Abstract
Immunomodulatory derivative (IMiD) CC-4047, a new analog of thalidomide, directly inhibits growth of B-cell malignancies in vivo and in vitro and exhibits stronger antiangiogenic activity than thalidomide. However, there is little information on whether CC-4047 affects normal hematopoiesis. Here we investigated the effect of CC-4047 on lineage commitment and differentiation of hematopoietic stem cells. We found that CC-4047 effectively inhibits erythroid cell colony formation from CD34+ cells and increases the frequency of myeloid colonies. We also demonstrate that development of both erythropoietin-independent and erythropoietin-dependent red cell progenitors was strongly inhibited by CC-4047, while terminal red cell differentiation was unaffected. DNA microarray analysis revealed that red cell transcription factors, including GATA-1, GATA-2, erythroid Kruppel-like factor (EKLF), and growth factor independence-1B (Gfi-1b), were down-regulated in CC-4047–treated CD34+ cells, while myeloid transcription factors such as CCAAT/enhancer binding protein-α (C/EBPα), C/EBPδ, and C/EBPϵ were induced. Analysis of cytokine secretion indicated that CC-4047 induced secretion of cytokines that enhance myelopoiesis and inhibit erythropoiesis. In conclusion, these data indicate that CC-4047 might directly influence lineage commitment of hematopoietic cells by increasing the propensity of stem and/or progenitor cells to undergo myeloid cell development and concomitantly inhibiting red cell development. Therefore, CC-4047 provides a valuable tool to study the mechanisms underlying lineage commitment.
Introduction
Differentiation of hematopoietic stem and progenitor cells into various lineages is controlled by a complex array of extrinsic and intrinsic factors.1-3 The molecular mechanisms controlling cellular commitment are still poorly defined.4 So far, there are no synthetic compounds that have the potential to determine lineage commitment of hematopoietic progenitor cells.
Thalidomide was originally developed as a sedative but was withdrawn from the market due to its association with teratogenicity.5 The recent discovery of its antimyeloma activity has led to an increased therapeutic use for multiple myeloma.6 To enhance the antimyeloma activity of thalidomide and reduce unwanted side effects, new immunomodulatory derivatives (IMiDs) of thalidomide like CC-4047 and CC-5013 have been developed.7,8 Our own data have shown that CC-4047 inhibits proliferation of B-cell neoplasias and angiogenesis in vitro and in vivo more potently than thalidomide and CC-5013.9,10 Because clinical trials with CC-4047 for the treatment of hematologic malignancies are ongoing, it is of special interest to evaluate the activity and toxicity of these drugs on the stem cell compartment and further differentiated cells.
Red blood cells represent one of the most abundant differentiated cell types of the human organism. Red cell development starts with the commitment of pluripotent hematopoietic stem cells into self-renewing lineage-specific progenitors, including erythroid burst-forming units (BFU-Es) and erythroid colony-forming units (CFU-Es), and results in fully mature hemoglobinized and enucleated erythrocytes. Various aspects of red cell development can be recapitulated in vitro under specific culture conditions.11,12
In this study, we analyzed the activity of IMiD CC-4047 on long-term culture-initiating cells (LTC-ICs) and on red cell development. We demonstrate that CC-4047 induces a shift in lineage commitment of CD34+ cells suppressing red cell development and promoting myelopoiesis. During this process red cell transcription factors are down-regulated while myeloid factors are induced. In addition, we show that CC-4047 induces secretion of cytokines that favor myeloid lineage commitment at the expense of cytokines for red cell development.
Materials and methods
Cells and cell culture
CD34+ cells were obtained from leukapheresis products from patients who were scheduled for autologous transplantation with enriched CD34+ cells. Leukapheresis products were subjected to positive selection with the ISOLEX 300 device.13 Subset analysis showed that CD34+ selected cells had predominantly mature phenotype (CD34+/CD38+ mean 95.5%, SD ± 5.3; CD34+/CD33+ mean 79.9%, SD ± 20.1; CD34+/DR+ mean 98.9%, SD ± 1.2).
Cells were stored in liquid nitrogen with 10% dimethyl sulfoxide (DMSO). CD34+ cells were cultured with an initial density of 1 × 105 to 3 × 105/mL in serum-free StemSpan medium (StemCell Technologies, Vancouver, BC, Canada) supplemented with stem cell factor (SCF) (100 ng/mL), interleukin-3 (IL-3) (10 ng/mL), and IL-6/soluble IL-6 receptor fusion protein (hyper–IL-6, 5 ng/mL) modified from Ju et al.14 Cytokines were added every second day, and cell density was maintained at 1 × 106 to 2 × 106/mL.
Alternatively, CD34+ cells were grown in culture medium for SCF/erythropoietin (SCF/Epo)–dependent erythroid progenitor cells (referred to as SCF/Epo cells)12 supplemented with SCF (100 ng/mL) and Epo (1 U/mL) to generate SCF/Epo cells. SCF/Epo cells were also obtained from cord blood (CB) and cultured with SCF plus Epo as described.12 To induce differentiation, cells were cultured with Epo (1 U/mL) and insulin (1 μg/mL) in differentiation medium (Dulbecco modified Eagle medium [DMEM] with 15% fetal calf serum [FCS], 1% bovine serum albumin [BSA], 0.1 mM β-mercaptoethanol, 0.128 mg/mL iron-saturated human transferrin, and 100 U/mL penicillin and streptomycin) modified from Panzenbock et al.12 Epo and insulin were added daily.
Thalidomide, CC-4047, and CC-5013 (Celgene, Warren, NJ) in DMSO were diluted in culture medium shortly before use and applied at 0.1, 1, 10, or 100 μM final concentration; 0.1% DMSO was used as a control. Compounds or DMSO were added in daily intervals.
To monitor cell growth, cells were counted at regular time intervals with an electronic cell counter device (CASY1; Schärfe Systems, Reutlingen, Germany), and cumulative cell numbers were determined. Erythroid differentiation was assessed by hemoglobin accumulation determined by neutral benzidine and Diff-Quick staining (Baxter, Dudingen, Switzerland) of cytospin preparations or by hemoglobin assay.15
Flow cytometry
Cells were analyzed for expression of specific surface antigens by flow cytometry as described.12,14 The following monoclonal antibodies were used: anti-CD11a, -CD33, -CD36 (BD Bioscience, San Jose, CA); anti–E-cadherin (R&D Systems, Minneapolis, MN); anti–band 3 and -glycophorin A/B (both Sigma-Aldrich, St Louis, MO); and anti-Epo receptor (Upstate Biotechnologies, Lake Placid, NY). Stained cells were analyzed by FACSCalibur flow cytometer and CELLQuest software (Becton Dickinson Immunocytometry Systems, San Diego, CA).
Apoptosis assay
Detection of apoptotic cells was performed by annexin/propidium iodide (annexin/PI) staining. Cells (5 × 105) were reacted with fluorescein isothiocyanate (FITC)–labeled annexin V (Roche Diagnostics, Mannheim, Germany) in the presence of PI and analyzed by flow cytometry according to the manufacturer's instructions. The ratios of living cells, apoptotic cells, and necrotic cells were determined.
Colony-forming assay
CD34+ cells were seeded onto the 35-mm plastic culture dishes (Corning, Acton, MA; 1.5 × 103 cells per dish) in 2 mL methylcellulose media (Methocult GF H4434; StemCell Technologies) and cultured in the presence of thalidomide, CC-4047 (0.1, 1, 10, and 100 μM), or DMSO 0.1% (control). Methocult GF H4434 contained the following hematopoietic growth factors: Epo 3 U/mL, recombinant human (rh) SCF 50 ng/mL, rh granulocyte-macrophage colony-stimulating factor (GM-CSF) 10 ng/mL, and rh IL-3 10 ng/mL. Cells were incubated in 5% CO2 with high humidity at 37°C for 14 days. At day 7 CFU-Es were evaluated, and at day 14 BFU-Es, CFU-GMs (granulocyte-macrophage colony-forming units), and CFU-Mix (mixed colony-forming units) were evaluated.
Cobblestone area forming cell (CAFC) assay
To analyze the influence of thalidomide or CC-4047 on the frequency of LTC-ICs of human CD34+ cells, cobblestone area forming cell (CAFC) assays were performed as described elsewhere.16 The FBMD-1 murine stromal cell line was overlaid by human CD34+ cells in a limiting dilution setup starting at 2000 cells per well down to 1 cell per well in 12 dilution steps. Sixteen replicates per dilution were used. The cultivation took place in long-term culture medium (StemCell Technologies) alone or in the presence of thalidomide (1 μM), CC-4047 (1, 10 μM), or DMSO (0.01%). The percentage of wells with at least 1 cobblestone area (1 phase-dark hematopoietic clone of at least 5 cells beneath the stromal layer) was determined after 6 weeks of culture, and the CAFC frequencies were calculated using Poisson statistics by the L-Calc software program (Stemsoft Software, Vancouver, BC, Canada).
Northern blotting
Western blotting
Western blotting was done as previously described.20 Briefly, cells were harvested, washed 3 times with phosphate-buffered saline (PBS), and lysed with lysis buffer (Nonidet P-40 [NP-40] 1%, dithiothreitol [DTT] 0.5 mM, sodium orthovanadate 1 mM, aprotinin 1 μg/mL, sodium fluoride 50 μM, phenylmethylsulfonyl fluoride [PMSF] 500 μM). Cell lysates were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond C super filters (Amersham, Arlington Heights, IL). The blots were probed with anti–GATA-1 antibody (N6; Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin antibody (Amersham), and immune complexes were detected using enhanced chemiluminescence (Amersham).
High-density oligonucleotide array expression analysis
Total RNA was isolated from 1 × 107 cells with the RNeasy kit (Qiagen). Complementary DNA synthesis was performed as described in the Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA) with 6 μg total RNA as template. Complementary RNA was generated with the BioArray High-Yield Transcript Labeling kit (Enzo Biochem, Farmingdale, NY). Target cRNA (10 μg) was hybridized to Affymetrix human U133A arrays. Hybridization, staining, washing, and scanning were carried out according to the manufacturer's protocol, and data analysis was performed with the Microarray Suite 5.0 software (Affymetrix). Arrays were scaled to an average intensity of 200.
Analysis of cytokine secretion
CD34+ cells (1 × 106/mL) were cultured with SCF, IL-3, and IL-6 in the presence of thalidomide (100 μM), CC-4047 (100 μM), or vehicle only (0.1% DMSO). New drugs were added, and partial media change was performed on day 3. Supernatants were collected on days 1, 3, and 6 and analyzed for IL-1β, IL-2, IL-4, IL-5, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), GM-CSF, G-CSF, monocyte chemotracting protein-1 (MCP-1), and macrophage inflammatory protein-1β (MIP-1β) by Bio-Plex Cytokine Assay (Bio-Rad Laboratories, Munich, Germany) according to the manufacturer's instructions. Intraassay variability, expressed as coefficient of variation (CV = standard deviation divided by the mean), was calculated based on determining quadruplicates of standards.21
Statistical analysis
To compare between the experimental groups, analysis of variance (ANOVA) was employed, followed by the Scheffe method as a post hoc test. P values less than .05 were considered statistically significant.
Results
CC-4047 inhibits the formation of erythroid colonies
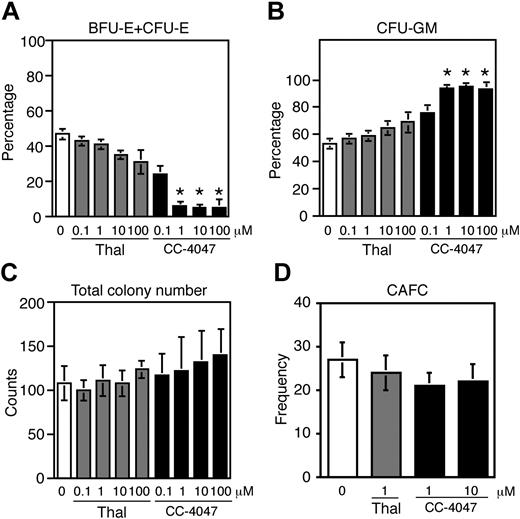
To determine the activity of thalidomide and its derivative CC-4047 on hematopoietic cells, we performed colony-forming and CAFC assays. In colony assays CC-4047 demonstrated a dramatic inhibitory activity on erythroid colony growth that was most pronounced at concentrations of 1 μM and above, whereas thalidomide showed only weak effects (Figure 1A). BFU-E and CFU-E colony formation was reduced by CC-4047 to the same extent. The number of CFU-GMs increased under CC-4047 (Figure 1B), resulting in an unaltered total colony number (Figure 1C). The number of CFU-Mix was also unaffected (data not shown). To analyze the impact of thalidomide and CC-4047 on the frequency of long-term culture-initiating cells (LTC-ICs), CAFC assays were performed. It was found that thalidomide and CC-4047 did not grossly affect the frequency of LTC-IC (Figure 1D).
Thus, thalidomide and, even more dramatically, its derivative CC-4047 exhibited a selective inhibitory activity on cells of the red cell lineage accompanied by enhanced myeloid differentiation.
CC-4047 influences lineage commitment by inhibiting development of early-stage erythroid cells
Colony assays are one-step continuous cultures that yield only limited cell numbers for more extensive biochemical and molecular studies. In addition, colony formation is, in most instances, the outcome of a limited number of cell divisions followed by commitment and terminal differentiation and thus does not permit study of the activity of compounds at distinct stages of development, proliferation, and differentiation separately. Most of these limitations can be overcome by liquid culture systems that recapitulate red cell development in vitro.
Activity of thalidomide and CC-4047 on erythroid colony formation and CAFCs. A total of 1.5 × 103 CD34+ cells were cultured in methylcellulose with SCF, IL-3, and hyper–IL-6 and increasing concentrations of thalidomide ( ) or CC-4047 (▪) as indicated. As vehicle control, 0.1% DMSO was used (□). CFU-E was evaluated at day 7 and BFU-E and CFU-GM at day 14. (A) Erythroid colonies (BFU-E + CFU-E) and (B) myeloid colonies (CFU-GM) were calculated as percentage of total colony numbers. (C) Total colony numbers were counted per plate. (A-C) Results are shown as means ± standard deviation from 3 independent experiments. *Significant change (P = .05) from control, calculated by ANOVA, followed by the Scheffe method as a post hoc test. (D) Cobblestone area forming cell (CAFC) assay. Frequencies of CD34+ cells required to form one cobblestone area under treatment with CC-4047 or thalidomide are shown. Data represent means ± standard error at 16 samples. The open bar indicates control (0.01% DMSO).
) or CC-4047 (▪) as indicated. As vehicle control, 0.1% DMSO was used (□). CFU-E was evaluated at day 7 and BFU-E and CFU-GM at day 14. (A) Erythroid colonies (BFU-E + CFU-E) and (B) myeloid colonies (CFU-GM) were calculated as percentage of total colony numbers. (C) Total colony numbers were counted per plate. (A-C) Results are shown as means ± standard deviation from 3 independent experiments. *Significant change (P = .05) from control, calculated by ANOVA, followed by the Scheffe method as a post hoc test. (D) Cobblestone area forming cell (CAFC) assay. Frequencies of CD34+ cells required to form one cobblestone area under treatment with CC-4047 or thalidomide are shown. Data represent means ± standard error at 16 samples. The open bar indicates control (0.01% DMSO).
Activity of thalidomide and CC-4047 on erythroid colony formation and CAFCs. A total of 1.5 × 103 CD34+ cells were cultured in methylcellulose with SCF, IL-3, and hyper–IL-6 and increasing concentrations of thalidomide ( ) or CC-4047 (▪) as indicated. As vehicle control, 0.1% DMSO was used (□). CFU-E was evaluated at day 7 and BFU-E and CFU-GM at day 14. (A) Erythroid colonies (BFU-E + CFU-E) and (B) myeloid colonies (CFU-GM) were calculated as percentage of total colony numbers. (C) Total colony numbers were counted per plate. (A-C) Results are shown as means ± standard deviation from 3 independent experiments. *Significant change (P = .05) from control, calculated by ANOVA, followed by the Scheffe method as a post hoc test. (D) Cobblestone area forming cell (CAFC) assay. Frequencies of CD34+ cells required to form one cobblestone area under treatment with CC-4047 or thalidomide are shown. Data represent means ± standard error at 16 samples. The open bar indicates control (0.01% DMSO).
) or CC-4047 (▪) as indicated. As vehicle control, 0.1% DMSO was used (□). CFU-E was evaluated at day 7 and BFU-E and CFU-GM at day 14. (A) Erythroid colonies (BFU-E + CFU-E) and (B) myeloid colonies (CFU-GM) were calculated as percentage of total colony numbers. (C) Total colony numbers were counted per plate. (A-C) Results are shown as means ± standard deviation from 3 independent experiments. *Significant change (P = .05) from control, calculated by ANOVA, followed by the Scheffe method as a post hoc test. (D) Cobblestone area forming cell (CAFC) assay. Frequencies of CD34+ cells required to form one cobblestone area under treatment with CC-4047 or thalidomide are shown. Data represent means ± standard error at 16 samples. The open bar indicates control (0.01% DMSO).
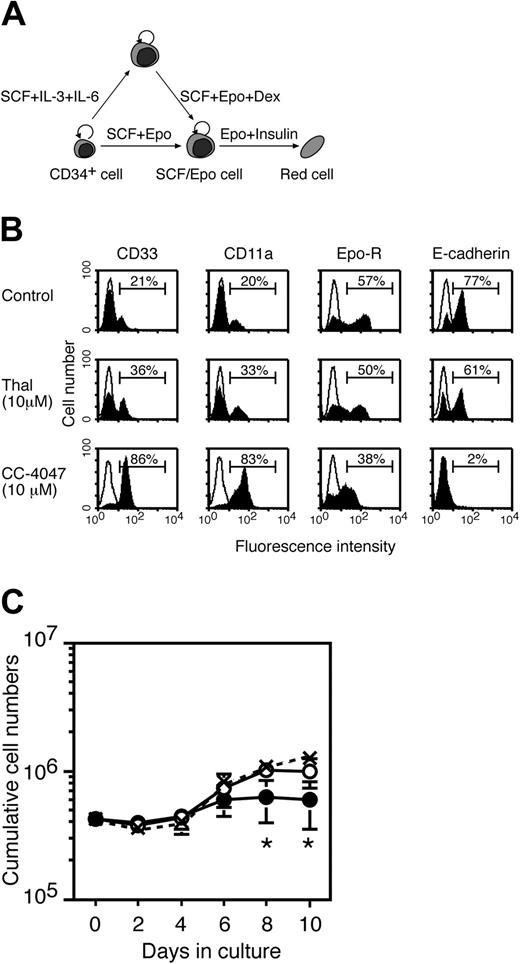
CC-4047 inhibits development of erythroid progenitor cells. (A) Schematic representation of the cell culture systems used in the study. CD34+ cells cultured with SCF plus Epo develop into SCF/Epo-dependent erythroid progenitors (SCF/Epo cells) that can be induced to further differentiate into fully mature red cells by Epo plus insulin. Alternatively, CD34+ cells are induced to develop first into Epo-independent progenitors with SCF plus IL-3 plus hyper–IL-6 (SI2 cells) and then induced to yield SCF/Epo cells by SCF plus Epo plus Dex. SI2 cells and SCF/Epo cells represent progenitors at the early and late stage of erythroid cell development, respectively. (B) A total of 4 × 105 CD34+ cells were cultured with SCF plus Epo to generate SCF/Epo cells in the presence of thalidomide (10 μM), CC-4047 (10 μM), or 0.1% DMSO (control). At day 10 in culture, cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. Horizontal lines represent the percent of positive cells for each staining. (C) The growth kinetics of cells from panel B are shown as cumulative cell numbers. ○ indicates thalidomide; •, CC-4047; and × with dotted line, control. Data represent means ± standard deviation from 3 independent experiments. *Significant change (P = .05) from control on each day.
CC-4047 inhibits development of erythroid progenitor cells. (A) Schematic representation of the cell culture systems used in the study. CD34+ cells cultured with SCF plus Epo develop into SCF/Epo-dependent erythroid progenitors (SCF/Epo cells) that can be induced to further differentiate into fully mature red cells by Epo plus insulin. Alternatively, CD34+ cells are induced to develop first into Epo-independent progenitors with SCF plus IL-3 plus hyper–IL-6 (SI2 cells) and then induced to yield SCF/Epo cells by SCF plus Epo plus Dex. SI2 cells and SCF/Epo cells represent progenitors at the early and late stage of erythroid cell development, respectively. (B) A total of 4 × 105 CD34+ cells were cultured with SCF plus Epo to generate SCF/Epo cells in the presence of thalidomide (10 μM), CC-4047 (10 μM), or 0.1% DMSO (control). At day 10 in culture, cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. Horizontal lines represent the percent of positive cells for each staining. (C) The growth kinetics of cells from panel B are shown as cumulative cell numbers. ○ indicates thalidomide; •, CC-4047; and × with dotted line, control. Data represent means ± standard deviation from 3 independent experiments. *Significant change (P = .05) from control on each day.
CD34+ cells cultured with SCF and Epo develop into SCF/Epo-dependent erythroid progenitor cells (referred to as SCF/Epo cells) that can be induced to differentiate further into fully mature red cells by Epo and insulin11,12 (Figure 2A). Alternatively, CD34+ cells can be induced to develop first into Epo-independent progenitors with SCF, IL-3, and hyper–IL-6 (referred to as SI2 cells by B.L., K.-R.K., and M.Z, manuscript in preparation) and then induced to yield SCF/Epo progenitors by SCF, Epo, and dexamethasone (Dex) treatment (Figure 2A). SI2 cells and SCF/Epo cells represent progenitors at early and late stages of erythroid cell development, respectively. Therefore, we employed these culture systems to investigate at which stage of red cell development thalidomide and/or CC-4047 might act.
CD34+ cells were cultured with SCF and Epo in the absence and presence of thalidomide or CC-4047. CC-4047 had a major impact on red cell differentiation as assessed by analysis of surface marker expression (Figure 2B). The number of cells expressing the erythroid markers Epo receptor and E-cadherin (B.L., K.-R.K., and M.Z., manuscript in preparation) was reduced while the number of myeloid CD33+ CD11a+ cells was increased. In contrast, the activity of thalidomide was only marginal, similar to what was observed in the colony assay (Figures 1A-C and 2B). Furthermore, CC-4047 reduced cell growth of developing SCF/Epo cells while there was no effect of thalidomide (Figure 2C).
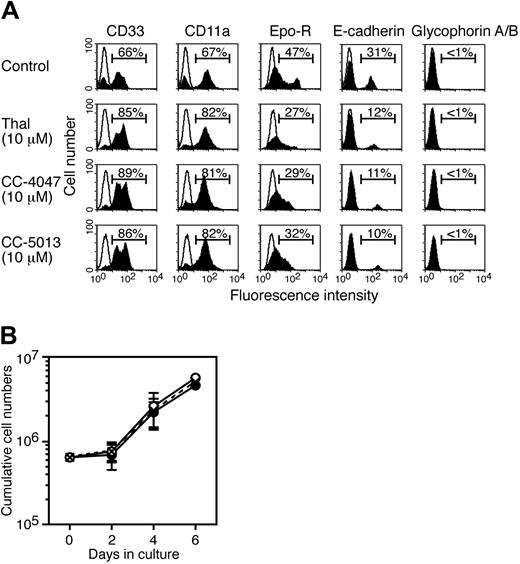
To determine whether CC-4047 affects early and/or late stages of erythroid development, the impact on differentiation into SI2 cells was analyzed. CD34+ cells were grown with SCF, IL-3, and hyper–IL-6 alone or in the presence of either thalidomide or CC-4047 (Figure 2A). Untreated cultures contained both myeloid and erythroid progenitor cells as determined by flow cytometry: About 70% of SI2 cells expressed the myeloid markers CD33 and CD11a, whereas about 30% to 40% of the cells had the erythroid markers Epo receptor and E-cadherin (Figure 3A). However, treatment with thalidomide or CC-4047 increased the number of myeloid cells while erythroid cell numbers were decreased. Glycophorin A/B, a marker of mature red cells, was not up-regulated by thalidomide or CC-4047 treatment (Figure 3A), indicating that the reduced growth potential of erythroid progenitors was not the result of differentiation induction by these compounds. We also tested the effects of CC-5013 on marker expression and obtained essentially the same results (Figure 3A). Treatment with thalidomide or CC-4047 had no effect on total cell numbers (Figure 3B).
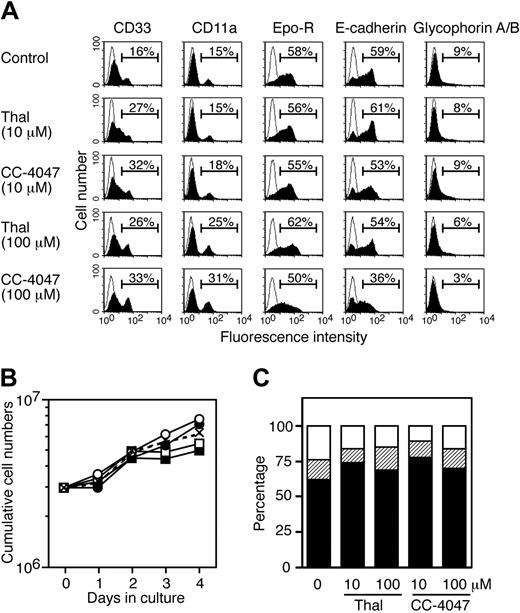
To investigate the activity of thalidomide and CC-4047 on later stages of erythroid progenitor development, SCF/Epo cells were generated from SI2 cells with SCF, Epo, and Dex (Figure 2A). Untreated cultures contained about 60% of SCF/Epo cells expressing the erythroid markers Epo receptor and E-cadherin. Concentrations of thalidomide or CC-4047 of 100 μM decreased numbers of SCF/Epo cells concomitantly with an increase of myeloid cells (Figure 4A). Thalidomide and CC-4047 reduced growth of SCF/Epo cells to some extent (Figure 4B), but neither compound affected cell survival even at high concentrations (100 μM) as determined by annexin (Figure 4C).
Activity of thalidomide and CC-4047 on development of early-stage erythroid progenitor cells. (A) A total of 6 × 105 CD34+ cells were cultured with SCF plus IL-3 plus hyper–IL-6 to yield SI2 cells as indicated in Figure 2A in the presence of thalidomide (10 μM), CC-4047 (10 μM), CC-5013 (10 μM), or 0.1% DMSO (control). At day 7 in culture, cells were analyzed for surface antigen expression by flow cytometry. The open area indicates staining with isotype control antibody. (B) The growth kinetics of cells from panel A are shown as cumulative cell numbers. ○ indicates thalidomide; •, CC-4047; and × with dotted line, control. Data represent means ± standard deviation.
Activity of thalidomide and CC-4047 on development of early-stage erythroid progenitor cells. (A) A total of 6 × 105 CD34+ cells were cultured with SCF plus IL-3 plus hyper–IL-6 to yield SI2 cells as indicated in Figure 2A in the presence of thalidomide (10 μM), CC-4047 (10 μM), CC-5013 (10 μM), or 0.1% DMSO (control). At day 7 in culture, cells were analyzed for surface antigen expression by flow cytometry. The open area indicates staining with isotype control antibody. (B) The growth kinetics of cells from panel A are shown as cumulative cell numbers. ○ indicates thalidomide; •, CC-4047; and × with dotted line, control. Data represent means ± standard deviation.
Activity of thalidomide and CC-4047 on development of late-stage erythroid progenitor cells. (A) SI2 cells were generated from CD34+ cells after 7 days of culture with SCF plus IL-3 plus hyper–IL-6 as indicated in Figure 2A. Then, 3 × 106 SI2 cells were induced to differentiate into SCF/Epo cells with SCF, Epo, and Dex in the presence of thalidomide (10, 100 μM), CC-4047 (10, 100 μM), or 0.1% DMSO (control). At day 4 in culture, cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. (B) The growth kinetics of cells from panel A are shown as cumulative cell numbers. ○ and • indicate 10 μM thalidomide and CC-4047, respectively; □and ▪, 100 μM thalidomide and CC-4047, respectively. × with dotted line indicates control. (C) At day 2 in culture, cells were subjected to apoptosis assay by double staining with annexin V and PI. Results are shown as the ratio of living cells (▪), apoptotic cells (▨), and necrotic cells (□) in the total cell numbers.
Activity of thalidomide and CC-4047 on development of late-stage erythroid progenitor cells. (A) SI2 cells were generated from CD34+ cells after 7 days of culture with SCF plus IL-3 plus hyper–IL-6 as indicated in Figure 2A. Then, 3 × 106 SI2 cells were induced to differentiate into SCF/Epo cells with SCF, Epo, and Dex in the presence of thalidomide (10, 100 μM), CC-4047 (10, 100 μM), or 0.1% DMSO (control). At day 4 in culture, cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. (B) The growth kinetics of cells from panel A are shown as cumulative cell numbers. ○ and • indicate 10 μM thalidomide and CC-4047, respectively; □and ▪, 100 μM thalidomide and CC-4047, respectively. × with dotted line indicates control. (C) At day 2 in culture, cells were subjected to apoptosis assay by double staining with annexin V and PI. Results are shown as the ratio of living cells (▪), apoptotic cells (▨), and necrotic cells (□) in the total cell numbers.
In summary, these data suggest that thalidomide and CC-4047 modulate hematopoiesis by inhibiting development of erythroid progenitors and by enhancing growth of myeloid progenitors. This effect is most pronounced in the early stage of differentiation.
CC-4047 has no inhibitory effect on terminal differentiation of erythroid cells
Next, we investigated whether thalidomide and CC-4047 inhibit terminal differentiation of red cells. SCF/Epo progenitor cells were induced to differentiate with Epo and insulin in the presence of thalidomide or CC-4047 (Figure 2A). Effective differentiation was controlled by measuring changes in cell size and morphology, hemoglobin accumulation, and expression of late cell-surface markers, such as glycophorin A/B and band 3. At standard concentrations (10 μM) the activity of thalidomide and CC-4047 was indistinguishable from medium-treated control. At 100 μM, CC-4047 resulted in a marginal reduction of growth while thalidomide was without effect (Figure 5A). For all other parameters analyzed, such as hemoglobin accumulation and cell survival, thalidomide- and CC-4047–treated cells were similar to control. Under treatment with CC-4047 and CC-5013, cell-surface marker expression of glycophorin A/B was unaffected and band 3 showed a minor reduction (Figure 5B-D).
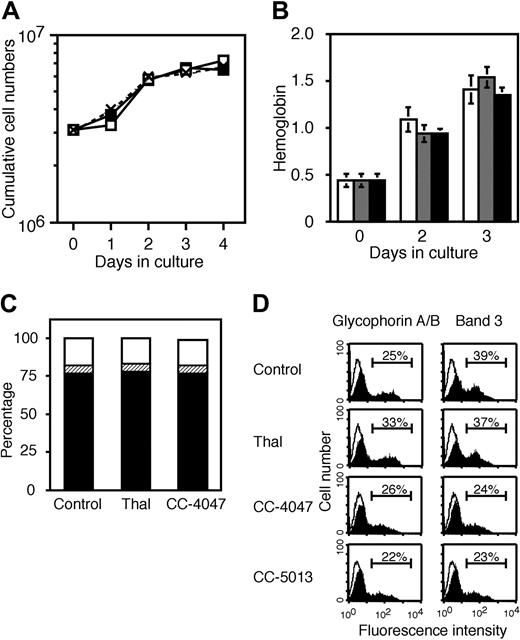
Thalidomide and CC-4047 do not affect terminal differentiation of erythroid progenitor cells. SCF/Epo erythroid progenitor cells were generated from SI2 cells after 3 to 4 days of culture with SCF plus Epo plus Dex as indicated in Figure 2A. Then, 3 × 106 SCF/Epo cells were induced to terminal differentiation with Epo plus insulin in the presence of thalidomide (100 μM), CC-4047 (100 μM), CC-5013 (10 μM), or 0.1% DMSO (control). (A) The growth kinetics are shown as cumulative cell numbers. Data represent means ± standard deviation from 3 different experiments. □ indicates thalidomide; ▪, CC-4047; and × with dotted line, control. (B) Cell hemoglobin levels were determined by hemoglobin assay and measured as optical density (OD 490). After 0, 2, and 3 days in the presence of thalidomide ( , 100 μM), CC-4047 (▪, 100 μM), or 0.1% DMSO (□, control). Data represent means ± standard deviation. (C) At day 2 in culture, cells were subjected to apoptosis assay by double staining with annexin V. Result is shown as the ratio of living cells (▪), apoptotic cells (▨), and necrotic cells (□) in the total cell numbers. (D) Cells at day 3 in culture. Cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. Horizontal bars indicate the percent of positive cells for each staining.
, 100 μM), CC-4047 (▪, 100 μM), or 0.1% DMSO (□, control). Data represent means ± standard deviation. (C) At day 2 in culture, cells were subjected to apoptosis assay by double staining with annexin V. Result is shown as the ratio of living cells (▪), apoptotic cells (▨), and necrotic cells (□) in the total cell numbers. (D) Cells at day 3 in culture. Cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. Horizontal bars indicate the percent of positive cells for each staining.
Thalidomide and CC-4047 do not affect terminal differentiation of erythroid progenitor cells. SCF/Epo erythroid progenitor cells were generated from SI2 cells after 3 to 4 days of culture with SCF plus Epo plus Dex as indicated in Figure 2A. Then, 3 × 106 SCF/Epo cells were induced to terminal differentiation with Epo plus insulin in the presence of thalidomide (100 μM), CC-4047 (100 μM), CC-5013 (10 μM), or 0.1% DMSO (control). (A) The growth kinetics are shown as cumulative cell numbers. Data represent means ± standard deviation from 3 different experiments. □ indicates thalidomide; ▪, CC-4047; and × with dotted line, control. (B) Cell hemoglobin levels were determined by hemoglobin assay and measured as optical density (OD 490). After 0, 2, and 3 days in the presence of thalidomide ( , 100 μM), CC-4047 (▪, 100 μM), or 0.1% DMSO (□, control). Data represent means ± standard deviation. (C) At day 2 in culture, cells were subjected to apoptosis assay by double staining with annexin V. Result is shown as the ratio of living cells (▪), apoptotic cells (▨), and necrotic cells (□) in the total cell numbers. (D) Cells at day 3 in culture. Cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. Horizontal bars indicate the percent of positive cells for each staining.
, 100 μM), CC-4047 (▪, 100 μM), or 0.1% DMSO (□, control). Data represent means ± standard deviation. (C) At day 2 in culture, cells were subjected to apoptosis assay by double staining with annexin V. Result is shown as the ratio of living cells (▪), apoptotic cells (▨), and necrotic cells (□) in the total cell numbers. (D) Cells at day 3 in culture. Cells were analyzed for surface antigen expression by flow cytometry (filled area). The open area indicates staining with isotype control antibody. Horizontal bars indicate the percent of positive cells for each staining.
In contrast to CD34+ cells, CB-derived SCF/Epo cells develop under Epo plus insulin condition more effectively into mature red cells (86% glycophorin A/B positive, 59% band 3 positive). Because we wanted to analyze especially the hemoglobinization and development of mature red cells, we performed additional experiments with cord blood cells. The treatment of cord blood cells with thalidomide and CC-4047 did not change marker expression, with 86% glycophorin A/B positive, 54% band 3 positive; and 84% glycophorin A/B positive, 54% band 3 positive, respectively. Hemoglobin accumulation and cell survival were essentially the same as for CD34+-derived cells (data not shown). Thus, we conclude that thalidomide and CC-4047 have no inhibitory effects on terminal differentiation of erythroid progenitor cells.
CC-4047 induces secretion of myeloid-specific cytokines
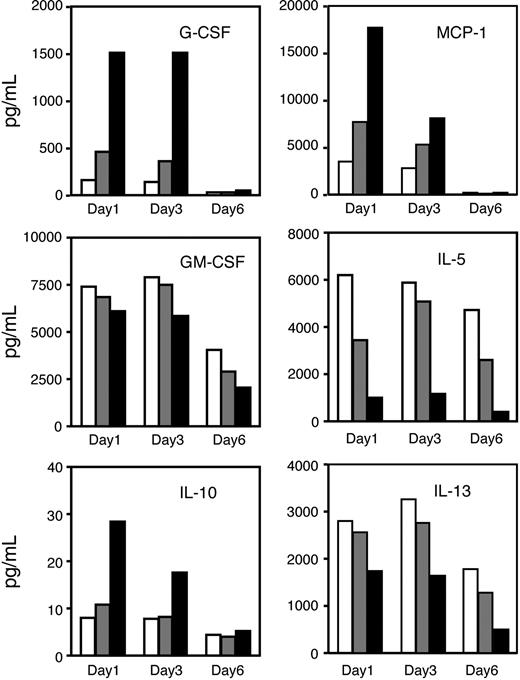
Hematopoiesis is regulated by cytokines that bind to lineage-specific receptors and thereby affect lineage fate. To investigate whether the activity of CC-4047 on hematopoietic cells might involve the production of specific cytokines, CD34+ cells were treated with thalidomide or CC-4047 for 1, 3, and 6 days and supernatants were analyzed by Bio-Plex Cytokine Assay. G-CSF, IL-10, and MCP-1 were effectively increased by CC-4047 at days 1 and 3 of treatment, while there was only a marginal increase by thalidomide (Figure 6). Conversely, levels of GM-CSF, IL-5, and IL-13 were reduced upon CC-4047 treatment, and this reduction was particularly prominent for IL-5. Cells also produce IL-1β, IL-2, IL-4, IL-7, IL-8, IL-12, IL-17, TNF-α, IFN-γ, and MIP-1β, but their expression was not affected by CC-4047 and thalidomide.
CC-4047 modulates expression of lineage-associated transcription factors
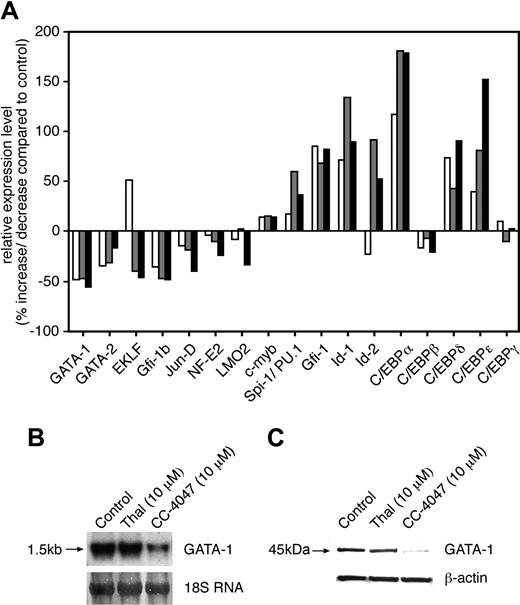
To obtain further insight into the molecular mechanisms that might be responsible for CC-4047 activity on hematopoiesis, we investigated the gene expression profile of CC-4047–treated CD34+ cells by oligonucleotide microarrays. CD34+ cells were treated with CC-4047 or vehicle alone, and expression analysis was performed after 1, 2, and 3 days of treatment. Figure 7A demonstrates the relative gene expression level in CC-4047–treated cells compared with control-treated cells. Because transcription factors have a determining function in lineage decision, we concentrated on changes in transcription factor expression in response to CC-4047. Several transcription factors with a function in red cell development were down-regulated by CC-4047, such as GATA-1, GATA-2, erythroid Kruppel-like factor (EKLF), growth factor independence-1B (Gfi-1b), Jun-D, nuclear factor–erythroid 2 (NF-E2), and Lim-only 2 (LMO2) (Figure 7A). Conversely, several transcription factors that are expressed in myeloid cells were strongly up-regulated, such as CCAAT/enhancer binding protein-α (C/EBPα), C/EBPδ, C/EBPϵ, and Spi-1/PU.1. Furthermore, we observed up-regulation of Gfi-1 and also an induction of the helix-loop-helix factors Id1 and Id2. Down-regulation of GATA-1 by CC-4047 was also observed by Northern and Western blotting (Figure 7B-C).
CC-4047 enhances secretion of myeloid-specific cytokines. CD34+ cells were cultured with SCF plus IL-3 plus IL-6 as indicated in Figure 2A in the presence of thalidomide (100 μM;  ), CC-4047 (100 μM; ▪), or 0.1% DMSO (control; □). Supernatants were analyzed for cytokine secretion (picograms per milliliter) after 1, 3, and 6 days of treatment by Bio-Plex Cytokine Assay. Intra-assay variability, expressed as coefficient of variation (CV = standard deviation divided by the mean), was calculated based on determining quadruplicates of standards. The CV for each experiment was less than 10%.
), CC-4047 (100 μM; ▪), or 0.1% DMSO (control; □). Supernatants were analyzed for cytokine secretion (picograms per milliliter) after 1, 3, and 6 days of treatment by Bio-Plex Cytokine Assay. Intra-assay variability, expressed as coefficient of variation (CV = standard deviation divided by the mean), was calculated based on determining quadruplicates of standards. The CV for each experiment was less than 10%.
CC-4047 enhances secretion of myeloid-specific cytokines. CD34+ cells were cultured with SCF plus IL-3 plus IL-6 as indicated in Figure 2A in the presence of thalidomide (100 μM;  ), CC-4047 (100 μM; ▪), or 0.1% DMSO (control; □). Supernatants were analyzed for cytokine secretion (picograms per milliliter) after 1, 3, and 6 days of treatment by Bio-Plex Cytokine Assay. Intra-assay variability, expressed as coefficient of variation (CV = standard deviation divided by the mean), was calculated based on determining quadruplicates of standards. The CV for each experiment was less than 10%.
), CC-4047 (100 μM; ▪), or 0.1% DMSO (control; □). Supernatants were analyzed for cytokine secretion (picograms per milliliter) after 1, 3, and 6 days of treatment by Bio-Plex Cytokine Assay. Intra-assay variability, expressed as coefficient of variation (CV = standard deviation divided by the mean), was calculated based on determining quadruplicates of standards. The CV for each experiment was less than 10%.
Gene expression profiles of transcription factors induced by CC-4047 in CD34+ cells. (A) CD34+ cells were cultured with SCF plus IL-3 plus IL-6 as indicated in Figure 2A in the presence of CC-4047 (100 μM) or 0.1% DMSO (control), followed by extraction of total RNA at day 1 (□), day 2 ( ), and day 3 (▪). Six micrograms of total RNA was subjected to DNA microarray analysis (see “Materials and methods”). The relative expression levels of transcription factors induced by CC-4047 are shown as percentage of increase or decrease (-) compared with control. (B) CD34+ cells were cultured with SCF plus IL-3 plus hyper–IL-6 (Figure 2A) in the presence of thalidomide (10 μM), CC-4047 (10 μM), or 0.1% DMSO (control), followed by extraction of total RNA (day 3) or protein lysates (day 7). RNA was analyzed for GATA-1 expression by Northern blotting. Methylene blue staining of 18S ribosomal RNA is shown as loading control. (C) The cell lysates were subjected to Western blotting to determine GATA-1 protein expression. β-actin expression served as loading control.
), and day 3 (▪). Six micrograms of total RNA was subjected to DNA microarray analysis (see “Materials and methods”). The relative expression levels of transcription factors induced by CC-4047 are shown as percentage of increase or decrease (-) compared with control. (B) CD34+ cells were cultured with SCF plus IL-3 plus hyper–IL-6 (Figure 2A) in the presence of thalidomide (10 μM), CC-4047 (10 μM), or 0.1% DMSO (control), followed by extraction of total RNA (day 3) or protein lysates (day 7). RNA was analyzed for GATA-1 expression by Northern blotting. Methylene blue staining of 18S ribosomal RNA is shown as loading control. (C) The cell lysates were subjected to Western blotting to determine GATA-1 protein expression. β-actin expression served as loading control.
Gene expression profiles of transcription factors induced by CC-4047 in CD34+ cells. (A) CD34+ cells were cultured with SCF plus IL-3 plus IL-6 as indicated in Figure 2A in the presence of CC-4047 (100 μM) or 0.1% DMSO (control), followed by extraction of total RNA at day 1 (□), day 2 ( ), and day 3 (▪). Six micrograms of total RNA was subjected to DNA microarray analysis (see “Materials and methods”). The relative expression levels of transcription factors induced by CC-4047 are shown as percentage of increase or decrease (-) compared with control. (B) CD34+ cells were cultured with SCF plus IL-3 plus hyper–IL-6 (Figure 2A) in the presence of thalidomide (10 μM), CC-4047 (10 μM), or 0.1% DMSO (control), followed by extraction of total RNA (day 3) or protein lysates (day 7). RNA was analyzed for GATA-1 expression by Northern blotting. Methylene blue staining of 18S ribosomal RNA is shown as loading control. (C) The cell lysates were subjected to Western blotting to determine GATA-1 protein expression. β-actin expression served as loading control.
), and day 3 (▪). Six micrograms of total RNA was subjected to DNA microarray analysis (see “Materials and methods”). The relative expression levels of transcription factors induced by CC-4047 are shown as percentage of increase or decrease (-) compared with control. (B) CD34+ cells were cultured with SCF plus IL-3 plus hyper–IL-6 (Figure 2A) in the presence of thalidomide (10 μM), CC-4047 (10 μM), or 0.1% DMSO (control), followed by extraction of total RNA (day 3) or protein lysates (day 7). RNA was analyzed for GATA-1 expression by Northern blotting. Methylene blue staining of 18S ribosomal RNA is shown as loading control. (C) The cell lysates were subjected to Western blotting to determine GATA-1 protein expression. β-actin expression served as loading control.
Discussion
This is the first study that investigates the effect of thalidomide and its immunomodulatory analog CC-4047 on human hematopoietic stem cells. We demonstrate that CC-4047 influences the lineage commitment of hematopoietic stem cells in vitro by impairing the generation of early erythroid progenitor cells and simultaneously enhancing myelopoiesis without any apparent toxicity on hematopoietic stem cells. Moreover, our study demonstrates that in contrast to the reduced erythroid progenitor cell production, both CC-4047 and thalidomide showed no inhibitory effect on terminal differentiation of erythroid progenitor cells.
To elucidate the effect of thalidomide and CC-4047 on hematopoietic progenitors, we demonstrated in CAFC assays that the number of CAFCs did not decrease under treatment with either thalidomide or CC-4047. These data suggest that CC-4047 as well as thalidomide have no toxic effects on hematopoietic precursors and do not impair self-renewal of hematopoietic stem cells. Remarkably, our colony assays showed that despite an increased number of total colonies the erythroid colonies almost disappeared under CC-4047 treatment, whereas CFU-GMs increased and CFU-Mix were unaffected. These results indicate that CC-4047 has a profound effect on lineage commitment. To determine which step in the process of lineage commitment is influenced by CC-4047, we used an in vitro culture system that has been established by our group (Figure 2A). The SCF/Epo erythroid progenitor cells can be generated directly from CD34+ cells with SCF and Epo. Treatment with CC-4047 during this stage induced down-regulation of erythroid progenitors and a very strong up-regulation of myeloid progenitors. Treatment of hematopoietic progenitors under SI2 conditions in the presence of CC-4047 resulted in a decrease of erythroid-committed cells and an increase of myeloid-committed cells. Because the SI2 condition supports the development into myeloid cells rather than into erythroid cells in this assay, we obtained more cells with myeloid markers. This might be the reason that in contrast to culture conditions including SCF and Epo, CC-4047–induced inhibition of erythroid commitment was less apparent under SI2 conditions.
The SI2 progenitor cell population contains erythropoietin-independent immature erythroid progenitor cells, which commit to erythropoietin-dependent mature erythroid progenitor cells (SCF/Epo progenitors) in the presence of SCF, Epo, and Dex. At this late stage of erythroid progenitor development, expression of myeloid markers increased and erythroid markers decreased under 100 μM CC-4047. In contrast, 1 μM CC-4047 already significantly inhibited erythroid colony formation compared with control (Figure 1A). The most remarkable difference between the colony assay and our liquid culture system is that the in vitro culture system enables us to confirm the stage-specific activity of CC-4047 on human erythropoiesis. In contrast, with the colony assay we cannot identify at which stage of erythropoiesis this compound showed inhibitory effect. The fact that CC-4047 at 10 μM did not affect the late stage of erythropoiesis from SI2 cells to SCF/Epo cells but showed inhibitory effect on the early stage of erythropoiesis (SI2 cell growth out of CD34+ cells) strongly suggests that the reduced number of erythroid colonies is mainly due to the inhibitory effect of CC-4047 on the early stage of erythropoiesis.
Finally, SCF/Epo progenitor cells can terminally differentiate into erythrocytes in the presence of Epo and insulin (Figure 2A). At this stage of terminal erythroid differentiation, treatment with CC-4047 or thalidomide had no effect on proliferation, surface marker expression, or hemoglobin synthesis. Because our in vitro culture system mimics the erythroid cell production in the bone marrow, we could show that CC-4047 influences the early lineage commitment by reducing erythropoiesis and enhancing myelopoiesis independently from erythropoietin. Neither thalidomide nor CC-4047 inhibits the further development of already committed erythroid progenitors.
CC-5013, another IMiD of thalidomide with similar structure and biologic effects as CC-4047, is already used in several clinical trials for the treatment of multiple myeloma22 and myelodysplastic syndrome (MDS).23 Therefore, we performed additional experiments with CC-5013. We examined early lineage commitment (SI2 pathway) and terminal differentiation and obtained essentially the same results (Figures 3A and 5D).
To examine the underlying mechanisms, we first analyzed the cytokine expression profile of CD34+ cells treated with thalidomide or CC-4047. The analysis revealed that cytokines supporting myelopoiesis increased very early. After CC-4047 stimulation, secretion of G-CSF increased within 24 hours 10-fold in comparison with control cells. Because G-CSF mediates enhanced development of myeloid progenitors,24 our data suggest that especially the up-regulation of G-CSF contributes to enhanced myelopoiesis. MCP-1, which is known to support predominantly the granulocytic lineage25 and to augment the clonal expansion of progenitor cells,26 increased also up to 500% on day 1 under CC-4047 treatment as compared with control. Secretion of IL-10, a proinflammatory cytokine known to inhibit erythropoiesis,27,28 was also strongly up-regulated on day 1 (350%) and on day 3 (226%) under CC-4047. In addition, IL-13, which favors the development of erythroid progenitors, was decreased by CC-4047 (day 1, 62%; day 3, 51%; day 6, 28%) compared with control. IL-13 has been described as increasing the number of immature erythroblasts and supporting BFU-Es and CFU-Es in the spleen of IL-13–treated mice.29 Our data show that treatment of CD34+ cells with CC-4047 induces a cytokine profile that favors myeloid and suppresses erythroid differentiation. This is an early event because these changes can be observed within the first 24 hours of CC-4047 treatment. In contrast, thalidomide induced much weaker changes in cytokine secretion. This is in line with our observation that thalidomide has only weak effects on lineage commitment.
Because a complex network of transcriptional regulators controls differentiation of hematopoietic stem cells and progenitors, we analyzed in a next step the influence of CC-4047 on gene expression. Gene array analysis revealed that CD34+ cells treated with CC-4047 show a significant decrease of GATA-1 mRNA expression. Interestingly, transcription factor GATA-1 is highly expressed during erythroid development and has been shown to be essential for normal erythropoiesis.30 In the absence of GATA-1, committed erythroid precursors fail to complete maturation and undergo apoptosis.31,32 In addition, we observed a down-regulation of the transcriptional regulators Gfi-1b and EKLF, which are required for proper red cell development.33,34 Spi-1, which blocks erythroid differentiation,35 was up-regulated. In contrast, several factors that govern myeloid differentiation were strongly induced (eg, C/EBPα, C/EBPϵ, and Gfi-1).36 Moreover, the enhanced expression of the helix-loop-helix protein Id1 has been shown to interfere with normal erythroid development, and Id2 is differentially expressed in erythroid and myeloid progenitors, being down-regulated in erythroid cells while remaining up-regulated in myeloid progenitors.37,38 Similar to our observation for cytokine expression, these effects of CC-4047 on transcription factor expression represent an early event that can be observed within the first 24 hours. This short time frame makes it very unlikely that the change of transcription factor levels is the result of a change in cell composition because differentiation processes take several days to occur.
Taken together, our findings indicate that CC-4047 can induce a profound change in the transcriptional program of hematopoietic stem cells, leading to a shift of the normal differentiation process toward myeloid development and inhibition of erythropoiesis. However, at this stage of analysis we cannot distinguish whether the altered expression of transcription factors is the cause or the consequence of CC-4047 activity.
Clinical trails have shown that thalidomide reduced splenomegaly and frequency of transfusion and increased platelets in patients with myelofibrosis.39 Sixty-two percent of MDS patients with symptomatic or transfusion-dependent anemia and treated with CC-5013 experienced an erythroid response with sustained transfusion independence or a rise in hemoglobin.23 CC-5013 induced grade III thrompocytopenia in 20% and grade III neutropenia in 60% of patients with multiple myeloma,22 while myeloma disease showed good remission. In contrast, our data demonstrate that CC-4047 inhibits erythropoiesis and enhances myelopoiesis. Essentially the same results were obtained with CC-5013. The most striking difference between clinical trials in which these effects were observed and our experiments is that we used hematopoietic progenitors from patients with nonhematologic malignancies. In accordance with our observation, clinical trials in which CC-5013 was applied for nonhematologic malignancies showed no neutropenia or thrombocytopenia,40 suggesting that there might be a fundamental difference in the action of IMiDs on diseased and nondiseased bone marrow.
The present study indicates that CC-4047 is a valuable tool to study the mechanisms underlying lineage commitment. Moreover, its strong effects on hematopoietic progenitors without showing toxicity on human hematopoietic stem cells could open new clinical aspects for the treatment of hematologic disorders. Further investigations are required to identify the mechanisms responsible for these effects.
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2004-03-0828.
Supported in part by a research grant from the Charité (89553182) and the National Genome Research Network (NGFN).
One of the authors (D.S.) is employed by a company or a competitor of a company (Celgene Corp) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Dr Ida Johanna Körner for excellent discussions; Margarete Gries, Kirstin Rautenberg, Renate Franke, and Brigitte Wollert-Wulf for outstanding technical assistance; and Dr Christian Zimmermann from Bio-Rad Laboratories Munich for assistance performing the cytokine analyses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal