Abstract
Embryonic stem (ES) cells can differentiate into many different somatic cells in culture. To better correlate hematopoietic and endothelial cell differentiation of ES cells in currently available protocols, we compared fetal liver kinase-1 (Flk-1)–, stem cell leukemia (Scl)–, and vascular endothelial–cadherin (VE-cadherin)–expressing cells generated in embryoid bodies (EBs) and on OP9 cells. We report that the kinetics of Scl and Flk-1 expression were similar in EBs and OP9 cells, although Flk-1 expression was extended on OP9 cells. CD45+ and Ter-119+ cells developed more efficiently in EBs, whereas VE-cadherin+ cells developed largely on OP9 cells. Cell sorting and replating studies showed that Scl+ cells, not Flk-1+ or VE-cadherin+ cells, were enriched for primitive and definitive hematopoietic progenitors. Our studies indicate that optimal hematopoietic and endothelial cell differentiation occur in EBs and on OP9 cells, respectively. Regardless of the culture systems used, Scl is the most relevant marker for enriching primitive and definitive hematopoietic progenitors.
Introduction
There is great interest in generating different types of somatic cells from in vitro–differentiated embryonic stem (ES) cells, as they can potentially be used for therapies for human diseases for which there are currently no effective treatments. Accordingly, many studies are aimed toward understanding mechanisms for maintaining the stem cell state and pathways leading to lineage specification. Successful generation and application of ES-derived somatic cells will require reproducible protocols to obtain desired cell types. There are currently 2 widely used protocols to generate blood and blood vessel cells in culture. In the first protocol, ES cells differentiate and form 3-dimensional cell masses called embryoid bodies (EBs).1-5 In the second protocol, ES cells differentiate on type IV collagen or stromal cells, such as OP9, in 2-dimensional sheets.6-8
In both the EB and OP9 culture systems, primitive erythroid cells develop prior to the definitive erythroid cell population.2,6 In addition, the putative common progenitor of hematopoietic and endothelial cells, the hemangioblast, has been reported to develop within EBs and on OP9 cells.7,9 Endothelial cells also develop in both culture systems.3-5,8 While these studies would argue that these 2 different methods could be used interchangeably, there has not yet been a systematic comparison of whether hematopoietic and endothelial cell development proceeds in parallel in these 2 culture systems. To better correlate hematopoietic and endothelial cell differentiation in EBs and the OP9 system, we compared fetal liver kinase-1 (Flk-1), stem cell leukemia (Scl), CD45, Ter-119, and vascular endothelial–cadherin (VE-cadherin) expression and performed cell sorting and hematopoietic replating studies. We demonstrate that hematopoietic cells develop more efficiently in EBs, whereas endothelial cell maturation is better supported by OP9 cells. We further demonstrate that Scl, not VE-cadherin, is ideal for isolating hematopoietic progenitors.
Study design
Scl+/hCD4 ES culture and hematopoietic colony assays were performed as described previously.10,11 EBs were formed in 20% fetal calf serum (FCS; preselected lot) in α–modified Eagle minimum essential medium (α-MEM) with ascorbic acid (50 μg/mL), l-glutamine (2 mM), and monothioglycerol (MTG; 4.5 × 10-4 M). OP9 differentiation was carried out in 20% FCS (preselected lot) in α-MEM.6 OP9 cells were treated with mitomycin C prior to ES seeding and differentiation, as the mitomycin C treatment consistently gave a higher number of differentiated cells (Supplemental Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Cell staining and fluorescence-activated cell sorter (FACS) analyses were carried out as described previously,10,11 except that differentiated cells were dissociated with collagenase (0.25%; Sigma, St Louis, MO) and anti-CD45 antibody used in this study was biotin labeled (Pharmingen, San Diego, CA).
Results and discussion
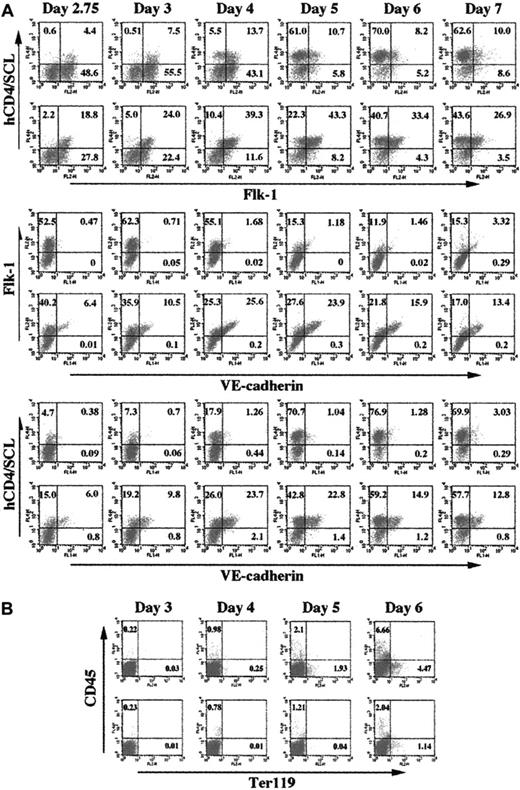
To compare hematopoietic and endothelial cell differentiation in EBs and the OP9 system, we used Scl+/hCD4 ES cells.10 As a first step, we compared the kinetics of Flk-1, Scl, and VE-cadherin expression. As previously reported10 and shown in Figure 1A, Flk-1 was readily detectable by day 2.75 in EBs. Flk-1+ cells continued to develop up to day 4 and declined thereafter. Cells expressing Flk-1 were also readily detectable on OP9 cells. A similar percentage of Flk-1+ cells was detected in EBs and OP9 cells until day 4. However, Flk-1 expression was prolonged when ES cells were differentiated on OP9 cells, such that Flk-1+ cells were still present at higher levels at later times: 13.4% (19.3% ± 5.1%) in day-6 EBs versus 37.7% (40.5% ± 3.4%) in OP9 cells (Student t test P value < .01). Scl-expressing (ie, human CD4 [hCD4]–expressing) cells developed slowly in EBs but expanded rapidly between days 4 and 5. About 60% to 70% of the total cells expressed hCD4 at days 5 to 7. On OP9, a higher percentage of cells expressed hCD4 at early time points: 8% (7.7% ± 1.8%) in day-3 EBs versus 29% (28.6% ± 6.6%) on OP9 cells (P < .05). However, the percentage of hCD4+ cells detected at later times (days 5-7) was similar in both EBs and OP9 cells. While most of hCD4+ cells present in days 5 to 7 EBs did not express Flk-1, a higher percentage of hCD4+ cells also expressed Flk-1 when ES cells were differentiated on OP9 cells. As hCD4–single-positive cells were more abundant in EB cells, we examined CD45 and Ter-119 expression patterns. As shown in Figure 1B, a higher percentage of CD45 (∼7% [7.1% ± 1.1%] in day-6 EBs vs ∼2.7% [3.5% ± 1.5%] in OP9 cells; P < .01) and Ter-119 (∼4.5% [3.9% ± 0.5%] in day-6 EBs vs ∼1.1% [1.9% ± 0.8%] in OP9-expressing cells; P < .05) was readily detectable in EBs. This suggests that hematopoietic differentiation was achieved more efficiently within EBs.
Kinetic analyses of Flk-1, Scl, VE-cadherin, CD45, and Ter-119 expression. In vitro–differentiated Scl+/hCD4 ES cells during EB and OP9 differentiation (from day 2.75 to day 7) were subjected to FACS analyses for Flk-1, hCD4, VE-cadherin (A) and CD45 and Ter119 (B) expression. Numbers in a given box indicate the percentage of each population. In each 2-row group of scatterplots, the top row shows EB differentiation while the bottom row shows OP9 differentiation.
Kinetic analyses of Flk-1, Scl, VE-cadherin, CD45, and Ter-119 expression. In vitro–differentiated Scl+/hCD4 ES cells during EB and OP9 differentiation (from day 2.75 to day 7) were subjected to FACS analyses for Flk-1, hCD4, VE-cadherin (A) and CD45 and Ter119 (B) expression. Numbers in a given box indicate the percentage of each population. In each 2-row group of scatterplots, the top row shows EB differentiation while the bottom row shows OP9 differentiation.
VE-cadherin–expressing cells developed very poorly in EBs. On OP9 cells, however, VE-cadherin expression was readily detectable. VE-cadherin was first detected at day 2.25 (data not shown) and about 10% (14.4% ± 3.6%) of the cells at day 3 (Figure 1A) expressed VE-cadherin. VE-cadherin expression increased up to day 5 and then gradually decreased. By day 6, about 16% (16.7% ± 2%) of the cells expressed VE-cadherin. More important, VE-cadherin+ cells at all stages (days 2.75-7) were always within the Flk-1+ hCD4+ cell population (Figures 1 and 2A). Our data suggest that endothelial progenitors will develop in EBs and OP9 cells, but that endothelial cell maturation, based on VE-cadherin expression, occurs more efficiently in the OP9 system. Thus, the Flk-1–expressing cells present at later times on OP9 cells would represent mature endothelial cells. Consistent with this interpretation, Flk-1+ cells, sorted from OP9 culture 6 days after differentiation, showed poor endothelial cell replating (data not shown).As our previous studies demonstrated that Flk-1+ cells sorted from day-6 EBs exhibited a robust endothelial cell differentiation,10 we propose that endothelial progenitors developing on OP9 cells concomitantly differentiate into VE-cadherin+ endothelial cells, whereas endothelial progenitors developing in EBs cannot. OP9-derived factor(s) could play a role in this process. Alternatively, a simple 2-dimensional culture condition could be sufficient.
Hematopoietic cells develop from Scl-expressing cells. (A) VE-cadherin–expressing cells also express Flk-1 and Scl. Scl+/hCD4 ES cells differentiated on OP9 for 6 days were subjected to FACS analyses for Flk-1, hCD4, and VE-cadherin expression. Based on the FACS data, the relationship between Flk-1–, Scl-, and VE-cadherin–expressing cells is presented in a Venn diagram (B). (C) VE-cadherin is not a marker for definitive hematopoietic cells. ES cells were differentiated on OP9 for 6 days, sorted for VE-cadherin- and VE-cadherin+ cells, and replated. Subsequently, erythroid/macrophage mixed colonies were subjected to globin gene analyses as described.10 As shown, erythroid cells developing from both VE-cadherin- and VE-cadherin+ cell populations expressed the β-major globin gene. N indicates H2O, negative control; P, RNA from day 3 EBs, positive control. L32, ribosomal subunit protein is shown as a loading control.
Hematopoietic cells develop from Scl-expressing cells. (A) VE-cadherin–expressing cells also express Flk-1 and Scl. Scl+/hCD4 ES cells differentiated on OP9 for 6 days were subjected to FACS analyses for Flk-1, hCD4, and VE-cadherin expression. Based on the FACS data, the relationship between Flk-1–, Scl-, and VE-cadherin–expressing cells is presented in a Venn diagram (B). (C) VE-cadherin is not a marker for definitive hematopoietic cells. ES cells were differentiated on OP9 for 6 days, sorted for VE-cadherin- and VE-cadherin+ cells, and replated. Subsequently, erythroid/macrophage mixed colonies were subjected to globin gene analyses as described.10 As shown, erythroid cells developing from both VE-cadherin- and VE-cadherin+ cell populations expressed the β-major globin gene. N indicates H2O, negative control; P, RNA from day 3 EBs, positive control. L32, ribosomal subunit protein is shown as a loading control.
To better define Scl and VE-cadherin expression in primitive erythroid and definitive hematopoietic progenitors,10,12 ES cells differentiated on OP9 cells were collected on day 4; sorted for hCD4+VE-cadherin+, hCD4+VE-cadherin-, or hCD4-VE-cadherin- cells; and replated for primitive erythroid colonies.As shown in Table 1, hCD4+VE-cadherin- cells, compared with hCD4+VE-cadherin+, generated a higher number of primitive erythroid colonies. To determine definitive hematopoietic potential, ES cells differentiated on OP9 cells for 6 days were initially sorted for VE-cadherin+ and VE-cadherin- cell populations and subjected to hematopoietic replating and globin gene analyses. As shown in Table 2 and Figure 2B, hematopoietic colonies developed from both cell populations. The β-major globin gene was also expressed in erythroid cells developed from VE-cadherin- cell populations (Figure 2C). Subsequently, hCD4+Flk-1+VE-cadherin+, hCD4+Flk-1+VE-cadherin-, hCD4+Flk-1-VE-cadherin-, hCD4-Flk-1+VE-cadherin-, and hCD4-Flk-1-VE-cadherin- cell populations, present in day-6 culture (Figures 1 and 2A), were sorted and replated. As shown in Table 3, definitive hematopoietic colonies developed from VE-cadherin-hCD4+Flk-1-, VE-cadherin-hCD4+Flk-1+, and VE-cadherin+hCD4+Flk-1+ cell populations. The common feature of these 3 cell populations was that they expressed hCD4. Thus, these studies suggest that Scl is the most relevant marker, not Flk-1 or VE-cadherin, for enriching primitive and definitive hematopoietic progenitors. Consistent with this interpretation, VE-cadherin knockout mice show relatively normal hematopoietic differentiation but defective endothelial cell development.13-15 Similarly, long-term repopulating potential of aorta-gonad-mesonephros (AGM)–derived cells was found in both VE-cadherin+ and VE-cadherin- cell populations.16 Finally, Scl expression prior to the generation of VE-cadherin–expressing cells could rescue hematopoietic defects in Scl-/- ES cells.17 Collectively, our studies indicate that VE-cadherin expression alone is not sufficient for isolating hematopoietic progenitors and that Scl is the most reliable marker for tracking hematopoietic differentiation.
Primitive erythroid colonies develop from Scl+ cells
. | Primitive erythroid colonies . |
|---|---|
| Nonfractionated | 425 ± 30 |
| hCD4-VE-cadherin- | 3 ± 2 |
| hCD4+VE-cadherin+ | 589 ± 61 |
| hCD4+VE-cadherin- | 1636 ± 48 |
. | Primitive erythroid colonies . |
|---|---|
| Nonfractionated | 425 ± 30 |
| hCD4-VE-cadherin- | 3 ± 2 |
| hCD4+VE-cadherin+ | 589 ± 61 |
| hCD4+VE-cadherin- | 1636 ± 48 |
Scl+/hCD4 ES cells were differentiated on OP9 for 4 days; sorted for hCD4-VE-cadherin-, hCD4+VE-cadherin+, or hCD4+VE-cadherin-; and replated for primitive erythroid colonies (6 × 104 cells/mL). The resulting primitive erythroid colonies were counted 4 days later. Data are expressed as mean values ± SD.
VE-cadherin is not a marker for definitive hematopoietic cells
. | E . | M . | E + M . |
|---|---|---|---|
| Nonfractionated | 1942 ± 82 | 206 ± 20 | 216 ± 40 |
| VE-cadherin- | 2010 ± 93 | 190 ± 59 | 280 ± 45 |
| VE-cadherin+ | 134 ± 20 | 468 ± 34 | 100 ± 6 |
. | E . | M . | E + M . |
|---|---|---|---|
| Nonfractionated | 1942 ± 82 | 206 ± 20 | 216 ± 40 |
| VE-cadherin- | 2010 ± 93 | 190 ± 59 | 280 ± 45 |
| VE-cadherin+ | 134 ± 20 | 468 ± 34 | 100 ± 6 |
Scl+/hCD4 ES cells were differentiated on OP9 for 6 days and FACS sorted for VE-cadherin+ and VE-cadherin- cells and subjected to hematopoietic replating (6 × 104 cells/mL). The resulting hematopoietic colonies were counted 6 days later. Data are expressed as mean values ± SD. Three independent sortings show similar results.
E indicates erythroid; M, macrophage; and E + M, erythroid/macrophage mixed colonies.
Definitive hematopoietic cells develop from Scl+ cells
. | M . | M + E . |
|---|---|---|
| VE-cadherin-hCD4-Flk- | 0 | 0 |
| VE-cadherin-hCD4-Flk- | 0 | 0 |
| VE-cadherin-hCD4+Flk- | 9 ± 2 | 13 ± 6 |
| VE-cadherin-hCD4+Flk+ | 15 ± 5 | 15 ± 2 |
| VE-cadherin+hCD4+Flk+ | 19 ± 2 | 23 ± 5 |
. | M . | M + E . |
|---|---|---|
| VE-cadherin-hCD4-Flk- | 0 | 0 |
| VE-cadherin-hCD4-Flk- | 0 | 0 |
| VE-cadherin-hCD4+Flk- | 9 ± 2 | 13 ± 6 |
| VE-cadherin-hCD4+Flk+ | 15 ± 5 | 15 ± 2 |
| VE-cadherin+hCD4+Flk+ | 19 ± 2 | 23 ± 5 |
Scl+/hCD4 ES cells were differentiated on OP9 for 6 days and FACS sorted for hCD4+Flk-1+VE-cadherin+, hCD4+Flk-1+VE- cadherin-, hCD4+Flk-1-VE-cadherin-, hCD4-Flk-1+VE-cadherin-, and hCD4-Flk-1-VE- cadherin- cells and subjected to hematopoietic replating (5 × 104 cells/mL). The resulting hematopoietic colonies were counted 6 days later. Data are expressed as mean values ± SD. Three independent sortings show similar results.
M indicates macrophage; and E + M, erythroid/macrophage mixed colonies.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2004-04-1306.
Supported by grants from the National Institutes of Health (National Heart, Lung, and Blood Institute [NHLBI]) R01 HL55337 and R01 HL63736 (K.C.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank the Choi lab members for critically reading the manuscript. We would also like to thank Toru Nakano for OP9 cells and Bill Eades for his excellent help with cell sorting.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal