Abstract
Diffuse large B-cell lymphoma (DLBCL) is a common and often fatal malignancy. Advances in the treatment of this disease will require the identification of novel therapeutic targets. We previously defined an expression signature of outcome in DLBCL and found that the phosphodiesterase PDE4B was overexpressed in fatal/refractory tumors. Phosphodiesterase 4B (PDE4B) inactivates the second messenger cyclic adenosine 3′,5′ monophosphate (cAMP) and abrogates its inhibitory effects in B lymphocytes. Hence, DLBCLs that express high PDE4B levels may be resistant to cAMP-induced apoptosis, contributing to their less favorable outcome. Herein, we confirmed the risk-related expression of PDE4B in an independent series of primary DLBCLs and defined the enzyme's role in modulating cAMP-induced apoptosis in parental DLBCL cell lines or those reconstituted with wild-type or mutant PDE4B. The cAMP-mediated apoptosis of DLBCLs was largely independent of the previously described cAMP effectors, protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC), but associated with inhibition of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. The central role of AKT in this process was confirmed by expressing constitutively active mutants of this kinase in DLBCL cells. Our findings highlight the important role of cAMP signaling in DLBCL and suggest that clinically relevant PDE4 and PI3K/AKT inhibitors might be useful in the treatment of DLBCL and additional B-lymphoid malignancies with increased PDE4B expression. (Blood. 2005;105:308-316)
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a common and heterogeneous B-lymphoid malignancy.1 Although a subset of patients with DLBCL are cured with combination chemotherapy, the remainder die of their disease. Therefore, additional understanding of the molecular and cellular heterogeneity of DLBCL and identification of potential rational therapeutic targets are needed. Recently, transcriptional profiling of DLBCL has been used to highlight similarities between subsets of tumors and normal B cells2 and to identify features associated with unfavorable responses to empiric combination chemotherapy.2,3 In a pilot study of DLBCL outcome signatures,3 one of the most prominently overexpressed genes in fatal/refractory DLBCL was the phosphodiesterase, PDE4B.
Phosphodiesterases (PDEs) catalyze the hydrolysis of cyclic adenosine 3′,5′ monophosphate (cAMP) and cyclic guanosine 3′,5′ monophosphate (cGMP), inactivating these second messengers.4 The PDE superfamily is subgrouped into 11 families that include at least 20 different genes and 50 unique isoforms. Of these PDE gene families, 3 are cAMP specific: PDE4, PDE7, and PDE8.5 Isoforms of the PDE4 family are the major inactivators of cellular cAMP and the predominant phosphodiesterases in lymphocytes.5,6 There are 4 PDE4 proteins, A to D, encoded by different genes and at least 3 different isoforms of PDE4B, PDE4B1, B2, and B3. These isoforms contain variable numbers of highly conserved amino terminal regions, PDE4B1 and B3 (long forms) including upstream conserved regions 1 and 2 (UCR1 and 2), and PDE4B2 (short form) containing only UCR2. The UCR1/UCR2 regions function as regulatory domains that control the conformation and activity of the catalytic domain, at least in part via the phosphorylation of a single serine residue at the amino terminus of UCR1 by protein kinase A (PKA).5,6 Further details on the structure and regulation of the PDE4 family can be found in recent reviews.5,6
The PDE4B substrate, cAMP, is a ubiquitous second messenger that regulates multiple cellular processes via activation of PKA,5-7 the exchange protein directly activated by cAMP (EPAC),8 and, additional, less well-characterized effector proteins.9 The consequences of cAMP signaling depend on both the cell type and the context of activation. In lymphocytes, cAMP-mediated effects are largely inhibitory in nature, and include cell cycle arrest and apoptosis.10-13 Because PDE4B terminates cAMP activity, it limits the growth-inhibitory effects of cAMP signaling. Hence, DLBCLs that express high levels of PDE4B may be resistant to cAMP-induced apoptosis, contributing to their less favorable outcome. Also, a better understanding of the mechanisms by which cAMP exerts its negative effects may reveal critical interactions between the cAMP effectors, PDE4B activity, and other survival pathways in DLBCL.
The cAMP signaling pathway and PDE4B activity are particularly relevant because several PDE4 inhibitors have recently entered clinical trials. Although these compounds were originally developed for reactive airway diseases,14,15 PDE4 inhibitors are also being evaluated in autoimmune diseases16 and 2 additional B-cell malignancies, chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL).17,18 In vitro analyses of primary CLL samples and ALL cell lines suggest that inhibition of PDE4 increases tumor cell apoptosis, underscoring the potential of this possible therapeutic target in DLBCL.
In this report, we confirm the risk-related expression of PDE4B in an independent series of primary DLBCLs and elucidate the roles of PDE4B and cAMP in modulating the viability of DLBCL. In addition, we show that cAMP-mediated apoptosis in DLBCL is largely independent of PKA and EPAC activation and associated with marked inhibition of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway.
Materials and methods
Cell lines, purified normal B cells, and primary DLBCLs
Human DLBCL cell lines (DHL4, DHL6, DHL7, DHL8, and DHL10) were cultured at 37°C in 5% CO2 in RPMI-1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS), whereas OCI-Ly3 and OCI-Ly10 were maintained in Iscoves modified Dulbecco media (Invitrogen, Grand Island, NY) with 20% human serum (Nabi, Miami, FL). Human embryonic kidney 293T cells were maintained in Dulbecco modified Eagle media (DMEM; Mediatech) with 10% FBS. PC12 cells were grown in DMEM containing 10% horse serum (Sigma, St Louis, MO) and 5% FBS on collagen-coated plates. All media contained penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (600 μg/mL).
Normal B-cell subpopulations (naive, germinal center [GC], centroblasts, GC centrocytes, and memory cells) were obtained from tonsillar cell suspensions as previously described.19
Frozen nodal tumor specimens from 112 newly diagnosed, untreated patients with DLBCL were studied according to an institution review board-approved protocol. All patients received adequate doses of cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP)-like combination chemotherapy for 6 or more cycles or until documented disease progression and had long-term follow-up.
Analysis of PDE4B expression by quantitative real-time RT-PCR and immunoblotting
RNAs from normal B-cell subpopulations, DLBCL cell lines, and primary tumors were isolated and used to generate cDNAs as previously described.20 To measure PDE4B expression, isoform-specific primers and probes for PDE4B1, PDE4B2, and PDE4B3 were used (sequences available upon request). These primer and probe sets were first validated by the amplification of isoform-specific plasmids. All reverse-transcription-polymerase chain reactions (RT-PCRs) were run in triplicate according to the TaqMan method (PE Applied Biosystems, Foster City, CA). The quantity of the target gene amplification product was calculated by entering the obtained cycle threshold (CT) values into a standard curve generated from known concentrations of the specific plasmid DNA. These values were normalized to those of a control gene (cyclophilin) for each sample. DLBCL cell lines were also analyzed for PDE4B protein expression by Western blot as previously described,21 using a PDE4B-specific antisera (a gift from Miles Houslay, University of Glasgow, United Kingdom). The PDE4B antisera were raised against a C-terminal region common to all PDE4B isoforms; showed no cross-reactivity with recombinant human PDE4A, PDE4C, or PDE4D forms; and were described in detail elsewhere.22
Intracellular cAMP quantification and PDE4 inhibition
cAMP was induced in the DLBCL cell lines by treatment with forskolin (10-40 μM; Sigma), an activator of adenyl cyclase. Intracellular cAMP levels were measured by Biotrak cAMP enzyme immunoassay (Amersham Pharmacia Biotech, Piscataway, NJ).
In certain experiments, DLBCLs were also treated with PDE4 inhibitors, rolipram (10-30 μM; Sigma) or PLX513 (1-20 μM; Plexxikon, Berkeley CA). PLX513 is a PDE4B-specific phosphodiesterase inhibitor identified by applying structure-guided screening and lead optimization methodology to target PDE4B activity.23 PLX513 is 10- to 100-fold more active against the catalytic domain of PDE4B than the catalytic domains of other PDE4 family members and additional PDEs (for supplemental data available on the Blood website, see the Supplemental Materials link at the top of the online article; specifically, see Table S1).
Analysis of cell proliferation and apoptosis
Cell proliferation was assessed by measuring MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium; Promega, Madison, WI) dye absorbance. Apoptosis was assessed with either propidium iodide staining as previously described21 or dual staining (annexin V and propidium iodide; Oncogene Research Products, San Diego, CA).
PDE4B and AKT retroviral constructs
Wild-type PDE4B2 (PDE4B2-WT) was RT-PCR amplified from normal mature B-cell cDNA using isoform-specific primers; subsequently PDE4B2-WT was cloned into the p3X-Flag-CMV-14 vector (Sigma). The PDE4B2-phosphodiesterase inactive (PI) mutant (PDE4B2-PI), containing a single amino acid substitution (H234S), was generated using Quick-Change Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Mutation of H234 and other conserved histidines in the phosphodiesterase catalytic domain abolishes the enzyme's activity in vitro, likely by interfering with metal binding and chelation, critical for PDE4 function.6,24 FLAG-tagged PDE4B2-WT and PDE4B2-PI constructs were subcloned into the retroviral vector MSCV-eGFP (a gift from Jon Aster, Brigham and Women's Hospital, Boston).
The constitutively active myristoylated forms of wild-type AKT (Myr-AKT-WT) and kinase-inactive AKT (triple mutant, K179M, T308A, and S473A; Myr-AKT-MAA) (gifts from W. Sellers, Dana-Farber Cancer Institute, Boston, MA) were also subcloned into MSCV-eGFP. The MSCV-eGFP vector contains an internal ribosomal entry sequence and a cDNA encoding enhanced green fluorescent protein (eGFP) 3′ of the multicloning site, allowing the expression of a single biscistronic mRNA encoding both the gene of interest (PDE4B2 or AKT) and eGFP.
MSCV-eGFP virus production and retroviral spin infections were performed as previously described.25 eGFP expression was analyzed after 48 hours to assess infection efficiency, and eGFP-positive cell enrichment was performed by fluorescence-activated cell sorting (FACS Vantage; Becton Dickinson, San Jose, CA). PDE4B2 expression in the DLBCL cells was also determined by Western blot using FLAG antibody (Sigma); total and phospho-AKT (pAKT) were assessed with the antitotal and phospho-AKT (Ser 473) antisera (Cell Signaling, Beverly, MA).
Protein kinase A analysis
A PepTag-Assay kit (Promega) was used to determine protein kinase A (PKA) activity in DLBCL cells exposed to forskolin and/or rolipram in the presence or absence of the PKA inhibitors H89 (10 μM; Sigma) or PKI (10 μM; Santa Cruz Biotechnology, Santa Cruz, CA).
EPAC analysis
A previously described semiquantitative RT-PCR approach26 was used to define the expression of EPAC1 in relevant DLBCL cell lines in the presence or absence of forskolin/rolipram. EPAC's role as a cAMP effector was assessed by measuring the accumulation of the guanosine triphosphate (GTP)-bound (active) form of RAP1 following treatment with forskolin/rolipram or an EPAC-specific cAMP analog (8-CPT-2′-0-Me-cAMP, 0.001-1 mM [Biolog, Bremen, Germany]). To detect GTP-bound RAP1, cell lysates were incubated with glutathione beads complexed with the RAS binding domain (RBD) of RALGDS-GST (a gift from V. Boussiotis, Dana-Farber Cancer Institute), which specifically pulls down GTP-bound RAP1.8 Following centrifugation, the glutathione-S-transferase (GST)-beads were washed, and bound proteins were eluted and immunoblotted with anti-RAP1 antisera (Santa Cruz Biotechnology).
Analysis of mitochondrial membrane potential, caspase-3 and caspase-9 activity, and BAD phosphorylation
Mitochondrial membrane potential was measured by incubating forskolin/rolipram-treated cells with the fluorescent dye 3, 3′-dihexyloxacarbocyanine iodide (DiOC6(3); Molecular Probes, Eugene, OR) and analyzing the cells by FACS. Caspase-3-like and caspase-9-like activity was defined against p-nitroanilide-linked substrates (Ac-DEVD-pNA, caspase-3 [Sigma] and Ac-LEHD-p-NA, caspase-9 [BIOMOL, Plymouth Meeting, PA]) in the presence or absence of specific caspase inhibitors. Total and phospho-BAD (pBAD) levels were analyzed by Western blot using the total and phospho-BAD (Ser 136) antisera (Cell Signaling).
PP2A assay
An Immunoprecipitation Phosphatase Assay Kit (Upstate Biotechnology, Charlottesville, VA) was used to measure protein phosphatase 2A (PP2A) activity in DLBCL cells exposed to forskolin and/or rolipram, in the presence or absence of H89 or okadaic acid (Sigma).
AKT kinase activity, phosphorylation of AKT substrates, and upstream regulators
Total AKT was immunoprecipitated from the relevant cell lysates, resuspended in kinase buffer supplemented with 200 μM cold adenosine triphosphate (ATP), and incubated with 1 μg of the recombinant AKT substrate, glycogen synthase kinase-3 (GSK-3), at 30°C for 30 minutes. AKT kinase activity was subsequently determined by Western blotting the reaction mixture for phosphorylated GSK-3α/β, using the phospho-GSK-3α/β (Ser21/9) antibody (Cell Signaling). The phospho-levels of the AKT substrates forkhead transcription factor (FKHR) and GSK-3β as well as of the AKT regulator, PTEN, were also analyzed by Western blot using their respective phospho-antibodies (Cell Signaling).
Phosphatidylinositol 3-kinase (PI3K) activity
PI3K kinase assays were performed as previously described27 in the presence or absence of forskolin/rolipram. In brief, relevant cell lysates were subjected to immunoprecipitation with the anti-phosphotyrosine 4G10 antibody (Upstate Biotechnology). Immune complex kinase assays were performed with a substrate mixture of 40% phosphatidylinositol (PI), 40% phosphatidyl-4,5-biphosphate (PIP2), and 20% of the phosphatidylserine carrier (Sigma). The lipid products were separated by thin-layer chromatography and visualized by autoradiography; radioactivity in the PtdIns-3,4,5-trisphosphate (PIP3) spot was quantified by densitometry. In additional experiments, DLBCL cell line proliferation was assessed in the presence or absence of forskolin/rolipram and PI3K inhibitors, LY249002 or wortmannin (Sigma).
Results
PDE4B2 is the major PDE4B isoform expressed in normal B-cell subpopulations and DLBCL cell lines
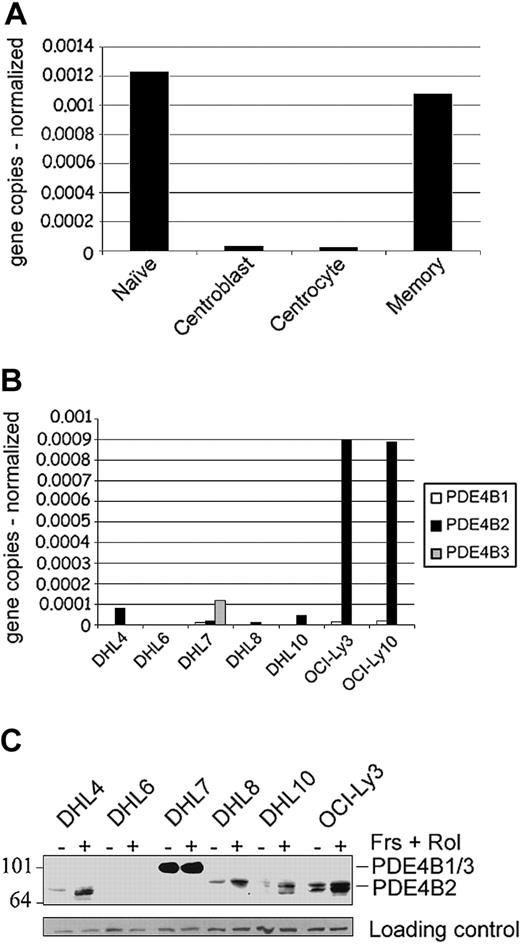
We initially used a quantitative real-time RT-PCR (Q-PCR) to define the levels of expression of the 3 known human PDE4B isoforms (B1-B3)22 in purified normal B-cell subpopulations and DLBCL cell lines (Figure 1A-B). PDE4B2 was the only PDE4B isoform expressed in normal B cells, with the highest levels in naive and memory B cells and much lower levels in centroblasts and centrocytes (Figure 1A). The DLBCL cell lines also primarily expressed PDE4B2 (Figure 1B), with the lowest levels in DHL6 and highest levels in OCI-Ly3 and OCI-Ly10. There were 3 DLBCL cell lines (DHL7, OCI-Ly3, and OCI-Ly10) that also expressed low levels of PDEB1, and 1 cell line (DHL7) also expressed PDE4B3. While analyzing PDE4B isoforms in normal B cells and DLBCL cell lines, we also cloned and characterized a novel PDE4B transcriptional unit, termed PDE4B5 (supplementary Figure S1).
PDE4B expression in normal and malignant B lymphocytes. (A-B) Quantitative real-time RT-PCR analyses of PDE4B isoforms in highly purified mature B-cell populations (naive, centroblasts, centrocytes, memory cells) and DLBCL cell lines. The expression of the target and control genes was calculated by entering the real-time RT-PCR CT values (average of triplicates) into a standard curve generated from plasmid DNA of known concentrations. Data represent PDE4B isoform copy number normalized to control gene for each sample (A, PDE4B2 only; B, PDE4B1, B2, and B3). (C) PDE4B protein expression in DLBCL cell lines. Cells were either untreated (-) or treated with 40 μM forskolin (Frs) and 10 μM rolipram (Rol) for 6 hours (+). Whole-cell lysates were immunoblotted with a pan-PDE4B antisera. PDE4B2 exhibits the characteristic up-regulation following an increase in intracellular cAMP. PDE4B1 and B3 comigrate at approximately 105 kDa, whereas the PDE4B2 doublet is 78 to 84 kDa. Size markers are indicated on the left in kilodaltons.
PDE4B expression in normal and malignant B lymphocytes. (A-B) Quantitative real-time RT-PCR analyses of PDE4B isoforms in highly purified mature B-cell populations (naive, centroblasts, centrocytes, memory cells) and DLBCL cell lines. The expression of the target and control genes was calculated by entering the real-time RT-PCR CT values (average of triplicates) into a standard curve generated from plasmid DNA of known concentrations. Data represent PDE4B isoform copy number normalized to control gene for each sample (A, PDE4B2 only; B, PDE4B1, B2, and B3). (C) PDE4B protein expression in DLBCL cell lines. Cells were either untreated (-) or treated with 40 μM forskolin (Frs) and 10 μM rolipram (Rol) for 6 hours (+). Whole-cell lysates were immunoblotted with a pan-PDE4B antisera. PDE4B2 exhibits the characteristic up-regulation following an increase in intracellular cAMP. PDE4B1 and B3 comigrate at approximately 105 kDa, whereas the PDE4B2 doublet is 78 to 84 kDa. Size markers are indicated on the left in kilodaltons.
To characterize PDE4B protein expression and assess PDE4B up-regulation following cAMP signaling, the DLBCL cell lines were cultured with forskolin and rolipram; thereafter, cell lysates were immunoblotted with a PDE4B-specific antisera. DLBCL cell lines with predominant PDE4B2 transcripts expressed the PDE4B2 doublet, which was up-regulated following forskolin treatment (Figure 1C).5,6,28,29 DHL7 expressed the larger PDE4B3 isoform, which is characteristically insensitive to intracellular cAMP levels.28 The documented range of PDE4B expression and cAMP-associated up-regulation of the major PDE4B isoform (PDE4B2) suggest that these DLBCL cell lines are useful models for studying the phosphodiesterase's role in this disease.
PDE4B2 is overexpressed in fatal/refractory DLBCL
Since PDE4B2 is the major isoform expressed by normal B cells and DLBCL cell lines, we used real-time RT-PCR to analyze PDE4B2 transcript abundance in an independent series of 112 newly diagnosed, previously untreated DLBCLs from patients with long-term clinical follow-up (57 cured patients and 55 patients with fatal/refractory disease). The previously described association between fatal/refractory DLBCL and increased PDE4B expression was confirmed in this independent DLBCL series (P = .02, 2-sided Wilcoxon rank sum test30 ). Overall 5-year survival for patients with PDE4B2 levels below and above median were 63% and 47%, respectively (P = .08).
PDE4B limits the growth-inhibitory effects of cAMP in DLBCL cell lines
After identifying the major PDE4B isoform in DLBCLs and confirming the association between increased PDE4B2 expression and fatal/refractory disease, we characterized the effects of cAMP signaling and PDE4B expression/activity on DLBCL growth and survival. In these experiments, we used a panel of 4 DLBCL cell lines with different levels of PDE4B expression. The DHL6 cell line expressed very low levels of PDE4B, whereas DHL7, OCILy3, and OCI-Ly10 had higher PDE4B levels (Figure 1B-C).
After forskolin treatment, proliferation of the PDE4B-low/negative cell line, DHL6, decreased to 20% of control levels (80% inhibition; Figure 2A). Conversely, in cell lines with higher levels of PDE4B, forskolin-associated decreases in proliferation were much less striking (Figure 2A). Thereafter, we compared the effects of forskolin alone versus forskolin combined with a general PDE4 inhibitor (rolipram) or the isoform-specific PDE4B inhibitor, PLX513. As expected, neither PDE inhibitor had a significant effect on cAMP-associated growth inhibition in PDE4B-low/negative DHL6 cells. In contrast, the isoform-specific PDE4B inhibitor, PLX513, significantly increased the growth-inhibitory effects of forskolin in cell lines with higher levels of PDE4B (DHL7, OCI-Ly3, and OCI-Ly10; Figure 2B). PLX513 effects were dose-dependent (supplemental Figure S7) and detectable only following forskolin treatment (Figure 2B), suggesting that PLX513 specifically augments cAMP effects by inhibiting PDE4B. The more pronounced activity of PLX513 when compared with rolipram might reflect its highly selective inhibition of PDE4B contrary to the pan-PDE4 inhibition mediated by rolipram (supplemental table S1).
PDE4B expression and activity limit the growth-inhibitory effects of cAMP. (A) Growth inhibition following forskolin treatment. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 24 hours. (B) Growth inhibition following treatment with PDE4 inhibitors. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 24 hours in the presence or absence of rolipram (10 μM), PLX513 (10 μM [DHL6] or 20 μM [DHL7, OCI-Ly3, and OCI-Ly10]), or with these PDE4 inhibitors alone. A 20-μM dose of PLX513 was cytotoxic for DHL6 cells regardless of forskolin treatment. Data in panels A-B represent the ratio of proliferation of treated cells to cells cultured with vehicle alone for the same time periods. Cell proliferation was measured by MTS assay. (C) cAMP induction following forskolin treatment. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 10 to 60 minutes and intracellular cAMP levels determined by ELISA. The cAMP induction in DHL7 and OCI-Ly10 cells was minimal (5 and 3 pmol/million cells, respectively), and it is not apparent in this display. (D) cAMP levels following inhibition of PDE4. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 10 to 60 minutes following preincubation with PLX513 for 30 minutes. Results are displayed as the percentage of increase in cAMP levels in cells treated with forskolin alone versus those treated with forskolin and PLX513. All proliferation and cAMP data are the mean and SD of 3 independent experiments. All individual experiments were performed in triplicate.
PDE4B expression and activity limit the growth-inhibitory effects of cAMP. (A) Growth inhibition following forskolin treatment. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 24 hours. (B) Growth inhibition following treatment with PDE4 inhibitors. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 24 hours in the presence or absence of rolipram (10 μM), PLX513 (10 μM [DHL6] or 20 μM [DHL7, OCI-Ly3, and OCI-Ly10]), or with these PDE4 inhibitors alone. A 20-μM dose of PLX513 was cytotoxic for DHL6 cells regardless of forskolin treatment. Data in panels A-B represent the ratio of proliferation of treated cells to cells cultured with vehicle alone for the same time periods. Cell proliferation was measured by MTS assay. (C) cAMP induction following forskolin treatment. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 10 to 60 minutes and intracellular cAMP levels determined by ELISA. The cAMP induction in DHL7 and OCI-Ly10 cells was minimal (5 and 3 pmol/million cells, respectively), and it is not apparent in this display. (D) cAMP levels following inhibition of PDE4. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 10 to 60 minutes following preincubation with PLX513 for 30 minutes. Results are displayed as the percentage of increase in cAMP levels in cells treated with forskolin alone versus those treated with forskolin and PLX513. All proliferation and cAMP data are the mean and SD of 3 independent experiments. All individual experiments were performed in triplicate.
To more specifically characterize the relationship between PDE4B expression and its activity in these DLBCL cell lines, we measured cAMP levels at baseline and following forskolin or forskolin/PDE4 inhibitor treatment (Figure 2C-D). All of the cell lines had low/undetectable baseline cAMP levels, consistent with low basal levels of adenylate cyclase activity.31 However, forskolin treatment markedly increased cAMP levels in PDE4B-low/negative DHL6 cells, but had more modest effects in cell lines with higher levels of PDE4B (Figure 2C). Expectedly, PDE4B inhibition had little effect on forskolin-associated cAMP levels in DHL6 cells (PDE4B-low/negative) (Figure 2D). In contrast, PDE4B inhibition with PLX513 markedly increased forskolin-associated cAMP levels in DHL7, OCI-Ly3, and OCI-Ly10 cells (PDE4B-high) (Figure 2D). In addition, in a direct comparison of the effects of PLX513 versus rolipram we found that the former induces a more prominent increase in intracellular cAMP following forskolin treatment (supplemental Figure S7), in agreement with the more evident growth-inhibitory effects of PLX513 (Figure 2B).
PDE4B2 reconstitution blocks cAMP-induced apoptosis in DLBCL cell lines
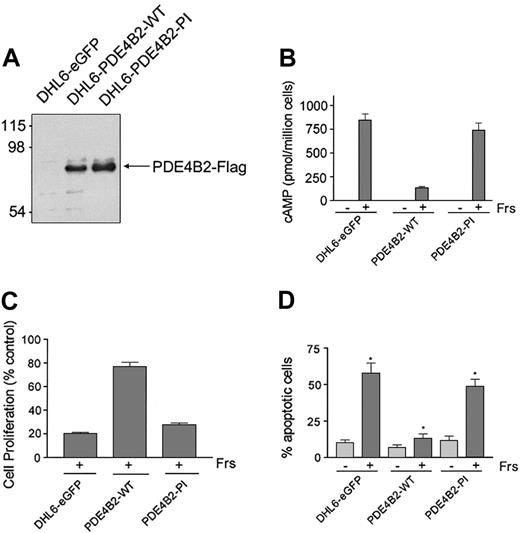
To directly establish the role of PDE4B in controlling the cAMP-inhibitory effects in DLBCL, we reconstituted PDE4B2 expression in the PDE4B-low/negative DHL6 cell line using a retroviral expression system. To confirm that the observed effects were due to PDE4B2 phosphodiesterase activity, we also generated cells expressing a phosphodiesterase inactive (PDE4B2-PI) mutant or a vector-only control (DHL6-eGFP). The FLAG-tagged PDE4B2-WT and PDE4B2-PI constructs were expressed at similar levels in the respective DHL6 cells (Figure 3A).
Reconstitution of PDE4B2 expression prevents cAMP-induced apoptosis. (A) Expression of PDE4B2-WT (wild type) and PDE4B2-PI (phosphodiesterase inactive) in retrovirally infected DHL6 cells. The expression of the indicated Flag-tagged constructs was analyzed with an anti-Flag immunoblot. Size markers are indicated on the left in kilodaltons. (B) cAMP induction in DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells following forskolin treatment. The indicated cells were incubated with 40 μM forskolin for 1 hour, and intracellular cAMP levels were analyzed by ELISA. (C) Growth inhibition following forskolin treatment. DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells were incubated with 40 μM forskolin for 24 hours. Data represent the ratio of proliferation of treated cells to cells cultured with vehicle alone for the same time period. Cell proliferation was measured by MTS assay. (D) Cellular apoptosis in DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells treated with forskolin. The indicated cells were incubated with 40 μM forskolin for 48 hours and analyzed by propidium iodide staining. *P < .05 when comparing rate of apoptosis following forskolin treatment in all 3 cell lines. Data in panels B-D represent the mean and SD of 3 independent experiments.
Reconstitution of PDE4B2 expression prevents cAMP-induced apoptosis. (A) Expression of PDE4B2-WT (wild type) and PDE4B2-PI (phosphodiesterase inactive) in retrovirally infected DHL6 cells. The expression of the indicated Flag-tagged constructs was analyzed with an anti-Flag immunoblot. Size markers are indicated on the left in kilodaltons. (B) cAMP induction in DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells following forskolin treatment. The indicated cells were incubated with 40 μM forskolin for 1 hour, and intracellular cAMP levels were analyzed by ELISA. (C) Growth inhibition following forskolin treatment. DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells were incubated with 40 μM forskolin for 24 hours. Data represent the ratio of proliferation of treated cells to cells cultured with vehicle alone for the same time period. Cell proliferation was measured by MTS assay. (D) Cellular apoptosis in DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells treated with forskolin. The indicated cells were incubated with 40 μM forskolin for 48 hours and analyzed by propidium iodide staining. *P < .05 when comparing rate of apoptosis following forskolin treatment in all 3 cell lines. Data in panels B-D represent the mean and SD of 3 independent experiments.
To define the specific activity of the transfected PDE4B2 constructs, we first measured cAMP levels in DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells at baseline and following forskolin treatment. Although forskolin led to a major increase in cAMP levels in DHL6-eGFP and DHL6-PDE4B2-PI cells, similar treatment of DHL6-PDE4B2-WT cells was much less effective (Figure 3B).
To confirm the link between cAMP levels, PDE4B2 activity, and cell survival, we compared the effects of forskolin on cellular viability of DHL6-eGFP, DHL6-PDE4B2-WT, and DHL6-PDE4B2-PI cells by measuring cell proliferation and apoptotic cellular fraction after treatment (Figure 3C-D). As expected, DHL6-PDE4B-WT cells were significantly more resistant to cAMP-associated apoptosis than DHL6-eGFP or DHL6-PDE4B-PI cells (Figure 3D; P < .05 comparing forskolin treatment in all 3 cell lines).
cAMP-associated growth inhibition in DLBCL is independent of PKA
Since cAMP is thought to mediate the majority of its effects via the serine-threonine protein kinase, PKA,4-7 we measured PKA enzymatic activity in DLBCL following forskolin treatment. In parental DHL6 cells, forskolin treatment significantly increased PKA activity and preincubation with a well-characterized PKA inhibitor, H89, largely blocked the forskolin-induced PKA activation (supplemental Figure S4). To determine whether PKA was responsible for the downstream effects of cAMP signaling in DLBCLs, we treated DHL6 cells with H89 prior to forskolin/rolipram stimulation. Although H89 inhibited most of the PKA activity, it did not limit cAMP-associated growth arrest (supplemental Figure S4). Importantly, H89 alone had only a minor effect on DHL6 proliferation. Similar results were obtained using a synthetic peptide corresponding to the conserved sequences of the naturally occurring PKA inhibitor, PKI (data not shown). Taken together, these data suggest that the inhibitory effects of cAMP signaling are largely independent of PKA.
cAMP-associated growth inhibition in DLBCL is independent of EPAC
Since the growth-inhibitory effects of cAMP in our DLBCL model appeared to be PKA independent, we explored alternative mechanisms including cAMP activation of EPAC, a guanine exchange factor (GEF), for the small GTPases RAP1 and RAP2. EPAC activation by cAMP leads to accumulation of the GTP-bound, active, form of RAP1.8 For these reasons, we measured the amounts of active RAP1 in the DLBCL cell lines following forskolin/rolipram treatment, using a classical RAP1 activation binding assay.8,32 Increasing intracellular levels of cAMP in these cells did not modify RAP1 activation, suggesting that EPAC was not mediating cAMP signaling (supplemental Figure S5). Conversely, B-cell receptor engagement, a known EPAC-independent activator of RAP1 in B lymphocytes33 clearly increased the amounts of GTP-bound RAP1 in these cells (supplemental Figure S5).
These findings and the recent suggestion that EPAC1 is differentially expressed in specific subtypes of mature B-lymphoid malignancies34 prompted us to characterize EPAC1 expression in a subset of relevant DLBCL cell lines with a semiquantitative RT-PCR assay. EPAC1 was expressed in most DLBCL cell lines analyzed, including DHL7 and OCI-Ly3, but was absent in DHL6 (supplemental Figure S5). The lack of EPAC1 expression in DHL6 cells, which are particularly sensitive to the inhibitory effects of cAMP, further indicates that EPAC is not a major cAMP effector in these cells. In agreement with these findings, EPAC-specific cAMP analogs did not modify RAP1 activation or cell proliferation in DLBCL cells (supplemental Figure S5).
Taken together, these studies on PKA and EPAC suggest that an additional novel cAMP effector9 may transduce cAMP inhibitory signals in DLBCL.
cAMP-mediated apoptosis involves mitochondrial membrane depolarization, activation of caspase-9 and caspase-3, and decreased BAD phosphorylation
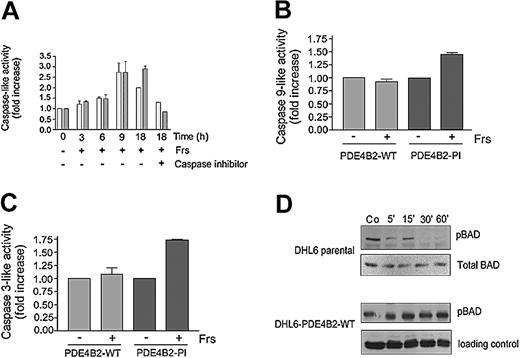
Mitochondrial membrane depolarization results in the opening of the mitochondrial permeability transition pores, release of cytochrome c, and subsequent activation of the intrinsic caspase cascade (caspase-9 and caspase-3).35,36 To determine whether cAMP signaling alters mitochondrial membrane potential, we performed FACS analysis of forskolin-treated DLBCL cell lines using the mitochondrial-potential sensitive dye, 3, 3′-dihexyloxacarbocyanine iodide (DiOC6(3)). Forskolin treatment of parental or DHL6-PDE4B2-PI cells markedly reduced mitochondrial membrane potential; in contrast, similar treatment of DHL6-PDE4B2-WT or OCI-Ly3 cells did not appreciably alter mitochondrial membrane integrity (data not shown). Consistent with these effects, cAMP signaling in parental DHL6 cells resulted in a time-dependent increase in caspase-9- and caspase-3-like activity (Figure 4A), whereas overexpression of wild-type, but not mutant, PDE4B2 inhibited cAMP-associated caspase-9 and caspase-3 activation (Figure 4B-C). In agreement with these findings, there was minimal increase in caspase activity in cell lines with high endogenous levels of PDE4B (data not shown).
PDE4B2 controls cAMP effects on caspase-9 and caspase-3 activity and BAD phosphorylation. (A) Caspase-9- and caspase-3-like activity in forskolin-treated parental DHL6 cells. After 40 μM forskolin treatment for the specified time periods, cell lysates were harvested and caspase-9-like (▦) and caspase-3-like (▪) activities were measured against 200 μM of their respective substrates (Ac-LEHD-p-NA and Ac-DEVD-pNA) in the presence or absence of their respective inhibitors (Ac-LEHD-CHO and Ac-DEVD-CHO). (B-C) Caspase-9- and caspase-3-like activity in PDE4B2-WT and PDE4B-PI cells in the presence or absence of forskolin. The cells were incubated with 10 μM forskolin, and caspase-9-like or caspase-3-like activity was determined at 9 and 18 hours, respectively. Data in panels A-C represent the mean and SD of 3 independent experiments. (D) BAD phosphorylation in DHL6-parental or DHL6-PDE4B-WT cells treated with forskolin/rolipram. The indicated cells were incubated with 40 μM forskolin and 10 μM rolipram for the specified time periods; cell lysates were harvested and immunoblotted for pBAD (Ser 136). Equal loading was established by reprobing with antibodies directed against total BAD or total AKT. Co indicates control.
PDE4B2 controls cAMP effects on caspase-9 and caspase-3 activity and BAD phosphorylation. (A) Caspase-9- and caspase-3-like activity in forskolin-treated parental DHL6 cells. After 40 μM forskolin treatment for the specified time periods, cell lysates were harvested and caspase-9-like (▦) and caspase-3-like (▪) activities were measured against 200 μM of their respective substrates (Ac-LEHD-p-NA and Ac-DEVD-pNA) in the presence or absence of their respective inhibitors (Ac-LEHD-CHO and Ac-DEVD-CHO). (B-C) Caspase-9- and caspase-3-like activity in PDE4B2-WT and PDE4B-PI cells in the presence or absence of forskolin. The cells were incubated with 10 μM forskolin, and caspase-9-like or caspase-3-like activity was determined at 9 and 18 hours, respectively. Data in panels A-C represent the mean and SD of 3 independent experiments. (D) BAD phosphorylation in DHL6-parental or DHL6-PDE4B-WT cells treated with forskolin/rolipram. The indicated cells were incubated with 40 μM forskolin and 10 μM rolipram for the specified time periods; cell lysates were harvested and immunoblotted for pBAD (Ser 136). Equal loading was established by reprobing with antibodies directed against total BAD or total AKT. Co indicates control.
Release of cytochrome c from the mitochondrial intermembrane space and activation of the intrinsic mitochondrial/cytochrome c caspase cascade is regulated in part by interactions of pro- and antiapoptotic BCL-2 family members, including BCL-2, Bcl-XL, BAX, and BAD.35,36 Therefore, we asked whether cAMP signaling modulated the phosphorylation levels and activity of BCL-2 family members, such as BAD. Forskolin treatment of the PDE4B-low (parental) DHL6 cells rapidly decreased BAD Ser-136 phosphorylation (Figure 4D); in contrast, no changes were seen in the DHL6 cells reconstituted with PDE4B2-WT (Figure 4D). Expectedly, preincubation with the PKA inhibitor H89 did not alter cAMP effects on BAD phosphorylation (data not shown)
cAMP signaling modulates BAD phosphorylation/activity via AKT
To determine how cAMP modulates BAD phosphorylation and activity, we assessed the effects of cAMP on kinases and phosphatases that regulate BAD phosphorylation, including AKT and protein phosphatase 2A (PP2A). In certain model systems, cAMP activates PP2A leading to the dephosphorylation of a range of target proteins including AKT and BAD.37,38 However, although PP2A activity was significantly increased by forskolin/rolipram in our DLBCL cell lines, PP2A did not modulate phospho-BAD levels, phospho-AKT, or cellular apoptosis (supplemental Figure S6).
To assess potential effects of cAMP on the AKT pathway in DLBCL, we assayed pAKT levels and AKT kinase activity in forskolin-treated PDE4B-low DHL6 cells and DHL6-PDE4B2-WT cells. In parental DHL6 cells, cAMP signaling markedly decreased pAKT without altering total AKT protein levels (Figure 5A). cAMP signaling also decreased AKT kinase activity and the phosphorylation of specific AKT substrates, FKHR and GSK3β (data not shown). In marked contrast, cAMP signaling did not alter pAKT levels in DHL6-PDE4B2-WT cells (Figure 5A).
AKT plays a central role in mediating cAMP inhibitory effects in DLBCL. (A) AKT phosphorylation in DHL6-parental or DHL6-PDE4B-WT cells treated with forskolin/rolipram. DHL6-parental or DHL6-PDE4B-WT cells were incubated with 40 μM forskolin and 10 μM rolipram for the specified time periods, and cell lysates were harvested and immunoblotted for pAKT (Ser 473) and total AKT as loading control. The Figure 4D filter was stripped and reprobed for pAKT and total AKT in this experiment. (B) Proliferation of forskolin-treated DHL6-eGFP, DHL6-Myr-AKT-WT, and DHL6-Myr-AKT-MAA cells. The indicated cells were incubated with 10 μM forskolin for 24 hours and cell proliferation was determined by MTS assay. Data represent the mean and SD of 3 independent experiments. (C) Total and phospho-AKT expression in DHL6-eGFP, DHL6-Myr-AKT-WT, and DHL6-Myr-AKT-MAA cells in the presence or absence of forskolin. The indicated cells were incubated with 10 μM forskolin for 15 minutes and cell lysates immunoblotted for pAKT (Ser 473) and total AKT. (D) AKT phosphorylation in PDE4B-high cell lines DHL7, OCI-Ly3, and OCI-Ly10. Cell lines were preincubated with 20 μM PLX513 for 30 minutes followed by treatment with 40 μM forskolin for the specified time periods and cell lysates were harvested and immunoblotted for pAKT.
AKT plays a central role in mediating cAMP inhibitory effects in DLBCL. (A) AKT phosphorylation in DHL6-parental or DHL6-PDE4B-WT cells treated with forskolin/rolipram. DHL6-parental or DHL6-PDE4B-WT cells were incubated with 40 μM forskolin and 10 μM rolipram for the specified time periods, and cell lysates were harvested and immunoblotted for pAKT (Ser 473) and total AKT as loading control. The Figure 4D filter was stripped and reprobed for pAKT and total AKT in this experiment. (B) Proliferation of forskolin-treated DHL6-eGFP, DHL6-Myr-AKT-WT, and DHL6-Myr-AKT-MAA cells. The indicated cells were incubated with 10 μM forskolin for 24 hours and cell proliferation was determined by MTS assay. Data represent the mean and SD of 3 independent experiments. (C) Total and phospho-AKT expression in DHL6-eGFP, DHL6-Myr-AKT-WT, and DHL6-Myr-AKT-MAA cells in the presence or absence of forskolin. The indicated cells were incubated with 10 μM forskolin for 15 minutes and cell lysates immunoblotted for pAKT (Ser 473) and total AKT. (D) AKT phosphorylation in PDE4B-high cell lines DHL7, OCI-Ly3, and OCI-Ly10. Cell lines were preincubated with 20 μM PLX513 for 30 minutes followed by treatment with 40 μM forskolin for the specified time periods and cell lysates were harvested and immunoblotted for pAKT.
Expression of constitutively active AKT protects DLBCL cell lines from cAMP-mediated growth inhibition
To confirm the central role of AKT in modulating cAMP inhibitory signals in DLBCL, we retrovirally infected DHL6 cells with a constitutively active myristoylated form of wild-type AKT (Myr-AKT-WT) or a kinase-inactive AKT mutant (Myr-AKT-MAA). Expression of constitutively active AKT protected DHL6 cells from cAMP-associated growth inhibition (Myr-AKT-WT, 85% baseline proliferation vs DHL6-eGFP cells, 49% baseline proliferation, P < .05; Figure 5B). In contrast, cells expressing the kinase-inactive AKT mutant responded to forskolin similarly to DHL6-eGFP cells. As expected, cAMP signaling was associated with a marked decrease in AKT phosphorylation in DHL6-eGFP and DHL6-Myr-AKT-MAA cells, but not DHL6-Myr-AKT-WT cells (Figure 5C). Importantly, DHL6-eGFP, DHL6-Myr-AKT-WT, and DHL6-Myr-AKT-MAA cells all proliferated at similar rates, indicating that the protective effects of AKT were not due to increased growth rates of DHL6-Myr-AKT-WT cells (data not shown). Taken together, these data confirm that the majority of the inhibitory cAMP signals in DHL6 cells are relayed by the AKT pathway.
cAMP signaling modulates AKT phosphorylation in multiple DLBCL cell lines
To determine whether AKT plays a comparable role in the additional DLBCL cell lines, we also analyzed pAKT levels in the PDE4B-high cell lines, DHL7, OCI-Ly3, and OCI-Ly10. As expected, treatment of these cell lines with forskolin alone had no impact on the pAKT levels (data not shown). However, when forskolin was combined with the isoform-specific PDE4B inhibitor, PLX513, cAMP signaling markedly decreased pAKT in DHL7 and OCI-Ly10 and moderately reduced pAKT in OCI-Ly3. Taken together, the data in the genetically reconstituted PDE4B-low and chemically inhibited PDE4B-high cell lines (Figure 5A,D) highlight the important role of AKT in mediating cAMP effects in DLBCL.
cAMP-PDE4B effects on AKT activity are mediated via PI3K
To determine how cAMP/PDE4B modulates AKT function, we assessed the effects of cAMP/PDE4B on 2 of the major AKT regulators, PTEN and the phosphatidylinositol 3-kinase, PI3K.39 Increasing the intracellular levels of cAMP did not modify the phospho-levels, or presumably the activity, of PTEN (data not shown). However, forskolin stimulation of the PDE4B-low (parental) DHL6 cell line significantly decreased PI3K activity toward its major lipid substrate, PIP2 (Figure 6).
cAMP signaling inhibits PI3K activity. DHL6 parental cells were treated with 40 μM forskolin and 10 μM rolipram for 5 minutes. Cell lysates were harvested and immunoprecipitated with anti-phosphotyrosine antibody (4G10). Precipitated proteins were incubated with phosphatidylinositol (PI), phosphatidyl-4,5-biphosphate (PIP2), and [γ-32P]ATP, followed by extraction of lipids, separation by thin layer chromatography, and exposure to an x-ray film (left panel, representative assay). PI3K activity toward its major lipid substrates (PIP2) was determined by densitometrically measuring the amounts of the phosphatidylinositol 3,4,5-trisphosphate (PIP3) produced in 3 independent reactions (right panel). Fold of inhibition (right panel) was determined by comparing the PIP3 intensity in forskolin/rolipram-treated and untreated cells and reported as the mean ± SD.
cAMP signaling inhibits PI3K activity. DHL6 parental cells were treated with 40 μM forskolin and 10 μM rolipram for 5 minutes. Cell lysates were harvested and immunoprecipitated with anti-phosphotyrosine antibody (4G10). Precipitated proteins were incubated with phosphatidylinositol (PI), phosphatidyl-4,5-biphosphate (PIP2), and [γ-32P]ATP, followed by extraction of lipids, separation by thin layer chromatography, and exposure to an x-ray film (left panel, representative assay). PI3K activity toward its major lipid substrates (PIP2) was determined by densitometrically measuring the amounts of the phosphatidylinositol 3,4,5-trisphosphate (PIP3) produced in 3 independent reactions (right panel). Fold of inhibition (right panel) was determined by comparing the PIP3 intensity in forskolin/rolipram-treated and untreated cells and reported as the mean ± SD.
Targeting the PI3K/AKT survival pathway in PDE4B-overexpressing cells
If cAMP effects in DLBCL are primarily mediated via PI3K/AKT, inhibition of this pathway might circumvent the resistance of PDE4B-overexpressing cells to cAMP-induced apoptosis. To explore this possibility, we stimulated DHL6-PDE4B2-WT or DHL6-PDE4B2-PI cells with forskolin in the presence or absence of P13K inhibitors (LY294002 and wortmannin). In DHL6-PDE4B2-PI cells, forskolin and LY249002 had similar effects on cellular proliferation (P > .1, unpaired, 2-tailed t test; Figure 7). In this in vitro model system, the combination of forskolin and LY249002 was not additive, again suggesting that the cAMP growth-inhibitory effects occur predominantly via the PI3K/AKT pathway. However, although PDE4B2-WT cells were resistant to the growth-inhibitory effects of forskolin, these cells were sensitive to P13K inhibition with LY249002 (Figure 7). Similar results were observed with wortmannin (data not shown). These findings suggest that targeting the PI3K/AKT pathway may render DLBCLs expressing high levels of PDE4B sensitive to the physiologic cAMP-inhibitory effects.
PI3K inhibition decreases cellular proliferation and overcomes the protective effects of PDE4B2 expression. DHL6-PDE4B2-WT or DHL6-PDE4B2-PI cells were incubated with 10 μM of the PI3K inhibitor LY249002 and/or 10 μM forskolin for 24 hours, and cell proliferation was determined by MTS assay. Data represent the means and SDs of 3 independent assays.
PI3K inhibition decreases cellular proliferation and overcomes the protective effects of PDE4B2 expression. DHL6-PDE4B2-WT or DHL6-PDE4B2-PI cells were incubated with 10 μM of the PI3K inhibitor LY249002 and/or 10 μM forskolin for 24 hours, and cell proliferation was determined by MTS assay. Data represent the means and SDs of 3 independent assays.
Discussion
In this report, we confirmed the risk-related expression of PDE4B in an independent series of primary DLBCLs and defined the enzyme's role in modulating cAMP-induced apoptosis in a panel of DLBCL cell lines. In PDE4B-low DLBCL cells, reconstitution of wild-type PDE4B limited cAMP signaling. In PDE4B-high DLBCL cell lines, chemical inhibition of the phosphodiesterase markedly enhanced cAMP signaling and associated growth inhibition. In DLBCL cell lines, cAMP-mediated apoptosis was associated with inhibition of the PI3K/AKT pathway and was largely independent of the activation of the cAMP effectors, PKA and EPAC.
We identified PDE4B2 as the predominant PDE4B isoform in normal B cells and DLBCLs, and found it to be developmentally regulated in mature B-cell subsets. PDE4B2 was expressed at lower levels in normal germinal center (GC) B cells (centroblasts and centrocytes), prompting speculation that DLBCLs with GC features2,40 might also express less PDE4B. These data suggest that certain normal and malignant B cells are less susceptible to cAMP-induced apoptosis because PDE4B limits cAMP effects. Consistent with this hypothesis, we found that high intracellular levels of cAMP induced DLBCL apoptosis and that these effects were largely inhibited by reconstitution of PDE4B2 expression. Accordingly, targeted PDE4B inhibition increased forskolin-induced apoptosis in a variety of DLBCL cell lines with high PDE4B levels. Our results together with those describing similar proapoptotic effects in 2 other B-cell malignancies, CLL17 and ALL,18 underscore the therapeutic potential of PDE4B inhibition in multiple B-cell malignancies.
All of the analyzed DLBCL cell lines had low/undetectable basal levels of cAMP. Therefore, we used forskolin or synthetic cAMP analogs to increase intracellular cAMP and study its inhibitory effects on DLBCLs in vitro. However, a variety of physiologic agonists increase cAMP levels in lymphocytes—prostaglandins,41 cytokines,42 as well as engagement of the immune cell receptors32,43 —highlighting the importance of the cAMP signals in vivo. The identification of the relevant agonists in normal and malignant B lymphocytes should help clarify the cAMP-dependent and -independent mechanisms associated with PDE4B overexpression in DLBCL.
The major known effectors of cAMP signals in mammalian cells are PKA and EPAC.7,8 However, our data indicate that in the analyzed DLBCL cell lines, EPAC and PKA are not the primary effectors of cAMP-associated apoptosis. Recently, similar findings were obtained in CLL where EPAC was expressed at high levels but did not mediate cAMP-associated apoptosis.44 In our model system, PKA inhibition did not limit cAMP-mediated cellular apoptosis and the associated effects on pAKT or pBAD. Although it is possible that residual PKA activity, not inhibited by H89 or PKI, might have contributed to the cAMP-mediated effects described here, the fact that all analyzed parameters were comparable in the presence or absence of H89 suggests otherwise. In other B-lymphoid malignancies where PDE4B inhibition plays a role in apoptosis, the role of PKA has not been directly investigated.17,18,44 Taken together, these findings indicate that in DLBCL as well as additional specialized tissues,45,46 the major cAMP effector proteins remain to be identified.
Although the proximal events associated with cAMP-mediated apoptosis in DLBCLs are still under study, we have now identified the critical downstream pathway, PI3K/AKT, involved in transducing cAMP inhibitory signals. AKT regulates multiple cellular processes such as glucose metabolism, transcription, apoptosis, cell proliferation, and angiogenesis39 ; the net result of AKT activation is cell survival. In our studies, cAMP signaling decreased AKT kinase activity and the phosphorylation of specific AKT substrates, including FKHR, GSK3β, and BAD, leading to apoptosis. Our results are of particular interest given the recent description of DLBCL in BAD-deficient mice.47 Taken together, these observations suggest that mature B cells with high PDE4B, limited cAMP signaling, and decreased BAD activity could have a functional profile similar to that of BAD-deficient B lymphocytes.
AKT activity is regulated by multiple signals and upstream molecules including production of the phospholipid PIP3 by PI3K. PI3K signaling, in turn, is limited by the actions of several phosphatases, including PTEN, a 3-position lipid phosphatase that converts PIP3 back to PIP2.39 We found that cAMP markedly decreases PI3K activity in DLBCL, resulting in restricted PIP3 generation and decreased AKT phosphorylation/activity. Although it is not yet clear how cAMP modifies PI3K activity, recent reports suggest that cAMP may impair the activity of upstream tyrosine kinases and, directly or indirectly, decrease RAS functions.32,43 Importantly, the cAMP modulation of PI3K activity has been recently described in other cell systems,48-50 suggesting a general role for this second messenger in controlling phospholipid signals.
These findings are particularly relevant because the PI3K/AKT pathway is abnormally active in multiple tumor types, including lymphoid malignancies,51,52 most frequently by inactivating mutations of PTEN but also by amplification and overexpression of PI3K and AKT.39 Our findings point to another novel regulatory mechanism for this critical pathway and suggest that in in vivo situations where PDE4B activity is elevated, the PI3K/AKT pathway might be inappropriately active.
Taken together, our findings suggest that PDE4B and the associated cAMP signaling pathway represent potential rational therapeutic targets in DLBCL. In this regard, the cross-talk between cAMP/PDE4B and PI3K/AKT is particularly important, given the recent progresses in targeting the PI3K/AKT pathway in human tumors.53 Therefore, the combination of PDE4B and PI3K/AKT inhibitors might act synergistically to decrease the resistance to apoptosis in DLBCL and additional B-lymphoid malignancies with increased PDE4B expression.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-01-0240.
Supported by a Translational Research Award (6179-02) from the Leukemia and Lymphoma Society of America (R.C.T.A.) and a Doris Duke Distinguished Clinical Scientist (DDCS) award (M.A.S.). R.C.T.A. is a V Foundation Scholar.
One of the authors (G.B.) is employed by a company (Plexxikon Inc, Berkeley, CA) whose potential product was studied in the present work.
The online version of the article contains supplemental data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Aster, V. Boussiotis, W. Sellers, M. Houslay, E. Fox, M. Hansen, K. Podar, J. Alberta, P. Dahia, J. Daley, and S. Lazo-Kallanian for reagents and technical support.

![Figure 2. PDE4B expression and activity limit the growth-inhibitory effects of cAMP. (A) Growth inhibition following forskolin treatment. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 24 hours. (B) Growth inhibition following treatment with PDE4 inhibitors. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 24 hours in the presence or absence of rolipram (10 μM), PLX513 (10 μM [DHL6] or 20 μM [DHL7, OCI-Ly3, and OCI-Ly10]), or with these PDE4 inhibitors alone. A 20-μM dose of PLX513 was cytotoxic for DHL6 cells regardless of forskolin treatment. Data in panels A-B represent the ratio of proliferation of treated cells to cells cultured with vehicle alone for the same time periods. Cell proliferation was measured by MTS assay. (C) cAMP induction following forskolin treatment. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 10 to 60 minutes and intracellular cAMP levels determined by ELISA. The cAMP induction in DHL7 and OCI-Ly10 cells was minimal (5 and 3 pmol/million cells, respectively), and it is not apparent in this display. (D) cAMP levels following inhibition of PDE4. DHL6, DHL7, OCI-Ly3, and OCI-Ly10 cells were incubated with 40 μM forskolin for 10 to 60 minutes following preincubation with PLX513 for 30 minutes. Results are displayed as the percentage of increase in cAMP levels in cells treated with forskolin alone versus those treated with forskolin and PLX513. All proliferation and cAMP data are the mean and SD of 3 independent experiments. All individual experiments were performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-01-0240/6/m_zh80010571880002.jpeg?Expires=1765895121&Signature=TGjXlh4YhIzJExF9KfuJTj6RJKqk6vLR5ty58tTT5O3Xtj9BnuPUSDGjQ2tN3GKErb43p9LINeqqD-E9-if3ygHEl7OU27Bq9YVUOqI-2uBfy4b1tn6gsLWrxmbciwB7hlsPTu3~lN1RVVueusjzTpZMll75WLct3sF5qddLf4~rUxQ1wq7AO5Eh2XeiP5QVFcMY2GarW43KRfFNTlcdCK257y0Tixq~f6eLqSF0WoXel7iheObIbPg8wIbkz5DBP~jrram9-h-HlM6XOezITj7qS2-kh1ONbIAbdZrSY1oILh0z5Y~Ca6oKx-76~L7KwduQ2aXZg5MTfThV0p3Wvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. cAMP signaling inhibits PI3K activity. DHL6 parental cells were treated with 40 μM forskolin and 10 μM rolipram for 5 minutes. Cell lysates were harvested and immunoprecipitated with anti-phosphotyrosine antibody (4G10). Precipitated proteins were incubated with phosphatidylinositol (PI), phosphatidyl-4,5-biphosphate (PIP2), and [γ-32P]ATP, followed by extraction of lipids, separation by thin layer chromatography, and exposure to an x-ray film (left panel, representative assay). PI3K activity toward its major lipid substrates (PIP2) was determined by densitometrically measuring the amounts of the phosphatidylinositol 3,4,5-trisphosphate (PIP3) produced in 3 independent reactions (right panel). Fold of inhibition (right panel) was determined by comparing the PIP3 intensity in forskolin/rolipram-treated and untreated cells and reported as the mean ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-01-0240/6/m_zh80010571880006.jpeg?Expires=1765895121&Signature=bmy9WvvXNGjuKJG94ruJLaDP2du6S5mCh1-7eWeZ8Zyz27J9-lf1HjoKGmhNQ7jhZyZ3uqtTd~08gShEOeI3C58j0GtXK9VBLt69K0lukCsX5TO5M7DFU2eBcXJciVgyg7p1yg8HUnXQqXfvStsLwPDZ9LmH1cP9XtB1NGlAIe57Q5F37zC5vzhTmyC1xqefO9EgOlmetG95ft9kA2afhDX8fVMWKF4XsD-TMdor~KCqZzB6rnDKBGPLnH5V1UPIiiUwTm17tmWw8AaCEAo3R~UZLbSbWLrq3ZO-U46SAWr2abPIHMItaNRdmNKR3f4cUyOgNhEmEkAryCirHDUsiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal