Abstract
Basophils, recruited from the blood to tissues, have been implicated by their presence in diverse allergic disorders including bronchial asthma, allergic rhinitis, and cutaneous contact hypersensitivity. We hypothesized that like other leukocytes involved in inflammatory responses, basophils would express members of the leukocyte immunoglobulin-like receptor (LIR) family of immuno-regulatory molecules on their cell surface. We identified LIR7, an activating member coupled to the common Fc receptor gamma chain, and LIR3, an inhibitory member containing cytoplasmic immunoreceptor tyrosine-based inhibitory motifs, on these cells from human peripheral blood. Cross-linking of LIR7 resulted in the concentration-dependent net release of histamine (29.8 ± 10.8%) and cysteinyl leukotrienes (cysLTs) (31.4 ± 8.7 ng/106 basophils) that were maximal at 30 minutes, and of interleukin-4 (IL-4) (410.2 ± 61.6 pg/106 basophils) that was maximal at 4 hours and comparable with the response initiated by cross-linking of the high-affinity receptor for immunoglobulin E (FcϵRI). Coligation of LIR3 to LIR7 or to FcϵRI by means of a second monoclonal antibody significantly inhibited net histamine release, cysLT production, and IL-4 generation. That LIR3 is profoundly counter-regulatory for both adaptive and innate receptors suggests a broad role in containment of the inflammatory response.
Introduction
Because they resemble mast cells in their panel of mediators and cell surface expression of the high-affinity receptor for the Fc portion of immunoglobulin E (FcϵRI), peripheral blood basophils have in the past often been studied as mast cell surrogates due to the relative ease with which they can be isolated. However, basophils differ importantly from mast cells in their developmental pathway and limited supply of effector molecules. Developmentally, basophils originate and mature in the bone marrow, circulating as terminally differentiated cells, while mast cells circulate as precursors and complete their development after transendothelial migration to specific tissues.1-3 Indeed, basophils bear a closer developmental relationship to eosinophils than to mast cells.4,5 A case report documents the absence of both eosinophils and basophils in a single patient,6 possibly secondary to destruction or dysfunction of a shared progenitor cell. Infection of Ws/Ws rats, which are deficient in mast cells due to a mutation in c-kit, with the parasite Nippostrongylus brasiliensis led to a more than 50-fold increase in peripheral blood basophils, equal to that observed in wild-type rats.7 Basophils also appear to have a limited capacity to provide inflammatory mediators in comparison with mast cells.8 Those currently recognized include histamine stored in preformed granules, leukotriene (LT) C4 generated from arachidonic acid with cell activation, and interleukin (IL) 4 induced also by activation.9 That basophils can provide IL-4 has led to the suggestion that these cells may reinforce T helper 2 (Th2) cell polarization.10-12 Basophils are also able to express CD40 ligand on their surface and have been shown to independently support immunoglobulin E (IgE) production by B cells in vitro.10
Basophils have been implicated in a number of human diseases including bronchial asthma, allergic rhinitis, and cutaneous contact hypersensitivity. Increased numbers of basophils, as detected by immunohistochemistry using monoclonal antibody (mAb) 2D7, are found in bronchial biopsy specimens from patients with fatal asthma.13 Compared with nonasthmatic controls, patients with allergic asthma had more basophils in airway biopsy tissue prior to inhaled allergen challenge, and there was a significant increase in tissue basophils 24 hours after challenge.14 In patients with allergic rhinitis, allergen challenge in the nose released histamine, cysteinyl leukotrienes, and prostaglandin (PG) D2 in the immediate phase response, and histamine and cysteinyl leukotrienes without PGD2 in the late phase response (LPR).15 That mast cells but not basophils generate PGD2 suggests that basophils are the likely source of histamine and cysteinyl leukotrienes in the LPR. This is supported by the 12-fold rise in Alcian blue-positive cells in nasal lavage fluids during the LPR, approximately 70% of which are basophils by morphologic criteria.16 Histologic studies of skin lesions from patients with allergic contact dermatitis to urushiol/oleoresin, responsible for poison ivy reactions, which is not IgE-dependent, demonstrate substantial infiltration of basophils, basophil degranulation, and evidence of increased vascular permeability.17-19 Serial biopsies of one patient showed that basophils enter the dermis after lymphocytes but prior to the arrival of eosinophils, and account for up to 1 in 6 of the inflammatory cells. Control biopsies from the same individual after antigen-independent skin trauma with liquid nitrogen did not evoke tissue basophilia. Thus, basophils accumulate and are activated in both IgE-mediated and T-cell-dependent allergic diseases.
Given their relatively low concentrations of preformed effector molecules, their ability to release immunomodulatory cytokines, their expression of costimulatory molecules, and their prominence in different classes of allergic reactions, we decided to determine the presence and function on basophils of a novel set of receptors with both activating and inhibitory members. The leukocyte immunoglobulin-like receptors (LIRs),20,21 also called immunoglobulin-like transcripts (ILTs)22-24 and recently given the CD designation CD85a-h,25 are a set of Ig superfamily proteins expressed by leukocytes. The LIRs are encoded on 2 loci separated by a region of approximately 200 kb within the leukocyte receptor complex on chromosome 19q13.4.26,27 Genes encoding LIR3, ILT8, LIR8, LIR2, LIR4, ILT11, and ILT7 lie in the centromeric cluster and genes encoding LIR7, LIR6, LIR1, and LIR5, and the pseudogenes ILT9 and ILT10 lie in the telomeric cluster, immediately adjacent to the genes encoding the killer cell Ig-like receptors (KIRs).28 The inhibitory LIRs have cytoplasmic domains containing 2 to 4 immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that recruit protein tyrosine phosphatases to inhibit cell activation signals.29 The activating LIRs have truncated cytoplasmic domains and possess a charged arginine residue in their transmembrane domain through which they associate with the immunoreceptor tyrosine-based activating motif (ITAM)-containing common Fc receptor gamma chain.30 We found that activation of LIR7, which like FcϵRI signals through the Fc receptor gamma chain, provides a comparable profile of mediators to activation through FcϵRI and that coligation of LIR3 to either FcϵRI or LIR7 down-regulates the response to cross-linking of each receptor.
Materials and methods
Reagents
PIPES (piperazine-N, N′-bis[2-ethanesulfonic acid]), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), human serum albumin (HSA), cycloheximide, and protein A sepharose were from Sigma (St Louis, MO). PIPES buffer was 25 mM PIPES, 110 mM NaCl, 5 mM KCl, pH 7.4. Percoll (Pharmacia, Uppsala, Sweden) was equilibrated 9:1 (vol/vol) with 10× PIPES buffer, and then diluted with PIPES buffer to a specific gravity of 1.0863. PAG buffer was PIPES buffer with 0.003% HSA and 0.1% dextrose (Fisher Scientific, Suwanee, GA). PAB buffer was 1× phosphate-buffered saline (PBS) with 0.05% NaN3 and 1% bovine serum albumin (BSA). RPMI medium was RPMI 1640 (Gibco, Invitrogen, Carlsbad, CA; or Cellgro, Mediatech), 10% heat-inactivated fetal bovine serum (Sigma), 100 U/mL penicillin, 100 μg/mL streptomycin, nonessential amino acids (Cellgro; Mediatech, Herndon, VA), and 25 mM HEPES. Recombinant human IL-3 was from Pierce Endogen (Woburn, MA) or from R and D Systems (Minneapolis, MN)
Monoclonal mouse IgG1 antibodies (mAbs) against LIRs 1, 2, 3, 5, 6, and 8 were previously described20,31 and are designated m401, m421, m431, m451, m467, and m481, respectively. We used 2 murine IgG1 antibodies directed against LIR7, designated m471 and m473. Mouse IgG1 against FcϵRI, clone 15A5,32 was provided by R. Chizzonite (Hoffmann-La Roche, Nutley, NJ). Mouse IgG1 of irrelevant specificity was from BioSource International (Camarillo, CA). Fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse IgG1 (F(ab′)2-specific) with minimal cross-reactivity to human, rat, and bovine serum; normal goat serum; goat F(ab′)2 anti-mouse IgG (heavy and light chain-specific, minimal cross-reactivity to human, rat, and horse serum); and rabbit anti-goat IgG (F(ab′)2-specific) were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Isolation and LIR epitope expression of human basophils
Basophils, but not other granulocytes, cosediment with human peripheral blood mononuclear cells (PBMCs) in density gradients. Therefore, venous blood was anticoagulated with EDTA (ethylenediaminetetraacetic acid), diluted with 0.9% NaCl, layered over Percoll, and centrifuged at 300g to separate the PBMC population. Highly purified basophils were isolated from PBMCs by negative selection with antibody-coated magnetic beads (Miltenyi Biotech, Auburn, CA). Purity of basophils was assessed by Alcian blue or Toluidine blue staining and was 92.3 ± 2.5% (n = 12).
LIR expression on purified human basophils was determined by flow cytometry using mAbs (10 μg/mL) to individual LIRs and FITC-conjugated goat F(ab′)2 anti-mouse IgG1 (F(ab′)2-specific) as described for human eosinophils.33 m471 was used to identify LIR7 expression. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Results were interpreted using Cell Quest software (Becton Dickinson). A unimodal shift in fluorescence intensity of cells stained with specific antibodies compared with the isotype-matched negative control IgG1 antibody was considered positive.
Stimulation of basophils for release of histamine, cysteinyl leukotrienes, and IL-4
For experiments in which release of histamine and LTC4 was measured, Fcγ receptors on PBMCs or purified basophils were blocked with 25% human IgG (Miltenyi Biotech) and pretreated with IL-3 (7.5 ng/mL) in PAG buffer, at 1 × 107 cells/mL on ice for 1 hour. Thereafter, portions of basophils (low or high purity, as indicated in the results) were incubated with mAbs to LIR7 (m473), to FcϵRI (clone 15A5), or with control IgG1 at 5 × 105 basophils/mL in PAG containing IL-3 (10 ng/mL) for 30 minutes on ice. Cells were then washed in ice-cold PAG, centrifuged at 4°C, resuspended at 5 × 105 basophils/mL in PAG containing IL-3 (10 ng/mL), 1 mM CaCl2, 1 mM MgCl2, and 10 μg/mL goat anti-mouse IgG that was prewarmed to 37°C, and incubated at 37°C for the indicated times. Goat anti-mouse IgG was used to cross-link anti-FcϵRI or anti-LIR7 and for coligation of LIR3 to either FcϵRI or LIR7 for methodologic consistency. The reaction was stopped by cooling in an ice slurry for 1 minute, followed by centrifugation at 200g at 4°C for 10 minutes. The supernatants were stored at –20°C until they were assayed for histamine and LTC4. To determine the requirement for IL-3 priming, a parallel sample of cells was processed and stimulated as described, except that IL-3 was omitted from each step. Initial experiments were performed with m471 at 1 μg/mL. Subsequent experiments performed with m473 at 0.1 μg/mL provided similar release of histamine and LTC4.
For experiments in which release of IL-4 was measured, purified basophils were blocked and primed under the same conditions as for histamine and LTC4 release except that RPMI medium was used instead of PAG buffer, and cells were stimulated at a 2-fold greater density of 1 × 106/mL. To determine the effects of cycloheximide on IL-4 secretion, cycloheximide (1 to 10 μg/mL) was added to the RPMI medium during blocking and all subsequent steps.
Supernatant concentrations of histamine were determined by enzyme-linked immunoassay (EIA) (Beckman Coulter, Miami, FL). In parallel with the experimental samples, a portion of 5 × 104 basophils (low or high purity, depending on the experiment) was blocked with IgG, incubated on ice in PAG containing IL-3 (10 ng/mL) without a primary antibody, washed, and then lysed with 1.6% perchloric acid prewarmed to 37°C. The supernatant from this acid lysate was neutralized with 0.8 M potassium tetraborate (Sigma) and assayed to determine the total basophil histamine content. The histamine released into supernatants was expressed as a percent of this value.
Cysteinyl leukotriene (cysLT) concentrations were routinely measured by EIA (Amersham Biosciences, Piscataway, NJ). To confirm the identity and absolute concentration of LTC4 and its active metabolites, LTD4 and LTE4, released from basophils, some supernatants were analyzed by reverse-phase high-pressure liquid chromatography (RP-HPLC) as previously described.34,35
In the assay for IL-4, the goat anti-mouse IgG (heavy and light chain specific) used to cross-link LIR7 or FcϵRI binds to both the capture and detection antibodies used in the IL-4 EIA (Beckman Coulter) leading to false high background values.36 Therefore, the supernatants to be assayed for IL-4 were cleared of the goat anti-mouse IgG. Protein A sepharose beads (500 μL) were incubated for 8 to 12 hours at 4°C with 1.5 mg rabbit anti-goat IgG F(ab′)2-specific, pelleted, and resuspended in 0.02 M NaH2PO4, 0.15 M NaCl, pH 8.0 to make a 50% slurry. Bead slurry (40 μL) and diluent 1 (380 μL) from the IL-4 EIA kit (Immunotech) were added to each supernatant and incubated for 8 to 12 hours at 4°C with continuous mixing. Protein A sepharose beads were then pelleted by centrifugation, and IL-4 concentrations in the supernatants were measured by EIA. Separate experiments demonstrated that the goat anti-mouse IgG does not affect the histamine and cysLT EIAs.
Statistical analysis
Data are expressed as means ± SE. Statistical analyses of dose-response curves and kinetics were done by analysis of variance. Statistical comparisons between 2 sets of data were done by paired t tests. Differences were considered significant for P less than .05.
Results
Cytofluorgraphic analysis of LIR expression by human peripheral blood basophils
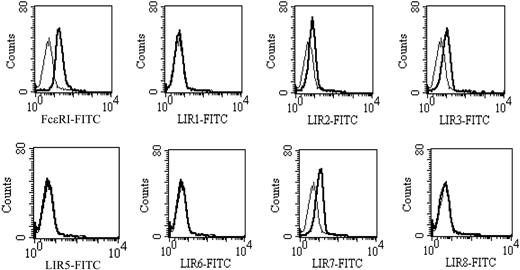
As a reference for an analysis of LIR expression on the basophil, a parallel study was conducted with monocytes (data not shown) that express each LIR for which there is a currently available mAb.20,33,37 Basophils from 7 of 7 donors invariably expressed a modest monophasic peak for LIR3 and LIR7, as illustrated for 1 donor (Figure 1) whose basophils also expressed minimal LIR2. Basophils from less than half the donor group of 7 individuals also expressed minimal LIR1 or LIR2. Mean fluorescence intensities for staining with control IgG1 and mAbs to LIR-3, LIR-7, and FcϵRI were 7.7 ± 1.2, 15.4 ± 1.4, 12.1 ± 1.5, and 19.4 ± 1.1, respectively (n = 7).
Cytofluorgraphic analysis of LIR expression on human basophils. Heavy lines depict staining with specific mAbs and light lines, their isotype controls (IgG1). mAb directed against FcϵRI was a positive control. Representative histograms from 1 of 7 donors are shown.
Cytofluorgraphic analysis of LIR expression on human basophils. Heavy lines depict staining with specific mAbs and light lines, their isotype controls (IgG1). mAb directed against FcϵRI was a positive control. Representative histograms from 1 of 7 donors are shown.
Cross-linking of LIR-7 on basophils in PBMCs or purified basophils
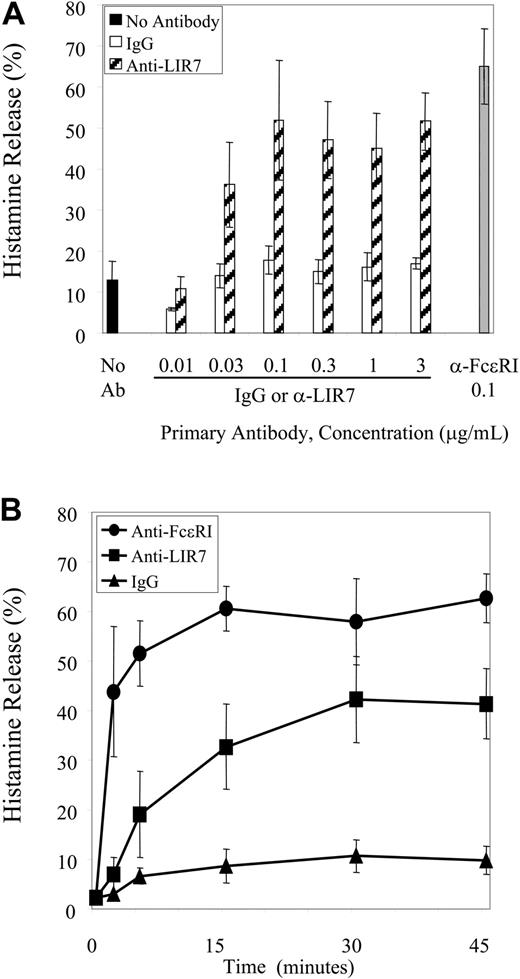
Because basophils are the only leukocytes in the PBMC fraction that contain histamine, the activation through LIR7 to elicit histamine release was examined using this mixed cell preparation. Basophils comprised 2.66% ± 0.37% of PBMCs in these experiments. Cross-linking of LIR7 elicited histamine release in a concentration-dependent manner (P < 10-4 versus IgG) with a plateau at 0.1 μg/mL (m473) (Figure 2A). The release of histamine following cross-linking of LIR7 began at the first time point studied with gradual progression to a plateau at 30 minutes (P < 10-5 versus IgG). These kinetics lagged the rapid histamine release observed with anti-FcϵRI that occurred within 2 minutes, with an early plateau between 5 and 15 minutes (Figure 2B). The maximal net release of histamine after incubation with 0.1 μg/mL anti-LIR7 and anti-FcϵRI for all 5 donors depicted in Figure 2 was 29.8 ± 10.8% and 50.1 ± 7.6%, respectively.
Concentration- and time-dependent release of histamine. (A) PBMCs were incubated with increasing concentrations of anti-LIR7 (m473) (▨), with an optimal concentration of anti-FcϵRI (0.1 μg/mL) (▦), or with control IgG1 (□), and then stimulated by addition of goat anti-mouse IgG for 45 minutes (n = 3). (B) PBMCs were incubated with 0.1 μg/mL anti-FcϵRI (•), anti-LIR7 (▪), or control IgG1 (▴) and then stimulated by addition of goat anti-mouse IgG for the indicated times (n = 4).
Concentration- and time-dependent release of histamine. (A) PBMCs were incubated with increasing concentrations of anti-LIR7 (m473) (▨), with an optimal concentration of anti-FcϵRI (0.1 μg/mL) (▦), or with control IgG1 (□), and then stimulated by addition of goat anti-mouse IgG for 45 minutes (n = 3). (B) PBMCs were incubated with 0.1 μg/mL anti-FcϵRI (•), anti-LIR7 (▪), or control IgG1 (▴) and then stimulated by addition of goat anti-mouse IgG for the indicated times (n = 4).
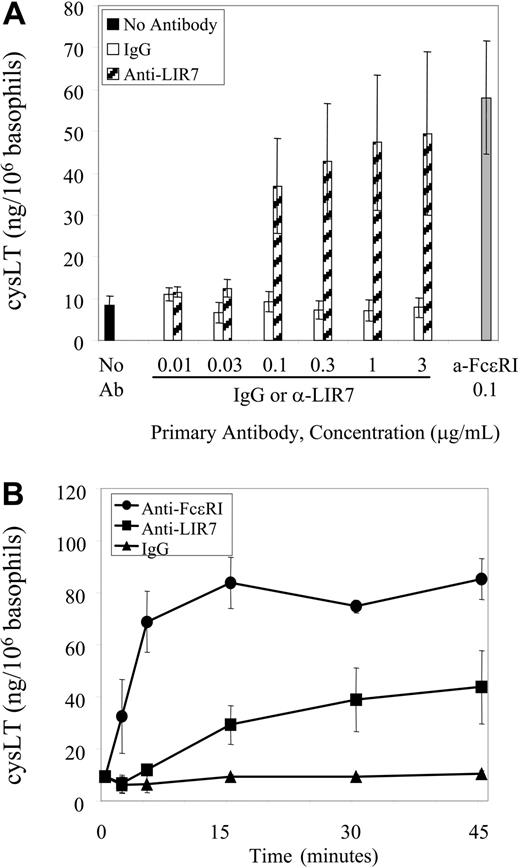
Cross-linking of LIR7 induced cysLT generation from the PBMC population in a concentration-dependent manner (P < .001 versus IgG) that was near maximal at 0.1 μg/mL (Figure 3A). Kinetic analysis revealed a definite lag for cysLT generation and release with initial detection at 5 minutes and progression to a plateau at 30 minutes (P < .001 versus IgG). In contrast, with anti-FcϵRI, initial detection was at 2 minutes with a plateau of cysLT generation by 5 minutes (Figure 3B). The maximal net release of cysLTs from PBMCs from all donors depicted in Figure 3 after incubation with 0.1 μg/mL anti-LIR7 and anti-FcϵRI was 31.4 ± 8.7 ng/106 basophils (n = 6) and 52.3 ± 11.4 ng/106 basophils (n = 5), respectively. As the EIA for cysLTs does not define whether the product is LTC4, LTD4, or LTE4, the supernatant from activation of purified basophils with anti-LIR7 cross-linking was analyzed by RP-HPLC. In 5 separate experiments, cross-linking of LIR7 on purified human basophils (> 99% purity) with 0.1 μg/mL anti-LIR7 and goat anti-mouse IgG for 45 minutes elicited the release of 40.9 ± 12.6 ng/106 basophils of only LTC4 that was not metabolized to LTD4 or to LTE4. However, RP-HPLC analysis of supernatants obtained after cross-linking of LIR7 on PBMCs under identical conditions demonstrated both LTC4 and its metabolite LTE4 (data not shown), suggesting that nonbasophil members of the PBMC population enzymatically converted basophil-derived LTC4.
Concentration- and time-dependent generation of cysteinyl leukotrienes. (A) PBMCs were incubated with increasing concentrations of anti-LIR7 (m473) (▨), with an optimal concentration of anti-FcϵRI (0.1 μg/mL) (▦), or with control IgG1 (□), and then stimulated by addition of goat anti-mouse IgG for 45 minutes (n = 3). (B) PBMCs were incubated with 0.1 μg/mL anti-FcϵRI (•), anti-LIR7 (▪), or control IgG1 (▴) and then stimulated by addition of goat anti-mouse IgG for the indicated times (n = 4).
Concentration- and time-dependent generation of cysteinyl leukotrienes. (A) PBMCs were incubated with increasing concentrations of anti-LIR7 (m473) (▨), with an optimal concentration of anti-FcϵRI (0.1 μg/mL) (▦), or with control IgG1 (□), and then stimulated by addition of goat anti-mouse IgG for 45 minutes (n = 3). (B) PBMCs were incubated with 0.1 μg/mL anti-FcϵRI (•), anti-LIR7 (▪), or control IgG1 (▴) and then stimulated by addition of goat anti-mouse IgG for the indicated times (n = 4).
To address the contribution of contaminating PBMCs to basophil histamine and leukotriene release, high-purity and low-purity basophils from the same donor were stimulated through LIR-7. For basophils of 98% and 5% purity the net release of histamine was 14.5% and 19.0%, respectively, and cysLT release was 5.7 and 4.6 ng/106 basophils, respectively. Therefore, basophils were the major if not exclusive source of histamine and cysLTs in the PBMC population stimulated by LIR7 cross-linking, and mediator release was due to direct stimulation of basophils through LIR-7 and not due to an indirect effect of cross-linking LIR-7 expressed on PBMCs.
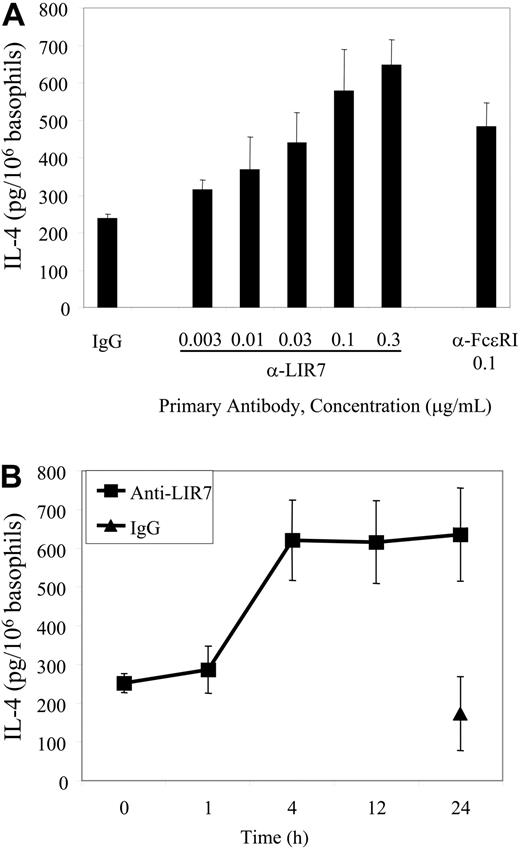
Because LIR7 was expressed by mononuclear cells in the PBMC population, IL-4 release in response to stimulation through LIR7 was analyzed using high-purity basophil preparations. In concentration response experiments, the onset of IL-4 secretion over the background was observed with 0.01 μg/mL anti-LIR7 and was maximal at 0.1 to 0.3 μg/mL (P = .004 versus IgG). The maximal net release of IL-4 from basophils in response to stimulation through LIR7 and FcϵRI was 410.2 ± 61.6 pg/106 basophils (n = 3) and 246.0 ± 51.3 pg/106 basophils (n = 3), respectively. Kinetic experiments showed a plateau at 4 hours (n = 3, Figure 4B). No detectable release of IL-4 was observed when LIR-7 was cross-linked on mixed PBMCs (∼ 4% basophils), indicating that contaminating PBMCs are not the source of IL-4 when LIR-7 is cross-linked.
Concentration- and time-dependent generation of IL-4. (A) High-purity basophils were incubated with the indicated concentrations of primary mAbs and then stimulated by addition of goat anti-mouse IgG for 4 hours (n = 3). (B) High-purity basophils were incubated with 0.1 μg/mL mAb to LIR7 or control IgG1 and stimulated by addition of goat anti-mouse IgG for 1 to 24 hours (n = 3). Supernatants were assayed for IL-4 by EIA after the goat anti-mouse IgG was cleared from the supernatants as described in “Materials and methods.”
Concentration- and time-dependent generation of IL-4. (A) High-purity basophils were incubated with the indicated concentrations of primary mAbs and then stimulated by addition of goat anti-mouse IgG for 4 hours (n = 3). (B) High-purity basophils were incubated with 0.1 μg/mL mAb to LIR7 or control IgG1 and stimulated by addition of goat anti-mouse IgG for 1 to 24 hours (n = 3). Supernatants were assayed for IL-4 by EIA after the goat anti-mouse IgG was cleared from the supernatants as described in “Materials and methods.”
IL-3 effects
Release of mediators from basophils in response to a number of different stimuli requires pretreatment of the cells with IL-3.38 We used IL-3 pretreatment routinely by convention. However, it seemed important to define whether IL-3 was necessary or just supplemental.
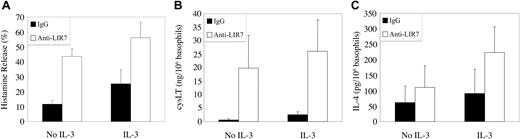
In 5 separate experiments, histamine release from unstimulated basophils in PBMCs was 12 ± 3% without IL-3 pretreatment, and the response to LIR7 stimulation provided a net gain of 32 ± 7% (P < .01; Figure 5A). IL-3 pretreatment alone increased histamine release to 25 ± 9%, and the net gain with cross-linking of LIR7 was 31 ± 7% and therefore no more than additive to IL-3. For cysLT generation (Figure 5B), there was release of less than 3 ng/106 unstimulated basophils in PBMCs with or without IL-3 pretreatment. Cross-linking of LIR7 elicited a net release of 19 ± 12 ng cysLT/106 basophils without IL-3 (n = 7, P = .05), which was not significantly different from the net release of 24 ± 16 ng cysLT/106 basophils with IL-3 pretreatment. In 3 separate experiments with purified basophils from different donors, the generation of IL-4 from unstimulated basophils in the absence of IL-3 was 61 ± 53 pg/106 cells, and this increased to 110 ± 71 pg/106 cells with cross-linking of LIR7 (P = .06 compared with IgG1 control). While this just failed to reach statistical significance, in each experiment anti-LIR7 cross-linking elicited IL-4 release greater than that observed with the IgG1-negative control. IL-3 pretreatment increased the increment in IL-4 generation (after subtraction of the value for the IgG1 control) in response to cross-linking of LIR7 from 49 ± 19 pg/106 basophils in the absence of IL-3 to 133 ± 8 pg/106 basophils with IL-3 pretreatment (P = .07 compared with no IL-3). While this did not reach statistical significance, the net release of IL-4 was greater for each donor when basophils were pretreated with IL-3.
Histamine release, cysteinyl leukotriene generation, and IL-4 secretion after stimulation through LIR7 with and without IL-3 priming. PBMCs (A-B) or highly purified (C) basophils were either incubated with 10 ng/mL IL-3 or not, incubated with IgG1 control antibody (▪) or with mAb to LIR7 (□) and stimulated by addition of goat anti-mouse IgG for 45 minutes (A-B) or 4 hours (C). Supernatants were assayed for (A) histamine (n = 3), (B) cysteinyl leukotrienes (CysLT, n = 3), and (C) IL-4 (n = 3) by EIA.
Histamine release, cysteinyl leukotriene generation, and IL-4 secretion after stimulation through LIR7 with and without IL-3 priming. PBMCs (A-B) or highly purified (C) basophils were either incubated with 10 ng/mL IL-3 or not, incubated with IgG1 control antibody (▪) or with mAb to LIR7 (□) and stimulated by addition of goat anti-mouse IgG for 45 minutes (A-B) or 4 hours (C). Supernatants were assayed for (A) histamine (n = 3), (B) cysteinyl leukotrienes (CysLT, n = 3), and (C) IL-4 (n = 3) by EIA.
These findings indicate that cross-linking of LIR7, like that of FcϵRI, directly elicits exocytosis, arachidonic acid metabolism, and IL-4 release without a requirement for IL-3 pretreatment.
Effect of cycloheximide on release of IL-4
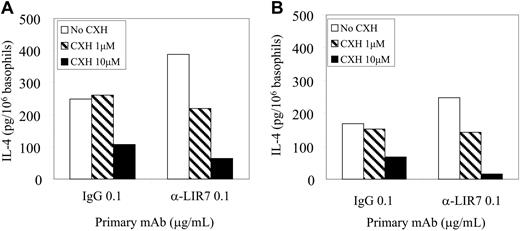
In order to determine if the IL-4 released by purified basophils with and without IL-3 pretreatment following stimulation through LIR7 was preformed or newly induced, we introduced the translation inhibitor, cycloheximide (Figure 6). At 4 hours, the IL-4 released with nonspecific IgG1, with or without IL-3 pretreatment, was unaltered by the low dose of 1 μM. The net increment in IL-4 release with LIR7 cross-linking was reduced to the baseline observed with control IgG1, with or without IL-3 pretreatment, by 1 μM cycloheximide, indicating induction of IL-4 synthesis rather than release from preformed stores. Cycloheximide (10 μM) reduced IL-4 release observed with control IgG1 and with cross-linking of LIR7 still further, again with no net release of IL-4 in response to LIR7 cross-linking. The findings for stimulation through FcϵRI were similar (data not shown) as previously reported.11,12 Basophil survival at 4 hours under all conditions was more than 92% and thus the falloff in IL-4 release after cycloheximide pretreatment is not attributable to cytotoxicity. Furthermore, at the earlier time point of 45 minutes, cycloheximide treatment did not attenuate histamine release in response to cross-linking of LIR-7, 50.2 ± 15.4% and 54.6 ± 12.6% in the absence and presence of 10 μM cycloheximide, respectively (n = 3).
Effect of cycloheximide on LIR7-mediated IL-4 generation. Highly purified basophils were either incubated with IL-3 (A) or not (B), incubated with 0.1 μg/mL mAb to LIR7 or control IgG1, and activated by addition of goat anti-mouse IgG for 4 hours in the absence (□) or presence of 1 μM (▨) or 10 μM (▪) cycloheximide. Goat anti-mouse IgG was cleared from the supernatants and IL-4 was assayed by EIA. A single representative experiment of 2 is depicted. CXH indicates cycloheximide.
Effect of cycloheximide on LIR7-mediated IL-4 generation. Highly purified basophils were either incubated with IL-3 (A) or not (B), incubated with 0.1 μg/mL mAb to LIR7 or control IgG1, and activated by addition of goat anti-mouse IgG for 4 hours in the absence (□) or presence of 1 μM (▨) or 10 μM (▪) cycloheximide. Goat anti-mouse IgG was cleared from the supernatants and IL-4 was assayed by EIA. A single representative experiment of 2 is depicted. CXH indicates cycloheximide.
Effect of coligation of LIR3 to the activating receptors LIR7 and FcϵRI
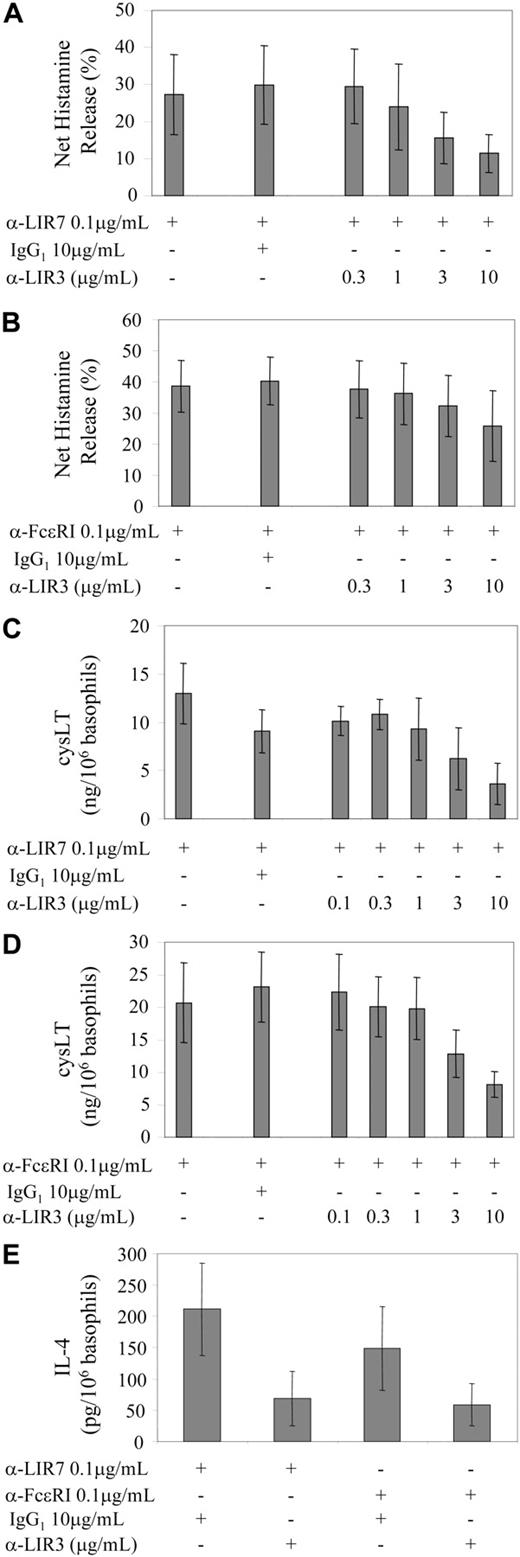
The ability of the ITIM-containing LIR3 to attenuate mediator release elicited by cross-linking of LIR7 or FcϵRI was examined for each basophil-derived mediator. Because the window for the LIR3 counter-regulatory effect rests between baseline and the net increment over baseline, the percent inhibition must be calculated from those parameters. Data are presented for basophils stimulated in the presence of IL-3. For histamine (Figure 7A) there was no difference in baseline release when mAb to LIR3 was substituted for nonspecific IgG. The net increment with cross-linking of 0.1 μg/mL anti-LIR7 was 27 ± 11% of the total cellular histamine, and this was suppressed in a concentration-dependent fashion by coligation to LIR3 (P = .005; Figure 7A). The dose response was similar when the activating ligand was 0.1 μg/mL anti-FcϵRI (P = .017; Figure 7B). For the 6 experiments performed with IL-3, the maximal inhibition with 10 μg/mL anti-LIR3 was 65 ± 10% and 47 ± 14% for LIR7- and FcϵRI-stimulated histamine release, respectively. Control nonspecific IgG or anti-CD154 in place of anti-LIR3 was not inhibitory at concentrations up to 10 μg/mL.
Effect of coligation of LIR3 with LIR7 or FcϵRI on activation of human basophils. PBMCs (A-D) or purified basophils (E) were treated with IL-3 and then incubated with 0.1 μg/mL anti-LIR7 (A, C, and E), anti-FcϵRI (B, D, and E), or control IgG1 (A-E), in the presence of increasing concentrations of anti-LIR3 or 10 μg/mL IgG1 control antibody. Basophils were then activated by the addition of 10 μg/mL goat anti-mouse IgG for 45 minutes (A-D) or 4 hours (E). Supernatants were assayed for histamine (A-B), cysteinyl leukotrienes (C-D), or IL-4 (E) by EIA.
Effect of coligation of LIR3 with LIR7 or FcϵRI on activation of human basophils. PBMCs (A-D) or purified basophils (E) were treated with IL-3 and then incubated with 0.1 μg/mL anti-LIR7 (A, C, and E), anti-FcϵRI (B, D, and E), or control IgG1 (A-E), in the presence of increasing concentrations of anti-LIR3 or 10 μg/mL IgG1 control antibody. Basophils were then activated by the addition of 10 μg/mL goat anti-mouse IgG for 45 minutes (A-D) or 4 hours (E). Supernatants were assayed for histamine (A-B), cysteinyl leukotrienes (C-D), or IL-4 (E) by EIA.
For the generation of cysLTs (Figure 7C-D), the baseline with or without IL-3 pretreatment plus control IgG1 or mAb to LIR3 was less than 5 ng/106 basophils. Cross-linking of LIR7 and of FcϵRI provided a net release of 13 ± 3 and 21 ± 6 ng cysLT/106 basophils, respectively. Coligation to LIR3 suppressed generation in response to cross-linking of LIR7 and of FcϵRI in a dose-related manner (P = .005 and P = .0001, respectively). For the experiments performed with IL-3, the maximal inhibition with 10 μg/mL anti-LIR3 was 76 ± 9% (n = 4) for LIR7 and 58 ± 6% (n = 5) for FcϵRI. Control nonspecific IgG and anti-CD154 in place of anti-LIR3 were not inhibitory at concentrations up to 10 μg/mL.
Because of the difficulty in obtaining large numbers of purified basophils, experiments in which LIR3 was coligated to either LIR7 or FcϵRI to inhibit release of IL-4 were performed using only the highest concentration of anti-LIR3 (10.0 μg/mL). Nonspecific IgG (10 μg/mL) provided a negative control for the mAb to LIR3. Coligation of LIR3 with LIR7 or with FcϵRI provided inhibition in each of 3 separate experiments. Inhibition of LIR7-induced net IL-4 release by coligation to LIR3 was 73 ± 8% (n = 3, P = .05), while inhibition of FcϵRI-induced net IL-4 release was 66 ± 16% (n = 3, P = .07) (Figure 7E).
Discussion
Using specific monoclonal antibodies we have demonstrated that human basophils consistently express LIR3 and LIR7, with additional expression of LIR1 and/or LIR2 on basophils of some donors (Figure 1). This pattern of LIR expression is identical to that on human eosinophils and neutrophils33 and is distinct from the profile on cells of monocytic origin that express all of the LIRs examined to date,20,33,37 with the exception that plasmacytoid dendritic cells lack LIR7 expression.39 These data suggest that the restricted expression of LIR3 and LIR7, with or without LIR1 or LIR2, is a pattern shared by myeloid cells of granulocytic lineage and are consistent with developmental regulation of LIR expression.
To examine the function of LIRs expressed on basophils, we cross-linked LIR7 using specific murine monoclonal antibodies followed by a goat anti-mouse IgG. Cross-linking of LIR7 elicited release of mediators from 3 compartments: preformed histamine from secretory granules, de novo synthesis of LTC4 from arachidonic acid, and IL-4 (Figures 2, 3, 4). The time course of IL-4 generation and its inhibition by cycloheximide (Figure 6) indicate that IL-4 was generated de novo by gene induction. The only other “complete” stimulus identified thus far, releasing all 3 compartments of mediators, is activation through the high-affinity IgE receptor.11,38,40-45 A 21-kDa histamine-releasing factor, whose action appears to be IgE-dependent, is also a complete stimulus for basophil mediator release, but it is active only on basophils from approximately 50% of individuals.46-48 Our study therefore not only identifies LIR7 as a novel signaling receptor on human basophils, but also identifies activation through LIR7 as the only presently identified IgE-independent complete stimulus for human basophils. For most adults with asthma and for many individuals with persistent asthmatic symptoms, the role of allergens and of IgE-dependent signaling is either partial or, in nonatopic asthma, nonexistent.49 The identification of LIR7 as an IgE-independent activating receptor for basophils suggests that it could respond to a stimulus for nonatopic, Th2-driven airway inflammation. The possibility that LIR7 could contribute to the Th2 phenotype of bronchial asthma is suggested by the recent report that LIR7 is overexpressed in skin lesions of lepromatous leprosy, in which the Th2 response is dominant, compared with tuberculoid leprosy, in which the Th1 response is dominant.50
Based entirely on in vitro studies, basophils respond to some stimuli de novo with release of only some of their potential mediators unless treated with IL-3 or are entirely dependent on IL-3 cotreatment.38 Thus, C5a43 and the chemokine monocyte chemotactic protein 1 (MCP-1)51,52 elicit basophil histamine release without exposure to IL-3, but require IL-3 to elicit LTC4 biosynthesis and modest IL-4 generation. While formyl-methionylleucyl-phenylalanine (fMLP) elicits histamine release and LTC4 biosynthesis from basophils without exposure to IL-3, it requires cytokines to effect IL-4 generation.53,54 C3a, platelet-activating factor (PAF), and chemokines other than MCP-1 that act through diverse chemokine receptors including CCR3, CCR1, and CXCR1 elicit modest histamine release and LTC4 generation only after IL-3 treatment.38 Although IL-3 at a concentration of 10 ng/mL has been characterized as a basophil priming agent for diverse stimuli,38 we found that IL-3 alone induced release and that costimulation of basophils by cross-linking LIR7 in the presence of IL-3 augmented mediator release from all 3 compartments, which was additive for histamine and cysLTs and possibly synergistic for IL-4 (Figure 5). Importantly, stimulation through LIR7, as through FcϵRI, can effect basophil mediator release from all 3 compartments without IL-3.
Basophils express not only the activating LIR7, but also the inhibitory LIR3. Indeed coligation of LIR3 with LIR7 or with FcϵRI substantially inhibited release of histamine, LTC4, and IL-4 (Figure 7). LIR3 is therefore able to inhibit the release of mediators from all 3 basophil compartments, antagonizing both IgE-dependent and IgE-independent activation. The balance between activating and inhibitory signals delivered by several families of receptors is critical for the regulated function of leukocytes in adaptive and innate immune responses.55-57 The functions of individual inhibitory receptors will depend in part upon their ligand(s) and sites of expression. Thus, the low-affinity inhibitory Fc receptor for IgG, FcγRIIB, terminates signaling initiated by antigen engagement of the B-cell receptor, contributing to peripheral B-cell tolerance and protection from autoimmune disease.58-60 FcγRIIb also potently down-regulates FcγRIII- and FcϵRI-dependent responses in mouse mast cells61 and FcϵRI-dependent responses in human basophils.62 This capacity for FcγRIIb to down-regulate IgE-dependent responses has been exploited through the development of a bispecific dimer of Fcϵ and Fcγ that down-regulated IgE-dependent basophil histamine release.63 Administration of this dimeric molecule to mice transgenic for the human FcϵRI alpha chain inhibited passive cutaneous anaphylactic responses.63 The capacity of FcγRIIb to temper mast cell and basophil activation appears confined to counter-regulation of immunoglobulin-dependent adaptive immune responses elicited through FcγRIII and FcϵRI. The possibility that LIR3 regulates both adaptive and innate immune responses is suggested by studies of mice with targeted disruption of the homologous mouse gp49B gene. Gp49B-null mice have exaggerated cutaneous mast cell responses to activation by IgE and hapten-specific antigen64 and to stem cell factor.65 Importantly, constitutive counter-regulation of innate immune responses by gp49B extends to the neutrophil, as the strain lacking gp49B is uniquely susceptible to the hemorrhagic Schwartzman-like microangiopathy mediated by intradermal lipopolysaccharide.66 Further elucidation of the immunomodulatory role of LIR3 requires identification of its ligand(s), and any appreciation of an in vivo role would depend upon recognition of a deletion in a clinical setting.
Prepublished online as Blood First Edition Paper, July 8, 2004; DOI 10.1182/blood-2004-01-0268.
Supported by National Institutes of Health (NIH) grants AI31599, AI 07306, and HL007718; an Arthritis Foundation postdoctoral fellowship; and a GlaxoSmithKline Allergy Fellowship Award.
One of the authors (L.B.) is employed by a company whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to gratefully acknowledge Bing Lam, PhD, for assistance with RP-HPLC analysis and Erin Rhode and Thomas Arm for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal