fold) than did supernatants obtained from control cultures, and in parallel an enhanced (∼2.0-fold) release of neopterin. Second, stimulation with M-CSF and IFN-γ increased kynurenine release and neopterin production in the CD14+ cells of control and post-HSCT cultures. However, while in proportion to the respective baseline levels the increase of neopterin release was similar in post-HSCT and control monocytes (3.0-fold and 2.8-fold, respectively), the absolute differences in kynurenine release were striking (Figure 3B, lower right). Even upon exposure to low doses of IFN-γ (25 U/mL), kynurenine in post-HSCT monocyte cultures rose from 1.7 μM (resting) to more than 9 μM, and reached a maximum of 11.6 μM upon exposure to 100 U/mL IFN-γ. In control cultures kynurenine concentrations only increased from 0.5 μM (resting) to 2.0 μM (stimulated). Finally, neither control nor post-HSCT CD14- cells were induced to release kynurenine or neopterin upon exposure to IFN-γ.
Several conclusions can be drawn: (1) significant tryptophan catabolic activity is confined to the CD14+ monocyte population in control and post-HSCT cells; (2) slightly higher kynurenine release by resting post-HSCT monocytes, in parallel with a higher neopterin level, suggests a state of enhanced activation of these cells, even without the addition of stimulating agents; (3) both normal and post-HSCT monocytes appear sensitive to activating stimuli (by neopterin release); but (4) post-HSCT monocytes respond with much higher kynurenine production, suggesting that stimulation of post-HSCT monocytes facilitates their conversion into suppressor cells, and in fact that post-HSCT monocytes are an inducible suppressor cell population.
IDO expression and activity by CD14+ monocyte population. (A) Total PBMCs obtained from normal controls or from HSCT recipients were cultured in medium supplemented with M-CSF (20 ng/mL) for 4 days, and IFN-γ (100 U/mL) that was added for the last 18 hours of culture and examined for IDO expression by flow cytometry as described in “Patients, materials, and methods.” Gray shaded histograms represent the CD14+ cell population; dark lines, the CD14- cell population; and light gray lines, negative control (second antibody only). (B) Total PBMCs were separated into a CD14+ (diamonds) and a CD14- (squares) cell population and were cultured in medium supplemented with M-CSF (20 ng/mL) for 4 days. During the last 18 hours of culture, graded amounts of IFN-γ were added as indicated. At the end of culture, concentrations of neopterin (top row) and of kynurenine (bottom row) were determined in cell culture supernatant by ELISA and HPLC, respectively. Results of one experiment, representative of 3 experiments, are shown.
IDO expression and activity by CD14+ monocyte population. (A) Total PBMCs obtained from normal controls or from HSCT recipients were cultured in medium supplemented with M-CSF (20 ng/mL) for 4 days, and IFN-γ (100 U/mL) that was added for the last 18 hours of culture and examined for IDO expression by flow cytometry as described in “Patients, materials, and methods.” Gray shaded histograms represent the CD14+ cell population; dark lines, the CD14- cell population; and light gray lines, negative control (second antibody only). (B) Total PBMCs were separated into a CD14+ (diamonds) and a CD14- (squares) cell population and were cultured in medium supplemented with M-CSF (20 ng/mL) for 4 days. During the last 18 hours of culture, graded amounts of IFN-γ were added as indicated. At the end of culture, concentrations of neopterin (top row) and of kynurenine (bottom row) were determined in cell culture supernatant by ELISA and HPLC, respectively. Results of one experiment, representative of 3 experiments, are shown.
Effect of IDO inhibition on T-cell proliferation
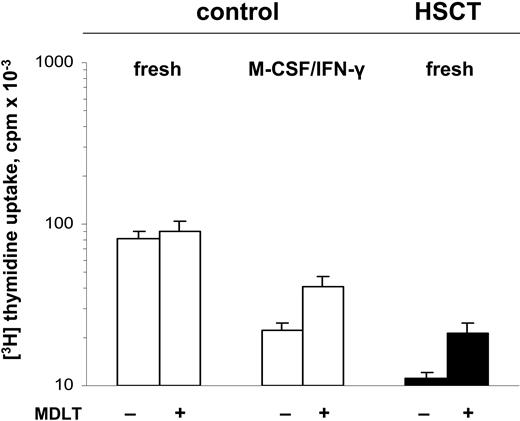
To address whether a direct link between increased tryptophan catabolism and inhibited proliferative responses could be detected, we inhibited IDO activity by supplementing cell cultures with the competitive inhibitor of IDO, MDLT (0.05 M to 0.5 M)31,48 , thereby reducing tryptophan catabolism, and then examined PBMC proliferative responses to PHA. In a representative experiment, the response of control PBMCs to PHA was essentially unaffected by the MDLT exposure (Figure 4). However, when control PBMCs were preincubated with M-CSF for 4 days and IFN-γ for the final 18 hours of culture, to induce a state of chronic activation, the proliferative responses were inhibited, and this inhibition was reversible in part by MDLT, as previously shown.31 In the case of post-HSCT PBMCs, even without pre-exposure to M-CSF and IFN-γ, there was an inhibited response to PHA that was also partially reversed by inhibition of IDO by MDLT. In a study of 4 patients the reduced responses of post-HSCT PBMCs were increased (by a median of 70%, n = 4, P < .05), by treatment of the PBMCs with MDLT. The significant recovery of the proliferative responses after MDLT inhibition of IDO activity strongly supports increased tryptophan catabolism as a factor contributing to depressed proliferative responses of post-HSCT cells.
Inhibition of post-HSCT PBMC proliferation is improved by blockade of IDO activity with MDLT. The effect of the addition of 0.5 M MDLT on the proliferative responses of control PBMCs (□) and of post-HSCT PBMCs (▪) to PHA was assessed. Cells were stimulated with PHA and, in parallel, exposed to MDLT, either immediately after separation from whole blood (fresh) or after pre-exposure to M-CSF (20 ng/mL) for 4 days, and IFN-γ (100 U/mL) that was added for the last 18 hours of culture. Error bars indicate SEM.
Inhibition of post-HSCT PBMC proliferation is improved by blockade of IDO activity with MDLT. The effect of the addition of 0.5 M MDLT on the proliferative responses of control PBMCs (□) and of post-HSCT PBMCs (▪) to PHA was assessed. Cells were stimulated with PHA and, in parallel, exposed to MDLT, either immediately after separation from whole blood (fresh) or after pre-exposure to M-CSF (20 ng/mL) for 4 days, and IFN-γ (100 U/mL) that was added for the last 18 hours of culture. Error bars indicate SEM.
Post-HSCT monocytes suppress proliferation and mediate apoptosis of susceptible T cells
To dissect the interaction between monocytes and T cells after HSCT, and to determine whether post-HSCT monocyte suppressor activity affects both normal and post-HSCT T cells, we conducted the following experiments. First, as shown in Figure 5A, comparing the response of control or post-HSCT PBMCs to an allogeneic challenge, the post-HSCT PBMC response to allogeneic normal monocytes in this MLR was markedly suppressed, as it was to anti-CD3 stimulation shown in Figure 1. To determine the suppressor effect specifically of post-HSCT monocytes, depleted of other non-T cells by MACS, we stimulated the separated and recombined highly enriched T cells and monocytes, of either a control or an HSCT recipient, also with control allogeneic cells (Figure 5B). Control T cells alone and control T cells cocultured with autologous monocytes were equally responsive to these allogeneic cells. In contrast, whereas post-HSCT T cells gave an equivalent response to control T cells, the addback of post-HSCT monocytes suppressed this T-cell response.
Post-HSCT monocyte suppressor activity affects post-HSCT T cells. (A) Total PBMCs obtained from a control (□) or an HSCT recipient (▪) were stimulated by highly enriched allogeneic control monocytes and proliferative responses were determined by [3H]thymidine incorporation after 5 days of culture. (B) Highly enriched T cells (Tc) or T cells cocultured with their autologous monocytes (Tc/m) of controls (□) or of HSCT recipients (▪) were stimulated by allogeneic cells and their proliferative response determined as in Figure 5A. (C) In a crossover experiment, control monocytes were stimulators of allogeneic post-HSCT T cells (□) and, vice versa, post-HSCT monocytes were stimulators of allogeneic control T cells (▪), and the respective proliferative responses were determined as in Figure 5A. Error bars indicate SEM.
Post-HSCT monocyte suppressor activity affects post-HSCT T cells. (A) Total PBMCs obtained from a control (□) or an HSCT recipient (▪) were stimulated by highly enriched allogeneic control monocytes and proliferative responses were determined by [3H]thymidine incorporation after 5 days of culture. (B) Highly enriched T cells (Tc) or T cells cocultured with their autologous monocytes (Tc/m) of controls (□) or of HSCT recipients (▪) were stimulated by allogeneic cells and their proliferative response determined as in Figure 5A. (C) In a crossover experiment, control monocytes were stimulators of allogeneic post-HSCT T cells (□) and, vice versa, post-HSCT monocytes were stimulators of allogeneic control T cells (▪), and the respective proliferative responses were determined as in Figure 5A. Error bars indicate SEM.
We also assessed T-cell susceptibility to suppression by post-HSCT monocytes in 2 ways. As shown in Figure 5C, we conducted a crossover experiment in which highly enriched T cells and monocytes of an HSCT recipient and of a control were recombined as shown. These data show that post-HSCT monocytes did not suppress the response of control T cells. In fact, in this particular experiment the control T-cell response to post-HSCT monocytes was higher, rather than lower, than the response of post-HSCT T cells to control monocytes.
The second line of evidence that normal T cells are resistant to the suppressor of effect of post-HSCT monocytes was obtained in an experiment in which T cells were purified from an HSCT recipient and that recipient's HLA-identical, MLR-nonreactive sibling donor. These purified normal donor T cells gave a similar proliferative response to anti-CD3, whether they were cultured alone (86 × 10-3 cpm) or in the presence of post-HSCT monocytes (91 × 10-3 cpm). Since control T-cell responses were not reduced by coculture with post-HSCT monocytes, we conclude that monocyte-mediated T-cell inhibition after HSCT involves both a suppressor effect of post-HSCT monocytes and a susceptibility of post-HSCT T cells to this suppression.
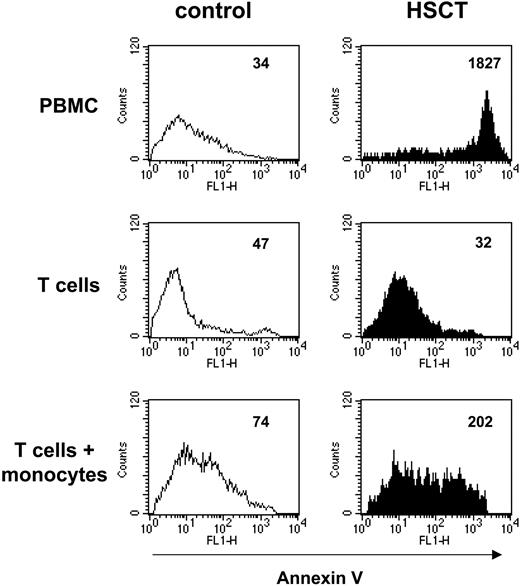
One prominent defect in T cells after HSCT is an increased susceptibility to apoptosis,20,25,26 and induction of T-cell apoptosis is one effect of accelerated tryptophan catabolism.28,49 Thus, we determined whether post-HSCT monocytes contributed to the enhanced rate of apoptosis in post-HSCT T cells. PBMCs, T cells alone, and T cells cocultured with monocytes were stimulated with PHA. After 3 days of culture, annexin V binding by CD3+ cells was determined by flow cytometry as a marker of apoptosis. As shown in Figure 6, upon PHA stimulation the proportion of apoptotic T cells in post-HSCT PBMCs was greatly increased, compared with controls. Enriched T cells (control and post-HSCT) did not exhibit apoptosis. However, addback of monocytes (40%) increased the level of annexin V binding by T cells in post-HSCT cultures to a much higher degree than in control cultures, indicating that post-HSCT monocytes augment post-HSCT T-cell apoptosis.
Post-HSCT monocytes mediate T-cell apoptosis. Total PBMCs or highly enriched T cells alone or highly enriched T cells cocultured with highly enriched autologous monocytes (40%), were stimulated with PHA for 3 days. T cells were identified as CD3+ and gated. Histogram plots depict apoptotic T cells identified as an annexin V-positive and propidium iodide-negative population. Assessment of the mean peak channel (mpc) in histogram analyses was used to detect differences in density of cell-surface binding of annexin V. Open histograms represent control cells; filled histograms, post-HSCT cells. MPC values are shown at the top right of each panel.
Post-HSCT monocytes mediate T-cell apoptosis. Total PBMCs or highly enriched T cells alone or highly enriched T cells cocultured with highly enriched autologous monocytes (40%), were stimulated with PHA for 3 days. T cells were identified as CD3+ and gated. Histogram plots depict apoptotic T cells identified as an annexin V-positive and propidium iodide-negative population. Assessment of the mean peak channel (mpc) in histogram analyses was used to detect differences in density of cell-surface binding of annexin V. Open histograms represent control cells; filled histograms, post-HSCT cells. MPC values are shown at the top right of each panel.
Discussion
The present study provides new insights into the mechanism of the frequently observed impaired T-cell function in the period following HSCT. Our data suggest that the T-cell population after HSCT may in itself have more intact proliferative capability than previously thought, but may be under the influence of inhibitory non-T-cell populations. While multiple cell populations (including CD14- cells; U.H. and A.H., unpublished data, September 2004) may act in this way, in our studies the monocyte population was identified as one that significantly exerts an inhibitory effect on T cells. These inhibitory monocytes, in a significant proportion of patients, released enhanced amounts of kynurenine, which specifically indicates augmented tryptophan catabolism via IDO activity,50,51 recognized to inhibit T-cell proliferation.52,53 Kynurenine release by post-HSCT monocytes was particularly sensitive to cellular activation, as compared with that of control monocytes.
Monocyte suppressor activity and enhanced monocyte tryptophan catabolism were common after HSCT even though our HSCT recipients encompassed a wide variety of transplant conditions (eg, various underlying diseases, allogeneic and autologous HSCT, different donor types, and different immunosuppression). This suggests that the alteration of immune responses may reflect a general effect of the immunomodulatory circumstances of HSCT (eg, T-cell depletion, transfusion of stem cells, phenomena of immune regeneration, etc). Findings that post-HSCT monocytes affected post-HSCT T cells but not control T cells and vice versa (control monocytes did not inhibit post-HSCT T cells) are intriguing. In fact, to inhibit control T cells by mechanisms associated with tryptophan catabolism (ie, sensitive to reversal by exposure to MDLT), vigorous pretreatment of control monocytes with M-CSF and IFN-γ was necessary, indicating that control T cells may be more resistant to monocyte-mediated inhibition by IDO activity.31 Indeed, the previously described state of sustained T-cell activation and exposure to a possibly altered environment54 may render post-HSCT T cells more susceptible to apoptosis,25 as we observed here by exposure to post-HSCT monocytes.
It will now be important to study in detail biochemical features of the enhanced tryptophan catabolism by post-HSCT monocytes. While depletion of tryptophan was unlikely to be involved in interfering with T-cell proliferation in the present study since a large excess of tryptophan was provided by the culture medium, tryptophan depletion has been shown to be one mechanism of the effect of IDO activity.31,54 Other possibilities to explore include whether enhanced kynurenine release occurs on the basis of increased IDO activity rather than quantity28 or on the basis of disturbed kynurenine degradation into further downstream products.55,56 In any case, kynurenine and further downstream tryptophan metabolites such as 3-hydroxykynurenine, 3-hydroxyanthranilic acid, and quinolinic acid are immunologically highly potent molecules that affect T-cell, B-cell, and natural killer cell function and survival.27,52,53 In general, the effect of enhanced tryptophan catabolism is believed to be to create local immunosuppression, and this is supported by our findings. This may include both effects on the cell itself (by tryptophan depletion) and on the microenvironment where monocytes and T cells interact (by the release of tryptophan catabolites).29 Additionally, it has been proposed that the interaction of dendritic cell CD80/CD86 and CTLA-451,57 causes IDO induction in the dendritic cell (DC).
Intriguingly, the generation of enhanced amounts of kynurenine after HSCT seems to be associated with an abnormal response of the monocytes to activation. Even without exogenous stimulation, monocytes generated and released moderately but nevertheless significantly elevated amounts of kynurenine. Together with the enhanced levels of neopterin observed in post-HSCT monocytes,58 this suggests that monocytes may be in a baseline state of sustained activation. Possibly even more significantly, upon stimulation with small doses of IFN-γ, which only slightly induced kynurenine secretion of control monocytes, the post-HSCT monocytes responded with a striking increase in kynurenine production and release, and post-HSCT lymphocyte cultures containing these monocytes responded with decreased lymphoproliferation. This observation was consistently made in the experiments comparing resting and stimulated (immobilized anti-CD3, M-CSF/IFN-γ, PHA) monocyte-containing cell populations, such as total PBMCs, cocultures of T cells with monocytes, and cultures of monocytes alone. Thus, after HSCT, monocytes appear to resemble a suppressor cell population rather than an accessory cell population.
The basic understanding of the physiologic role of tryptophan metabolism in immunity proposes that the breakdown of tryptophan along the kynurenine pathway mediated by IDO activity in monocytes, macrophages, or dendritic cells, is induced by immune activation to serve as a regulatory mechanism to limit the extent of immune responses to tissue injury or infection.27 Normally, tryptophan catabolism is induced upon cellular activation by molecules that otherwise participate in the induction of effector immune responses,59,60 for example, LPS61 or M-CSF. A prominent example is IFN-γ, synthesized and secreted by activated T cells.62,63 Augmented tryptophan catabolism to counterbalance recently induced effector immune reactions may then prevent unwanted immune reactions as well as contribute to induction and/or maintenance of tolerance (for a conceptual view see Finger and Bluestone64 ).
After HSCT, immune activation during the phase of immune recovery may be caused by multiple factors.65 These include the HSCT preparative regimen, which uniformly includes cytotoxic therapies (such as chemotherapy, irradiation, or lymphocytotoxic antibodies), and thus will induce tissue injury and initiate tissue repair mechanisms. Other possible activating stimuli may be the infusion of hematopoietic stem cells per se, as well as the exposure to multiple environmental or infectious pathogens, because of the breakdown of physiologic barriers such as the mucous membranes. All these factors will initiate immune activation, including the activation of monocytes, as demonstrated by an increased release of neopterin.46,58 Our data are compatible with a concept viewing the time period following HSCT as a state of a sustained immune activation that finally results in the observed (negative) deviation of the immune balance in HSCT recipients.
On the other hand, the hyperactivation of tryptophan catabolism, which we detected in the monocyte population but possibly also characterizes other cell populations, such as bone marrow stromal cells,54 by its potential to dampen T-cell effector pathways, may shift the balance of the immune system toward exerting a protective function in allogeneic HSCT by supporting the development of tolerance and the prevention of GVHD.66,67
In conclusion, we propose the following conceptual synthesis of our findings of enhanced kynurenine release by post-HSCT monocytes and decreased lymphoproliferative responses: monocytes regenerating after HSCT are susceptible to factors causing cellular activation. This susceptibility is evidenced by increased kynurenine release in vitro, even without the addition of exogenous activating agents but possibly resulting from immune activating processes associated with HSCT. Subsequently, in response to even mild activating signals, tryptophan catabolism is greatly magnified, and this reflects a dysfunction of the monocyte. The subsequent exaggerated release of kynurenine and other tryptophan catabolites by monocytes interacting with particularly vulnerable T cells in vivo then impairs the T-cell responses, as we observe in vitro. Thus, the generation and release of enhanced amounts of kynurenine by monocytes may on a molecular level represent one pathway linking the unexplained phenomena of enduring immune activation and immunosuppression after HSCT.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-05-1726.
Supported by the Austrian Federal Ministry of Social Affairs and Generations, by Austrian Science Foundation grant no. P16764-B13 (U.H.), the Children's Cancer Society of Vorarlberg, Tyrol, and South Tyrol (P.O.), and by National Institutes of Health grant CA42361 (S.L).
U.H. and P.O. contributed equally to the work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the medical staff from the transplantation units of the University Children's Hospital Innsbruck and of the University Hospital, Vienna, Austria, especially to Gabriele Kropshofer, MD, and to Felix Kreil, MD, respectively, for blood sampling from HSCT recipients.

![Figure 1. Monocytes mediate inhibition of T-cell proliferative responses after HSCT. (A) Total PBMCs or T cells highly enriched from total PBMCs obtained from healthy controls (□) or HSCT recipients (▪) were stimulated by immobilized anti-CD3 (250 ng/mL) for 72 hours and the proliferative response was quantified by net [3H]thymidine incorporation. Each bar depicts the median proliferative response of 15 experiments. Proliferation of post-HSCT PBMCs was significantly lower than that of control PBMCs (P < .001). The asterisk indicates that in HSCT recipients proliferation of enriched T cells was significantly increased over that of total PBMCs (P < .001). (B) T cells were cocultured with graded proportions of autologous monocytes as indicated and stimulated as in panel A. The bars depict net [3H]thymidine incorporation of control cultures (□) and cultures obtained from HSCT recipients (▪) as percent of the response of the respective purified T cells alone (horizontal line = 100%). *Significant (P < .05) differences of post-HSCT and control cultures. Error bars indicate standard error of the mean (SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-05-1726/6/m_zh80100578350001.jpeg?Expires=1765150301&Signature=GX-LTW2Y4c53m8BfhIGnNxnTo-2yCDS4rtnp23wC-lgiknHof6YwTiNxT~LBmfwwxPh4T~lBZRCDRPlIYk62Sk4blgplbBBYzS58JPxyDISGz3WKcALBYgogZn3Sll25zpAFhFlm48F1I8-mjYnzwMES0EmFshf4k6EqApwVaeJjmSQv4BN9WYgOP-GbmNFuPl1MyXQM2SJAbgoVGZRapIxeKed5ozXY87QaRB2UUBuRbnrQ-jF~kbLJlxBE5lq8DsQpzGaHzifuRdz1Rl535ztRdVn1UWdsmiWLv94RqHJ5ExDjkFVuaut6cmLJfPJNMlvlAtsqKayCy1OGsR3vKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Post-HSCT monocyte suppressor activity affects post-HSCT T cells. (A) Total PBMCs obtained from a control (□) or an HSCT recipient (▪) were stimulated by highly enriched allogeneic control monocytes and proliferative responses were determined by [3H]thymidine incorporation after 5 days of culture. (B) Highly enriched T cells (Tc) or T cells cocultured with their autologous monocytes (Tc/m) of controls (□) or of HSCT recipients (▪) were stimulated by allogeneic cells and their proliferative response determined as in Figure 5A. (C) In a crossover experiment, control monocytes were stimulators of allogeneic post-HSCT T cells (□) and, vice versa, post-HSCT monocytes were stimulators of allogeneic control T cells (▪), and the respective proliferative responses were determined as in Figure 5A. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-05-1726/6/m_zh80100578350005.jpeg?Expires=1765150301&Signature=x3xy3Prm6CrzqBcVF1DDEwhM-~TZ1kd8kB3sRcomaZyswmMoNrXqXPeWBiYFlU-l0kMeYlLByKsyQhzNPixFhLu18v-p31~zqdEHf3BCDENtA49IZvrHrkOob-abgPq4KpBfOZPWeTbjci5Y2E1cZU6sUxR9ZvFIXLwOYV81JSh1eVMzpbc0r0W1DB7pHQgAY~4vbOx~uGXnkRg7aAnH9~XiyjtO68N0aISbhBtW4LJLujCAObFKQqRQWYfnJnkqUdovIIdnUAzQjt0zFIc7jJacTc4vwhTn~W1qiPfW3mfg2-BJ~2BAXQ2Sfho6vcBQbHBt0z~HYP~oqfw0awoMqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal