Abstract
Several characteristics of the immunoglobulin (Ig) repertoire in fetuses and adults set them apart from each other. Functionally, this translates into differences in the affinity and effectiveness of the humoral immune response between adults and the very young. At least 2 possibilities could explain these differences: (1) fetal and adult lymphocyte progenitors differ significantly in their potential to form a diverse repertoire, and (2) factors extrinsic to the immunoglobulin locus are more influential to the character of the repertoire. To address this we used nonobese diabetic-severe combined immunodeficient-β2 microglobulin knockout (NOD/SCID/β2m-/-) mice reconstituted with human B-cell progenitors to compare the immunoglobulin repertoire potential of human fetal, cord blood, and adult sources. We found nearly identical VH and JH gene segment use and only modest differences in the third complementarity determining region of the immunoglobulin heavy chain (HCDR3). We conclude that the repertoire potential is remarkably similar regardless of the age of the individual from which progenitors are derived. Age-related differences in the immunoglobulin repertoire and variance of B-cell responses to immunization appear to arise from selection rather than from changes in recombination of the immunoglobulin locus itself. From the standpoint of the Ig repertoire, an immune system reconstituted from fetal or neonatal stem cells would likely be as diverse as one generated from adult bone marrow.
Introduction
Age-related changes in the human humoral immune system have long been known to occur and involve the nature of the antibodies that form the primary effectors of B-cell-mediated immunity. Both fetuses and older adults exhibit properties in their immunoglobulin (Ig) repertoire that differ from that seen in young adults.
Neonates are vulnerable to certain pathogens and respond poorly to immunization, even though they (and fetuses) display some degree of immunologic competence.1-3 This could reflect the immaturity of lymphocytes and antigen-presenting cells, or could be a result of persisting immunosuppressive aspects of the fetal environment.4-6 In general the fetal repertoire is more restricted than the adult repertoire.7-9 Several studies have shown that there are significant differences between the fetal, cord blood, and adult immunoglobulin repertoires in mice and humans.8,10-21 In humans, the VH4 family is represented throughout life but continues to increase as a function of age (particularly the VH4-34 and VH4-59 gene segments).16 Developmental age-related changes in the third complementarity determining region of the immunoglobulin heavy chain (HCDR3) are even more dramatic, as length, for example, increases with age. The DHQ52 gene segment is commonly used in fetal sequences but is rarely found in adults. HCDR3 length is markedly reduced in fetal compared with adult sequences.7,14,20,22 D and J gene segment preferences, as well as N nucleotide additions and exonuclease activity, account for this difference. These findings suggest that the antigen receptors on fetal B lymphocytes have a much more restricted range of specificities than those present in adults. Some experimental observations suggest that early waves of lympho-hematopoietic cells may be intrinsically different from their adult counterparts.23 However, the potential of lymphocytes may simply reflect different signals they receive or the differing environments they encounter.
As individuals approach older ages, other changes occur in the characteristics of their humoral immune system. The sera of elderly individuals have long been noted to harbor more autoantibodies.24 In addition, older individuals do not respond to immunization as efficiently as younger adults.25 These differences in the character of the repertoire have been postulated to result either from different potentials of hematopoietic stem cells themselves or from changes in other selective processes including tolerance mechanisms.
Hematopoietic cell transplantation represents an important therapy for many diseases, and umbilical cord blood is increasingly used as a source when sufficient autologous stem cells are unavailable.26 However, the time required for regeneration of humoral immunity is problematic, and there are conflicting reports concerning the degree to which repertoire development is influenced by the age of the hematopoietic stem cell source.22,27,28 Thus, it is important to know whether an immune system reconstituted from fetal/neonatal stem cells would be as effective as one generated from adult bone marrow.
Several types of immunodeficient mice have successfully received transplants of human cells (reviewed in Greiner et al29 ). Hematopoietic chimerism from transplanted stem cells has been particularly impressive when nonobese diabetic-severe combined immunodeficiency (NOD/SCID) or the more natural killer cell-deficient NOD/SCID/β2m-/- strains were used.30,31 Indeed, we recently found that all stages of B-cell progenitors were normally represented in the marrow of chimeric mice, and newly formed B cells expressed a diverse repertoire of antigen receptors.32 A rationale for the present study followed from the fact that the lymphocyte differentiation potential of hematopoietic stem cells from diverse human sources can be compared in a common environment using this model. Here we show that B cells emerging from mice that received transplants of fetal, cord blood, or adult stem cell sources express a comparable immunoglobulin repertoire with respect to VH4 and JH segments and show very similar yet not identical HCDR3 characteristics. Thus, the dramatic differences noted by many studies are likely due to environmental factors (ie, antigen exposure, selection, paracrine signals in the bone marrow milieu, hormonal influences, etc) and accessory molecules (terminal deoxynucleotidyl transferase [TDT], exonucleases, etc) that influence the humoral immune system and generation of the Ig repertoire following transplantation rather than from differences arising from the immunoglobulin locus in the hematopoietic stem cells themselves. These results have important implications for hematopoietic reconstitution in humans, which has become a common clinical procedure.
Materials and methods
Animals
NOD/SCID and NOD/SCID/β2m-/- were obtained from a breeding colony established at the Laboratory Animal Resource Center at the Oklahoma Medical Research Foundation (OMRF, Oklahoma City, OK) from breeding pairs that were provided by Dr Leonard D. Schultz (The Jackson Laboratory, Bar Harbor, ME). Animals were housed in microisolator cages in a restricted barrier facility.
Cell sources
Cord blood samples were obtained from patients at St Anthony Hospital (Oklahoma City, OK). One pool of cord blood was used for a single transplantation experiment, and 2 independent experiments were performed using single cord blood donors. Adult bone marrow samples were collected from discarded material from hip replacement surgery at Bone and Joint Hospital (Oklahoma City, OK) from a pool of 2 patients (37 and 85 years of age) and a single donor aged 75 years. Pooled fetal bone marrow between 16 and 24 weeks of gestation was obtained from the Anatomic Gift Foundation (Laurel, MD). Use of human tissue was approved by the institutional review boards of St Anthony Hospital, University of Oklahoma Health Sciences Center, and Oklahoma Medical Research Foundation. Guidelines and informed consent procedures were provided according to Declaration of Helsinki guidelines.
Transplantation of human cells
Adult and fetal engrafted samples were treated according to Rossi et al32 with the addition of a CD34 Magnetic Acquisition Cell Sorting isolation step (Miltenyi Biotec, Auburn, CA) in which CD34+ progenitor cells were separated from CD34- cells. In the latter case, 2 × 105 CD34+ cells were injected with 5 × 106 irradiated (adult bone marrow cells) or unirradiated (fetal bone marrow cells) CD34- cells in a volume of 200 μL into each irradiated 6-week-old mouse (1 Gy; 4-5 mice per transplantation). Cord blood experiments involved the injection of total cord blood mononuclear cells (20 × 106) or isolated CD34+ cells with irradiated CD34- cells as described for isolated CD34+ cells. Animals were screened after 6 weeks for engraftment of human cells and killed at 8 weeks for the harvest of human cells from the bone marrow and the spleen.
Antibodies for flow cytometry
The following antibodies were either biotinylated or conjugated for flow cytometry with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC): anti-CD5 (clone UCHT2) (Becton Dickinson, San Jose, CA), anti-IgM (goat anti-human Fab fragment) from Southern Biotechnology Associates (Birmingham, AL), anti-CD34 (HPCA-2) (Becton Dickinson), and anti-CD19 (clone SJ25-C1) from Caltag Laboratories (Burlingame, CA). The biotinylated antibodies were stained with a second step using Streptavidin Red 613 (Gibco/BRL, Rockville, MD).
Purified monoclonal antibody LC1 at 1 mg/mL was a gift from Ritzgar Mageed. LC1 was conjugated to FITC using standard protocols. Monoclonal antibody 9G4 was obtained from Frida Stevenson, and was purified from cell culture supernatants using a HiTrap Protein G column (Amersham Biosciences, Buckinghamshire, United Kingdom) according to the manufacturer's instructions and concentrated to 1 mg/mL. FITC anti-rat IgG1/2a (G28-5) obtained from Becton Dickinson was used to detect the 9G4 antibody.
Cell sorting
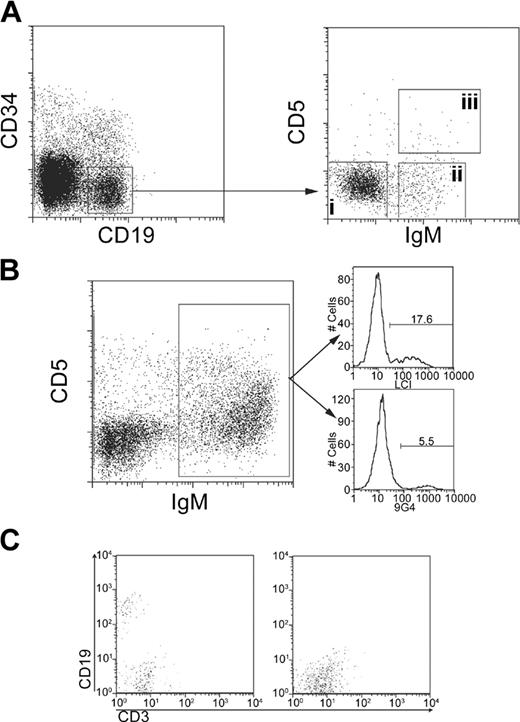
Bone marrow and spleens were harvested when the animals were killed after 8 weeks of engraftment, and antibodies to lineage markers (Mac-1, Gr-1, Ter119, murine CD19, CD2) and magnetic beads were used to deplete murine cells from the resulting cell suspensions prior to sorting into 5 CD34-CD19+ subpopulations (Figure 1A). That is, the enriched human cell suspensions were incubated with anti-human IgM-FITC, anti-human CD5-PE, and anti-human CD19-APC. CD34-CD19+ IgM- or CD34-CD19+ IgM+ CD5+, or CD34-CD19+ IgM+ CD5- cells from the bone marrow and splenic CD34-CD19+ IgM+ CD5+ or CD34-CD19+ IgM+ CD5- cells were sorted using a MoFlo (Cytomation, Fort Collins, CO) cell sorter in bulk into separate collection tubes for cDNA preparation and cloning. The purity of the sorted populations was more than 95%. VH4-expressing lymphocytes were also evaluated by staining with monoclonal LC1-FITC or 9G4-FITC antibodies. These reagents were used to stain human B cells derived from adult bone marrow engrafted mice.
Cell sorting of transplanted cell populations. (A) Cells were obtained for immunoglobulin sequencing. At 8 weeks after transplantation, cells were collected from the spleen and bone marrow of chimeric mice that had received transplants of CD34+ lymphoid progenitors (in the case of fetal and adult bone marrow) or cord blood mononuclear cells. Cells were enriched for human cells by depleting mouse myeloid and macrophage lineage positive cells. CD34-CD19+ cells (left box) were sorted into surface IgM- CD5- (i), IgM+ CD5- (ii), or IgM+ CD5+ (iii) populations (right box) in the bone marrow and spleen (the latter with the exception of surface IgM- cells). (B) Anti-V gene antibodies were used to determine the contribution of the VH4 family to the chimeric repertoire. Surface IgM+ cells (left) were stained with monoclonal antibodies that together recognize all VH4 family members. 9G4 recognizes VH4-34 immunoglobulins and LC1 recognizes all VH4 family members except VH4-34. Thus 17.6% of the repertoire was LC1 positive and 5.5% of the repertoire was positive for 9G4 binding, which combined equals 23.1% of cells that express an immunoglobulin using a member of the VH4 family. These monoclonal antibodies were also used to determine the contributions of VH4-34 (recognized by 9G4) relative to the other VH4 family members (recognized by LC1). 9G4 staining cells comprised 23% of cells that stain for any member of the VH4 family (not related to the percentage of VH4 staining cells among the entire cell population). (C) CD34- cells from adult bone marrow do not stably engraft NOD/SCID mice. Mice received transplants of either adult bone marrow CD34+ cells (left) or cord blood CD34- cells (right). Engraftment was compared by screening the peripheral blood first for human CD45+ cells followed by CD19 and CD3.
Cell sorting of transplanted cell populations. (A) Cells were obtained for immunoglobulin sequencing. At 8 weeks after transplantation, cells were collected from the spleen and bone marrow of chimeric mice that had received transplants of CD34+ lymphoid progenitors (in the case of fetal and adult bone marrow) or cord blood mononuclear cells. Cells were enriched for human cells by depleting mouse myeloid and macrophage lineage positive cells. CD34-CD19+ cells (left box) were sorted into surface IgM- CD5- (i), IgM+ CD5- (ii), or IgM+ CD5+ (iii) populations (right box) in the bone marrow and spleen (the latter with the exception of surface IgM- cells). (B) Anti-V gene antibodies were used to determine the contribution of the VH4 family to the chimeric repertoire. Surface IgM+ cells (left) were stained with monoclonal antibodies that together recognize all VH4 family members. 9G4 recognizes VH4-34 immunoglobulins and LC1 recognizes all VH4 family members except VH4-34. Thus 17.6% of the repertoire was LC1 positive and 5.5% of the repertoire was positive for 9G4 binding, which combined equals 23.1% of cells that express an immunoglobulin using a member of the VH4 family. These monoclonal antibodies were also used to determine the contributions of VH4-34 (recognized by 9G4) relative to the other VH4 family members (recognized by LC1). 9G4 staining cells comprised 23% of cells that stain for any member of the VH4 family (not related to the percentage of VH4 staining cells among the entire cell population). (C) CD34- cells from adult bone marrow do not stably engraft NOD/SCID mice. Mice received transplants of either adult bone marrow CD34+ cells (left) or cord blood CD34- cells (right). Engraftment was compared by screening the peripheral blood first for human CD45+ cells followed by CD19 and CD3.
Cloning and sequencing of Ig VH4 gene segment cDNAs
Cloning and sequencing of VH cDNAs were carried out according to methods described previously.32,33 Either the TOPO-TA polymerase chain reaction (PCR) Cloning Kit (Invitrogen, Carlsbad, CA) or Qiagen PCR Cloning Kit (Qiagen, Valencia, CA) was used for fetal bone marrow and adult bone marrow engraftment experiments. The following were compared from chimeric mice that underwent transplantation: 240 unique sequences from fetal bone marrow, 571 unique sequences from cord blood (255 of which were reported previously), and 289 unique sequences from adult bone marrow. Thus, in total, nearly 1100 unique Ig VH transcripts were sequenced in these studies. The sequences used for this study have been deposited in GenBank with accession numbers AY670707 to AY671786.
Heavy chain CDR3 lengths are defined as the amino acids between the cystine of the VH gene and the tryptophan of the JH gene. D gene calls were accepted when 7 consecutive nucleotides matched a germ-line gene or 8 nucleotides were interrupted by a single difference.
Computer analysis and statistics
Sequences were analyzed using Sequencher (GeneCodes, Ann Arbor, MI) and the ImMunoGeneTics (IMGT) database (http://imgt.cines.fr).34 Chi square and t tests were performed using JMP Stat for Macintosh (SAS Institute, Cary, NC). We assessed the significance of the qualitative and quantitative variables using Chi square and analysis of variance (ANOVA) tests, respectively. Post hoc pairwise comparisons were also performed to examine the differences between groups. Finally, the nonzero correlation or linear trend between a specific category (eg, 4-31) and age (fetal, cord blood, adult) was assessed using a Mantel-Haenszel Chi square statistic.
Results
The VH4 family of gene segments makes up a substantial part of the repertoire of chimeric animals
Monoclonal antibodies directed to VH4-34 (the 9G4 antibody) or other members of the VH4 family (the LC1 antibody) together stained 23.1% of human lymphocytes in animals that received transplants of adult bone marrow (Figure 1B). When we compare 9G4-stained cells to all cells that stain with either 9G4 or LC1 to obtain the percent of the VH4 family antibodies that use VH4-34 gene segment, we find that they make up (coincidently and unrelated to the discussion of VH4 contribution to all antibody-expressing cells) 23%. Therefore, a substantial fraction of the total B-cell repertoire can be evaluated by focusing on this well-studied VH family. Pooled CD34+ cells from fetal (16-24 weeks gestation), adult (pooled ages 37 and 85 or a single donor age 75) bone marrow, or cord blood (single donor) were used to engraft NOD/SCID/β2m-/- mice at 5 to 6 weeks of age. Pooled cord blood experiments were described previously.32 A third single donor cord blood transplantation in NOD/SCID mice was also performed. The animals were killed 8 weeks later and 5 lymphocyte subsets were isolated as detailed in “Materials and methods” (and Figure 1A). Human CD45+ cells made up a substantial proportion of cells in the bone marrow of chimeric mice. Mice that underwent cord blood transplantation had 1.57% ± 0.94% (mean % ± SD, n = 10 in 2 independent experiments) and mice that underwent adult bone marrow transplantation had 0.82% ± 0.35% (mean % ± SD, n = 5 in 2 independent experiments) CD45+ cells in total bone marrow. Mice that underwent fetal bone marrow transplantation had 2.14% ± 1.17% (mean % ± SD, n = 5 in 2 independent experiments). Human cells from mice that underwent transplantation contained mostly CD19+ B cells. Immunoglobulin transcripts from the recovered cells were then sequenced to evaluate the relationship between transplantation source and Ig diversity. While the data were separately analyzed within each subset, for the purpose of this study the results obtained from all lymphocyte subsets were combined and are shown as sequences from cells expressing IgM or not expressing IgM but containing IgM transcripts (Figures 2-3).
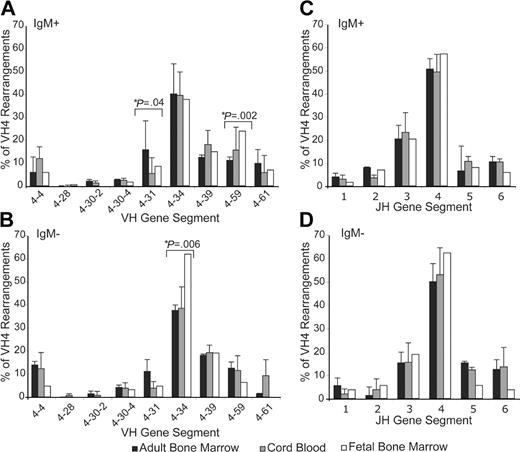
VH4 and JH gene segment use as a function of the source of transplanted hematopoietic precursors. VH4 leader and μ constant region primers were used to amplify VH4 from cDNA derived from each population studied. The products were then cloned and sequences of colony picks were obtained. Data shown represent immunoglobulin genes derived from adult (▪), cord blood (▦), or fetal (□) progenitors. The representation of the VH4 and JH gene segments from sIgM+ cells (A,C) and sIgM- cells (B,D) are shown. Data represent the percent of each family found in 2 experiments for adult bone marrow (sIgM+,n = 135 + 36; sIgM-, n = 50 + 56) and cord blood (sIgM+, n = 172 + 129 + 114; sIgM-, n = 87 + 31 + 43) LP sources and 1 experiment for the fetal LP source (sIgM+, n = 188; sIgM-, n = 53). Because of low numbers, VH4-28 and VH4-30-2 were considered together in statistical tests. The difference in VH4-34 in sIgM- cells among LP sources is significant for an age-related trend (P = .006) and VH4-31 and VH4-59 in sIgM+ cells among LP sources (P = .04 and P = .002) were significant for an age-related trend, but other differences were not significant by Chi square analysis. No significant differences were found in JH gene segments. Error bars indicate standard error.
VH4 and JH gene segment use as a function of the source of transplanted hematopoietic precursors. VH4 leader and μ constant region primers were used to amplify VH4 from cDNA derived from each population studied. The products were then cloned and sequences of colony picks were obtained. Data shown represent immunoglobulin genes derived from adult (▪), cord blood (▦), or fetal (□) progenitors. The representation of the VH4 and JH gene segments from sIgM+ cells (A,C) and sIgM- cells (B,D) are shown. Data represent the percent of each family found in 2 experiments for adult bone marrow (sIgM+,n = 135 + 36; sIgM-, n = 50 + 56) and cord blood (sIgM+, n = 172 + 129 + 114; sIgM-, n = 87 + 31 + 43) LP sources and 1 experiment for the fetal LP source (sIgM+, n = 188; sIgM-, n = 53). Because of low numbers, VH4-28 and VH4-30-2 were considered together in statistical tests. The difference in VH4-34 in sIgM- cells among LP sources is significant for an age-related trend (P = .006) and VH4-31 and VH4-59 in sIgM+ cells among LP sources (P = .04 and P = .002) were significant for an age-related trend, but other differences were not significant by Chi square analysis. No significant differences were found in JH gene segments. Error bars indicate standard error.
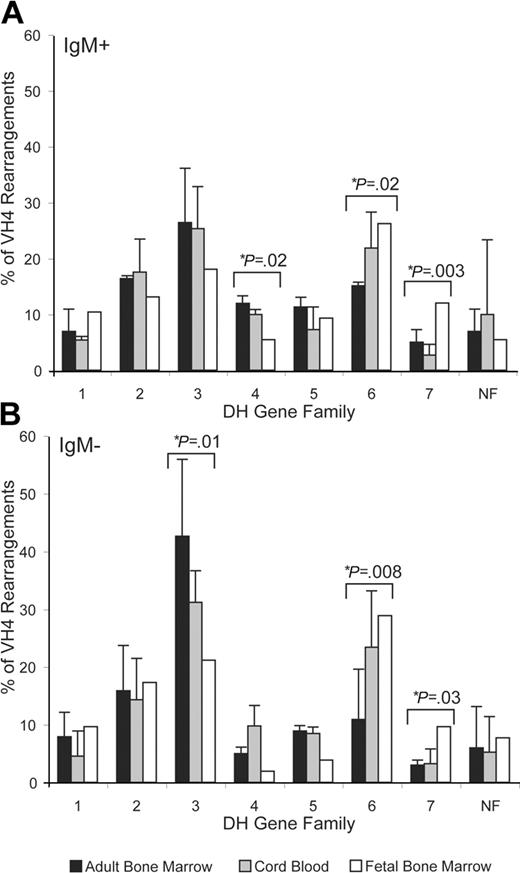
DH gene segment use in transplanted sources of hematopoietic precursors. DH gene segment use for sIgM+ (A) and sIgM- (B) cells from adult bone marrow (▪), cord blood (▦), and fetal (□) sources are shown for DH gene use. For adult bone marrow (sIgM+, n = 135 + 28; sIgM-, n = 50 + 49) and cord blood (sIgM+, n = 172 + 87 + 112; sIgM-, n = 87 + 19 + 48) LP sources and one experiment for the fetal LP source (sIgM+, n = 173; sIgM-, n = 51, 2 experiments). DH6 (P = .03 and P = .003) and DH7 (P = .008 and P = .02) in sIgM- and sIgM+ cells were significant for an age-related trend. In addition DH4 (P = .02) in sIgM+ and DH3 (P = .01) in sIgM- cells showed a significant age-related trend. Error bars indicate standard error.
DH gene segment use in transplanted sources of hematopoietic precursors. DH gene segment use for sIgM+ (A) and sIgM- (B) cells from adult bone marrow (▪), cord blood (▦), and fetal (□) sources are shown for DH gene use. For adult bone marrow (sIgM+, n = 135 + 28; sIgM-, n = 50 + 49) and cord blood (sIgM+, n = 172 + 87 + 112; sIgM-, n = 87 + 19 + 48) LP sources and one experiment for the fetal LP source (sIgM+, n = 173; sIgM-, n = 51, 2 experiments). DH6 (P = .03 and P = .003) and DH7 (P = .008 and P = .02) in sIgM- and sIgM+ cells were significant for an age-related trend. In addition DH4 (P = .02) in sIgM+ and DH3 (P = .01) in sIgM- cells showed a significant age-related trend. Error bars indicate standard error.
The frequencies of VH4 gene segment use by B cells arising from fetal, cord blood, and adult progenitor transplants were comparable (Figure 2). VH4-34 was the most highly represented gene segment of the VH4 family, being found in 30% to 35% of sequences studied. These values are typical for the repertoire found in normal naive B cells in human tonsils.35 They also compare favorably with the ratio of cells staining with the 9G4 and LC1 monoclonal antibodies that together detect all VH4 family antibodies (Figure 1B).
In addition we used CD34- cells from cord blood in order to determine the ability of more mature cells to stably engraft the NOD/SCID mouse over an 8-week time period. We screened peripheral blood from each of 5 mice with antibodies to CD45, CD19, and CD3 in order to determine the level of engraftment present in these mice. We found no evidence of engraftment in these mice at the end of 8 weeks following transplantation (Figure 1C). Thus we can be confident that the B-cell repertoire observed in this chimeric mouse system is the result of the development of B cells from progenitors rather than mature human cells that took up residence in the mouse tissues. Furthermore this repertoire does not reflect a skewed distribution of heavy chain uses when examined at the level of immunoglobulin receptor expression on human cells and is consequently useful for the comparison of the repertoire from progenitors of various ages.
VH4 and JH gene segment use is remarkably similar among progenitor sources
In order to gain statistical power, we compared the VH4 and JH gene segment use patterns of 1100 unique Ig transcripts among the fetal and adult bone marrow progenitor cell sources (heretofore lymphoid progenitors or LPs) as well as with cord blood sources (255 sequences of which have been reported previously in Rossi et al32 ) (Figure 2). Transplantation experiments involved the use of the following groups in independent experiments: (1) a group of pooled fetal lymphocyte progenitor (LP) sources, (2) a pool of 2 adult patients, (3) a single adult patient, (4) a pool of cord blood patients, and (5-6) 2 individual cord blood patients. The VH4 gene segment use pattern was remarkably similar among these populations for both surface IgM- (sIgM-) and surface IgM+ (sIgM+) cells. The differences that followed an age-related trend occurred in the VH4-34 gene segment of surface IgM- cells as well as VH4-59 and VH4-31 in sIgM+ populations. However, these statistical differences were not present across sIgM- and sIgM+ expression.
No significant differences in JH use were found in either surface IgM+ or IgM- sequences from chimeric animals in spite of the varying ages of progenitors. The JH distribution was dominated by the use of the JH4 gene segment, commonly used in cord blood and fetal repertoires. The VH4 and JH gene segment use is reflective of what is seen in naive B-cell repertoires that have been reported for children.35
The HCDR3s from cells derived from chimeric mice that received transplants of different progenitor sources exhibit some modest differences
We compared the length of heavy chain complementarity determining region 3 (HCDR3) among sequences based upon the age of the transplanted LP source from cells grouped into either sIgM- or sIgM+ categories (Table 1). In sIgM- cells we found a difference of approximately 3 amino acids on average between adult LP and cord blood or fetal LP sources (P = .0003). This difference in lengths was reduced in sIgM+ cells to 1 amino acid (which was not considered significant at P = .0142 because of the large data set and single variable per age).
CDR3 characteristics of sequences isolated from human LP-mouse chimeras, by source
. | Adult, mean length ± SE (no.) . | Cord blood, mean length ± SE (no.) . | Fetal, mean length ± SE (no.) . | ANOVA† . | P . |
|---|---|---|---|---|---|
| CDR3* | |||||
| sIgM- | 18.0 ± 0.6 (67) | 15.4 ± 0.4 (154) | 14.9 ± 0.6 (52) | F(2 270) = 8.24 | .0003 |
| sIgM+ | 15.3 ± 0.4 (140) | 14.5 ± 0.2 (385) | 14.0 ± 0.2 (186) | F(2 708) = 4.61 | .0142 |
| N nucleotide | |||||
| sIgM- | 15.9 ± 1.1 (67) | 13.4 ± 1.1 (70) | 10.5 ± 1.3 (49) | F(2 183) = 4.99 | .0078 |
| sIgM+ | 12 ± 0.6 (148) | 13.3 ± 0.6 (179) | 8.7 ± 0.6 (177) | F(2 501) = 16.29 | < .0001 |
| V-DJ N nucleotides, sIgM- | 8.0 ± 0.8 (67) | 7.1 ± 0.8 (40) | 7.0 ± 1.0 (49) | F(2 153) = 0.4343 | .6485 |
| D-J N nucleotides, sIgM- | 6.2 ± 0.6 (67) | 6.9 ± 0.6 (40) | 3.0 ± 0.7 (49) | F(2 153) = 9.60 | .0001 |
. | Adult, mean length ± SE (no.) . | Cord blood, mean length ± SE (no.) . | Fetal, mean length ± SE (no.) . | ANOVA† . | P . |
|---|---|---|---|---|---|
| CDR3* | |||||
| sIgM- | 18.0 ± 0.6 (67) | 15.4 ± 0.4 (154) | 14.9 ± 0.6 (52) | F(2 270) = 8.24 | .0003 |
| sIgM+ | 15.3 ± 0.4 (140) | 14.5 ± 0.2 (385) | 14.0 ± 0.2 (186) | F(2 708) = 4.61 | .0142 |
| N nucleotide | |||||
| sIgM- | 15.9 ± 1.1 (67) | 13.4 ± 1.1 (70) | 10.5 ± 1.3 (49) | F(2 183) = 4.99 | .0078 |
| sIgM+ | 12 ± 0.6 (148) | 13.3 ± 0.6 (179) | 8.7 ± 0.6 (177) | F(2 501) = 16.29 | < .0001 |
| V-DJ N nucleotides, sIgM- | 8.0 ± 0.8 (67) | 7.1 ± 0.8 (40) | 7.0 ± 1.0 (49) | F(2 153) = 0.4343 | .6485 |
| D-J N nucleotides, sIgM- | 6.2 ± 0.6 (67) | 6.9 ± 0.6 (40) | 3.0 ± 0.7 (49) | F(2 153) = 9.60 | .0001 |
Amino acids
ANOVA for all 3 age groups
Seeking an explanation for the difference in HCDR3 lengths from the construction of the HCDR3 we compared the use of D gene segment families (Figure 3). We found that there were significant differences among sequences from sIgM- cells that involved the use of the DH6 (DN) and DH7 (DHQ52) family (P = .008 and P = .03, respectively). These trends were also repeated in sIgM+ cells (P = .02 and P = .003, respectively). Other differences were not consistently significant between sIgM- and sIgM+ populations.
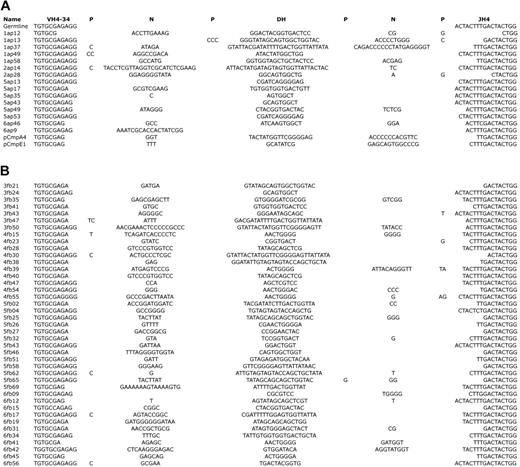
While the differential use of DH6 and DH3 families in the fetal and adult LP chimeric mice partially explains the length difference in HCDR3, another contributor was the extent of TDT activity, which adds N nucleotides to the junctions of the rearrangements. We observed a difference of 5 nucleotide additions on average in sIgM- cells between fetal and adult chimeric mice that underwent transplantation (Table 1). This was reduced to 3 nucleotide additions however in sIgM+ cells. In comparing the number of nucleotides in each junction, we found fetal chimeras in general had less N addition at the D-J junction in comparison with adult chimeras (3.0 vs 6.2). Furthermore we examined exonuclease activity as indexed by comparison with the germ-line VH4-34 and JH4 gene segments in sequences from fetal and adult chimeric groups (Figure 4). In sIgM+ cells there was, on average, greater germline-encoded nucleotide removal in sequences from fetal chimeras in comparison with the adult chimeras at both the V-D (1.3 vs 1.2 nucleotides, respectively) and especially D-J (4.1 vs 3.6 nucleotides, respectively) junctions. In addition, half of the P nucleotides were found in sIgM+ cells from fetal chimeras in comparison with adult chimeras (0.3 and 0.6 nucleotides, respectively).
Junctional nucleotide distribution and exonuclease activity. HCDR3s for VH4-34 with JH4 rearrangements are shown for sIgM+ adult (A) and fetal (B) LP chimera sequences. Germline sequences for comparison are shown along with N and P nucleotides and DH segments.
Junctional nucleotide distribution and exonuclease activity. HCDR3s for VH4-34 with JH4 rearrangements are shown for sIgM+ adult (A) and fetal (B) LP chimera sequences. Germline sequences for comparison are shown along with N and P nucleotides and DH segments.
Discussion
A diverse Ig repertoire is transcribed and expressed
Our examination of the VH4 repertoire in immune-deficient mice that were reconstituted with human lymphocyte progenitors from fetal and adult age groups provides insights into the intrinsic potential of B-cell progenitors to produce a normal repertoire regardless of age. We found that VH4-expressing cells made up a total of 23% of the human lymphocytes using the anti-VH antibodies LC1 and 9G4. The VH4 component of the peripheral blood immunoglobulin repertoire has been reported to range from 19% in young to 32% in elderly adults.10 Thus our findings fell within this range. The VH family distribution was of significant concern since it was possible that the repertoire could have been skewed toward a single family or a single immunoglobulin gene segment. A normal representation of the VH4 family allowed us to use it for a more in-depth analysis of individual VH, D, and JH gene segments with the confidence that the members of the VH4 family do not represent a minority or a skewed majority. The VH4 family has a single, nondegenerate priming site in the leader sequence, which, combined with a Cμ heavy chain region site, eliminates priming biases in the survey of this family.
Most members (with some minor alleles absent) of the VH4 family were represented in the chimeric animals studied. In addition a wide diversity of VH3 rearrangements was found (data not shown). Regardless of the source of the progenitors that were used for transplantation into the immunodeficient mice, we obtained a diverse VH4 Ig repertoire with no evidence of pauci or oligoclonality. This result is different from that reported in some human bone marrow transplantations in which an oligoclonal population was produced followed by eventual resolution to polyclonality.36
Similar Ig repertoires are produced regardless of the age of their source
Using progenitors from fetal, cord blood, and adult bone marrow, we were able to address the important question, “Do progenitors derived from these respective sources have the intrinsic ability to produce the same repertoire?” Previous studies of differences between young and older adult repertoires (based on peripheral blood cells) have shown that older individuals have a greater representation of VH4-34 and VH4-59 gene segments than younger adults.10 In addition, patients who have had bone marrow transplantations initially have restricted repertoires.22
The fetal, neonatal, and adult repertoires can usually be distinguished by several parameters (see the Supplemental Table link at the top of the online article on the Blood website). The JH gene segment use in fetal and neonatal repertoires is normally dominated by the use of JH4. While naive adult repertoires also use this gene segment most frequently, the use of other JH segments occurs with greater overall frequency than seen in the repertoires of the very young.7 In addition the HCDR3 length in adults is considerably longer than that found in developing humans. Indeed distinct patterns of N nucleotide addition usually occur in this region. As well, the prominent use of the DH7-27 (DQ52) gene segment in fetal repertoires compared with adults is also a common finding.14,20
Upon examination of the immunoglobulins of adult and fetal bone marrow as well as cord blood LP-derived cells in an immunodeficient mouse environment, we found the VH4 and JH gene segment uses to be nearly indistinguishable once IgM was expressed on the surface of the cells. This striking feature of the repertoires leads us to conclude that the patterns of gene segment use in progenitors are roughly the same when placed in the same maturational environment. This similarity among the LP sources extends to both sIgM- and sIgM+ cells. Indeed none of the other statistically significant differences in VH gene segment use were consistent between sIgM- and sIgM+ populations. Particularly, one would have expected the range of JH4 use to be more of a distinguishing feature among the chimeric repertoires as in primary cells from the human.7 Differences have been reported in the peripheral blood repertoire between fetuses and adults in the VH4 family, although studies in the aged are limited. In our own studies, we have found child and adult repertoires exhibit distinct VH4 gene segment repertoires (data not shown). These differences generally involve the use of VH4-59 and VH4-34.10
The mean HCDR3 lengths between adult and fetal or cord blood LP sources were modestly different in sIgM- cells, but not to the degree that one would expect for individuals of these respective ages. While the adult and cord blood ranges fell within the parameters expected,14 we found that the fetal lengths were higher. This may be due to a programmed maturation of fetal B-cell HCDR3 lengths from the second trimester (the age of transplantation) to the age of birth (one month longer than our date of harvest) that some have suggested.20 The mean HCDR3 lengths resolved to one amino acid after surface expression of IgM, however, reflecting a selective process between IgM- and IgM+ stages that greatly impacts the structural constraints of the antibodies. While it is possible that the mouse environment may be specifically selective for HCDR3s of this length, our previous studies with human minilocus transgenic mice revealed HCDR3 lengths that are much shorter.37 Indeed others have shown similar shorter length restrictions in normal mice.38 It is likely that human B cells producing immunoglobulins with the HCDR3 lengths observed in the chimeras have a selective advantage.
Our division of sequences based upon surface IgM expression roughly corresponds to the observation of the repertoire just before and during the expression of surrogate light chain on the surface of the pre-B cell (surface IgM levels are too low to be detected by conventional means during pre-B-cell receptor expression). We are also able to observe the repertoire after surrogate light chain-heavy chain pair appears on the surface (the majority of the cells, including immature B cells). Sequence differences of enriched populations were therefore examined. The selective pressures that are observed are likely to arise from 1 of 2 places: first, the selection of human surrogate light chain itself in the human B cells for those heavy chains that will pair successfully with the regular light chain; and secondly, the effects of any mouse stromal selective influences for autoantigens, and so forth, in the bone marrow on the human cells. Since there is no T-cell help in this murine system and the mice are raised in specific pathogen-free (SPF) environments, later selective influences are not likely to be present.
We examined the HCDR3s of the sequences along D gene segment use and N nucleotide addition parameters. The use of D gene segments differed only in 3 of the 7 families, but only 2 of these were statistically significant. The most important family for the distinction of fetal and adult immunoglobulin repertoires is the DH7-27 segment (also referred to as DQ52). In our studies it was used more in fetal than adult. However many of the sequences in which a D gene could not be determined—particularly for the adult LP chimeras—have a stretch of nucleotides that could indeed be attributable to the DH7-27 segment but did not fit our criteria for inclusion. While the DH6 (DN) gene segment family is not classically identified as a cardinal difference in adult and fetal repertoires, our studies did indeed show a pattern in the use of this family that varied among the LP sources. The DH3 and DH4 families showed statistically significant differences but were not consistent between sIgM- and sIgM+ populations. The DH3 family contains the longest D gene segments, which partially contribute to the difference in HCDR3 length found between fetal and adult LP chimeras.
Another means of extending the HCDR3 length in immunoglobulin variable regions is through the activity of TDT. We examined the difference of N nucleotide additions between experimental groups and found a distinction among them. The difference of N nucleotide additions in the D-J junction of sIgM- cells made up nearly 2 amino acids (2/3 of the HCDR3 length discrepancy) but was reduced to 1 amino acid difference in the sIgM+ cells, which would explain the difference in HCDR3 length in this group.
In our studies we find that the repertoires produced from fetal, cord blood, and adult lymphocyte progenitors are strikingly similar particularly once IgM is expressed on the surface of the cells. Even before this stage, the VH and JH gene segment use is nearly identical with the only distinguishing characteristics being located in the HCDR3. In particular, this includes the overall length of the HCDR3, which is influenced by the combination of slightly more frequent use of longer D gene segments, but moreover by differences in TDT activity in the junction (in particular the D-JH junction). These differences in HCDR3 length and overall N addition are nearly eliminated after the expression of IgM on the surface of the cells. While some statistical differences do exist between the immunoglobulin repertoires of cells derived from progenitors from individuals of different ages, only part of these differences fit known patterns between repertoires from young and older individuals, and most were eliminated once immunoglobulin was expressed on the surface of cells. Consequently, the overall similarity of the Ig repertoire in these studies appears to be the biologically significant finding.
From the standpoint of the immunoglobulin repertoire, it appears that the use of cord blood for transplantation in humans is a promising alternative to adult bone marrow without any loss of repertoire potential. Considering the relative abundance of cord blood, and consequently the library of diverse HLA types that may be available, and its engraftment potential, this source may become increasingly important in transplantation. It is consequently vital to pursue studies on the means to expand the number of progenitor cells (which are in relatively low number) in cord blood samples in order make this promising transplant source a viable option.
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2003-11-3961.
Supported by National Institutes of Health grants AI45864, RR15577, and AI12127.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work was greatly influenced and assisted by the consultation of Paul Kincade of the Oklahoma Medical Research Foundation (OMRF). We gratefully acknowledge the technical work performed by Darshna Mehta and Ken Wilson as well as that done by Sheryl Christofferson, Diana Edwards, and Viji Dandapani in the sequencing and flow cytometry core facilities.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal