Abstract
Signaling by transmembrane immunoglobulin G (IgG)-Fc receptors (FcγRs) in response to ligand involves association with membrane microdomains that contain glycosyl phosphatidylinositol (GPI)-anchored proteins. Recent in vitro studies showed enhancement of FcγR signaling by forced monoclonal antibody-mediated cocrosslinking with various GPI-anchored proteins. Here, the possibility that GPI-anchored proteins are involved in normal physiologic FcγR effector functions in response to a model ligand was studied using myeloid-specific GPI-anchor-deficient mice, generated by Cre-loxP conditional targeting. GPI-anchor-deficient primary myeloid cells exhibited normal FcγR expression and binding or endocytosis of IgG-immune complexes (IgG-ICs). Strikingly, after stimulation with IgG-ICs, tumor necrosis factor-α release, dendritic cell maturation, and antigen presentation were strongly reduced by GPI-anchor deficiency. Tyrosine phosphorylation of the FcR γ-chain in response to IgG-IC was impaired in GPI-anchor-deficient cells. Myeloid GPI-anchor deficiency resulted in attenuated in vivo inflammatory processes during IgG-IC-mediated alveolitis. This study provides the first genetic evidence for an essential role of GPI-anchored proteins in physiologic FcγR effector functions in vitro and in vivo. (Blood. 2004;104:2825-2831)
Introduction
Leukocytes express a number of surface proteins linked to the cell membrane through the glycosyl phosphatidylinositol (GPI)-anchor.1 Recently, GPI-anchored proteins received increased attention since they were found highly concentrated in detergent-insoluble membranes or lipid rafts.2,3 In the hematologic disorder paroxysmal nocturnal hemoglobinuria (PNH), the GPI-anchor is absent on varying numbers of all types of leukocytes. This is caused by a somatic mutation in the PIG-A gene,4,5 which is essential for the biosynthesis of the GPI-anchor,6 in hematopoietic stem cells. One of the main causes of death in PNH is bacterial infection,7 raising the possibility that GPI-anchor deficiency causes impairment of certain antimicrobial functions of the immune system.
Myeloid cells, the main effector cells in inflammation and antigen presentation, express immunoglobulin G (IgG)-Fc receptors (FcγRs), which mediate a wide variety of cellular responses to complexed IgG.8,9 In mouse myeloid cells, 2 activatory FcγRs (ie, FcγRI and FcγRIII) and 1 inhibitory FcγR (ie, FcγRII) have been identified. These murine FcγRs are all transmembrane receptors; no GPI-anchored murine FcγR has been identified. Human FcγRs are all transmembrane except for the GPI-anchored FcγRIIIB on neutrophils. Signaling by transmembrane FcγRs involves association with lipid rafts.10,11 Recent studies reported a physical association between transmembrane FcγRs and different GPI-anchored proteins.12-14 Monoclonal antibody-mediated cocrosslinking of transmembrane FcγRs with different GPI-anchored proteins (ie, CD59, CD48, or human FcγRIIIB) can synergistically enhance FcγR signaling in vitro.15-17 The biologic significance of the latter findings, potentially relevant for FcγR function during physiologic responses to natural ligands, has not been clarified.
The purpose of this study was to investigate the possible role of GPI-anchored proteins in physiologic FcγR effector functions, using newly generated myeloid-specific GPI-anchor-deficient mice. These mice were generated by Cre-loxP-mediated disruption of the Pig-a gene. Analysis of FcγR functions, using IgG-immune complexes (IgG-ICs) as a model ligand, in these mice revealed an unexpectedly strong cooperation between GPI-anchored proteins and FcγRs. The presence of the GPI-anchor was found to be indispensable for IgG-IC-triggered tumor necrosis factor α (TNF-α) release, dendritic cell (DC) maturation and antigen presentation, FcR γ-chain tyrosine phosphorylation, and in vivo inflammatory processes.
Materials and methods
Mice
Myeloid-specific Pig-a-targeted (LysMCre/Pig-aflox) mice were generated using Cre-loxP conditional targeting.18 Specific disruption of Pig-a, an X-linked gene essential for the GPI-anchor biosynthesis,6 in the myeloid lineage prevented embryonic lethality observed previously after Pig-a disruption in the germ line,19 and allowed examination of myeloid effector functions in vivo. Pig-a was targeted by introducing loxP sites flanking exon 6 of this gene (loxP-Pig-a),20 in combination with myeloid-specific expression of Cre recombinase driven by the LysM promoter.21 Female mice carrying loxP-Pig-a were crossed with male mice expressing Cre recombinase, both strains being bred in a C57Bl/6-129Sv genetic background. In the male offspring, polymerase chain reaction (PCR) analysis was used to detect the presence of Cre using the Cre-specific primers 5′-CATCGCCATCTTCCAGCAG-3′ and 5′-CAATTTACTGACCGTACAC-3′, or to detect the presence of loxP as well as disruption of Pig-a as described.20 DNA from male mice positive for both Cre and loxP consistently demonstrated disruption of the Pig-a locus by PCR, and these mice were used as myeloid-specific Pig-a-targeted mice in further experiments, at the age of 2 to 5 months. The expression of GPI-anchored proteins on various myeloid cells from these mice was examined by flow cytometry as described in the next paragraph. Age-matched male mice positive for Cre and negative for loxP were used as littermate control mice. In none of the functional experiments were significant differences observed between mice carrying Cre only, mice carrying loxP only, or C57Bl/6 inbred mice. Mouse experiments have been approved by the institutional review board of the Research Institute for Microbial Diseases of Osaka University.
Isolation and stimulation of alveolar macrophages (AMs)
Mice were killed and AMs were isolated by bronchoalveolar lavage (BAL) through 3 intratracheal injections with 1 mL ice-cold phosphate-buffered saline (PBS) using a 20-gauge Angiocath cathether (Becton Dickinson, Sandy, UT). Typically, these cell populations contained at least 92% AMs, identified by staining with the macrophage-specific marker F4/8022 (Serotec, Oxford, England) (not shown). The phenotype of these cells was analyzed by flow cytometry after staining with monoclonal antibodies (mAbs) specific for heat-stable antigen (HSA; BD Pharmingen, San Diego, CA), CD48 (Immunotech, Marseille, France), or mAb 2.4G2 specific for FcγRII/III (BD Pharmingen). To analyze FcγRI expression, cells were stained with monomeric murine IgG2a anti-trinitrophenol (kindly provided by Dr Sjef Verbeek, Leiden University, the Netherlands), followed by secondary polyclonal sheep antimouse (Cappel ICN, Aurora, OH). AMs were allowed to adhere in Terasaki microwell plates (Nunc, Roskilde, Denmark) by incubation of 5 × 103 cells per well in RPMI-1640 medium (Sigma, St Louis, MO) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL, Grand Island, NY), 100 U/mL penicillin, and 0.1 mg/mL streptomycin, for 2 hours at 37°C, followed by 3 washes with PBS.
Adherent AMs were stimulated with lipopolysaccharide (LPS) from Salmonella minnesota Re 595 (Sigma) in the same medium for 18 hours at 37°C. Stimulation of adherent AMs with IgG-IC was performed by incubation of the cells for 4 hours at 37°C with IgG-ICs in RPMI-1640 supplemented with 50 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma) and 2 mM l-glutamine (Gibco BRL), and without serum to avoid interference by exogenous complement. These IgG-ICs were preformed as described23 by incubation of polyclonal rabbit IgG anti-ovalbumin (OVA; Cappel ICN) at a fixed concentration of 25 μg/mL and different indicated concentrations of OVA (Sigma) for 30 minutes at 37°C, in RPMI-1640 without serum. For some experiments, large insoluble IgG-ICs (IICs) were prepared as described24 using polyclonal rabbit IgG anti-bovine serum albumin (BSA; Cappel ICN) and BSA (Sigma). All antibodies and antigens used to prepare IgG-ICs tested LPS negative using a Limulus amebocyte lysate assay (Sigma). Concentrations of TNF-α in the AM culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Generation and stimulation of GPI-anchor-deficient dendritic cells (DCs)
Long-term myeloid DC cultures were generated from the spleen according to a previously described method.25 In brief, splenocytes from LysMCre/Pigaflox or littermate control mice were cultured for at least 5 weeks in Dulbecco modified Eagle medium (DMEM; Sigma) supplemented with 10% FBS, 30% supernatant of confluent NIH-3T3 fibroblast cultures, and 1250 U/mL recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Pharmingen). These cells uniformly stained positive with mAb against CD11c (Pharmingen) and CD11b (Pharmingen), negative with anti-CD8 mAb (Pharmingen), and weak with mAb against CD86 (Pharmingen) and major histocompatibility complex (MHC) class II (Pharmingen) by flow cytometry, confirming that they had differentiated into immature myeloid DCs. In Terasaki culture plates, 7.5 × 103 DCs per well were stimulated with LPS from Escherichia coli 026:B6 (Sigma) as described in the previous paragraph. Alternatively, the cells were stimulated with phorbol 12-myristate acetate (PMA; Sigma) for 1 hour at 37°C, or with IgG-IC in RPMI-1640 supplemented with 50 mM Hepes and 2 mM l-glutamine and without serum as described in the previous paragraph. Incubation of DCs with IgG-ICs did not affect their viability as assessed by trypan blue staining. TNF-α release into the culture supernatants was measured as described in the previous paragraph.
Maturation of DCs was induced by incubating 7.5 × 104 DCs per well of 96-well tissue culture plates (Iwaki, Funabashi, Japan) at 37°C in the presence of IgG-ICs prepared as described in the previous paragraph, in DMEM supplemented with 10% heat-inactivated FBS and 2 mM l-glutamine. The expression of CD86 on these DCs, as a maturation marker, was determined by flow cytometry 2 days later. To examine antigen presentation in response to IgG-ICs, after 1 day these IgG-IC-pulsed DCs were irradiated with 30 Gray, and 105 lymph node cells of OVA-specific T-cell receptor-transgenic OT-II mice26 were added to each well. The cells were cultured for another day, in the presence of 1 μCi (0.037 MBq) [3H]-thymidine per well during the last 18 hours, and the radioactivity of the cells served as a measure for the proliferation of the T cells.
Binding and endocytosis of IgG-ICs
To examine binding of IgG-ICs, AMs or DCs were incubated with IgG-ICs prepared with rabbit IgG anti-OVA (25 μg/mL) and OVA (10 μg/mL or 1 μg/mL when assessing blocking by 2.4G2) for 30 minutes on ice, followed by washing. Bound IgG-ICs were detected using flow cytometry after a second incubation with phycoerythrin-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA).
To assess endocytosis, IgG-ICs were prepared with rabbit IgG anti-OVA (25 μg/mL) and fluorescein isothiocyanate (FITC)-conjugated OVA (1 μg/mL; Sigma) as described in the previous paragraph, and were allowed to bind DCs by incubation for 30 minutes on ice. After washing and resuspension of the cells in ice-cold medium, incubation was continued either on ice or for 20 or 30 minutes at 37°C. Cells were next incubated with phycoerythrin-conjugated anti-rabbit IgG on ice to detect extracellularly bound, noninternalized IgG-ICs. Fluorescence through the FL1 channel represented the total cell-associated IgG-ICs. A decrease in FL2 fluorescence with unchanged FL1 fluorescence after incubation at 37°C, in comparison with incubation on ice, served as a measure for endocytosis of the immune complexes.
Immunoblot analysis of FcR γ-chain tyrosine phosphorylation
DCs were serum starved by overnight culture in DMEM containing 0.1% BSA, 10 mM Hepes, and 2 mM l-glutamine (pH 7.4). The cells were suspended in the same medium at a concentration of 1 × 106/mL, and incubated for 20 minutes on ice with or without IgG-ICs, which were prepared with rabbit IgG anti-OVA (25 μg/mL) and OVA (5 μg/mL), and then incubated again for 2 minutes at 37°C. After 2 washes with ice-cold PBS, the cells were lysed by incubation for 30 minutes on ice in 20 mM Tris (tris(hydroxymethyl)aminomethane)-HCl containing 150 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), 1% nonidet P-40 (NP-40), 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, and 10 μg/mL leupeptin (pH 7.4). The FcR γ-chain was immunoprecipitated from the lysates using protein G-Sepharose (Pharmacia, Uppsala, Sweden) coated with rabbit IgG anti-FcR γ-chain (Upstate, Lake Placid, NY). The precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), and detected by mouse antiphosphotyrosine IgG (4G10; Upstate) or rabbit IgG anti-FcR γ-chain. After incubation with horseradish peroxidase-conjugated secondary antibodies against mouse IgG (Pharmacia) or rabbit IgG (Pharmacia), respectively, the blots were developed using chemiluminescence reagent (Perkin Elmer, Boston, MA).
In vivo IgG-IC alveolitis
IgG-IC-mediated alveolitis in mice was performed as described,27 by an intravenous injection with 500 μg OVA (Sigma) and an intratracheal injection with 200 μg rabbit IgG anti-OVA (Cappel ICN). Some mice were injected intravenously with OVA and intratracheally with saline as a control. Then 4 hours later, the BAL fluids of these mice were harvested as described for AM isolation. Concentrations of TNF-α and interleukin-6 (IL-6) in the BAL fluids were measured by ELISA (R&D Systems, Minneapolis, MN). The numbers of infiltrating neutrophils and the numbers of erythrocytes (as a measure for hemorrhage) in the BAL fluids were determined by morphologic analysis of cytospin preparations using light microscopy (Axiovert 100M microscope [Zeiss, Oberkochen, Germany]; 40×/1.30 objective; acquisition by LSM 510).
Statistical analysis
Data were analyzed using the unpaired t test.
Results
Phenotypic analysis of myeloid-specific Pig-a-targeted mice
Myeloid-specific targeting of the X-linked Pig-a gene was performed by crossing mice carrying loxP-containing Pig-a20 with “knock-in” mice expressing Cre recombinase driven by the myeloid-specific LysM promoter.21 PCR analysis of DNA isolated from bone marrow, blood, or peritoneal macrophages of male offspring positive for both Cre and loxP (LysMCre/Pig-aflox mice) confirmed disruption of Pig-a (not shown). AMs from LysMCre/Pig-aflox mice almost completely lacked the GPI-anchored proteins HSA (Figure 1A left) and CD48 (not shown) at their surface, indicating a GPI-anchor-negative phenotype. Expression of GPI-anchored proteins on nonmyeloid leukocytes was unaffected, as indicated by similar expression levels of CD48 (Figure 1A, right) or HSA (not shown) on peripheral blood lymphocytes. Other myeloid cell types exhibited an unaltered or only partially decreased expression of GPI-anchored proteins (not shown). This is likely due to a rather low turnover rate of GPI-anchored proteins28 together with relatively late expression of Cre and subsequent Pig-a disruption (ie, when most myeloid cells remain nonproliferating). AMs may have a higher membrane turnover, due to continuous internalization of air-derived particles and pulmonary surfactant,29 in addition to a continued local proliferation in lung tissue30 resulting in a GPI-anchor-negative phenotype in vivo.
GPI-anchor-deficient phenotype of alveolar macrophages and dendritic cells. (A) Alveolar macrophages (AM; left) or dendritic cells (DC; middle) cultured for 6 weeks were stained with mAb anti-HSA. Peripheral blood leukocytes gated for lymphocytes (PBL) were stained with mAb anti-CD48 (right), which is expressed on both T and B lymphocytes. Shown are fluorescence intensity plots of cells from littermate control (thin dotted lines) or LysMCre/Pig-aflox mice (bold lines), and antibody controls (dashed lines). (B-C) Responses to LPS. Control (wild type [WT]; •) or GPI-anchor-deficient (knock out [KO]; ○) AMs (B) or DCs (C) were stimulated with LPS; mean concentrations of TNF-α released of at least 3 independent experiments are shown.
GPI-anchor-deficient phenotype of alveolar macrophages and dendritic cells. (A) Alveolar macrophages (AM; left) or dendritic cells (DC; middle) cultured for 6 weeks were stained with mAb anti-HSA. Peripheral blood leukocytes gated for lymphocytes (PBL) were stained with mAb anti-CD48 (right), which is expressed on both T and B lymphocytes. Shown are fluorescence intensity plots of cells from littermate control (thin dotted lines) or LysMCre/Pig-aflox mice (bold lines), and antibody controls (dashed lines). (B-C) Responses to LPS. Control (wild type [WT]; •) or GPI-anchor-deficient (knock out [KO]; ○) AMs (B) or DCs (C) were stimulated with LPS; mean concentrations of TNF-α released of at least 3 independent experiments are shown.
The latter assumption was supported by the analysis of long-term in vitro culture of myeloid DCs from LysMCre/Pig-aflox mice. It was anticipated that multiple proliferation cycles during culture of myeloid cells from these mice would generate a GPI-anchor-negative phenotype, due to a gradual dilution of the amount of GPI-anchor present during disruption of the Pig-a gene. Splenocytes were cultured with GM-CSF and fibroblast-derived growth factors to establish long-term myeloid DC lines.25 At the start of the culture, expression of GPI-anchored proteins on DCs from LysMCre/Pig-aflox mice was similar to that on cells from littermate controls (not shown). During 5 to 6 weeks of culture, expression of HSA (Figure 1A, middle) and CD48 (not shown) on DCs from LysMCre/Pig-aflox mice had gradually decreased to almost background levels. PCR analysis confirmed almost complete disruption of the Pig-a gene in these cells (not shown).
After stimulation of GPI-anchor-deficient AMs or DCs with LPS, particularly at a low concentration, release of TNF-α (Figure 1B-C) and up-regulation of the DC activation marker CD86 (not shown) were impaired, consistent with previous studies using macrophages deficient in the GPI-anchored protein CD14,31 and confirming their GPI-anchor-negative phenotype.
Thus, both freshly isolated AMs and cultured DCs from LysMCre/Pig-aflox mice exhibited a clear GPI-anchor-deficient phenotype, allowing further functional analysis of these myeloid cell types.
Normal binding and endocytosis of IgG-ICs by GPI-anchor-deficient AMs or DCs
Analysis by flow cytometry revealed that staining of GPI-anchor-deficient AMs or DCs with anti-FcγRII/III mAb 2.4G2 (Figure 2A, upper panels), with monomeric mouse IgG2a as a marker for FcγRI (Figure 2A, bottom panels), or with IgG-ICs (Figure 2B; Figure 2D, left panels) was similar to that of control cells. Binding of IgG-ICs to control DCs could be blocked by preincubation with mAb 2.4G2 (Figure 2C, middle), but not by an isotype-matched control mAb anti-CD11b (Figure 2C, right) or monomeric murine IgG2a (not shown), confirming their specific interaction with FcγR, in particular FcγRII/III. The percentages of GPI-anchor-deficient or control DCs that had endocytosed IgG-ICs were similar (Figure 2D). Thus, neither FcγR expression nor binding or endocytosis of IgG-ICs was affected by GPI-anchor deficiency.
Normal binding and endocytosis of IgG-immune complexes (IgG-ICs) by GPI-anchor-deficient DCs. (A) Staining of AMs (left panels) or DCs (right panels) with anti-FcγRII/III mAb 2.4G2 (top panels) or monomeric mouse IgG2a as a marker for FcγRI (bottom panels). (B) Staining of DCs with IgG-ICs; mean fluorescence intensity for WT cells was 111 and for KO cells was 165. (A-B) Shown are fluorescence intensity plots of cells from littermate control (thin lines) or LysMCre/Pig-aflox mice (bold lines), and antibody controls (dotted lines). (C) Blocking of IgG-IC binding by 2.4G2. Control DCs were preincubated with medium (left), 2.4G2 (25 μg/mL; middle), or isotype-matched control mAb anti-CD11b (M1/70; 25 μg/mL; right) for 30 minutes on ice, and next stained with IgG-ICs (solid line) or medium (dotted line). (D) Endocytosis of IgG-ICs. Control (WT, top panels) or GPI-anchor-deficient (KO, bottom panels) DCs were incubated on ice with FITC-containing IgGICs, washed, and kept on ice (4°C; left panels) or incubated at 37°C for 20 minutes (middle panels) or 30 minutes (right panels). Percentages of cells having endocytosed IgG-ICs (ie, gated cells exhibiting decreased extracellular IgG-IC staining [FL2] and unchanged FL1 signal) are shown in the upper right quadrants.
Normal binding and endocytosis of IgG-immune complexes (IgG-ICs) by GPI-anchor-deficient DCs. (A) Staining of AMs (left panels) or DCs (right panels) with anti-FcγRII/III mAb 2.4G2 (top panels) or monomeric mouse IgG2a as a marker for FcγRI (bottom panels). (B) Staining of DCs with IgG-ICs; mean fluorescence intensity for WT cells was 111 and for KO cells was 165. (A-B) Shown are fluorescence intensity plots of cells from littermate control (thin lines) or LysMCre/Pig-aflox mice (bold lines), and antibody controls (dotted lines). (C) Blocking of IgG-IC binding by 2.4G2. Control DCs were preincubated with medium (left), 2.4G2 (25 μg/mL; middle), or isotype-matched control mAb anti-CD11b (M1/70; 25 μg/mL; right) for 30 minutes on ice, and next stained with IgG-ICs (solid line) or medium (dotted line). (D) Endocytosis of IgG-ICs. Control (WT, top panels) or GPI-anchor-deficient (KO, bottom panels) DCs were incubated on ice with FITC-containing IgGICs, washed, and kept on ice (4°C; left panels) or incubated at 37°C for 20 minutes (middle panels) or 30 minutes (right panels). Percentages of cells having endocytosed IgG-ICs (ie, gated cells exhibiting decreased extracellular IgG-IC staining [FL2] and unchanged FL1 signal) are shown in the upper right quadrants.
Impaired effector functions of GPI-anchor-deficient AMs and DCs in response to IgG-IC stimulation
Next, the effect of GPI-anchor deficiency on the proinflammatory response of AMs to IgG-IC stimulation was investigated. While control AMs stimulated with IgG-ICs released high levels of TNF-α, almost no TNF-α could be detected in the supernatants of GPI-anchor-deficient AMs, at either antibody-antigen ratio used (Figure 3A). Similar results were obtained when using larger insoluble IgG-ICs prepared from rabbit IgG anti-BSA and BSA (Figure 3B). Thus, the GPI-anchor is critical for the release of TNF-α by AMs in response to IgG-ICs.
Impaired responses of AMs or DCs to IgG-IC stimulation. (A-B) AMs were stimulated with (A) IgG-ICs preformed with a fixed concentration of rabbit IgG anti-OVA and 0.1 (IC-0.1), 1 (IC-1), or 10 (IC-10) μg/mL OVA or with (B) insoluble IgG-ICs (IIC), preformed using rabbit IgG anti-BSA and BSA, at different concentrations. (C-D) DCs were stimulated with (C) IgG-ICs prepared as in panel A or with (D) different concentrations of PMA. (A-D) Shown are mean concentrations of TNF-α released ± SEM of at least 3 independent experiments. (E) CD86 expression after incubation of DCs with IgG-ICs. Broken lines indicate control DCs; and bold lines, GPI-anchor-deficient DCs; shown are fluorescence intensity plots representative of 3 experiments. (F) IgG-IC induced presentation of OVA epitopes to OVA-specific T cells. DCs were incubated with medium (med), IgG-ICs prepared as for panel A, or 10 μg/mL OVA alone (ova-10) as control, and proliferation of OVA-specific T cells was assessed by measuring [3H]-thymidine uptake; shown are mean triplicate values ± SEM of a representative experiment. (A-D,F) ▪ indicates control cells (WT); ▦, GPI-anchor-deficient cells (KO). *Significant differences (P < .05) with values for WT cells.
Impaired responses of AMs or DCs to IgG-IC stimulation. (A-B) AMs were stimulated with (A) IgG-ICs preformed with a fixed concentration of rabbit IgG anti-OVA and 0.1 (IC-0.1), 1 (IC-1), or 10 (IC-10) μg/mL OVA or with (B) insoluble IgG-ICs (IIC), preformed using rabbit IgG anti-BSA and BSA, at different concentrations. (C-D) DCs were stimulated with (C) IgG-ICs prepared as in panel A or with (D) different concentrations of PMA. (A-D) Shown are mean concentrations of TNF-α released ± SEM of at least 3 independent experiments. (E) CD86 expression after incubation of DCs with IgG-ICs. Broken lines indicate control DCs; and bold lines, GPI-anchor-deficient DCs; shown are fluorescence intensity plots representative of 3 experiments. (F) IgG-IC induced presentation of OVA epitopes to OVA-specific T cells. DCs were incubated with medium (med), IgG-ICs prepared as for panel A, or 10 μg/mL OVA alone (ova-10) as control, and proliferation of OVA-specific T cells was assessed by measuring [3H]-thymidine uptake; shown are mean triplicate values ± SEM of a representative experiment. (A-D,F) ▪ indicates control cells (WT); ▦, GPI-anchor-deficient cells (KO). *Significant differences (P < .05) with values for WT cells.
Immature DCs must undergo a maturation step to acquire a full antigen-presenting capacity,32 characterized by the production of TNF-α,25 and up-regulation of costimulatory molecules such as CD86. IgG-ICs can induce maturation of DCs in an FcγR-dependent manner.33 Incubation of GPI-anchor-deficient DCs with IgG-ICs resulted in strongly impaired levels of released TNF-α compared with control DCs (Figure 3C). PMA, a protein kinase C activator, induced similar levels of TNF-α released by control or GPI-anchor-deficient DCs (Figure 3D), indicating that GPI-anchor-deficient cells have the intrinsic capacity to produce TNF-α when triggered through a signal downstream of FcγR.
IgG-IC-mediated up-regulation of the maturation marker CD86 on GPI-anchor-deficient DCs was markedly impaired (Figure 3E). Presentation of IgG-IC-derived OVA epitopes to OVA-specific T cells was less efficient when GPI-anchor-deficient DCs were used than when control DCs were used as antigen-presenting cells (Figure 3F). Thus, IgG-IC-mediated maturation and antigen presentation by DCs require the presence of the GPI-anchor.
Requirement of the GPI-anchor for FcR γ-chain tyrosine phosphorylation
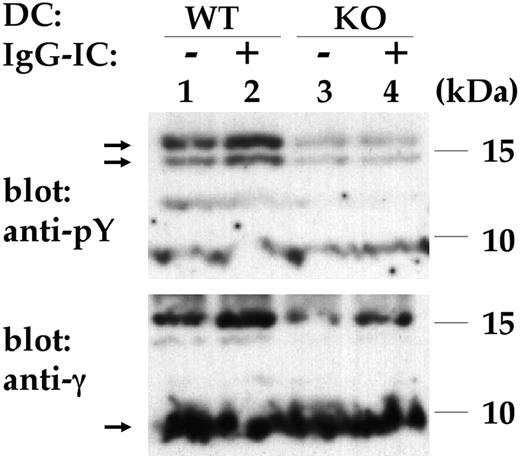
Next, tyrosine phosphorylation of the FcR γ-chain, an initial FcγR intracellular signal, was studied. The FcR γ-chain was immunoprecipitated from lysates of resting or IgG-IC-stimulated DCs, and detected by antiphosphotyrosine and anti-γ chain Abs using immunoblotting. Stimulation of control DCs with IgG-ICs induced significant tyrosine phosphorylation of the FcR γ-chain (Figure 4, lanes 1-2). Phosphorylated FcR γ-chain had a reduced mobility, consistent with previous observations.34 In contrast, incubation of GPI-anchor-deficient cells with IgG-ICs did not result in enhanced FcR γ-chain tyrosine phosphorylation (Figure 4, lanes 3-4). This demonstrates the requirement of the GPI-anchor for tyrosine phosphorylation of the FcR γ-chain in response to IgG-ICs. Diminished FcR γ-chain tyrosine phosphorylation in GPI-anchor-deficient cells is likely the main cause for the impaired downstream FcγR effector functions observed.
Impaired FcR γ-chain tyrosine phosphorylation. Control (WT) or GPI-anchor-deficient (KO) DCs were incubated without (-) or with (+) IgG-ICs and lysed. The FcR γ-chain was immunoprecipitated and was detected by antiphosphotyrosine (anti-pY; top blot) and anti γ-chain (anti-γ; bottom blot) Abs using immunoblotting. Lane 1 indicates unstimulated WT DCs; lane 2, WT DCs stimulated with IgG-ICs; lane 3, unstimulated KO DCs; and lane 4, KO DCs with IgG-ICs. Nonphosphorylated γ-chain migrated around approximately 9 to 10 kDa, typically appearing as a rather blurry band (arrow in bottom blot), phosphorylated γ-chain appeared as 2 bands around 15 kDa (arrows in top blot). IgG-IC-induced FcR γ-chain phosphorylation is impaired in GPI-anchor-deficient cells. Shown is the result of 1 representative experiment of 3.
Impaired FcR γ-chain tyrosine phosphorylation. Control (WT) or GPI-anchor-deficient (KO) DCs were incubated without (-) or with (+) IgG-ICs and lysed. The FcR γ-chain was immunoprecipitated and was detected by antiphosphotyrosine (anti-pY; top blot) and anti γ-chain (anti-γ; bottom blot) Abs using immunoblotting. Lane 1 indicates unstimulated WT DCs; lane 2, WT DCs stimulated with IgG-ICs; lane 3, unstimulated KO DCs; and lane 4, KO DCs with IgG-ICs. Nonphosphorylated γ-chain migrated around approximately 9 to 10 kDa, typically appearing as a rather blurry band (arrow in bottom blot), phosphorylated γ-chain appeared as 2 bands around 15 kDa (arrows in top blot). IgG-IC-induced FcR γ-chain phosphorylation is impaired in GPI-anchor-deficient cells. Shown is the result of 1 representative experiment of 3.
Attenuated IgG-IC alveolitis in LysMCre/Pig-aflox mice
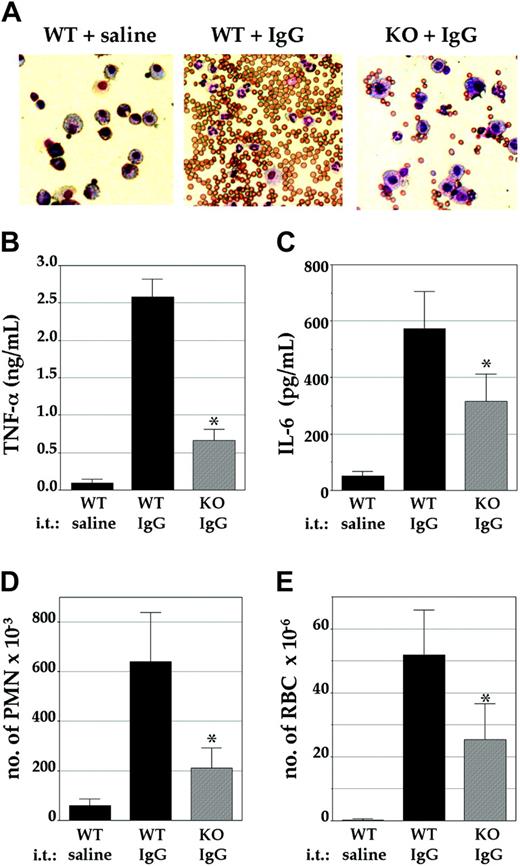
Finally, the severity of IgG-IC-mediated alveolitis, an in vivo model for inflammation involving FcγR, was studied in these mice. This reaction is initiated by the local formation of IgG-ICs, which stimulate AMs to release proinflammatory mediators followed by an influx of inflammatory neutrophils and hemorrhage.27 After intravenous injection of littermate control mice with OVA and intratracheal injection with rabbit IgG anti-OVA, a vigorous inflammatory reaction was observed, as visualized by the dominant appearance of neutrophils and red blood cells in the BAL fluids (Figure 5A, middle). Examination of BAL fluids from LysMCre/Pigaflox mice revealed a markedly weaker inflammatory reaction (Figure 5A, right). Quantitative analysis demonstrated a significant impairment in local release of the proinflammatory cytokines TNF-α and IL-6 (Figure 5B-C), numbers of inflammatory neutrophils (Figure 5D), and numbers of red blood cells (Figure 5E), a measure for hemorrhage, in the BAL fluids of these mice. The numbers of AMs in the BAL fluids of LysMCre/Pig-aflox mice and in littermate controls, before and after the induction of alveolitis, were similar (not shown). Since neutrophils from LysMCre/Pigaflox mice were found to have a normal expression of GPI-anchored proteins (ie, CD48, HSA, and Gr-1 [not shown]), the impaired neutrophil influx is likely caused by the reduced production of proinflammatory mediators by GPI-anchor-deficient AMs. Together, these results indicate that the severity of IgG-IC-induced alveolitis is significantly reduced in LysMCre/Pig-aflox mice.
Attenuated IgG-IC-induced alveolar inflammation. Mice were injected intravenously with OVA, and intratracheally (i.t.) with either rabbit IgG anti-OVA or saline as control. Then, 4 hours later, the bronchoalveolar (BAL) fluids were isolated for further analysis. (A) The cellular content of the BAL fluids was examined by microscopy. (Left) Control mice injected intratracheally with saline. (Middle) Control mice injected intratracheally with IgG anti-OVA. (Right) LysMCre/Pig-aflox mice injected intratracheally with IgG anti-OVA. Concentrations of (B) TNF-α and (C) IL-6, and numbers of (D) inflammatory neutrophils (PMN) and (E) red blood cells (RBC) in the BAL fluids were determined. (B-D) Shown are mean values ± SEM of at least 3 independent experiments in control mice (WT; ▪) or LysMCre/Pig-aflox mice (KO; ▦). *Significant differences between WT and KO mice (P < .05).
Attenuated IgG-IC-induced alveolar inflammation. Mice were injected intravenously with OVA, and intratracheally (i.t.) with either rabbit IgG anti-OVA or saline as control. Then, 4 hours later, the bronchoalveolar (BAL) fluids were isolated for further analysis. (A) The cellular content of the BAL fluids was examined by microscopy. (Left) Control mice injected intratracheally with saline. (Middle) Control mice injected intratracheally with IgG anti-OVA. (Right) LysMCre/Pig-aflox mice injected intratracheally with IgG anti-OVA. Concentrations of (B) TNF-α and (C) IL-6, and numbers of (D) inflammatory neutrophils (PMN) and (E) red blood cells (RBC) in the BAL fluids were determined. (B-D) Shown are mean values ± SEM of at least 3 independent experiments in control mice (WT; ▪) or LysMCre/Pig-aflox mice (KO; ▦). *Significant differences between WT and KO mice (P < .05).
Discussion
The present study reveals an as-yet-unrecognized strong and physiologically relevant cooperation between GPI-anchored proteins and transmembrane FcγRs. Myeloid GPI-anchor deficiency caused impairment in several FcγR effector functions in response to IgG-ICs, such as TNF-α production, DC maturation and antigen presentation, and FcR γ-chain tyrosine phosphorylation. In vivo relevance of these defective responses to IgG-ICs was demonstrated by impaired inflammatory responses during alveolitis, an in vivo model for IgG-IC-mediated inflammation, in myeloid-specific GPI-anchor-deficient mice. These findings provide genetic evidence for an indispensable role of GPI-anchored proteins in biologically relevant FcγR effector functions in response to the model FcγR ligand IgG-IC in vitro and in vivo. These results extend, and affirm the biologic significance of, previous in vitro studies showing a synergistic enhancement of transmembrane FcγR signaling by forced cocrosslinking with GPI-anchored proteins using monoclonal antibodies.15-17
The present experiments were performed using primary murine myeloid cells, which express transmembrane FcγR. Control or GPI-anchor-deficient AMs and DCs expressed similar high levels of FcγRII/III and low levels of FcγRI (Figure 2A). Binding of IgG-ICs could be blocked by anti-FcγRII/III mAb 2.4G2 (Figure 2C), while murine IgG2a had no effect. Thus, these IgG-ICs interact predominantly with FcγRII/III on these cells, while FcγRI plays no significant role. Since FcγRIII positively mediates IgG-IC-induced inflammatory responses,8,35 while FcγRII inhibits those responses,8,36 it is likely that FcγRIII cooperates with GPI-anchored proteins. This is supported by impaired tyrosine phosphorylation of the FcR γ-chain, essential for FcγRIII but not FcγRII function, observed in GPI-anchor-deficient cells. The observation that endocytosis of IgG-ICs was not affected by GPI-anchor deficiency (Figure 2D) may reflect either the role of FcγRII in ingestion35,37 or a GPI-anchor-independent role for FcγRIII in endocytosis.
A previous study17 reported a decrease in phosphorylation of transfected human FcγRIIA upon forced cocrosslinking with GPI-anchored proteins, in contrast to the GPI-anchored protein-mediated enhanced phosphorylation of the FcR γ-chain suggested by the present observations. This difference may be attributed to different experimental conditions in the previous study, such as overexpression or monoclonal antibody-induced cocrosslinking of FcγR and GPI-anchored proteins in Jurkat T cells; alternatively, it is possible that human FcγRIIA, which has its own immunoreceptor tyrosine-based activation motif and does not depend on the γ-chain for signaling, and murine γ-chain-associated FcγR use different initial signaling pathways upon interaction with GPI-anchored proteins.
It remains to be established whether the GPI-lipid anchor itself, which is common among all GPI-anchored proteins, or the protein portion, which is unique, is critical for FcγR function. At least 5 different GPI-anchored proteins have been shown to physically or functionally associate with transmembrane FcγR.12-17 This suggests that in principle any GPI-anchored protein may associate with FcγR, and makes a role for the lipid anchor in cooperation with FcγR most likely. This is further supported by the observation that human FcγRIIIB, which is normally GPI-anchored, lost its ability to synergistically enhance transmembrane FcγRIIA signaling upon cocrosslinking when its GPI-anchor was replaced by a transmembrane anchor.17
Recently, GPI-anchored proteins have received increased attention since they were found highly concentrated in detergent-insoluble membranes or lipid rafts.2,3 Since these rafts concentrate various surface receptors upon extracellular crosslinking by their ligands and intracellular signaling components including Src tyrosine kinases, they have been proposed to function as signaling platforms.38 Analogous to other multichain immune recognition receptors, including the high-affinity IgE receptor FcϵRI, the IgA receptor FcαRI, and the T-cell receptor (TCR), FcγRs require association with lipid rafts for ligand-induced signaling through Src kinases.10,11,39 The mechanism of association of FcγRs with lipid rafts remains unclear. Since FcγRs and GPI-anchored proteins can physically associate,12-14 it is possible that GPI-anchored proteins are involved in stabilizing association of FcγRs with lipid rafts necessary for downstream signaling. Inefficient raft association would prevent productive contact of FcγRs with intracellular kinases, consistent with the impaired tyrosine phosphorylation of the FcR γ-chain and further downstream effector functions observed here in GPI-anchor-deficient cells.
Cooperation with GPI-anchored proteins may not be restricted to FcγRs, and may apply to other lipid raft-targeting multichain immune recognition receptors. It is noteworthy that coengagement of the TCR and the GPI-anchored protein CD48 enhances TCR association with rafts and phosphorylation of the TCR ζ-chain.40 Further insight into the structural aspects of the interactions between GPI-anchored proteins and FcγR may identify new means to attenuate FcγR-mediated inflammatory functions, being relevant for therapeutic intervention in immune complex-mediated inflammatory diseases.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-02-0671.
Supported by the Japanese Society for the Promotion of Science, The Novartis Foundation in Japan, and the Ministry of Education, Science, Sports, Culture and Technology of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to express their gratitude to I. Förster for kindly providing the LysMCre mouse strain; H. Morisaki for help with artwork; and R. van Furth, A. Ioan-Facsinay, A. Kumanogoh, Y. Maeda, Y. Murakami, J. S. Verbeek, J. A. Villadangos, and J. G. J. van de Winkel for helpful discussions.

![Figure 1. GPI-anchor-deficient phenotype of alveolar macrophages and dendritic cells. (A) Alveolar macrophages (AM; left) or dendritic cells (DC; middle) cultured for 6 weeks were stained with mAb anti-HSA. Peripheral blood leukocytes gated for lymphocytes (PBL) were stained with mAb anti-CD48 (right), which is expressed on both T and B lymphocytes. Shown are fluorescence intensity plots of cells from littermate control (thin dotted lines) or LysMCre/Pig-aflox mice (bold lines), and antibody controls (dashed lines). (B-C) Responses to LPS. Control (wild type [WT]; •) or GPI-anchor-deficient (knock out [KO]; ○) AMs (B) or DCs (C) were stimulated with LPS; mean concentrations of TNF-α released of at least 3 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-02-0671/6/m_zh80210468490001.jpeg?Expires=1767742536&Signature=xGj0to7DHXuoTvFhTEeXYjyO1GtTT4mI3EV2medsiGQjRDBkd7M~tKiXhcH1ZogQpuow4nxhqtr0xAGo5gBzri-6DI58XC-XRbScFk1JQtY~dqV4B~AqmZWv4etmHfbfBSb9w-EKpEnNrLI~WTK04TW30wh-2j2sX3qNAhV1PXLUO5yWSGlYJc8FpcMq6GH81~4mE-fDsgD1JkXoo2JljFfEkAFsmz5uu6s-HFxFnyg8XDWI4X7KfGvBLId1VTPJHC6AbHNSV~MY8XvrolE~iSimsHNIcqDBmS9Rdfl1Dhn0LL00H7eev0wxvdLN46GDiBFf0-4oDEyMgzHTEBbzvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Normal binding and endocytosis of IgG-immune complexes (IgG-ICs) by GPI-anchor-deficient DCs. (A) Staining of AMs (left panels) or DCs (right panels) with anti-FcγRII/III mAb 2.4G2 (top panels) or monomeric mouse IgG2a as a marker for FcγRI (bottom panels). (B) Staining of DCs with IgG-ICs; mean fluorescence intensity for WT cells was 111 and for KO cells was 165. (A-B) Shown are fluorescence intensity plots of cells from littermate control (thin lines) or LysMCre/Pig-aflox mice (bold lines), and antibody controls (dotted lines). (C) Blocking of IgG-IC binding by 2.4G2. Control DCs were preincubated with medium (left), 2.4G2 (25 μg/mL; middle), or isotype-matched control mAb anti-CD11b (M1/70; 25 μg/mL; right) for 30 minutes on ice, and next stained with IgG-ICs (solid line) or medium (dotted line). (D) Endocytosis of IgG-ICs. Control (WT, top panels) or GPI-anchor-deficient (KO, bottom panels) DCs were incubated on ice with FITC-containing IgGICs, washed, and kept on ice (4°C; left panels) or incubated at 37°C for 20 minutes (middle panels) or 30 minutes (right panels). Percentages of cells having endocytosed IgG-ICs (ie, gated cells exhibiting decreased extracellular IgG-IC staining [FL2] and unchanged FL1 signal) are shown in the upper right quadrants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-02-0671/6/m_zh80210468490002.jpeg?Expires=1767742536&Signature=p-ZbredzOWNG8sc9VlCr1LrqA-HAP78ue1Uh3uER-MdmnECIgKN8LOAS~FTGzkO7-tbXRFAOcoeqhed-7Ve-~40HshxbVBdr03SDChIq4YjXlxDUzQCoONH5WNvs650NcfUudONXaDuyvKwW-Knrb7O02eySOqHL0-c~r5wNy2s0BQqOPBj4wsTbZtV9DvQ~ojJNaEbxgDn8JF-WZBJz9Z~CaJYBVAZw~EycGO4ljVBB9iTNw7nPKNzd3ta4JvyiteaUT7staKht-DD17vzrOxNN2wvT-k4Q3kD5XPhuor7toTkbZZjMcRP~4fqyniaMSis8JFFMqgALt6YA6RY~Jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Impaired responses of AMs or DCs to IgG-IC stimulation. (A-B) AMs were stimulated with (A) IgG-ICs preformed with a fixed concentration of rabbit IgG anti-OVA and 0.1 (IC-0.1), 1 (IC-1), or 10 (IC-10) μg/mL OVA or with (B) insoluble IgG-ICs (IIC), preformed using rabbit IgG anti-BSA and BSA, at different concentrations. (C-D) DCs were stimulated with (C) IgG-ICs prepared as in panel A or with (D) different concentrations of PMA. (A-D) Shown are mean concentrations of TNF-α released ± SEM of at least 3 independent experiments. (E) CD86 expression after incubation of DCs with IgG-ICs. Broken lines indicate control DCs; and bold lines, GPI-anchor-deficient DCs; shown are fluorescence intensity plots representative of 3 experiments. (F) IgG-IC induced presentation of OVA epitopes to OVA-specific T cells. DCs were incubated with medium (med), IgG-ICs prepared as for panel A, or 10 μg/mL OVA alone (ova-10) as control, and proliferation of OVA-specific T cells was assessed by measuring [3H]-thymidine uptake; shown are mean triplicate values ± SEM of a representative experiment. (A-D,F) ▪ indicates control cells (WT); ▦, GPI-anchor-deficient cells (KO). *Significant differences (P < .05) with values for WT cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-02-0671/6/m_zh80210468490003.jpeg?Expires=1767742536&Signature=w6a-OaxwqbBqP~v65dxG0jj17-JDub3CQ6jWTdMcoiyKPSoj7lTx4Lp-sCsxhcDQWchTTG5YBp6fXK9-CHhd5s3bMs1q3ABv6nhICeWoND3wgWhJ0knYVszSkT5DC15lkSZiDBZhMkRyrk1NEFmCNP~yB4XXZm26hK9qqMg7qkZNroEy4j2-HmTIGOhyoeyZhqhaRSL3DcDUDakibUCmKR2FSIJ5iktg5v~u6RqlMR73JhAYvqCfXRAY-5bvyRR3OC2MlpPn2FSK72oRCN2zzEriJUM8L0lW6LFxHD4pmQj6CoRsrC6D0fzGlnksI1UEodwj0xTuDTkBgrfuW2SB6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal