Abstract
We systematically compared cytokine-mediated increases or decreases in proliferation with globin gene and protein expression in adult human erythroblasts. Despite their opposite effects on growth, stem cell factor (SCF) and transforming growth factorbeta (TGF-B) had synergistic effects with respect to fetal hemoglobin (HbF): average HbF/HbF + adult hemoglobin (HbA) ratio in erythropoietin (EPO) = 1.4 ± 1.0%; EPO + TGF-B = 10.8 ± 1.9%; EPO + SCF = 19.1 ± 6.2%; and EPO + SCF + TGF-B (EST) = 39.3 ± 6.3%. Polymerase chain reaction (PCR) revealed significant increases in gamma-globin transcripts that were balanced by reduced beta-globin transcripts. Single-cell quantitative PCR demonstrated a complete reversal of gamma-globin gene silencing with detectable gamma-globin mRNA in more than 95% of the cells. Immunostaining with HbF antibodies also showed a pancellular distribution in EST (96.2 ± 0.01% HbF positive) compared with a heterocellular distribution in EPO (42.9 ± 0.01% HbF positive). As shown here for the first time, a robust and pancellular reversal of gamma-globin gene silencing among hemoglobinized erythroblasts from adult humans may be achieved in the absence of hereditary mutation or direct genomic manipulation. (Blood. 2005;105:387-393)
Introduction
Well-defined mutations in the beta-globin locus on the short arm of chromosome 11 cause sickle cell disease and beta-thalassemia. Those hemoglobinopathies are manifested soon after birth when fetal hemoglobin (HbF) expression is silenced and replaced by expression of adult hemoglobin (HbA). In some individuals with a hereditary persistence of fetal hemoglobin (HPFH), the fetal hemoglobin silencing phenomenon is prevented by other mutations within the beta-globin locus.1,2 Individuals with adequate levels of fetal hemoglobin are partially protected from the clinical sequelae of sickle cell disease or beta-thalassemia. The HPFH mutations in the beta-globin locus include deletional and nondeletional subtypes that cause either a heterocellular or pancellular expression of HbF. HPFH also occurs in the absence of known mutations within the beta-globin locus.3 In those families, other genomic regions are being investigated. Based upon the proven clinical benefits of HbF, understanding how specific HPFH mutations lead to dysregulated globin gene transcription remains a fundamental research goal.
In contrast to the extremely rare cases of HPFH, humans more generally activate HbF expression in the setting of acute erythroid stress. Erythroid stress is characterized by increased erythropoiesis in response to tissue hypoxia, and acute stress has more profound effects upon HbF.4 A molecular mechanism for stress-related increases in HbF has not yet been fully defined. Historically, studies of stress erythropoiesis led to the proposal that increased HbF results from proliferation of a “fetal clone.”5 Alternatively, it was suggested that increased HbF results primarily from accelerated differentiation of developmentally immature erythroblasts that were thought to normally express high levels of gamma-globin.6
Mechanistic studies of growth- or signaling-related modulation of human fetal hemoglobin are currently not possible in murine models due to well-described differences in the erythroid stress response7 as well as the inability to detect globin gene or protein expression in the circulating blood of nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice that received transplants of human bone marrow.8 For these reasons, cultures of primary human erythroblasts are the method of choice for defining relationships between erythroid growth, differentiation, and HbF. In the presence of erythropoietin (EPO) alone, low levels of HbF are detectable in a majority of proerythroblasts. As those cells undergo terminal maturation, detectable HbF is usually lost. Stem cell factor (SCF) increases erythroblast growth and fetal hemoglobin.9-11 Inhibitor studies suggest that the HbF-modulating effects of SCF require activation of the same signal transduction cascade involving mitogen-activated/extracellular signal-regulated kinase (MEK) that is exploited by SCF to increase erythroid growth.12 SCF modulation of HbF depends largely upon the regulated expression pattern of the SCF receptor (CD117, c-kit).13 CD117 peaks at the proerythroblast stage of differentiation, and is thereafter lost during the final stages of maturation. As a result, SCF has significantly less effect on HbF in mature erythroblasts. Furthermore, when SCF is added to progenitor populations, it inhibits their maturation.13-15 Because of these reasons, SCF alone may not be sufficient to signal robust increases in HbF that persist during terminal maturation.
While not as well-characterized as SCF, transforming growth factor-beta (TGF-B) also signals increases in HbF among adult human erythroblasts.16 However, TGF-B has distinctly opposite effects to SCF on erythroid growth and differentiation. TGF-B inhibits erythroid proliferation, and it is cytotoxic at higher concentrations. It also accelerates the differentiation of erythroid progenitors.17,18 We hypothesized that SCF and TGF-B together may have balanced effects on erythropoiesis and synergistic effects on HbF. As demonstrated here, the combination of SCF and TGF-B signaling resulted in the persistent, pancellular expression of gamma-globin mRNA and protein at high levels during adult human erythropoiesis.
Materials and methods
Primary erythroblast cultures
Informed consent was obtained for the collection of peripheral blood CD34+ cells. The cells were enumerated using an electronic cell counter (Beckman Coulter, Hialeah, FL) and cultured in medium containing 4 U/mL EPO (Amgen, Thousand Oaks, CA) as described previously.11 The medium was supplemented with 50 ng/mL SCF (R&D Systems, Minneapolis, MN) or 1.25 ng/mL TGF-B1 (R&D Systems), or both in matched cultures. The concentrations of SCF and TGF-B used in this study were based upon dose-titration analyses over a 2- to 3-log range (data not shown). Cells obtained from at least 3 separate donors were used for all comparisons of growth and hemoglobin content.
Phenotyping studies
Cytospins were performed on days 0, 2, 4, 6, 8, 10, 12, and 14 and cell types were identified by morphology after Giemsa staining. Cell imaging was performed using an Axiophot microscope equipped with an oil medium objective of 63 × magnification and a numerical aperture of 1.25 (Zeiss, Jena, Germany). An HQ digital camera (Photometrics, Tucson, AZ) and Scanalytics version 3.6 software (IP Labs, Billerica, MA) were used to capture images. Immunostaining with antibodies directed against glycophorin A (GPA), CD71 (Beckman Coulter), HbF (Caltag Laboratories, Burlingame, CA), and HbA (Perkin Elmer Wallac, Norton, OH) were performed. A minimum of 5000 cells were analyzed using an EPICS ELITE ESP flow cytometer (Beckman Coulter). Positive staining was defined by fluorescence at levels more than 2 standard deviations above the isotypic controls.
Cell-cycle analyses
CD34+ cells from 3 donors cultured in EPO and EPO + SCF + TGF-B were harvested on day 7 and day 14. The cell pellet was stained using Nucycl PI kit (Exalpha Biologicals, Watertown, MA), according to the manufacturer's protocol. For each sample, more than 20 000 cells were analyzed using EPICS ELITE ESP flow cytometer (Beckman Coulter).
Hemoglobin and carbonic anhydrase analyses
Aliquots containing approximately 1.5 million cells were washed in Dulbecco phosphate-buffered saline (PBS, CellGro; MediaTech, Herndon, VA). Cellular lysates were analyzed for HbF and HbA content using a 20 × 4-mm POLYCATA column (Poly LC, Columbia, MD) fitted to a Gilson HPLC system (Gilson, Middleton, WI) as previously described.12 Cellulose acetate gel electrophoresis was performed according to the manufacturer's protocol for detection of carbonic anhydrase (Helena, Beaumont, TX).
Western blotting
For phosphorylation analyses, the cells were initially cultured in EPO. After 1 week, 50 ng/mL SCF or 1.25 ng/mL TGF-B was added to the medium, and cellular lysates were prepared at 0, 10, and 30 minutes. Protein was extracted using mammalian protein extraction reagent (M-PER) (Pierce Biotechnology, Rockford, IL) as recommended by the manufacturer. The protein extracts were electrophoresed (25 μg/lane) and transferred to nitrocellulose membranes. The membranes were probed for total and phosphorylated forms of MEK1/2 (Cell Signaling Technology, Beverly, MA) as well as for total and phosphorylated Sma- and Mad-related protein 2 (SMAD2; Cell Signaling Technology) using secondary horseradish peroxidase (HRP)-conjugated antibodies (Amersham Pharmacia, Piscataway, NJ).
Quantitative PCR for gamma- and beta-globin expression
RNA was extracted from erythroblast pools using RNeasy Mini kit (Qiagen, Valencia, CA) as described previously.12 cDNA was generated using Superscript II (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. For single-cell studies, each well of a 96-well round-bottom plate (Corning, Corning, NY) was preloaded with 7 μL of Cells-to-cDNA II Lysis Buffer (Ambion, Austin, TX) on ice. Using flow cytometry, single cells were sorted from the main population according to forward and side scatter parameters. The cellular lysates were then transferred to polymerase chain reaction (PCR) tubes (Applied Biosystems, Foster City, CA) and incubated at 75°C for 15 minutes and then treated with 1 U DNase I (Ambion) at 37°C for 15 minutes. The DNase I was heat inactivated at 75°C for 5 minutes, and 4 μL deoxynucleoside triphosphate (dNTP; Ambion) and 2 μL oligo-dT (Ambion) were added. Incubation at 70°C for 3 minutes was followed by immediate transfer to ice for 1 minute. RNase- and DNase-free water, 10 × reverse-transcriptase (RT) buffer (Ambion), 20 U RNaseOut (Invitrogen), and 200 U Moloney murine leukemia virus (M-MLV) reverse transcriptase were added to the mixture (20 μL total volume) and incubated at 42°C for 1 hour. The reverse-transcriptase enzyme was heat inactivated at 94°C for 10 minutes. The reaction mixture was then divided in half for beta-globin versus gamma-globin mRNA quantitation.
A quantitative real-time RT-PCR assay was carried out with gene-specific TaqMan probes for both gamma- and beta-globin labeled with 6-carboxy fluorescein (FAM) and 6-carboxytetramethyl rhodamine (TAMRA) dyes in a 7700 Sequence Dectector (Applied Biosystems). The PCR conditions were described previously.12 A plasmid DNA encoding gamma- and beta-globin templates was used to generate the standard curve (20 to 2 000 000 copies) for determination of copy number. The control plasmid DNA copy number was calculated based upon the molecular weight of DNA. Templates with fewer than 20 copies were scored as below the detection limit of this assay. Statistical significance for all experiments was determined by Student paired t test analyses.
Results
Maturation patterns of cells cultured in EPO versus EST
While SCF has major effects upon gamma-globin gene expression in proerythroblasts, it also inhibits the maturation of those cells.13-15 Therefore, we hypothesized that additional signals may be necessary to overcome the inhibited maturation associated with SCF. TGF-B was chosen to test this hypothesis due to its ability to accelerate erythroid differentiation.17,18 As shown in Figures 1, 2, the addition of TGF-B to SCF resulted in a pattern of differentiation nearly identical to that seen in EPO alone. In EPO versus EPO + SCF + TGF-B (EST), a uniform population of proliferating proerythroblasts was observed after 1 week. By day 14, hemoglobinized normoblasts became the predominant population under both conditions.
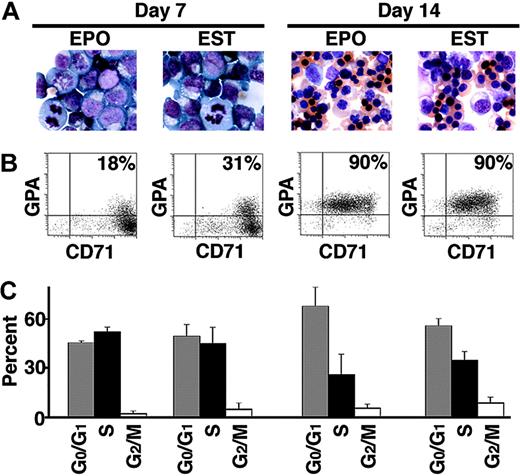
Appearance of erythroid progenitors cultured in EPO and EST on day 7 and day 14. (A) Representative fields of Giemsa-stained erythroid cells. Identical magnification (× 63) was used for all the microscopic fields to demonstrate the decrease in erythroblast size associated with terminal differentiation. (B) Flow cytometric analyses of cells simultaneously stained with anti-transferrin receptor (CD71) and anti-glycophorin A (GPA) antibodies. The percentage in the right upper quadrant of each panel describes the population staining positive for GPA and CD71. (C) Summary of cell-cycle analyses. Bars with standard deviation denote mean percentage values for G0/G1 (▦), S (▪), and G2/M (□) populations from experiments performed on 3 separate donors.
Appearance of erythroid progenitors cultured in EPO and EST on day 7 and day 14. (A) Representative fields of Giemsa-stained erythroid cells. Identical magnification (× 63) was used for all the microscopic fields to demonstrate the decrease in erythroblast size associated with terminal differentiation. (B) Flow cytometric analyses of cells simultaneously stained with anti-transferrin receptor (CD71) and anti-glycophorin A (GPA) antibodies. The percentage in the right upper quadrant of each panel describes the population staining positive for GPA and CD71. (C) Summary of cell-cycle analyses. Bars with standard deviation denote mean percentage values for G0/G1 (▦), S (▪), and G2/M (□) populations from experiments performed on 3 separate donors.
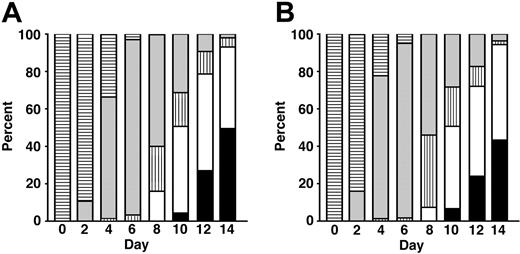
Morphology-based erythrokinetics. CD34+ cells from 3 separate donors were cultured in EPO (A) versus EST (B). On days 0 to 14 (x-axis), differential counts of Giemsa-stained cells were enumerated. The mean percentages of each cell type are shown on the y-axis. Undefined blasts (▤), preproerythroblast/proerythroblast (▦), basophilic normoblasts (▥), polychromatic normoblast (□), and orthochromatic normoblasts (▪) are indicated.
Morphology-based erythrokinetics. CD34+ cells from 3 separate donors were cultured in EPO (A) versus EST (B). On days 0 to 14 (x-axis), differential counts of Giemsa-stained cells were enumerated. The mean percentages of each cell type are shown on the y-axis. Undefined blasts (▤), preproerythroblast/proerythroblast (▦), basophilic normoblasts (▥), polychromatic normoblast (□), and orthochromatic normoblasts (▪) are indicated.
In a separate comparison of erythroid maturation, matched cultures were examined by flow cytometry. Transferrin receptor (CD71) and glycophorin A (GPA) were compared on days 7 and 14. Those 2 membrane proteins are well-recognized markers of erythroid maturation.19 Representative flow cytometric dot plots are in Figure 1B. On culture day 7, EPO and EST cultures both demonstrated expression of CD71 at very high levels. Low levels of GPA were also detected in some cells. A slightly higher percentage of cells expressed GPA on day 7 in EST compared with EPO alone (23.8 ± 9.5% in EPO versus 28.8 ± 5.1% in EST; P = .1; 6 donors). By day 14, both culture conditions promoted GPA expression in 90% of the erythroblasts. The high-level expression of CD71 was uniformly detected on day 7, but was later reduced as the cells underwent terminal differentiation. Consistent with the similarities in morphology, the immunophenotyping studies demonstrated nearly identical maturation of erythroblasts in EPO versus EST over the 14-day culture period. Cell-cycle analyses further demonstrated similar erythrokinetics in EPO and EST cultures. Mean values from more than 20 000 cells analyzed from 3 separate donors are shown in Figure 1C. On days 7 and 14, no significant differences were observed in the percentages of G0/G1, S, or G2/M populations in EPO versus EST (P > .05).
Growth-related patterns of hemoglobin production
Based upon the overall similarities of the erythroblasts generated after 14 days in EPO versus EST, analyses of cell proliferation and hemoglobin production were performed. As shown in Figure 3A, TGF-B significantly inhibited the proliferation of the cells compared with EPO alone (average cell counts in EPO = 3.1 × 105 cells/mL; EPO + TGF-B = 6.2 × 104 cells/mL; P ≤ .001). Due to its toxicity, measurable erythropoiesis was not detected in higher concentrations of TGF-B (data not shown). Adding SCF to the cultures had an opposite effect on growth. SCF significantly increased cell proliferation (EPO + SCF = 2.7 × 106 cells/mL; P ≤ .001). The combination of EPO + SCF + TGF-B resulted in proliferation of the erythroblasts to slightly higher levels than those found in EPO alone (EST = 4.5 × 105 cells/mL; P = .03). Hence, the divergent effects of SCF and TGF-B upon erythropoiesis appeared to be balanced when the 2 cytokines were added together. This result further supports the data in Figures 1, 2 in suggesting that erythroid growth and maturation in EPO versus EST were nearly identical.
Cytokine effects on proliferation and hemoglobin expression. CD34+ cells were cultured for 14 days in EPO (E), EPO + TGF-B (ET), EPO + SCF (ES), and EPO + SCF + TGF-B (EST). (A) Cell counts and (B) hemoglobin expression levels calculated from HPLC analyses on day 14 are shown. The cell counts represent the number of cells enumerated per 105 CD34+ cells placed in culture on day 0. The mean values ± standard deviation bars are from separate experiments with cells from 4 donors. *Significant (P ≤ .001) changes relative to cells cultured in EPO alone. (C) HPLC tracings from cells achieving HbF dominance in EST. Controls were used to identify the hemoglobin peaks as HbF (F), HbA (A), or HbA2 (A2). The percentages above the HbF peaks represent HbF/HbF + HbA ratios. (mV indicates millivolts; elution time is represented on the x-axis.)
Cytokine effects on proliferation and hemoglobin expression. CD34+ cells were cultured for 14 days in EPO (E), EPO + TGF-B (ET), EPO + SCF (ES), and EPO + SCF + TGF-B (EST). (A) Cell counts and (B) hemoglobin expression levels calculated from HPLC analyses on day 14 are shown. The cell counts represent the number of cells enumerated per 105 CD34+ cells placed in culture on day 0. The mean values ± standard deviation bars are from separate experiments with cells from 4 donors. *Significant (P ≤ .001) changes relative to cells cultured in EPO alone. (C) HPLC tracings from cells achieving HbF dominance in EST. Controls were used to identify the hemoglobin peaks as HbF (F), HbA (A), or HbA2 (A2). The percentages above the HbF peaks represent HbF/HbF + HbA ratios. (mV indicates millivolts; elution time is represented on the x-axis.)
While SCF and TGF-B are both known to increase HbF in adult erythroblasts, a quantitative comparison of their growth and globin-modulating effects has not been previously reported. Control populations grown in EPO had an average HbF/HbF + HbA content of 1.4 ± 1.0% (Figure 3B). In association with the decrease in proliferation, the HbF/HbF + HbA increased to 10.8 ± 1.9% (P ≤ .001) with the addition of TGF-B. A greater effect on HbF was noted with SCF. In those cultures, the dramatic rise in proliferation was associated with HbF/HbF + HbA levels of 19.1 ± 6.2% (P ≤ .001). EST had the greatest effect on HbF, with HbF/HbF + HbA levels averaging 39.3 ± 6.3% (P ≤ .001). This surprising result demonstrated synergy between SCF and TGF-B with respect to fetal hemoglobin expression despite their opposite effects on growth. In erythroblasts from 2 donors, an HbF-dominant phenotype was detected (HbF/HbF + HbA ratio: donor 1: EPO = 1.8% versus EST = 71.2%; donor 2: EPO = 1.1% versus EST = 54.2%; HPLC tracings from donor 2 are shown in Figure 3C).
Carbonic anhydrase production
Carbonic anhydrase I (CAI) expression has been classically used to distinguish fetal and adult erythroid cells since its production closely follows the gamma- to beta-globin switch.20 CAI bands are absent after electrophoresis of fetal cell lysates in cellulose acetate gels. Therefore, we compared CAI levels among cells grown in EPO versus EST to determine if the increase in HbF was associated with a change in CAI (Figure 4). As expected, no CAI band was present in the control (lane 1; loaded with the HbF + HbA standard). In cultures grown in EPO alone (lane 2), CAI and HbA bands were both identified. In matched lysates from cells cultured in EST (lane 3), the single HbA band was replaced with equivalent HbA and HbF bands, but the CAI band remained unchanged. In addition to confirming the robust increase in HbF demonstrated earlier by HPLC, this analysis suggests that significant changes in CAI expression were not associated with the increased HbF.
Cellulose acetate electrophoresis. Cell lysates from control (lane 1), EPO (lane 2), and EST (lane 3) samples from matched cultures are shown. The locations of carbonic anhydrase (CAI), fetal hemoglobin (HbF), and adult hemoglobin (HbA) bands are indicated on the right.
Cellulose acetate electrophoresis. Cell lysates from control (lane 1), EPO (lane 2), and EST (lane 3) samples from matched cultures are shown. The locations of carbonic anhydrase (CAI), fetal hemoglobin (HbF), and adult hemoglobin (HbA) bands are indicated on the right.
MEK and SMAD2 phosphorylation studies
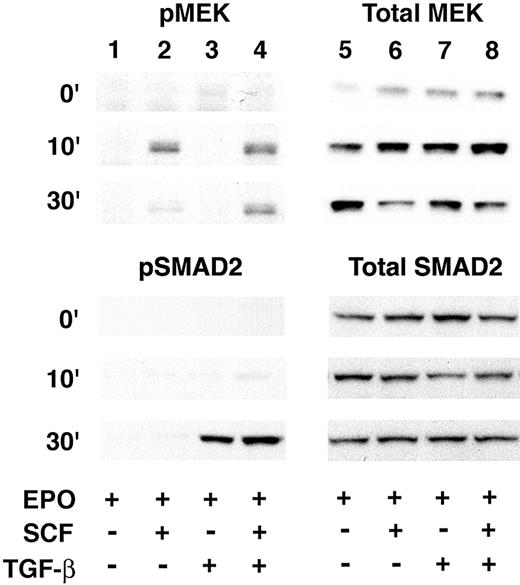
Activation of the MEK was previously associated with an SCF-mediated increase of HbF.12 Therefore, MEK phosphorylation studies were performed to investigate if TGF-B induction of HbF is also mediated through activation of that kinase. On day 7, SCF or TGF-B, or both were added and cellular extracts prepared at 0, 10, and 30 minutes. As demonstrated in Figure 5, SCF caused an increase in MEK phosphorylation within 10 minutes that was reduced by 30 minutes. Significant MEK phosphorylation was not detected after the addition of TGF-B. The combination of SCF and TGF-B resulted in a pattern of MEK phosphorylation similar to that seen after the addition of SCF alone.
MEK phosphorylation studies. Western analyses for phosphorylated MEK1/2 (pMEK; upper left), total MEK1/2 (Total MEK; upper right), phosphorylated SMAD2 (pSMAD2; lower left), or total SMAD2 (Total SMAD2; lower right) are shown. Total protein (25 μg/well) was extracted from culture day-7 cells harvested at 0 (top row), 10 (middle row), and 30 (bottom row) minutes after the addition of SCF or TGF-B (denoted by + at the bottom of the figure).
MEK phosphorylation studies. Western analyses for phosphorylated MEK1/2 (pMEK; upper left), total MEK1/2 (Total MEK; upper right), phosphorylated SMAD2 (pSMAD2; lower left), or total SMAD2 (Total SMAD2; lower right) are shown. Total protein (25 μg/well) was extracted from culture day-7 cells harvested at 0 (top row), 10 (middle row), and 30 (bottom row) minutes after the addition of SCF or TGF-B (denoted by + at the bottom of the figure).
As the primary target of TGF-B signaling, SMAD2 phosphorylation was also investigated to examine activation of that transcription factor in this culture system (Figure 5). Phosphorylated SMAD2 associates with SMAD4 and translocates as a multicomplex to the nucleus where the heteromeric SMAD complex regulates transcription through its ability to interact with DNA binding and non-DNA binding transcription factors and cofactors.21 SMAD2 was interrogated by Western analyses on cellular extracts under the same conditions as those used for MEK. In EPO alone or in EPO + SCF, SMAD2 phosphorylation was detected at low or background levels. When TGF-B was added to EPO or EPO + SCF, strong phosphorylation of SMAD2 was detected after 30 minutes.
Gamma-globin mRNA expression patterns
In order to investigate the pattern of beta- and the gamma-globin transcription during the TGF-B-mediated maturation of the cells, we initially performed quantitative PCR for comparative analyses on cell pools sampled on days 7 and 14. Mean values from matched cultures from 5 separate donors are in Table 1. As shown, the average levels of beta- and gamma-globin mRNA were reduced as the cells underwent terminal differentiation between days 7 and 14. Cells grown in EST demonstrated a highly significant increase in the gamma/total globin percentage at day 7 (53.9 ± 11.5%; P ≤ .001) and day 14 (25.8 ± 20.9%; P ≤ .001) compared with 4.6 ± 5.3% on day 7 and 1.4 ± 1.1% on day 14 in EPO. On day 7, the increase in the gamma/total globin mRNA was due to an increase in gamma-globin mRNA and a significant reduction in beta-globin mRNA. On day 14, the relative decrease in beta-globin mRNA was no longer detected. While the total globin levels were increased in EPO versus EST, those increases did not achieve statistical significance.
Quantitative PCR measurement of beta-globin and gamma-globin mRNA expression among pooled samples
. | EPO . | EST . | P . |
|---|---|---|---|
| Day 7 | |||
| Gamma-globin mRNA | 1 960 ± 1280 | 12 100 ± 8530 | .02 |
| Beta-globin mRNA | 41 200 ± 22900 | 10 400 ± 8050 | .02 |
| Total globin mRNA | 43 100 ± 24200 | 22 500 ± 16600 | .1 |
| Gamma/total, % | 4.9 ± 5.3 | 53.9 ± 11.5 | .00001 |
| Day 14 | |||
| Gamma-globin mRNA | 49.1 ± 36.8 | 1 590 ± 1120 | .01 |
| Beta-globin mRNA | 3 540 ± 3260 | 4 580 ± 4230 | .4 |
| Total globin mRNA | 3 590 ± 3300 | 6 170 ± 5350 | .2 |
| Gamma/total, % | 1.4 ± 1.1 | 25.8 ± 20.9 | .00001 |
. | EPO . | EST . | P . |
|---|---|---|---|
| Day 7 | |||
| Gamma-globin mRNA | 1 960 ± 1280 | 12 100 ± 8530 | .02 |
| Beta-globin mRNA | 41 200 ± 22900 | 10 400 ± 8050 | .02 |
| Total globin mRNA | 43 100 ± 24200 | 22 500 ± 16600 | .1 |
| Gamma/total, % | 4.9 ± 5.3 | 53.9 ± 11.5 | .00001 |
| Day 14 | |||
| Gamma-globin mRNA | 49.1 ± 36.8 | 1 590 ± 1120 | .01 |
| Beta-globin mRNA | 3 540 ± 3260 | 4 580 ± 4230 | .4 |
| Total globin mRNA | 3 590 ± 3300 | 6 170 ± 5350 | .2 |
| Gamma/total, % | 1.4 ± 1.1 | 25.8 ± 20.9 | .00001 |
Messenger RNA samples from erythroblasts cultured in EPO and EST isolated on days 7 and 14 were examined for beta- and gamma-globin mRNA expression using quantitative PCR. The mean gamma- and beta-globin and total (gamma + beta) mRNA copy numbers per cell with the percentage of gamma/total globin are shown. The mean ± standard deviation values from experiments performed on cells from 5 separate donors are shown. The P values comparing the EPO and EST data are on the right.
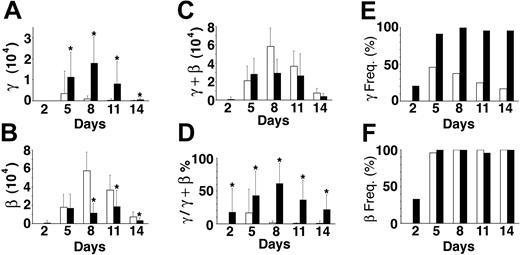
In addition to studies of mRNA from pooled cells, we examined mRNA from one donor's sorted single cells on days 2, 5, 8, 11, and 14 using real-time PCR (Figure 6). On each of those days, mRNA from 48 sorted cells (24 cultured in EPO plus 24 cultured in EST; 240 cells in total) was studied. The patterns were consistent with those in Table 1 showing a rise in globin mRNA levels during the first week followed by progressively lower levels associated with terminal differentiation during the second week. By day 14, gamma-globin mRNA in EPO averaged only 60.7 ± 176 molecules/cell compared with beta-globin mRNA averaging 7450 ± 5060 molecules/cell. By comparison, gamma-globin in EST reached levels of 17 900 ± 12 500 molecules/cell on day 8, and then declined to an average of 655 ± 624 molecules/cell on day 14. As with the pools described in Table 1, the significant increase in gamma-globin mRNA detected midway through the culture was associated with a significant decrease in beta-globin mRNA. The divergent changes in the absolute levels of gamma- and beta-globin mRNA on culture day 8 (Figure 6A-B) caused profound changes in the gamma/total globin ratio from 1.4 ± 3.2% in EPO to 61.5 ± 30.7% in EST (Figure 6D). As a result of the decrease in beta-globin transcripts, the total globin mRNA levels were not significantly different in EPO versus EST (Figure 6C).
Quantitative PCR analyses of single cells. Cells from matched (EPO versus EST) cultures were sorted and analyzed on the days shown (x-axis). The copy numbers of mRNA amplified from cells cultured in EPO (□) versus EST (▪) are shown. (A) Average gamma-globin mRNA copy number per cell. (B) Average beta-globin mRNA copy numbers per cell. (C) Average total (gamma + beta) globin mRNA copy numbers per cell. (D) The ratio of gamma to total globin mRNA copies expressed as a percentage. (E) Frequency of cells having gamma-globin transcripts expressed as a percentage (γ Freq). (F) Frequency of cells having beta-globin transcripts expressed as a percentage (β Freq). The bars demonstrate the mean ± standard deviation (vertical line) of 24 single cells analyzed for each culture condition on each culture day. *Statistical significance (P ≤ .01) between EPO versus EST cultures.
Quantitative PCR analyses of single cells. Cells from matched (EPO versus EST) cultures were sorted and analyzed on the days shown (x-axis). The copy numbers of mRNA amplified from cells cultured in EPO (□) versus EST (▪) are shown. (A) Average gamma-globin mRNA copy number per cell. (B) Average beta-globin mRNA copy numbers per cell. (C) Average total (gamma + beta) globin mRNA copy numbers per cell. (D) The ratio of gamma to total globin mRNA copies expressed as a percentage. (E) Frequency of cells having gamma-globin transcripts expressed as a percentage (γ Freq). (F) Frequency of cells having beta-globin transcripts expressed as a percentage (β Freq). The bars demonstrate the mean ± standard deviation (vertical line) of 24 single cells analyzed for each culture condition on each culture day. *Statistical significance (P ≤ .01) between EPO versus EST cultures.
A major advantage for examining mRNA in single cells is the ability to directly determine the distributions of gamma- and beta-globin gene expression within the population. As shown in Figure 6F, pancellular beta-globin mRNA was detected in EPO and EST. Unlike beta-globin mRNA, gamma-globin mRNA was not detected in a pancellular distribution on any of the days in cultures supplemented with EPO alone. The maximum percentage (46% of cells examined expressing gamma-globin mRNA) was reached on day 5 in EPO, and then progressively declined to 17% by day 14. In EST, the distribution of gamma-globin mRNA was quite similar to that of beta-globin mRNA (Figure 6E-F). Detection became pancellular by day 5, and that pattern persisted for the remainder of the culture period. Hence, expression of gamma-globin mRNA in a majority of cells was entirely cytokine dependent.
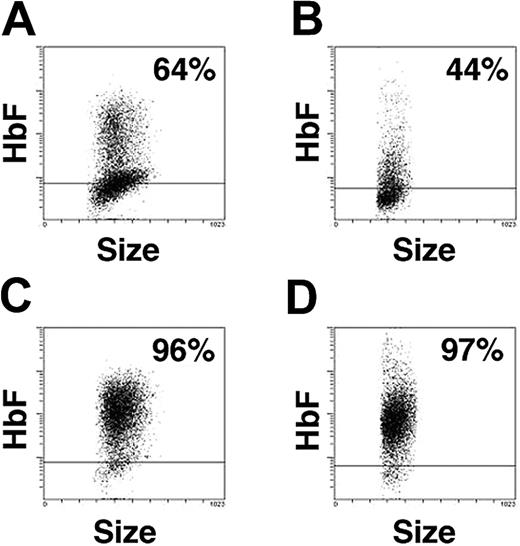
Distribution of fetal hemoglobin
The pancellular expression of gamma-globin mRNA prompted us to study the distribution of HbF-expressing cells during erythroid maturation. Representative HbF-based histograms from day-7 and day-14 analyses from several donors are in Figure 7. In EPO alone, HbF was detected in 64% of the cells on day 7, and by day 14, HbF was reduced to 44%. The expression of HbF in EPO was generally low with fluorescence at or near the negative level (defined by isotypic controls) in most cells. By comparison, more than 95% of erythroblasts grown in EST-supplemented medium demonstrated expression of HbF. In those populations, higher levels of HbF-based fluorescence were detected on day 7 and persisted among the more mature cells on day 14. In contrast to EPO, the positive population grown in EST expressed HbF at consistently higher levels. These results further support the HPLC (Figure 3) and starch electrophoresis (Figure 4) studies in demonstrating increased HbF. These results are also consistent with the quantitative RT-PCR studies (Table 1; Figure 6) in demonstrating the pancellular nature of signaling on gamma-globin expression in EST, even among the mature population on day 14.
Cellular distribution of HbF. Flow cytometric analysis of cells cultured in EPO for (A) 7 days or (B) 14 days versus matched cultures in EST for (C) 7 days or (D) 14 days. The x-axis indicates the forward scatter of cells (size) and y-axis, the HbF fluorescence from the cells stained with anti-HbF antibodies. The horizontal bar marks the level of fluorescence 2 standard deviations above that of the isotypic controls. At least 5000 cells were analyzed in each sample. The percentage of HbF-positive cells is shown in the top right corner of each panel.
Cellular distribution of HbF. Flow cytometric analysis of cells cultured in EPO for (A) 7 days or (B) 14 days versus matched cultures in EST for (C) 7 days or (D) 14 days. The x-axis indicates the forward scatter of cells (size) and y-axis, the HbF fluorescence from the cells stained with anti-HbF antibodies. The horizontal bar marks the level of fluorescence 2 standard deviations above that of the isotypic controls. At least 5000 cells were analyzed in each sample. The percentage of HbF-positive cells is shown in the top right corner of each panel.
Discussion
Based upon the growing body of evidence presented here and elsewhere, we propose that expression of gamma-globin mRNA and HbF in normal adult human erythroblasts is controlled by growth and related signal transduction. We previously identified EPO-supplemented culture conditions that lead to production of hemoglobinized populations of erythroblasts with low levels of HbF.13 In that study, HbF production was silenced in a majority of the cells as they underwent a major growth transition at the proerythroblast stage toward terminal differentiation. To further examine the possible relationship between erythroblast growth and HbF, additional growth-promoting cytokines were screened. SCF promoted the growth of CD117+ proerythroblasts and increased the level and distribution of HbF in those cells. However, SCF inhibited the maturation of those cells. In this study, we determined that TGF-B also increases HbF production, albeit to a lower extent than SCF. In distinct contrast to SCF, TGF-B inhibited the proliferation of the cells. When added together, SCF and TGF-B signaled balanced effects on growth, and the erythroblast populations were nearly identical to those arising in cultures containing EPO alone. Despite the similarities in cell number, immunophenotype, and morphology, dramatic differences were identified in the globin expression patterns in EPO versus EST. EST signaled high-level expression of HbF that persisted among the entire population of mature, hemoglobinized cells.
We also determined that the significant rise in HbF was associated with changes in globin mRNA levels using quantitative PCR. In EPO alone, beta-globin mRNAlevels rose to extremely high levels during the first culture week, but declined as the cells underwent terminal maturation. Interestingly, the biphasic pattern of beta-globin mRNA resembled that previously demonstrated for the pattern of cells in the S-phase of cell cycle.11 The maximum levels of cell cycling and globin gene expression coincided with the proerythroblast stage of differentiation. In contrast to beta-globin mRNA, gamma-globin mRNAlevels and distribution were lower in EPO alone. When SCF and TGF-B were added to the culture medium, several significant changes in the globin mRNAlevels were observed. The gamma-globin mRNAlevels rose and became dominant in proerythroblasts. The robust increase in gamma-globin mRNA was associated with a significant decrease in beta-globin mRNA. The balanced changes in gamma- and beta-globin mRNA levels may be indicative of a mechanism that controls the total level of transcription with resulting competition between the 2 genes. The combined levels of gamma- and beta-globin mRNA were not significantly different under the 2 culture conditions. Importantly, gamma-globin mRNAwas present in more than 90% of the cells cultured in EST from day 5 onward. Therefore, molecular mechanisms must exist for a signaled activation of pancellular gamma-globin mRNA expression to be maintained throughout several mitotic divisions. Hence, chance and inheritance are not the sole determinants of gamma-globin gene activation in primary adult human erythroblasts.22
These signaled increases in gamma-globin mRNA and fetal hemoglobin were closely associated with events that encompass erythropoiesis. At the cellular level, we found no evidence that the combination of SCF and TGF-B act by recruiting a separate population of fetal cells. Instead, their effects appear to be mediated by balanced increases in growth and differentiation. It was previously demonstrated that SCF acts primarily by increasing gamma-globin mRNA levels at the proerythroblast stage of development.12 Our data suggest that TGF-B permitted or promoted a reversal of SCF-inhibited differentiation. With regard to signal transduction, it was previously shown that MEK activation is necessary for SCF-mediated increases in HbF. Here we demonstrated that SCF + TGF-B-signaled increases in HbF are associated with a combination of MEK and SMAD2 phosphorylation. In addition to its primary mechanism of activating SMAD transcription factors,21 TGF-B may also act by increasing cyclic adenosine monophosphate (cAMP) levels.23 In the nucleus, both cytokines have considerable epigenetic effects that result in new gene transcription. SCF increases HbF primarily through mitogen-activated protein kinase (MAPK) signaling, and that pathway has been implicated in the regulation of nuclear factor-erythroid 2 (NF-E2) and the locus control region of the beta-globin gene cluster on chromosome 11.24,25 TGF-B is known to activate a transcription factor called TGF-B-inducible early response gene 2 (TIEG2).26 Of note, TIEG2 was later determined to be involved in fetal globin gene transcription and renamed FKLF.27 SCF and TGF-B both have additional effects upon the regulation of cell cycle and erythrokinetics. Thus, SCF and TGF-B likely modulate transcription at the globin locus through complex but integrated mechanisms.
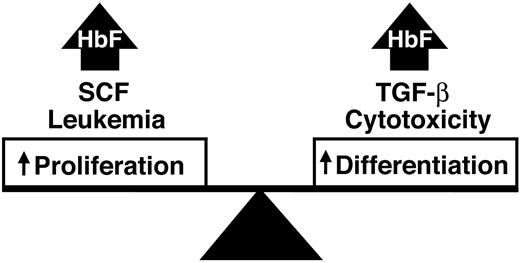
Our study suggests that balanced increases in growth and differentiation beyond steady-state levels lead to persistent expression of HbF in adult erythroid precursor cells. Analogies between these cytokine-signaling effects and well-defined clinical scenarios may also exist (Figure 8). In healthy adult individuals, erythropoiesis at steady state may be associated with a default program of globin gene expression that results in low levels of HbF in terminally differentiated cells. We propose that increased erythroblast proliferation or differentiation above those steady-state levels may result in increased gamma-globin gene and protein expression. Erythroleukemia and SCF both increase HbF, and those increases are accomplished through increased proliferation associated with inhibited differentiation. In contrast, cytotoxic drugs and TGF-B act by increasing HbF in the setting of increased differentiation associated with decreased proliferation. We further propose that stress-related elevations of HbF result from simultaneous increases in erythroblast growth and differentiation like those demonstrated here by the combination of SCF and TGF-B. This dual mechanism for stress erythropoiesis provides for a rapid and sustainable response to tissue hypoxia.
A model for stress-related or other elevations of HbF driven by a balanced increase in erythroid proliferation and differentiation.
A model for stress-related or other elevations of HbF driven by a balanced increase in erythroid proliferation and differentiation.
Maintenance of increased erythroblast growth and differentiation may also be important for the therapeutic modulation of HbF in patients with sickle cell diseases and beta-thalassemias. Just as TGF-B has less effect on HbF in the absence of SCF, cytotoxic drugs may have suboptimal effects on HbF in subjects who do not manifest robust increases in proliferation. Phlebotomy-related increases in growth may be essential for the HbF response to hydroxyurea in primates.28 This reasoning is also pertinent in patients who are treated with transfused erythrocytes or oxygen, because those therapies blunt hypoxia-driven proliferation.29,30 Low reticulocyte counts indicate suboptimal growth in sickle cell patients treated with cytotoxic drugs. Hydroxyurea-treated patients with lower reticulocyte counts also demonstrate lower HbF and increased mortality.31 In those patients, supplemental therapies may be worth considering for augmentation of erythroblast proliferation. A more intriguing postulate is that targeted modulation of erythroblast signal transduction in the absence of other interventions may be useful for increasing HbF in patients with beta-hemoglobinopathies. Since SCF and TGF-B have undesirable effects on nonerythroid cells in vivo, additional signaling molecules should be identified and investigated in this context.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-04-1599.
N.V.B. and T.A.T. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the National Institutes of Health (NIH) Department of Transfusion Medicine for cell processing and Natalie Murray for assistance with hemoglobin analyses.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal