Abstract
CD40-ligand (CD154) is expressed on activated CD4+ T lymphocytes and is essential for the T cell–dependent activation of B lymphocytes. CD154 is also expressed at the activated platelet surface. In this study, we show that platelet-associated CD154 is increased in immune thrombocytopenic purpura (ITP), a disease characterized by an autoimmune response against proteins of the platelet membrane. CD154 and its messenger RNA were also present in increased amounts in the megakaryocytes of patients with ITP. We found that platelet-associated CD154 is competent to induce the CD40-dependent proliferation of B lymphocytes, and we observed an in vitro CD154-dependent production of antibodies to the GPIIb/IIIa complex (integrin αIIbβ3) when platelets and peripheral blood B lymphocytes from ITP patients with circulating anti-GPIIb/IIIa antibody were cultured together. Therefore, platelet-associated CD154 expression is increased in ITP and is able to drive the activation of autoreactive B lymphocytes in this disease.
Introduction
Immune (idiopathic) thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by reduced platelet counts and normal or increased numbers of megakaryocytes in the bone marrow. Circulating antiplatelet autoantibodies are frequently detected. The most common target of antiplatelet antibodies is the platelet glycoprotein (GP) GPIIb/IIIa complex (integrin αIIbβ3).1 It is believed that T-cell activation is a critical event in ITP.1,2 T-cell help for B-cell activation relies on the interaction between CD154 and CD40. Interaction of CD154 on activated T cells, with its receptor CD40 on B cells, is essential for B-cell proliferation, differentiation, isotype switch, memory B-cell generation, and germinal center formation.3-6 CD154 is expressed intracellularly in platelets. Normally, surface levels are low, but these increase after activation.7,8 Platelet CD154 has been studied mostly in its relation with the biology of the vascular endothelium and inflammation.9 Expression of CD154 by platelets could be critical in ITP because it would mean the coexistence at the platelet surface of potential target antigens, such as GPIIb/IIIa, and an essential costimulus of B-cell activation. Therefore, we studied the expression and immunologic function of platelet-associated CD154 in ITP patients.
Patients, materials, and methods
Patients
A cohort of 22 patients was included using criteria generally accepted for the clinical diagnosis of ITP. All patients had thrombocytopenia (platelet count less than 50 × 109/L), normal or increased numbers of bone marrow megakaryocytes without morphologic evidence for dysplasia, and no secondary immune- or nonimmune-associated disease that could account for the thrombocytopenia. A second group of 11 patients with low platelet counts (less than 80 × 109/L) of nonimmune origin included 3 with myelodysplasia, 3 with multiple myeloma, 2 with myelofibrosis and myeloid metaplasia, 1 with chronic lymphocytic leukemia, and 1 with hypersplenism related to cirrhosis. For in vitro anti-GPIIb/IIIa antibody production, a third group of 6 patients with autoimmune diseases but without thrombocytopenia was also studied; it included 3 patients with systemic lupus erythematosus, 1 patient with multiple sclerosis, 1 patient with systemic scleroderma, and 1 patient with rheumatoid arthritis. A final group of 10 healthy subjects consisted of medical staff volunteers from our department. Samples were obtained according to recommendations from the Comité de Protection des Personnes dans la Recherche Biomédicale de Bordeaux. Patients gave informed consent to participate in the study.
Platelets
Platelets were isolated as described, and samples were activated with 0.5 U/mL thrombin (Ortho Diagnostic, Raritan, NJ) for 5 minutes at 37°C.10 The suspension of activated platelets was then used for B-lymphocyte proliferation and in vitro assays of anti-GPIIb/IIIa production. For dosing CD154, platelets were lysed in 1% (vol/vol) Triton X-100 (Sigma, Saint-Quentin Fallaviers, France) containing leupeptin (Sigma) (20 μg/mL) for 30 minutes at 4°C.
Megakaryocytes
Bone marrow was obtained from 6 ITP patients undergoing marrow aspiration for diagnostic purposes. Control samples were from patients undergoing orthopedic surgery, or they were purchased (BioWhittaker, Verviers, Belgium). Megakaryocytes were enriched from bone marrow with CD61-coated microbeads, as described by the manufacturer (Miltenyi Biotec, Paris, France).
B lymphocytes
Tonsillar B lymphocytes. B lymphocytes were isolated from tonsils, as described.11
Peripheral blood B lymphocytes. Peripheral blood was drawn into EDTA (ethylenediaminetetraacetic acid)–anticoagulant. CD19 B cells were sorted from buffy coats on an Epics Elite (Beckman-Coulter, Paris, France) cell sorter. When necessary, cells were sorted a second time to achieve greater than 95% purity.
B-lymphocyte proliferation
Fifty thousand tonsillar B lymphocytes were cultured for 4 days with 5 × 106 activated platelets in a total volume of 200 μL in 96-well, flat-bottomed microtiter plates in the presence of interleukin-2 (IL-2; Chiron, Paris, France) (100 IU/mL) and IL-10 (gift from Schering-Plough, Dardilly, France) (50 ng/mL). Controls included B cells alone, B cells grown in the presence of thrombin, B cells grown in the presence of 5 × 103 mouse fibroblastic L cells, or 5 × 103 L cells that had been stably transfected with recombinant CD154 (gift from Schering-Plough). Cultures were grown either in or not in the presence of 200 ng/mL blocking anti-CD40 monoclonal antibody (mAb89) (gift from Schering-Plough12 ). Cell proliferation was quantitated by [3H]thymidine incorporation.
In vitro anti-GPIIb/IIIa production
Five thousand B cells from ITP patients or controls were grown in the presence of 5 × 106 activated autologous platelets and IL-2 (10 IU/mL) plus IL-10 (50 ng/mL). Cultures were grown either in or not in the presence of monoclonal antibody (mAb) 89, as described in “B-lymphocyte proliferation.” Controls included B cells from ITP patients plus activated platelets from healthy donors, B cells from healthy donors plus platelets from ITP patients or healthy donors, B cells from ITP patients alone, or B cells from healthy donors alone. On day 10, the presence of anti-GPIIb/IIIa antibodies was measured by monoclonal antibody immobilization of platelet antigen II (MAIPA II) assay.13
CD154 ELISA
CD154 measurements were performed on platelet lysates using enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, United Kingdom).
Flow cytometry
Platelets were activated or not activated with thrombin as described in “B-lymphocyte proliferation,” fixed with 1% paraformaldehyde (PFA), and analyzed by flow cytometry, as described previously.14
Immunofluorescence
Megakaryocytes were layered onto glass slides by slow spin (10g, 15 minutes). Cells were fixed with 3.7% PFA for 30 minutes and were permeabilized for 5 minutes in phosphate-buffered saline (PBS)/0.1% Triton X-100 buffer. After saturation with PBS/bovine serum albumin (BSA) (1%), samples were incubated for 1 hour with murine mAb anti-CD154 (5 μg/mL; MK13A4; Alexis Biochemical, Paris, France), and goat polyclonal anti-CD41 (6 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature. Samples were washed and stained with fluorescein isothiocyanate (FITC)–conjugated antimouse immunoglobulin G (IgG) and Cy5-conjugated antigoat IgG for 1 hour. Slides were washed and dried for 15 minutes and mounted. Control samples were treated as described here, but the primary antibodies were omitted. Stained cells were observed under a PCM 2000 confocal microscope equipped with a 60 ×/1.40 oil WD0.21 objective lens (Nikon, Champigny, France). Imaging medium solution was Slowfade (Molecular Probes, Eugene, OR). Images were acquired using EZ2000 software (Nikon).
In situ hybridization
Three oligonucleotide probes complementary to CD154 mRNA were used (nucleotides 61-110, 781-830, and 841-890; human CD40-ligand sequence, EMBL accession number L-07414.1). Labeling of oligoprobes was performed using Ulysis labeling kit (Kreatech, Amsterdam, The Netherlands). In situ hybridization was carried out on cytospins, as previously described.15
Results and discussion
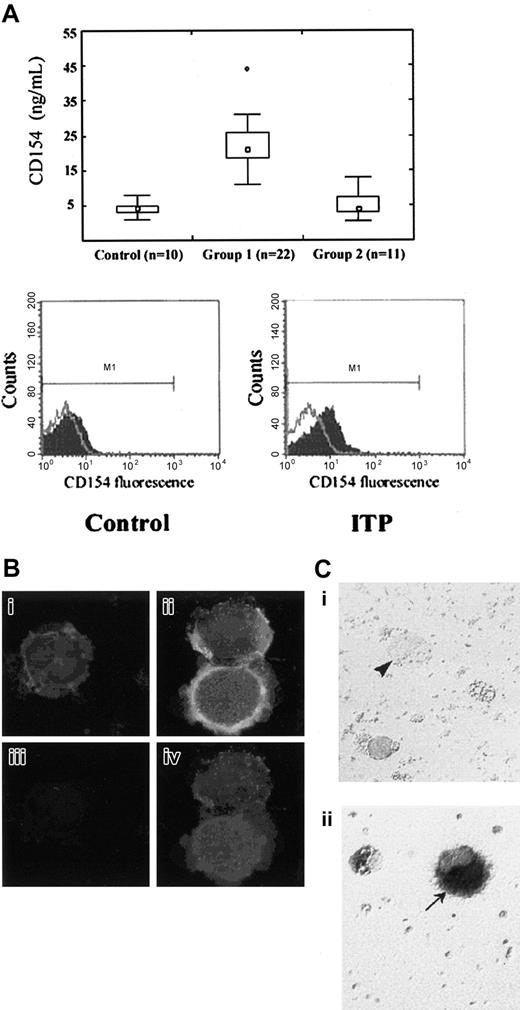
We first examined the expression of platelet-associated CD154 in a cohort of 22 ITP patients. ELISA measurements showed significantly increased amounts of total platelet CD154 compared with healthy donors or patients with nonimmune thrombocytopenia (Figure 1A). Flow cytometry on activated and fixed platelets also showed increased surface expression of platelet-associated CD154 in ITP patients (Figure 1A). Levels were also increased on unactivated platelets, but the difference was less apparent, perhaps because of proteolysis and the release of soluble CD154 from the surface (for a review, see Andre et al9 ). Immunofluorescence studies on permeabilized cells showed that CD154 could be detected within megakaryocytes from ITP patients but was only weakly seen in cells from control donors (Figure 1B). Here, megakaryocytes were identified by double labeling with anti–GPIIb-IIIa. In situ hybridization experiments also showed the expression of CD154 mRNA in ITP megakaryocytes (Figure 1C). Lack of detection of the CD154 signal in normal megakaryocytes is likely to result from a low level of expression that is in agreement with the low level of CD154 in normal platelets. Therefore, the megakaryocyte is the source for the platelet-associated CD154 in ITP platelets; our results suggested an augmented synthesis. We next investigated the immunologic function of platelet-associated CD154. Naive and memory B lymphocytes have previously been shown to proliferate when cultured on cells transfected with CD154.16,17 As expected, tonsillar B lymphocytes proliferated in the presence of CD154 transfectants, and they also proliferated in the presence of activated platelets from 4 selected ITP patients and did so as efficiently as with CD154 transfectants (Figure 2A). In all cases, B-lymphocyte proliferation was impaired by antibody-induced neutralization of CD40 (Figure 2A). Therefore, platelet-associated CD154 was competent to induce the proliferation of B lymphocytes, which is a previously unrecognized property of platelet-associated CD154. In contrast, activated platelets from healthy donors were less competent in inducing tonsillar B-lymphocyte proliferation.
Platelet-associated CD154 in ITP. (A) (top panel) CD154 was quantitated by ELISA in whole platelet lysates from healthy donors (control), ITP patients (group 1), and patients with nonimmune thrombocytopenia (group 2). Median value is indicated by □. Boxes indicate 25th and 75th percentiles; error bars, 10th and 90th percentiles. ○ indicates values outside these ranges. Statistical comparison (Mann-Whitney U test) shows a significant difference between group 1 and control (P < .001) or group 2 (P < .001) platelet CD154 levels. (Bottom panel) Surface expression of CD154 on activated platelets from an ITP patient compared with a healthy donor (control). Flow cytometry profile representative of 4 experiments. (B) CD154 detection by immunofluorescence in ITP megakaryocytes. Red fluorescence corresponds to GPIIb/IIIa. Green fluorescence corresponds to CD154. Yellow fluorescence corresponds to the superposition of red and green fluorescences. (i,iii) Control megakaryocytes. (ii, iv) ITP megakaryocytes. (i, ii) Double GPIIb/IIIa plus CD154 staining. (iii, iv) CD154 staining only. (C) Detection of CD154 mRNA by in situ hybridization in ITP megakaryocytes. Highlighted (arrows) are (i) a control megakaryocyte (arrowhead) and (ii) an ITP megakaryocyte (arrow).
Platelet-associated CD154 in ITP. (A) (top panel) CD154 was quantitated by ELISA in whole platelet lysates from healthy donors (control), ITP patients (group 1), and patients with nonimmune thrombocytopenia (group 2). Median value is indicated by □. Boxes indicate 25th and 75th percentiles; error bars, 10th and 90th percentiles. ○ indicates values outside these ranges. Statistical comparison (Mann-Whitney U test) shows a significant difference between group 1 and control (P < .001) or group 2 (P < .001) platelet CD154 levels. (Bottom panel) Surface expression of CD154 on activated platelets from an ITP patient compared with a healthy donor (control). Flow cytometry profile representative of 4 experiments. (B) CD154 detection by immunofluorescence in ITP megakaryocytes. Red fluorescence corresponds to GPIIb/IIIa. Green fluorescence corresponds to CD154. Yellow fluorescence corresponds to the superposition of red and green fluorescences. (i,iii) Control megakaryocytes. (ii, iv) ITP megakaryocytes. (i, ii) Double GPIIb/IIIa plus CD154 staining. (iii, iv) CD154 staining only. (C) Detection of CD154 mRNA by in situ hybridization in ITP megakaryocytes. Highlighted (arrows) are (i) a control megakaryocyte (arrowhead) and (ii) an ITP megakaryocyte (arrow).
Activation of B lymphocytes by platelet-associated CD154. (A) Platelet-associated CD154 is competent to induce the proliferation of B lymphocytes. Activated platelets from 4 selected healthy donors or ITP patients were cocultured for 4 days with tonsillar B lymphocytes. In parallel wells, CD40 was blocked (anti-CD40) or not blocked. Other controls included incubating tonsillar B lymphocytes with L-cell CD154 transfectants (CD154 tr). The proliferation ratio was obtained by dividing the thymidine incorporation obtained from tested sample (B lymphocytes plus activated platelets) by the thymidine incorporation obtained from thrombin-treated B lymphocytes alone. (B) Platelets from ITP patients drive the production of anti-GPIIb/IIIa antibody (Ab) in a CD154-dependent manner. Activated platelets from healthy donors (control), ITP patients (group 1), and patients with non-ITP autoimmune diseases (group 2) were cultured in the presence of autologous B lymphocytes. The production of anti-GPIIb/IIIa antibodies was measured using a MAIPA assay. Ratio (R) values were obtained according to the formula, R = mean test OD/mean control OD of supernatants. Threshold of positivity was calculated from 4 controls (control B cells + control platelets) as the mean ± 3 SD. For each patient, all control wells included were below the threshold of positivity. Statistical significance (P < .05; Student t test) was observed on comparing group 1 (no antibody) with group 1 (+ anti-CD40 antibody), group 1 (no antibody) and controls (no antibody), and group 1 (no antibody) and group 2 (no antibody).
Activation of B lymphocytes by platelet-associated CD154. (A) Platelet-associated CD154 is competent to induce the proliferation of B lymphocytes. Activated platelets from 4 selected healthy donors or ITP patients were cocultured for 4 days with tonsillar B lymphocytes. In parallel wells, CD40 was blocked (anti-CD40) or not blocked. Other controls included incubating tonsillar B lymphocytes with L-cell CD154 transfectants (CD154 tr). The proliferation ratio was obtained by dividing the thymidine incorporation obtained from tested sample (B lymphocytes plus activated platelets) by the thymidine incorporation obtained from thrombin-treated B lymphocytes alone. (B) Platelets from ITP patients drive the production of anti-GPIIb/IIIa antibody (Ab) in a CD154-dependent manner. Activated platelets from healthy donors (control), ITP patients (group 1), and patients with non-ITP autoimmune diseases (group 2) were cultured in the presence of autologous B lymphocytes. The production of anti-GPIIb/IIIa antibodies was measured using a MAIPA assay. Ratio (R) values were obtained according to the formula, R = mean test OD/mean control OD of supernatants. Threshold of positivity was calculated from 4 controls (control B cells + control platelets) as the mean ± 3 SD. For each patient, all control wells included were below the threshold of positivity. Statistical significance (P < .05; Student t test) was observed on comparing group 1 (no antibody) with group 1 (+ anti-CD40 antibody), group 1 (no antibody) and controls (no antibody), and group 1 (no antibody) and group 2 (no antibody).
We then studied whether platelet-associated CD154 could drive the activation of autoreactive B lymphocytes in ITP. Autoreactive B lymphocytes that produce antiplatelet antibodies are present in the peripheral blood and spleens of patients with ITP,2 and CD154 is implicated in antiplatelet antibody production in ITP.18 We studied 6 patients for whom circulating anti-GPIIb/IIIa antibody had been detected by MAIPA. We observed an in vitro production of anti-GPIIb/IIIa antibody when the peripheral blood B lymphocytes of these ITP patients were cultured in the presence of autologous activated platelets but not in the presence of platelets from healthy donors (not shown). This production was blocked by the neutralization of CD40. Production of anti-GPIIb/IIIa antibody was not observed using peripheral blood B lymphocytes from either control group (Figure 2B). Therefore, platelet-associated CD154 could be instrumental in driving the activation of autoreactive B lymphocytes in ITP. Platelet involvement in the production of pathogenic antibodies in ITP raises the question of where the interactions between activated platelets and B lymphocytes may take place in vivo. The spleen is considered to be a primary site for antiplatelet antibody production in ITP2,19,20 and could be where the necessary contact interactions between platelets and B lymphocytes take place. Interestingly, GPIIb/IIIa can be detected within the white pulp follicles in ITP.21 Because soluble CD154 levels are known to be increased in acute coronary syndromes,22,23 it would also be interesting to know whether CD154 plays a role in such diseases as heparin-induced thrombocytopenias.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2003-07-2367.
Supported by a Protocole Hospitalier de Recherche Clinique grant from the Centre Hospitalier Universitaire de Bordeaux.
A.S., J.M.P., and J.F.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal