Abstract
The K1 gene of Kaposi sarcoma–associated herpesvirus (KSHV) encodes a transmembrane glycoprotein bearing a functional immunoreceptor tyrosine-based activation motif (ITAM). Previously, we reported that the K1 protein induced plasmablastic lymphomas in K1 transgenic mice, and that these lymphomas showed enhanced Lyn kinase activity. Here, we report that systemic administration of the nuclear factor kappa B (NF-κB) inhibitor Bay 11-7085 or an anti–vascular endothelial growth factor (VEGF) antibody significantly reduced K1 lymphoma growth in nude mice. Furthermore, in KVL-1 cells, a cell line derived from a K1 lymphoma, inhibition of Lyn kinase activity by the Src kinase inhibitor PP2 decreased VEGF induction, NF-κB activity, and the cell proliferation index by 50% to 75%. In contrast, human B-cell lymphoma BJAB cells expressing K1, but not the ITAM sequence–deleted mutant K1, showed a marked increase in Lyn kinase activity with concomitant VEGF induction and NF-κB activation, indicating that ITAM sequences were required for the Lyn kinase–mediated activation of these factors. Our results suggested that K1-mediated constitutive Lyn kinase activation in K1 lymphoma cells is crucial for the production of VEGF and NF-κB activation, both strongly implicated in the development of KSHV-induced lymphoproliferative disorders.
Introduction
Kaposi sarcoma (KS)–associated herpesvirus (KSHV), also known as human herpesvirus 8, is a gamma-2 herpesvirus.1 KSHV has been associated with all forms of KS and with certain lymphoproliferative disorders, such as primary effusion lymphomas (PELs) and multicentric Castleman disease (MCD).2-4 The KSHV genome encodes more than 85 open reading frames, several of which have been implicated in transformation, proliferation, signaling, immunomodulation, and inhibition of apoptosis.5 However, the molecular mechanisms by which infection with KSHV leads to the development of different diseases remain unclear.
Among the KSHV open reading frames, K1 is a transmembrane glycoprotein related to the immunoglobulin receptor family and is similar to the B-cell receptor (BCR).6 The cytoplasmic region contains a functional immunoreceptor tyrosine-based activation motif (ITAM). ITAMs are capable of coupling extracellular signals to downstream intracellular signaling pathways to elicit cellular activation events.7 However, unlike the BCR, K1 signaling occurs constitutively in the absence of exogenous ligands, presumably through the multimerization of its cysteine-rich ectodomain, which results in phosphorylation of the tyrosine residues in the ITAM and recruitment of B-cell–specific Syk kinase.8 This recruitment initiates a cascade of downstream signaling events in B lymphocytes, resulting in calcium mobilization and induction of activator protein 1 (AP1)–, nuclear factor kappa B (NF-κB)–, and nuclear factor of activated T cell (NFAT)–dependent promoter activities which in turn contribute to inflammatory responses and growth deregulation.8,9 There is also evidence showed that K1 expression in B lymphocytes enhances cell survival signals and protects cells from forkhead transcription factor–and Fas-mediated apoptosis.10 In an earlier report, we showed that K1 expression in human B-cell lymphoma BJAB cells suppresses anti-Fas antibody–mediated apoptosis.11
Strong evidence of a role for K1 in KSHV pathogenesis first emerged from studies conducted by Lee et al,12 who showed that K1 expression transforms rodent fibroblasts in vitro, and that recombinant herpesvirus saimiri strains in which the saimiri transformation protein had been replaced with the K1 gene induce lymphomas in vivo. Moreover, K1 expression has been detected in MCD tissues, and in PEL cells during the lytic viral life cycle on induction with 12-O-tetradecanoylphorbol-13-acetate (TPA).9,13,14 It is possible that K1 produced during lytic replication has a lasting role in the development of KSHV-associated lymphoproliferative disorders. This is further supported by the finding that ITAM-dependent signaling by K1, although not critical, moderately augments lytic replication in B lymphocytes.8
Vascular endothelial growth factor (VEGF) and NF-κB, which have been implicated in the pathogenesis of KS, PEL, and MCD, are also upregulated in KSHV-infected cells.9,15-21 PEL-derived cell lines produce high levels of VEGF, and the neutralization of VEGF in mice abrogates tumor growth and ascites production.16 VEGF is also important in the pathogenesis of MCD.19
We previously showed that transgenic mice expressing K1 develop plasmablastic lymphomas.22 These lymphomas, which are most likely derived from B lymphocytes, show constitutive activation of NF-κB and Oct-2 transcription factors as well as enhanced activity of Lyn, a protein tyrosine kinase of the Src family. To gain further insight into the pathogenetic function of K1, in our current study we investigated the role of K1 expression on VEGF expression and NF-κB activity in B-lineage cells. We showed that K1 signaling leads to the induction of VEGF and activation of NF-κB in an ITAM-dependent manner and that neutralization of VEGF in K1-induced lymphoma-bearing mice efficiently suppressed tumor growth. We also found that more than 90% of K1 transgenic mice developed hyperplastic lymph nodes that produced high levels of VEGF, suggesting that VEGF might be involved in the vascular proliferation of lymph nodes in K1 mice.
Materials and methods
Generation of the lymphoma-derived cell line KVL-1
The production of transgenic mice expressing the K1 gene under the transcriptional control of the SV40 promoter has been described previously.22 Tumor tissues from a K1-induced B-cell–type lymphoma in a K1 transgenic mouse were minced into 2-mm3 to 3-mm3 pieces and treated with collagenase type 1 (Worthington Biochemicals, Freehold, NJ) in RPMI 1640 at 37°C in 5% CO2. The dissociated cells were centrifuged at 500g and resuspended in growth medium, RPMI 1640 supplemented with 10% fetal calf serum (Life Technologies, Grand Island, NY), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells growing in suspension were maintained at 37°C in 5% CO2, and the culture medium was replaced every 3 to 4 days. When cells approached confluence, they were diluted 1:3 with fresh medium and recultured until use. The KVL-1 cells maintained expression of K1 mRNA and had enhanced Lyn kinase activity.
Establishment of K1 lymphomas in mice and treatment with anti–VEGF antibody or the NF-κB inhibitor BAY 11-7085
K1 lymphoma subtransplants were generated by subcutaneously injecting tumor fragments (∼2 mm3) from primary lymphomas in K1 transgenic mice into anesthetized BALB/c nu/nu mice22 and were maintained by subsequent transplants in nude mice. To test the effect of an anti–VEGF antibody on tumor growth, tumor-bearing mice were intraperitoneally injected with rabbit polyclonal antibody raised against an amino-terminal peptide of human VEGF or with nonimmune rabbit immunoglobulin G (IgG; 15 μg/mouse each week; Santa Cruz Biotechnology, Santa Cruz, CA). To test the effect of the NF-κB inhibitor BAY 11-7085 (BIOMOL, Plymouth, PA), mice were intraperitoneally injected with the inhibitor (100 μg/0.1 mL of dimethyl sulfoxide [DMSO]) or DMSO alone twice weekly. All animals were killed after 3 weeks by cervical dislocation, and the tumors were recovered and weighed.
Histologic and immunohistochemical evaluation of lymph nodes from K1 transgenic and nontransgenic mice
For histologic examination, tissues were fixed in 10% buffered formalin and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin, and their pathologic features were examined by light microscopy. VEGF expression in the lymph nodes was assessed by immunofluorescence staining of the paraffin sections. Anti–VEGF rabbit polyclonal antibody (A-20; Santa Cruz Biotechnology) raised against an amino-terminal peptide of human VEGF was used as the primary antibody, and goat anti–rabbit IgG labeled with green fluorescent Alexa Flour 488 dye (Molecular Probes, Eugene, OR) was used as the secondary antibody. The tissues were also stained with the F-actin probe phalloidin conjugated with red-orange fluorescent dye Alexa Flour 568 and with blue fluorescent nucleic acid stain 4′,6-diamidino-2-phenylindole dihydrochloride (Molecular Probes). The images of the stained tissue sections were captured with a deconvolution microscope (Axiovert 200; Zeiss, Gottingen, Germany) using SlideBook software (Intelligent Imaging Innovations, Denver, CO).
Quantification of VEGF
VEGF in 50 μL aliquots of mouse sera or culture supernatants was measured with an enzyme-linked immunosorbent assay (ELISA) using a mouse or human Quantikine kit (R&D Systems, Minneapolis, MN) and following the manufacturer's instructions.
[3H]-thymidine incorporation assay
KVL-1 cells (1 × 106) were cultured in 6-well tissue-culture plates in complete medium in the presence of different concentrations of the Src tyrosine kinase inhibitor PP2, its inactive analog PP3, or the Syk tyrosine kinase inhibitor piceatannol23 or equivalent concentrations of solvent (DMSO). After 16 hours, each well was pulsed for 4 hours with 1 μCi (37 kBq) of [3H]-thymidine, the cells were harvested, and the radioactivity that was incorporated into the trichloroacetic acid–precipitable material was quantified in a β counter, as previously described.24
Cell-cycle analysis
KVL-1 cells were cultured in 6-well tissue-culture plates at a density of 1 × 106 cells per well in complete medium in the presence of different concentrations of PP2. After 16 hours, the cells were fixed and stained with propidium iodide, as described previously.25 Cell-cycle distribution was determined by flow cytometric analysis with the use of a Becton Dickinson (Franklin Lakes, NJ) FACS Caliber flow cytometer equipped with Becton Dickinson Cell Quest software. The percentages of cells in G0G1,G2M, and S phases of the cell cycle were obtained from DNA histograms using ModFit software (Varsity Software House, Topsham, ME). The proliferation indexes were quantified from SG2/G0G1 ratios.
Western blot and immunoprecipitation analysis of B lymphocytes for activation of protein tyrosine kinases
Activation of protein tyrosine kinases Lyn and Syk by hyperphosphorylation was detected essentially as described previously.22 Briefly, cells were harvested and lysed in lysis buffer containing protease inhibitors. Cell-free lysates containing 100 μg protein were resolved on sodium dodecyl sulfate–polyacrylamide gels by electrophoresis and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Total Lyn and Syk were detected using anti–Lyn mouse monoclonal antibody (H-6) and anti–Syk rabbit polyclonal antibody (LR) as the primary antibodies (Santa Cruz Biotechnology) and horseradish peroxidase–linked sheep anti–mouse IgG and anti–rabbit IgG (Amersham Pharmacia Biotech, Piscataway, NJ) as the secondary antibodies. The ECL PLUS chemiluminescent system (Amersham Pharmacia Biotech) was used to visualize antibody-bound proteins on Western blots.
Tyrosyl phosphorylation of Lyn and Syk was detected by subjecting these proteins immunoprecipitated with agarose-conjugated anti–Lyn or anti–Syk antibody (H-6 and LR, respectively) from extracts containing 500 μg protein to electrophoresis and immunoblot analysis, using mouse monoclonal anti–p-Tyr as the primary antibody (Amersham Pharmacia Biotech) and horseradish peroxidase–linked sheep anti–mouse IgG as the secondary antibody.
In vitro kinase assay
In vitro Lyn kinase activity was determined by immunoprecipitating Lyn from cell lysates essentially as described previously.22 The immunoprecipitates were incubated in kinase buffer containing the tyrosine kinase substrate Raytide (Oncogene Research Products, Cambridge, MA) and [γ-32P]ATP, and the radioactivity incorporated into the substrate was estimated using a filter assay. When the inhibition of Lyn kinase activation was required, the cells were treated with PP2 (13 μM) for 1 to 2 hours.
Transfections and reporter-gene analysis
The human Burkitt lymphoma cell line Raji was obtained from the American Type Culture Collection (Manassas, VA). Human B-cell lymphoma BJAB cells that constitutively expressed K1 (BJAB-K1), the ITAM sequence–deleted mutant K1 (BJAB-K1m), and vector control cells (BJAB-XS) were generated by retroviral transfection. Briefly, the K1 and K1m DNAs were subcloned into the pLXSN retroviral vector along with a reporter gene (alkaline phosphatase). The plasmids were purified on a cesium chloride gradient and transfected using FuGENE 6 (Roche, Basel, Switzerland) into the PT67 NIH3T3 packaging cell line (RetroPack PT67; BD Biosciences, Palo Alto, CA). After 48 hours, the supernatants were clarified and passed through 0.45 μM filters. Titers were routinely 1 × 106 to 3 × 106 multiplicities of infection. BJAB cells (American Type Culture Collection) were infected by mixing cells with virus-containing supernatants (105/mL multiplicities of infection) in the presence of 12 μg/mL polybrene (Sigma-Aldrich, St Louis, MO) for 2 hours. Cell selection was made with 1 mg/mL geneticin (Gibco Invitrogen, Grand Island, NY), and the cells were pooled for analysis. K1 and K1m in these cells are expressed under the transcriptional control of the Moloney murine leukemia virus long terminal repeat. The cell lines were propagated in complete RPMI 1640 medium. K1 expression in BJAB-K1 cells and K1m expression in BJAB-K1m cells were confirmed by reverse transcription–polymerase chain reaction analysis (not shown).
Unless stated otherwise, approximately 2 × 106 cells were transfected by electroporation, as previously described,22 with 10 μg K1 expression plasmid pSG5-K1,22 or KSHV G protein–coupled receptor (vGPCR) expression plasmid vGPCR-pSG5,26 and cotransfected with 5μg to 10 μg of NF-κB–luciferase reporter construct (Stratagene, La Jolla, CA) or human VEGF promoter–luciferase construct pGL3-V2274.27 Cells were then resuspended in 10 mL medium and incubated for 24 hours before harvesting for the quantification of luciferase activity by using the Luciferase Assay System (Promega, Madison, WI) and a luminometer (Turner Designs, Sunnyvale, CA). Transfection efficiencies were normalized by cotransfection with a reporter plasmid containing the β-galactosidase gene under the transcriptional control of the cytomegalovirus promoter pCMVβ-gal, and β-galactosidase assays were carried out according to the manufacturer's instructions (Promega). All experiments were repeated at least 3 times, and assays were performed in triplicate. When required, the Src kinase inhibitor PP2, the Syk kinase inhibitor piceatannol, and the phosphatidylinositol 3-kinase (PI3-K) inhibitor LY294002 (Calbiochem, La Jolla, CA) were added to the cell culture medium at the indicated concentrations 4 hours before harvest.
Statistical analysis
Results are presented as the means plus or minus the standard deviations (SDs) for each group. Comparisons between groups were made using the Student t test. Differences at P = .05 were considered significant.
Results
Activation of Src tyrosine kinase Lyn is crucial for VEGF production and NF-κB activation in KVL-1 cells derived from a K1-induced lymphoma
Previously, we reported that Lyn, a Src family tyrosine kinase, was substantially activated in B lymphocytes from KSHV K1 transgenic mice, as revealed by phosphorylation and enhanced in vitro catalytic activity.22 Furthermore, Lyn kinase activity was significantly higher in a malignant plasmablastic lymphoma from a K1 transgenic mouse than in K1-expressing B lymphocytes, which suggested that Lyn kinase activation plays a role in K1-induced lymphomagenesis. A cell line (KVL-1) derived from one of the lymphomas also showed a marked increase in Lyn kinase activity (not shown).
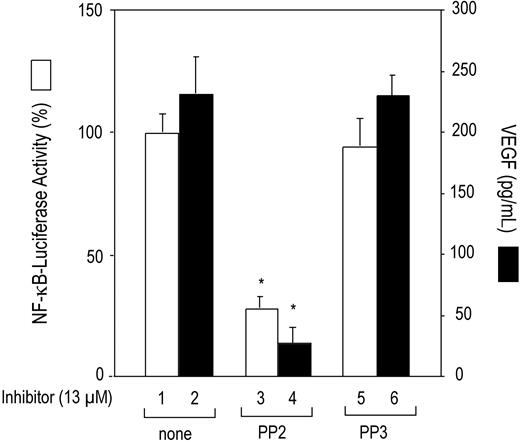
VEGF and NF-κB also have been associated with the KSHV-mediated pathogenesis of lymphoproliferative disorders.16,19,28 We investigated whether Lyn kinase activation induces constitutive NF-κB transcription factor activation and VEGF expression in K1-induced lymphomas. To ascertain whether NF-κB was activated, KVL-1 cells were transfected with the NF-κB–luciferase reporter construct, and luciferase activity was measured. As shown in Figure 1, the KVL-1 cells showed high basal NF-κB promoter activity (bar 1), which was significantly inhibited by treating the cells with the Src protein tyrosine kinase inhibitor PP2 (bar 3), but not with the inactive compound PP3 (bar 5). The KVL-1 cells also produced high levels of VEGF. The average VEGF concentration in the conditioned medium (1 × 106 cells/mL) after 24 hours of incubation was 240 ± 30 pg/mL (bar 2). Although not shown, the VEGF concentration in the conditioned medium varied proportionally with the number of cells. Treatment of these cells with PP2 (bar 4) but not with PP3 (bar 6) significantly decreased (∼90%) VEGF production.
To further assess the involvement of Lyn kinase activation in the growth of KVL-1 cells, we examined the effect of the Lyn kinase inhibitor PP2 on cell proliferation by [3H]-thymidine incorporation assay. As shown in Figure 2A, the incorporation of [3H]-thymidine decreased significantly (> 80%) in response to treatment with 2.5 μM or 5.0 μM PP2 (compare bars 1, 2, and 3). In contrast, only 10% and 15% decreases were observed when the cells were exposed to PP3 (compare bars 1, 4, and 5) or the Syk tyrosine kinase inhibitor, piceatannol (compare bars 1, 6, and 7). PP2 was 5- to 10-fold more effective in inhibiting cell proliferation than PP3 and piceatannol at similar concentrations. The effect of Lyn kinase inhibition on the proliferation of the KVL-1 cells was also examined by flow cytometry. As shown in Figure 2B, PP2 treatment decreased the number of cells in the S phase, and increased the number of cells in the G0G1 phase, which resulted in a 50% to 75% decrease in the proliferation index (SG2/G0G1). Taken together, our results suggested that constitutive activation of Lyn kinase was involved in the growth and survival of KVL-1 cells.
Inhibition of constitutive NF-κB promoter activity and VEGF production by Src kinase inhibitor PP2 in K1 lymphoma–derived KVL-1 cells. KVL-1 cells (2 × 106) that express K1 were transfected with 10 μg of NF-κB–luciferase reporter construct. After 20 hours, the cells were treated with the Src kinase inhibitor PP2 (13 μM) or its inactive analog PP3 (13 μM) for 4 hours, harvested, and assayed for NF-κB activity (□) in cell extracts and for VEGF production (▪) in culture supernatants as described in “Materials and methods.” Luciferase values are expressed as a percentage of activities observed in the absence of inhibitors (bar 1). Data are presented as the means (± SD) of 3 experiments. There were statistically significant (*P < .001) decreases in NF-κB–luciferase activity and VEGF production in the cells treated with PP2.
Inhibition of constitutive NF-κB promoter activity and VEGF production by Src kinase inhibitor PP2 in K1 lymphoma–derived KVL-1 cells. KVL-1 cells (2 × 106) that express K1 were transfected with 10 μg of NF-κB–luciferase reporter construct. After 20 hours, the cells were treated with the Src kinase inhibitor PP2 (13 μM) or its inactive analog PP3 (13 μM) for 4 hours, harvested, and assayed for NF-κB activity (□) in cell extracts and for VEGF production (▪) in culture supernatants as described in “Materials and methods.” Luciferase values are expressed as a percentage of activities observed in the absence of inhibitors (bar 1). Data are presented as the means (± SD) of 3 experiments. There were statistically significant (*P < .001) decreases in NF-κB–luciferase activity and VEGF production in the cells treated with PP2.
K1 expression in human B-cell lymphoma BJAB cells induces ITAM-mediated Lyn kinase activity
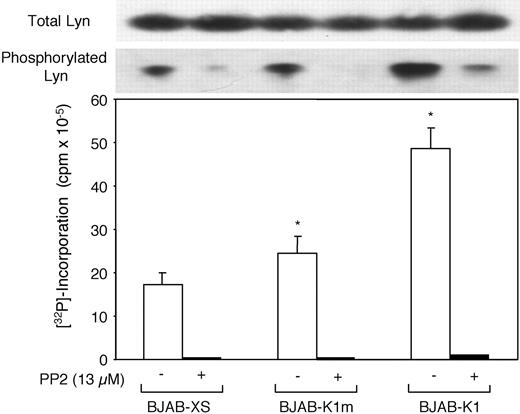
Because we previously showed that K1 expression in mouse lymphoma cells stimulated Lyn kinase activity,22 we examined whether K1 expression in human B-cell lymphoma BJAB cells also activates Lyn kinase. Furthermore, because ITAMs are crucial for the coupling of extracellular signals to intracellular signaling pathways through the activation of Lyn and other Src family protein tyrosine kinases, we also examined the involvement of K1 ITAMs in the activation of Lyn kinase on the basis of phosphorylated Lyn expression levels and in vitro Lyn kinase assay. BJAB cells that constitutively expressed K1 (BJAB-K1), mutant K1 (BJAB-K1m), and vector control (BJAB-XS) cells were used. Total Lyn expression and tyrosine phosphorylated Lyn expression in cell lysates were detected by anti–Lyn and anti–p-Tyr monoclonal antibodies. Although total Lyn expression was comparable in the 3 cell lines, tyrosine-phosphorylated Lyn expression was higher in BJAB-K1 cells than in BJAB-XS or BJAB-K1m cells (Figure 3). In parallel experiments, treatment of these cells with the Src kinase inhibitor PP2 almost completely inhibited tyrosine phosphorylation but did not alter total Lyn expression. The results from an in vitro Lyn kinase assay with equivalent amounts of Lyn immunoprecipitates indicated almost 3 times as much Lyn kinase activity in BJAB-K1 cells as in the BJAB-XS cells and almost twice that in BJAB-K1m cells (Figure 3). Treating cells with PP2 decreased the Lyn kinase activity in the 3 cell lines markedly below the level of BJAB-K1 cells. Similar results were obtained when K1 and mutant K1 were transiently expressed under the control of the SV40 promoter in human Burkitt lymphoma Raji cells and the cell extracts were analyzed for in vitro Lyn kinase activity (data not shown). Taken together, our results indicated that K1 expression led to the activation of Lyn kinase, and that ITAM sequences were essential for the effect.
Src kinase inhibitor PP2 blocked the incorporation of [3H]-thymidine into KVL-1 cells and caused the reduction of cells in the S phase of the cell cycle. (A) KVL-1 cells (1 × 106) were treated with the Src kinase inhibitor PP2, its inactive analog PP3, or the Syk kinase inhibitor piceatannol (all 2.5 μMor5.0 μM), in a growth medium. After 16 hours, the cells were pulsed with [3H]-thymidine for 4 hours, and the radioactivity incorporated into the cells was measured as described in “Materials and methods.” Results represent the means (± SD) of 3 separate experiments. Marked decreases in [3H]-thymidine incorporation were observed only with PP2 (P < .001). (B) KVL-1 cells (1 × 106) were treated with 2.5 μM or 5.0 μM PP2 for 20 hours. Cell cycle profiles of the propidium iodide–stained cells were analyzed by flow cytometry, and the percentages of the cells in the G0G1,G2M, and S phases of cell cycle were calculated using the ModFit software program. Proliferation index (PI) was calculated from the SG2/G0G1 ratios. Results are representative of 3 independent experiments. DNA content (FL2-A) is depicted on the x axis and the number of cells after staining with propidium iodide is depicted on the y axis.
Src kinase inhibitor PP2 blocked the incorporation of [3H]-thymidine into KVL-1 cells and caused the reduction of cells in the S phase of the cell cycle. (A) KVL-1 cells (1 × 106) were treated with the Src kinase inhibitor PP2, its inactive analog PP3, or the Syk kinase inhibitor piceatannol (all 2.5 μMor5.0 μM), in a growth medium. After 16 hours, the cells were pulsed with [3H]-thymidine for 4 hours, and the radioactivity incorporated into the cells was measured as described in “Materials and methods.” Results represent the means (± SD) of 3 separate experiments. Marked decreases in [3H]-thymidine incorporation were observed only with PP2 (P < .001). (B) KVL-1 cells (1 × 106) were treated with 2.5 μM or 5.0 μM PP2 for 20 hours. Cell cycle profiles of the propidium iodide–stained cells were analyzed by flow cytometry, and the percentages of the cells in the G0G1,G2M, and S phases of cell cycle were calculated using the ModFit software program. Proliferation index (PI) was calculated from the SG2/G0G1 ratios. Results are representative of 3 independent experiments. DNA content (FL2-A) is depicted on the x axis and the number of cells after staining with propidium iodide is depicted on the y axis.
K1-induced Lyn hyperphosphorylation and in vitro Lyn kinase activity in BJAB-K1 cells. Hyperphosphorylation and in vitro kinase activity of Lyn tyrosine kinase in BJAB-K1, BJAB-K1m, and BJAB-XS human B-lymphoma cells in the presence and absence of the Src protein tyrosine kinase inhibitor PP2 were determined as described in “Materials and methods.” Compared with BJAB-XS cells, the differences in Lyn kinase activity were significant in BJAB-K1m cells (*P = .007) and BJAB-K1 (*P = .01) cells.
K1-induced Lyn hyperphosphorylation and in vitro Lyn kinase activity in BJAB-K1 cells. Hyperphosphorylation and in vitro kinase activity of Lyn tyrosine kinase in BJAB-K1, BJAB-K1m, and BJAB-XS human B-lymphoma cells in the presence and absence of the Src protein tyrosine kinase inhibitor PP2 were determined as described in “Materials and methods.” Compared with BJAB-XS cells, the differences in Lyn kinase activity were significant in BJAB-K1m cells (*P = .007) and BJAB-K1 (*P = .01) cells.
ITAM sequences are essential for K1-mediated activation of VEGF and NF-κB in human B-lymphoma cells
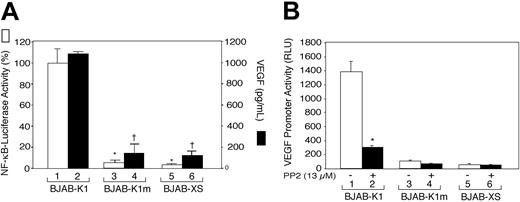
Because K1 ITAM-induced signaling is involved in the activation of Lyn kinase in human B-cell lymphoma BJAB cells, we determined whether activation of a Src kinase was linked to activation of signal transduction pathways and thereby activation of VEGF and NF-κB. BJAB-K1, BJAB-K1m, and BJAB-XS cells (1 × 107/mL) were cultured for 20 to 24 hours in growth medium, and VEGF concentrations in culture supernatants were determined by ELISA. As shown in Figure 4A, BJAB-K1 cells produced almost 10 times more VEGF in the culture supernatants than did the BJAB-K1m or BJAB-XS cells; likewise, NF-κB promoter activity was markedly increased in the BJAB-K1 cells but not in BJAB-K1m or BJAB-XS cells. Treatment of the cells with the Src kinase inhibitor PP2 almost completely inhibited K1-induced VEGF production and NF-κB promoter activity (not shown). These results suggested that VEGF production and NF-κB activation were linked to ITAM signaling via Lyn kinase activation.
The immunoreceptor tyrosine-based activation motif of the K1 protein and Lyn kinase activation are required for K1-induced NF-κB activity and VEGF production. (A). BJAB-K1, BJAB-K1m, and BJAB-XS cells were transfected with 5μg NF-κB–luciferase reporter construct. After 24 hours, the cells were harvested for luciferase activity (□), and VEGF in culture supernatants (▪) was quantified as described in “Materials and methods.” Results represent the means (± SD) of 3 experiments. There were statistically significant decreases in NF-κB promoter activity (*P < .001) and VEGF production (†P < .001) in BJAB-K1m and BJAB-XS cells compared with BJAB-K1 cells. (B) BJAB-K1, BJAB-K1m, and BJAB-XS cells (2 × 106) were transfected with 5 μg of a human VEGF promoter–luciferase construct. After 20 hours, the cells were treated with PP2 (13 μM; ▪) or not (□) for 4 hours and harvested for a luciferase assay as described in “Materials and methods.” Results represent the means (± SD) of 3 experiments. Statistically significant decrease (*P = .005) in VEGF promoter activity versus no treatment with PP2 was observed in BJAB-K1 cells.
The immunoreceptor tyrosine-based activation motif of the K1 protein and Lyn kinase activation are required for K1-induced NF-κB activity and VEGF production. (A). BJAB-K1, BJAB-K1m, and BJAB-XS cells were transfected with 5μg NF-κB–luciferase reporter construct. After 24 hours, the cells were harvested for luciferase activity (□), and VEGF in culture supernatants (▪) was quantified as described in “Materials and methods.” Results represent the means (± SD) of 3 experiments. There were statistically significant decreases in NF-κB promoter activity (*P < .001) and VEGF production (†P < .001) in BJAB-K1m and BJAB-XS cells compared with BJAB-K1 cells. (B) BJAB-K1, BJAB-K1m, and BJAB-XS cells (2 × 106) were transfected with 5 μg of a human VEGF promoter–luciferase construct. After 20 hours, the cells were treated with PP2 (13 μM; ▪) or not (□) for 4 hours and harvested for a luciferase assay as described in “Materials and methods.” Results represent the means (± SD) of 3 experiments. Statistically significant decrease (*P = .005) in VEGF promoter activity versus no treatment with PP2 was observed in BJAB-K1 cells.
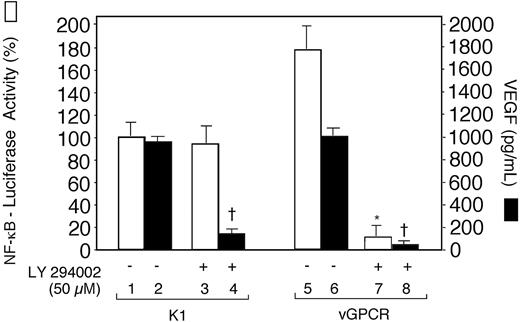
The phosphatidylinositol 3-kinase inhibitor LY294 002 inhibited NF-κB promoter activity and the production of VEGF in Raji cells transiently expressing KSHV G protein–coupled receptor (vGPCR), but only inhibition of VEGF production in K1-transfected cells. Raji cells were transfected with 10 μg pSG5-K1 or vGPCR-pSG5 and cotransfected with 5 μg of the NF-κB–luciferase reporter construct. After 20 hours, the cells were treated with LY294002 (50 μM) for 4 hours and harvested for luciferase activity in cell extracts (□) and VEGF production in culture supernatants (▪) as described in “Materials and methods.” Luciferase values are expressed as a percentage of the value observed in the absence of LY294002 in K1-transfected cells (bar 1). Results represent the means (± SD) of 3 experiments. There was a statistically significant decrease in NF-κB promoter activity in vGPCR-transfected cells (*P < .001, compare bars 5 and 7), but not in K1-transfected cells (P = .3, compare bars 1 and 3). On the other hand, statistically significant decreases were observed in the production of VEGF in cells transfected with K1 (†P < .001, compare bars 2 and 4) and with vGPCR (†P < .001, compare bars 6 and 8).
The phosphatidylinositol 3-kinase inhibitor LY294 002 inhibited NF-κB promoter activity and the production of VEGF in Raji cells transiently expressing KSHV G protein–coupled receptor (vGPCR), but only inhibition of VEGF production in K1-transfected cells. Raji cells were transfected with 10 μg pSG5-K1 or vGPCR-pSG5 and cotransfected with 5 μg of the NF-κB–luciferase reporter construct. After 20 hours, the cells were treated with LY294002 (50 μM) for 4 hours and harvested for luciferase activity in cell extracts (□) and VEGF production in culture supernatants (▪) as described in “Materials and methods.” Luciferase values are expressed as a percentage of the value observed in the absence of LY294002 in K1-transfected cells (bar 1). Results represent the means (± SD) of 3 experiments. There was a statistically significant decrease in NF-κB promoter activity in vGPCR-transfected cells (*P < .001, compare bars 5 and 7), but not in K1-transfected cells (P = .3, compare bars 1 and 3). On the other hand, statistically significant decreases were observed in the production of VEGF in cells transfected with K1 (†P < .001, compare bars 2 and 4) and with vGPCR (†P < .001, compare bars 6 and 8).
Having shown that K1-expressing mouse and human B-cell lymphoma cells constitutively produce high levels of VEGF, we then investigated whether K1 regulates VEGF production at the level of VEGF gene expression. BJAB-K1, BJAB-K1m, and BJAB-XS cells were transiently transfected by electroporation with the human VEGF promoter–luciferase construct pGL3-V227427 and assayed for luciferase activity. As shown in Figure 4B, BJAB-K1 cells showed a nearly 25-fold higher VEGF promoter activity than the control BJAB-XS cells did (compare bars 1 and 5), and a nearly 15-fold higher activity than the BJAB-K1m cells did (compare bars 1 and 3). To examine the functional role of Lyn kinase in activating the VEGF promoter, transfected cells in parallel experiments were treated with PP2 for 4 hours before harvesting for luciferase activity. PP2 decreased the VEGF promoter activity by 80% in BJAB-K1 cells (Figure 4B, compare bars 1 and 2). Taken together, our results suggested that Lyn kinase activation and ITAM sequences were required for K1-induced activation of the VEGF promoter.
KSHV K1 activates VEGF and NF-κB by independent signaling pathways
An earlier study showed that the PI3-K/Akt pathway was involved in the activation of NF-κB and VEGF production by vGPCR.26 To determine whether this pathway is involved in the K1-mediated activation of VEGF and NF-κB, we transfected human Raji B cells with pSG5-K1 and NF-κB–luciferase reporter constructs. The NF-κB reporter activities in cell extracts and VEGF concentrations in culture supernatants were estimated in PI3-K inhibitor LY294002-treated cells and untreated cells. In K1-transfected cells, LY294002 inhibited VEGF production but not NF-κB promoter activity. This finding suggested that activation of VEGF and NF-κB by K1 was mediated by independent pathways or at least through pathways that partly diverged at the level of PI3-K involvement. Similar results were obtained with BJAB-K1 cells and BJAB-XS cells transiently transfected with NF-κB reporter plasmid (not shown). On the other hand, consistent with the earlier report,26 LY294002 inhibited the activation of both VEGF and NF-κB in vGPCRtransfected Raji cells (Figure 5).
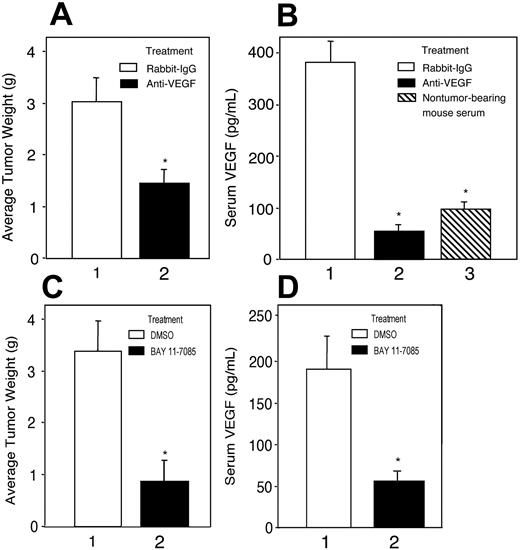
Treatment with anti–VEGF antibody or NF-κB inhibitor suppresses growth of K1 lymphomas in nude mice
Because the K1 lymphoma–derived cell line KVL-1 consistently produced high levels of VEGF and had substantial NF-κB activity, we investigated potential roles of VEGF and NF-κB in the growth of K1 lymphomas. BALB/c nude mice were subcutaneously injected with a single fragment (∼2mm3) of a K1 lymphoma in the dorsal scapular region. The tumor fragments grew into tumors with diameters of approximately 2.5 cm in about 3 weeks. Intraperitoneal administration of an anti–VEGF antibody but not a control isotype (at 15 μg/mouse each week) for 3 weeks in tumor-bearing mice decreased mean tumor weight by 45% to 50% (Figure 6A, compare bars 1 and 2). Tumor-bearing mice showed a 3- to 4-fold increase in serum VEGF concentrations compared with the mean VEGF concentrations in nontumor-bearing control animals (Figure 6B, compare bars 1 and 3), indicating that the tumors produced VEGF. Anti–VEGF antibody treatment of the tumor-bearing animals decreased the serum VEGF concentrations to below the baseline concentrations (Figure 6B, compare bars 2 and 3).
Bay 11-7085 is an irreversible inhibitor of IκBα phosphorylation,29 which is required for the phosphorylation of NF-κB, and has been shown to induce apoptosis of KSHV-infected PEL cells.27 Treating tumor-bearing mice with Bay 11-7085 (100 μg/mouse, twice weekly) for 3 weeks suppressed tumor growth as much as 75% compared with growth in solvent-treated mice (Figure 6C, compare bars 1 and 2). Furthermore, decrease in the growth of tumors correlated with decreased VEGF concentrations in the sera of Bay 11-7085–treated tumor-bearing mice (Figure 6D, compare bars 1 and 2). The results of this in vivo study suggested that constitutive activation of NF-κB and VEGF production were necessary for the growth of K1 lymphomas in nude mice.
Anti–VEGF antibody and NF-κB inhibitor Bay 11-7085 suppressed tumor growth and serum VEGF levels in K1 lymphoma–bearing BALB/c nu/nu mice. (A,C) K1 lymphoma subtransplants were generated by subcutaneously injecting tumor fragments (∼2 mm3) into BALB/c nu/nu mice from a primary lymphoma in a K1 transgenic mouse. To ascertain the effect of VEGF on tumor growth each tumor-bearing mouse (n = 6 per group) was given an anti–VEGF antibody (A, ▪) or normal rabbit IgG (15 μg/mouse each week; A, □) intraperitoneally. To ascertain the effect of NF-κB on tumor growth, tumor-bearing mice (n = 6 per group) were given Bay 11-7085 (100 μg/mouse; C, ▪) in 0.1 mL of DMSO, or DMSO alone (C, □), twice weekly intraperitoneally. The animals were killed after 3 weeks, and the tumors were recovered and weighed. (B,D) VEGF concentration in 50-μL aliquots of mouse sera was measured by ELISA. Statistically significant differences in tumor growth (A and C, bars 1 and 2) and serum VEGF levels (B and D) in tumor-bearing mice (bar 1) versus tumor-bearing mice treated with anti–VEGF antibody (B, bar 2) or Bay 11-7085 (C, bar 2), and nontumor-bearing mice (B, ▧) are shown with an asterisk (*).
Anti–VEGF antibody and NF-κB inhibitor Bay 11-7085 suppressed tumor growth and serum VEGF levels in K1 lymphoma–bearing BALB/c nu/nu mice. (A,C) K1 lymphoma subtransplants were generated by subcutaneously injecting tumor fragments (∼2 mm3) into BALB/c nu/nu mice from a primary lymphoma in a K1 transgenic mouse. To ascertain the effect of VEGF on tumor growth each tumor-bearing mouse (n = 6 per group) was given an anti–VEGF antibody (A, ▪) or normal rabbit IgG (15 μg/mouse each week; A, □) intraperitoneally. To ascertain the effect of NF-κB on tumor growth, tumor-bearing mice (n = 6 per group) were given Bay 11-7085 (100 μg/mouse; C, ▪) in 0.1 mL of DMSO, or DMSO alone (C, □), twice weekly intraperitoneally. The animals were killed after 3 weeks, and the tumors were recovered and weighed. (B,D) VEGF concentration in 50-μL aliquots of mouse sera was measured by ELISA. Statistically significant differences in tumor growth (A and C, bars 1 and 2) and serum VEGF levels (B and D) in tumor-bearing mice (bar 1) versus tumor-bearing mice treated with anti–VEGF antibody (B, bar 2) or Bay 11-7085 (C, bar 2), and nontumor-bearing mice (B, ▧) are shown with an asterisk (*).
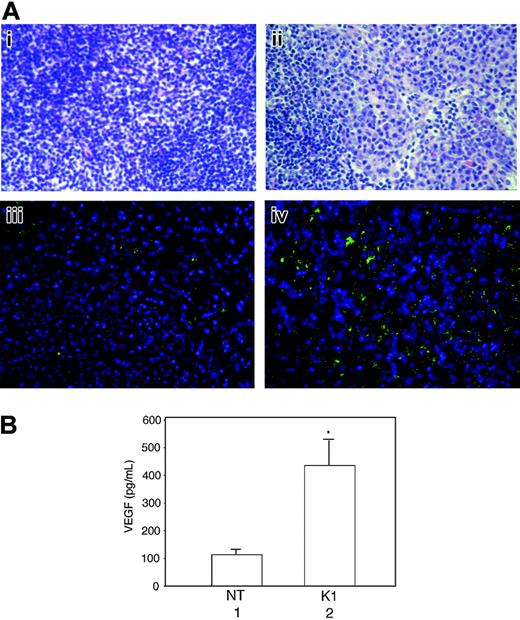
Lymph nodes of K1 transgenic mice exhibit hyperplasia and VEGF overexpression
At about 1 year of age more than 90% of K1 mice showed enlargement of the lymph nodes. Histologic examination revealed marked lymphadenopathy with distinct loss in normal nodal architecture, and an abundance of plasma cells, whereas a few scattered plasma cells were seen in the lymph nodes of nontransgenic mice (Figure 7Ai-ii). To evaluate the possible involvement of VEGF in lymph node hyperplasia, we compared VEGF expression in the lymph nodes of nontransgenic and K1 transgenic mice by using immunohistochemical analysis and in the supernatants of cultured lymph nodes by ELISA. A large number of plasma cells in the lymph node of a K1 transgenic mouse were positively stained with an anti–VEGF antibody, whereas VEGF expression in the normal lymph node was restricted to a very small number of cells (Figure 7Aiii-iv). Furthermore, the lymph nodes from the K1 transgenic mice produced more than 4 times as much VEGF as nontransgenic mice did (Figure 7B). The results suggested that K1-mediated VEGF induction was involved in the development of lymphoproliferative disorders in K1 mice.
Lymph nodes from K1 transgenic mice showing sheets of plasma cells and loss of normal architecture and increased VEGF production as detected by immunohistochemical staining and ELISA. (A) Lymph node tissue sections from a nontransgenic mouse (Ai) and a K1 transgenic mouse (Aii) were stained with hematoxylin and eosin and examined under a light microscope. Immunohistochemically stained sections of a lymph node from a nontransgenic mouse (Aiii) and a K1 transgenic mouse (Aiv) with anti–VEGF antibody were examined under a deconvolution microscope as described in “Materials and methods.” The original magnification was ×400. Results are representative of 3 separate experiments. (B) Equal weights of minced lymph nodes from nontransgenic (NT) and K1 transgenic mice were cultured overnight in 1 mL growth medium, and the VEGF levels were analyzed in 50-μL aliquots of culture supernatants by ELISA. Results represent the means (± SD) of VEGF concentrations in lymph nodes from 6 mice per group. There were statistically significant increases in VEGF concentration in the lymph nodes from K1 mice (*P = .009).
Lymph nodes from K1 transgenic mice showing sheets of plasma cells and loss of normal architecture and increased VEGF production as detected by immunohistochemical staining and ELISA. (A) Lymph node tissue sections from a nontransgenic mouse (Ai) and a K1 transgenic mouse (Aii) were stained with hematoxylin and eosin and examined under a light microscope. Immunohistochemically stained sections of a lymph node from a nontransgenic mouse (Aiii) and a K1 transgenic mouse (Aiv) with anti–VEGF antibody were examined under a deconvolution microscope as described in “Materials and methods.” The original magnification was ×400. Results are representative of 3 separate experiments. (B) Equal weights of minced lymph nodes from nontransgenic (NT) and K1 transgenic mice were cultured overnight in 1 mL growth medium, and the VEGF levels were analyzed in 50-μL aliquots of culture supernatants by ELISA. Results represent the means (± SD) of VEGF concentrations in lymph nodes from 6 mice per group. There were statistically significant increases in VEGF concentration in the lymph nodes from K1 mice (*P = .009).
Discussion
In this study, we found that the transcription factor NF-κB and the VEGF promoter are constitutively activated in K1-expressing mouse and human cells. This activation appears to be mediated primarily by the Src kinase Lyn because the specifically acting Lyn kinase inhibitor PP2 abrogated K1-dependent NF-κB activation and VEGF expression and blocked cellular proliferation. The stimulatory effects of K1 on NF-κB and VEGF can be blocked in cell culture and in vivo by using an inhibitor of IκB phosphorylation and anti–VEGF antibodies.
The concept that K1 contributes to KSHV-associated lymphoproliferative disorders is supported by several observations. Structurally, K1 markedly resembles Igα and Igβ of the B-cell receptor. In addition, it contains a functional ITAM in its cytoplasmic region, which in the absence of ligands efficiently induces signal transduction in B lymphocytes to elicit activation, which underlies growth deregulation.6,30,31 K1 expression induced lymphomas in vivo in common marmosets infected with recombinant herpesvirus saimiri in which the saimiri transformation protein oncogene had been replaced with the K1 gene.12 In addition, we earlier reported that K1 induced plasmablastic lymphomas in transgenic mice expressing K1 under the transcriptional control of the SV40 early promoter.22 These lymphomas had certain characteristics in common with KSHV-associated PEL in humans; for example, like PEL cells, a large percentage of K1 lymphoma cells expressed the plasma cell marker CD138, showed plasma cell morphology, and lacked common T- and B-cell markers.22
The Src family tyrosine kinase Lyn is expressed preferentially in B cells and other hematopoietic cells but not in T cells.32,33 Following activation by antigen binding, Lyn associates with the B-cell antigen receptor and triggers antigen-mediated signal transduction.34,35 Several studies have suggested that Lyn is a negative regulator of apoptosis.36-39 In addition, overexpression and activation of Lyn kinase have been shown to play an important role in the proliferation and survival of hematopoietic progenitor cells and B-lineage cells.40-42 Lyn kinase is also highly overexpressed and activated in chronic myelogenous leukemia cells resistant to the tyrosine kinase inhibitor STI571 (Gleevec; Novartis AG, Basel, Switzerland).43 Inhibition of Lyn with antisense treatment significantly reduced their proliferation and survival. Our current study showed that activation of Lyn kinase appears to be crucial for the proliferation of K1 lymphoma KVL-1 cells. This conclusion is based on our studies with Src kinase inhibitor PP2, which showed that the inhibition of Lyn kinase has a dominant effect on the growth of these cells. Although a previous study showed that ITAM-based signaling by K1 involves protein tyrosine kinase Syk,30 our tyrosyl phosphorylation analysis did not show Syk activation in KVL-1 cells or BJAB-K1 cells (data not shown). Consistent with these observations, piceatannol, a potent inhibitor of protein tyrosine kinases p56lck and Syk,44 only modestly inhibited the proliferation of KVL-1 cells.
VEGF stimulation of vascular proliferation and permeability may be critical to the pathogenesis of PEL. For example, PEL cell lines have been found to produce high levels of VEGF, and PEL cells inoculated into the peritoneal cavity of mice with severe combined immunodeficiency produced effusion lymphomas; neutralization of VEGF in these mice by intraperitoneal administration of an anti–VEGF antibody inhibited the formation of these lymphomas.16 In our study, we observed that nude mice bearing K1-induced lymphomas had very high serum VEGF concentrations, and the neutralization of serum VEGF markedly decreased tumor growth. Interestingly, the treatment of KVL-1 cells with anti–VEGF antibody had negligible effect on the proliferation of these cells in vitro (data not shown). Thus, our observations are also consistent with an earlier study showing that VEGF was critical for the growth of tumors in mice, likely as a result of its effect on the formation of blood vessels.16
The lymphoid hyperplasia that occurs in MCD is often associated with paracrine VEGF production by plasma cells and with vascular proliferation in the lymph nodes.18,19,45 The VEGF concentrations in the sera and in the supernatants of cultured lymph nodes from patients with the plasma cell type of MCD are significantly higher than those of control subjects.18,19 Consistent with these observations in humans, we found that the VEGF concentration in the supernatants of cultured lymph nodes from K1 mice was 4 to 5 times higher than that of control mice. We also observed that the serum VEGF concentrations in K1 mice were approximately 3 times greater than those in nontransgenic mice (data not shown).
NF-κB, which is constitutively expressed during all developmental stages of B-lineage cells, regulates the proliferative and survival responses of normal and transformed B cells.46-48 Constitutive NF-κB activation is essential for the growth and survival of Hodgkin lymphoma cells.49 In fact, NF-κB is constitutively activated in PEL cell lines and PEL primary clinical specimens and the survival of these cells depends on NF-κB activity.28 Inhibiting NF-κB with Bay 11-7082 blocked NF-κB activation and induced apoptosis. We have reported that K1 induced NF-κB in B lymphocytes from K1 mice.22 In the current study, we further found that K1 expression in transgenic mice constitutively activated NF-κB in plasmablastic lymphoma-derived KVL-1 cells, and in BJAB cells. Targeting NF-κB with Bay 11-7085 in K1 lymphoma–bearing nude mice drastically suppressed tumor growth, suggesting that NF-κB is essential for tumor development. In addition, our findings showed that NF-κB activation was mediated by ITAM-induced Lyn kinase activation because either a mutation in the ITAM sequences or inhibition of Lyn kinase activity suppressed NF-κB–dependent promoter activity. Thus, our observation that K1 induces NF-κB activation and tumor development lends support to the concept that chronic NF-κB activation eventually leads to cell transformation. The K1 transgenic mice showed chronic NF-κB activation in lymphocytes months before the appearance of lymphomas and lymph node hyperplasia, suggesting that the lymphoma and hyperplasia are the direct result of NF-κB stimulation. Thus, in addition to PEL, NF-κB activation is anticipated to play a key role in the development of MCD. Further studies into the early steps of K1 activation of NF-κB will be important in the design of cancer treatment and prevention strategies.
In B lymphocytes, BCR engagement activates PI3-K, which in turn activates serine threonine kinase Akt, which is an important regulator of cell proliferation, cell differentiation, antiapoptosis, and oncogenesis.50-52 The inhibition of PI3-K abrogates BCR-induced Akt activation and enhances apoptosis.51 Previous studies have shown that the PI3-K/Akt pathway is involved in KSHV vGPCR-mediated activation of NF-κB and VEGF production in human KS–derived endothelial cells, because the PI3-K inhibitor LY294002 inhibited both pathways.26,53 We found that VEGF production but not NF-κB activation was inhibited in the cells expressing K1, although LY294 002 inhibited NF-κB activation and VEGF production in vGPCR-transfected Raji cells. These observations suggest that different pathways might be involved, at least in part, in the activation of VEGF and NF-κB by K1.
In conclusion, our findings suggest a critical role of K1 in deregulating B-cell–specific signaling that may underlie the development of PEL and MCD. Through episodic lytic replication and continuous low-level spontaneous KSHV lytic gene expression in KS, PEL, and MCD, K1 expression can have a lasting role in the pathogenesis of these diseases.54 Thus, K1 expression during the early lytic cycle of viral replication or during nonlytic expression triggered by certain cellular factors does not rule out its later involvement in the development of KSHV-induced lymphoproliferative disorders. This idea is supported by our unpublished observations that induction of the viral lytic cycle in TPA-stimulated KS-1 and BC-3 PEL cells induced Lyn kinase–mediated NF-κB activation and VEGF production (O.P., O.R.S., X.P., and F.S., November 2004). In addition to K1, other KSHV-encoded lytic gene products may participate in pathogenesis. For example, viral interleukin 6 (vIL-6) is detected in the serum of most patients with PEL and MCD and some patients with KS.55 IL-6 is a B-cell growth and differentiation factor and is expressed in many types of B-cell malignancies.19,56,57 The finding that several other lytic genes have growth-regulatory properties provides further support for their role in KSHV-associated malignancies.5,54,58 Taken together, our data provide strong evidence that the B-cell–specific protein K1 is capable of inducing lymphoproliferative disorders in KSHV-infected individuals.
Prepublished online as Blood First Edition Paper, January 21, 2005; DOI 10.1182/blood-2004-07-2781.
Supported in part by a grant from the AIDS Crisis Trust (O.P.), the Cancer Association of Greater New Orleans (X.P.), and National Cancer Institute grants CA-16672 and CA-098412 (F.S).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Marvin Reitz for providing the vGPCR expression plasmid vGPCR-pSG5 and to Dr Keping Xie for providing the VEGF promoter–luciferase construct pGL3-V2274. We thank Roy Coleman for his expert technical assistance in the animal studies and Carol Thouron for her assistance in the immunohistochemical analysis.

![Figure 2. Src kinase inhibitor PP2 blocked the incorporation of [3H]-thymidine into KVL-1 cells and caused the reduction of cells in the S phase of the cell cycle. (A) KVL-1 cells (1 × 106) were treated with the Src kinase inhibitor PP2, its inactive analog PP3, or the Syk kinase inhibitor piceatannol (all 2.5 μMor5.0 μM), in a growth medium. After 16 hours, the cells were pulsed with [3H]-thymidine for 4 hours, and the radioactivity incorporated into the cells was measured as described in “Materials and methods.” Results represent the means (± SD) of 3 separate experiments. Marked decreases in [3H]-thymidine incorporation were observed only with PP2 (P < .001). (B) KVL-1 cells (1 × 106) were treated with 2.5 μM or 5.0 μM PP2 for 20 hours. Cell cycle profiles of the propidium iodide–stained cells were analyzed by flow cytometry, and the percentages of the cells in the G0G1,G2M, and S phases of cell cycle were calculated using the ModFit software program. Proliferation index (PI) was calculated from the SG2/G0G1 ratios. Results are representative of 3 independent experiments. DNA content (FL2-A) is depicted on the x axis and the number of cells after staining with propidium iodide is depicted on the y axis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-07-2781/6/m_zh80100578690002.jpeg?Expires=1767699346&Signature=ZIy97AKT1WFY49dML0OiMjExvuur9~L2NtKVx5M2K1bphpwf4ubM-kAnCNAvwgsZOzOSmvzvuuAbvWNnxOGa6DyZDowhAUfMdOsHdRimWrm9qAW24iBFWSXeTvvppUddGF1ahSyJLnHONz-Ay-cY4Wi7g9ucd8UM9xhsbD~HYjKxn3em~HImx~Xi6uDEwVUzxxXph7t1AronvBOfvcZ6HT1ksUGWs5w7kgHAk-JVVTWK8JbHFZLQS9mhEDXtVZwTvIuh3uyPIbKQV9Jbk0t5yXGaIxfe~oi1FenRsi5nx8k06KQQhHqfT8DognZhtuRToT1VXONkrjfz27BzTQnYDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal