Abstract
Cytogenetic abnormalities, evaluated either by karyotype or by fluorescence in situ hybridization (FISH), are considered the most important prognostic factor in multiple myeloma (MM). However, there is no information about the prognostic impact of genomic changes detected by comparative genomic hybridization (CGH). We have analyzed the frequency and prognostic impact of genetic changes as detected by CGH and evaluated the relationship between these chromosomal imbalances and IGH translocation, analyzed by FISH, in 74 patients with newly diagnosed MM. Genomic changes were identified in 51 (69%) of the 74 MM patients. The most recurrent abnormalities among the cases with genomic changes were gains on chromosome regions 1q (45%), 5q (24%), 9q (24%), 11q (22%), 15q (22%), 3q (16%), and 7q (14%), while losses mainly involved chromosomes 13 (39%), 16q (18%), 6q (10%), and 8p (10%). Remarkably, the 6 patients with gains on 11q had IGH translocations. Multivariate analysis selected chromosomal losses, 11q gains, age, and type of treatment (conventional chemotherapy vs autologous transplantation) as independent parameters for predicting survival. Genomic losses retained the prognostic value irrespective of treatment approach. According to these results, losses of chromosomal material evaluated by CGH represent a powerful prognostic factor in MM patients. (Blood. 2004;104:2661-2666)
Introduction
The clinical application of conventional cytogenetics in multiple myeloma (MM) patients has been hampered by the low proliferative index of plasma cells (PCs) as well as the limited extent of bone marrow involvement, which reduces the number of analyzable metaphases.1-3 Many of the limitations of conventional cytogenetic analysis have been overcome with the development of fluorescence in situ hybridization (FISH).4-7 However, the information provided by this technique is confined to specific target regions, and abnormalities will be undetected unless a comprehensive FISH analysis with several probes is performed. Comparative genomic hybridization (CGH) allows analysis of chromosomal copy number changes without the requirement of tumoral metaphases and has been demonstrated to be a useful technology for identifying gains and losses of DNA sequences in tumors with a low proliferative index, such as MM.8 While the major advantage of CGH is its ability to examine the whole tumor cell genome in a single hybridization experiment, the potential of this method to detect genomic changes may be limited by the proportion of cells bearing the abnormality. Thus, chromosomal imbalances present in a low percentage of cells cannot be detected by CGH.9
Despite the difficulties in detecting and characterizing myeloma genetic aberrations, several studies have demonstrated that almost all MM patients display chromosomal abnormalities.10 Moreover, similarly to leukemia, some of these abnormalities may influence disease outcome. Thus, chromosomal abnormalities such as IGH translocations or monosomy 13 as well as the presence of hypodiploidy have been shown to be powerful prognostic indicators.11-15 More recently, a close relationship between nonhyperdiploid MM and IGH translocations and monosomy 13 has been described.14,16,17 This finding may explain why both hypodiploidy and specific genetic aberrations are associated with poor survival. Nevertheless, it remains to be elucidated whether IGH translocations or monosomy 13 is the main contributor to the adverse outcome observed in hypodiploid MM.18
CGH studies from our group and others have shown that genomic changes may affect almost all chromosomes, and therefore CGH would be a more appropriate tool than interphase FISH for examining DNA gains and losses globally and to assess the impact of genomic imbalances on survival.19-22 Accordingly, the present study was designed to explore the prognostic impact of genetic changes as detected by CGH and evaluate the relationship between these changes and IGH translocation analyzed by FISH.
Patients, materials, and methods
Patients
A total of 74 patients with previously untreated MM were included in the study. The median age was 60 years (range, 45-87 years). Patients were treated according to the Spanish Pethema/Grupo Español de Mieloma (GEM) protocols: 33 received polychemotherapy followed by high-dose chemotherapy (melphalan, 200 mg/m2) with autologous stem cell transplantation (ASCT), while the remaining 41 were treated with conventional chemotherapy. Table 1 shows the base-line characteristics of the 74 patients. There were no significant differences, except for age, between patients undergoing ASCT and those treated with just conventional therapy. The study was approved by the local research ethics committee of the University Hospital of Salamanca, Spain, and written informed consent was obtained from all patients.
Clinical and biologic characteristics of the 74 patients
Characteristics . | All patients . | Conventional chemotherapy . | ASCT . |
|---|---|---|---|
| Older than 70 y, no. | 13 | 12 | 1 |
| Sex, no. | |||
| Male | 41 | 21 | 20 |
| Female | 33 | 20 | 13 |
| Stage, no. of patients | |||
| I/II | 21 | 13 | 8 |
| III | 53 | 28 | 25 |
| Ig subtype, no. of patients | |||
| IgG | 33 | 19 | 14 |
| IgA | 17 | 8 | 9 |
| IgD | 1 | 1 | 0 |
| Bence Jones protein | 22 | 12 | 10 |
| Nonsecretory | 1 | 1 | 0 |
| S-phase plasma cells, % (range) | 1.79 (0-14.4) | 1.96 (0-14.4) | 1.5 (0.1-5.9) |
| Albumin, g/L (range) | 35 (15-49) | 34 (15-49) | 35 (21-45) |
| β2-microglobulin, nM/L (range) | 440.7 (25.4-4661.2) | 440.7 (25.4-4661.2) | 389.8 (144.1-1669.6) |
| Calcium, mM (range) | 2.4 (1.9-4.2) | 2.4 (1.9-3.9) | 2.5 (2.0-4.2) |
| Creatinine, μM (range) | 88 (53-955) | 106 (53-955) | 88 (53-902) |
| Hemoglobin, g/L (range) | 92 (58-135) | 94 (59-135) | 92 (58-135) |
| C-reactive protein, mg/L (range) | 18 (1-1200) | 25 (1-1200) | 13 (1-379) |
| Lactate dehydrogenase, IU/L (range) | 337 (140-2322) | 313 (159-2322) | 345 (140-2223) |
Characteristics . | All patients . | Conventional chemotherapy . | ASCT . |
|---|---|---|---|
| Older than 70 y, no. | 13 | 12 | 1 |
| Sex, no. | |||
| Male | 41 | 21 | 20 |
| Female | 33 | 20 | 13 |
| Stage, no. of patients | |||
| I/II | 21 | 13 | 8 |
| III | 53 | 28 | 25 |
| Ig subtype, no. of patients | |||
| IgG | 33 | 19 | 14 |
| IgA | 17 | 8 | 9 |
| IgD | 1 | 1 | 0 |
| Bence Jones protein | 22 | 12 | 10 |
| Nonsecretory | 1 | 1 | 0 |
| S-phase plasma cells, % (range) | 1.79 (0-14.4) | 1.96 (0-14.4) | 1.5 (0.1-5.9) |
| Albumin, g/L (range) | 35 (15-49) | 34 (15-49) | 35 (21-45) |
| β2-microglobulin, nM/L (range) | 440.7 (25.4-4661.2) | 440.7 (25.4-4661.2) | 389.8 (144.1-1669.6) |
| Calcium, mM (range) | 2.4 (1.9-4.2) | 2.4 (1.9-3.9) | 2.5 (2.0-4.2) |
| Creatinine, μM (range) | 88 (53-955) | 106 (53-955) | 88 (53-902) |
| Hemoglobin, g/L (range) | 92 (58-135) | 94 (59-135) | 92 (58-135) |
| C-reactive protein, mg/L (range) | 18 (1-1200) | 25 (1-1200) | 13 (1-379) |
| Lactate dehydrogenase, IU/L (range) | 337 (140-2322) | 313 (159-2322) | 345 (140-2223) |
Values are expressed as medians.
Autologous stem cell transplantation.
The median overall survival (OS) for all patients was 30 months (95% CI, 4.6-54.3 months), and the median follow-up for survivors was 32 months (range, 10-94 months). At the time of the study, 33 patients remained alive. The following clinical and laboratory variables were examined at diagnosis in each patient: sex, age, Eastern Cooperative Oncology Group (ECOG) performance status, Durie and Salmon stage, creatinine, albumin, calcium, hemoglobin, β2-microglobulin, lactate dehydrogenase (LDH), C-reactive protein (CRP), number of plasma cells in S phase, and DNA content by flow cytometry.
Bone marrow samples were used for molecular cytogenetic analysis. In those cases with a low proportion of bone marrow plasma cells (< 50%), an enrichment of plasma cells was performed using immunomagnetic separation with the monoclonal antibody BB4 conjugated with immunomagnetic beads.
Comparative genomic hybridization
CGH was performed as previously described.22 Calculation of the tumor DNA to normal DNA fluorescence ratios along the length of each chromosome was performed by means of an automated CGH software package (Applied Imaging, Sunderland, United Kingdom). Ratio values obtained from at least 10 metaphase cells for each case were averaged. Ratio values more than 1.25 and less than 0.75 were considered to represent chromosomal gain and loss, respectively. These thresholds correspond to the theoretic value that would be expected for a trisomy or a monosomy of a given chromosome in 50% of the test cells.9 Overrepresentations were defined as high-level amplifications when the profiles exceeded the cut-off value of 1.5. Chromosomal gains exceeding 1.5 involving the whole chromosome or large areas of a chromosomal arm were not considered as high-level DNA amplification. Negative control experiments were performed using differentially labeled male versus male DNA, and female versus female DNA. Additional control experiments included the interchange of the digoxigenin-deoxyuridine triphosphate (dUTP) and biotin-dUTP labels between normal and tumor. Of the 74 patients, 25 have been previously reported.22
Fluorescence in situ hybridization
Cells from 51 of the 74 patients were available for interphase FISH studies. In order to detect IGH rearrangements, a specific probe for IGH region (14q32) was used: LSI IGH Dual Color, break apart rearrangement probe (Vysis, Downers Grove, IL). Patients with IGH translocations were analyzed for 11q13 partner (CCND1) by means of the probe LSI IGH/CCND1, dual color, dual fusion translocation probe (Vysis). Moreover, losses on 13q were explored using a specific probe for 13q14 (RB): LSI 13 (RB1) probe (Vysis). The FISH procedure has been previously described in detail.23 A total of 500 interphase nuclei were analyzed using Vysis scoring criteria. Based on the results using these probes in 10 healthy controls, the cut-off point for the identification of alteration was set at more than 10% cells with abnormal signal.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (SPSS, Chicago, IL). The Fisher exact test was used to test for associations between genomic changes as well as between genomic changes and other categoric variables. For continuous variables, the Wilcoxon rank sum test was used. Overall survival was estimated using the Kaplan-Meier method, and the difference in survival curves was tested for statistical significance using the log-rank test. P values less than .05 were considered to reflect statistical significance. Multivariate analysis of survival was performed using the Cox proportional hazards model (stepwise regression approach). All the parameters reaching significance in the univariate analysis were included in the multivariate analysis. Factors were retained in the model if they were statistically significant at P ≤ .05.
Results
Description of genomic changes detected by CGH
Chromosomal imbalances using CGH were identified in 51 (69%) of the 74 MM patients. Overall, a total of 243 DNA copy number changes were detected with a median of 4 imbalances per abnormal case (range, 1-17 imbalances): 161 gains, 78 losses, and 4 amplifications. All but 3 abnormal cases showed chromosomal gains. By contrast, there were 19 abnormal cases without losses.
The most frequent aberrations among the cases with genomic changes were gains on chromosome regions 1q (45%), 5q (24%), 9q (24%), 11q (22%), 15q (22%), 3q (16%), and 7q (14%), while losses mainly involved chromosomes 13q (39%), 16q (18%), 6q (10%), and 8p (10%). When we focused only on gains affecting whole chromosomes, the most frequent trisomies were 9 (14%), 15 (14%), 19 (14%), 5 (12%), and 3 (10%). Table 2 shows all the genomic changes distributed according to chromosome, and Figure 1 depicts the chromosomal imbalances of the most frequently affected chromosomes. There were interesting associations between chromosomal abnormalities. Thus, the most frequent losses (13q, 16q, and 8p) were associated with each other (P < .03). In a similar way, gains on different chromosomes (3q, 5q, 7q, 9q, 11q, and 15q), with the exception of 1q, also tended to be associated. Interestingly, cases with gains on 1q displayed a significantly higher incidence of losses on 13q and 16q (P < .04), but were not related to other chromosomal gains.
Genomic changes distributed according to chromosome regions
. | Gains . | . | . | Losses . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome . | Whole chromosome . | p arm . | q arm . | Whole chromosome . | p arm . | q arm . | ||||
| 1 | 0 | 5 | 23 | 0 | 5 | 0 | ||||
| 2 | 1 | 2 | 0 | 0 | 0 | 2 | ||||
| 3 | 5 | 4 | 3 | 0 | 0 | 0 | ||||
| 4 | 1 | 0 | 4 | 0 | 0 | 1 | ||||
| 5 | 6 | 1 | 6 | 0 | 0 | 1 | ||||
| 6 | 0 | 6 | 2 | 0 | 1 | 5 | ||||
| 7 | 3 | 2 | 4 | 0 | 0 | 0 | ||||
| 8 | 0 | 1 | 5 | 0 | 5 | 1 | ||||
| 9 | 7 | 2 | 5 | 0 | 0 | 1 | ||||
| 10 | 0 | 0 | 0 | 0 | 1 | 0 | ||||
| 11 | 3 | 0 | 8 | 0 | 0 | 0 | ||||
| 12 | 0 | 0 | 5 | 1 | 2 | 3 | ||||
| 13 | 0 | 0 | 1 | 14 | 0 | 6 | ||||
| 14 | 0 | 0 | 2 | 4 | 0 | 2 | ||||
| 15 | 7 | 0 | 4 | 0 | 0 | 0 | ||||
| 16 | 1 | 3 | 1 | 3 | 0 | 6 | ||||
| 17 | 2 | 1 | 3 | 0 | 4 | 1 | ||||
| 18 | 0 | 1 | 1 | 0 | 1 | 1 | ||||
| 19 | 7 | 2 | 2 | 0 | 1 | 0 | ||||
| 20 | 0 | 0 | 1 | 0 | 0 | 3 | ||||
| 21 | 2 | 0 | 0 | 0 | 0 | 0 | ||||
| 22 | 5 | 0 | 0 | 1 | 0 | 1 | ||||
| X | 0 | 0 | 5 | 1 | 0 | 0 | ||||
. | Gains . | . | . | Losses . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome . | Whole chromosome . | p arm . | q arm . | Whole chromosome . | p arm . | q arm . | ||||
| 1 | 0 | 5 | 23 | 0 | 5 | 0 | ||||
| 2 | 1 | 2 | 0 | 0 | 0 | 2 | ||||
| 3 | 5 | 4 | 3 | 0 | 0 | 0 | ||||
| 4 | 1 | 0 | 4 | 0 | 0 | 1 | ||||
| 5 | 6 | 1 | 6 | 0 | 0 | 1 | ||||
| 6 | 0 | 6 | 2 | 0 | 1 | 5 | ||||
| 7 | 3 | 2 | 4 | 0 | 0 | 0 | ||||
| 8 | 0 | 1 | 5 | 0 | 5 | 1 | ||||
| 9 | 7 | 2 | 5 | 0 | 0 | 1 | ||||
| 10 | 0 | 0 | 0 | 0 | 1 | 0 | ||||
| 11 | 3 | 0 | 8 | 0 | 0 | 0 | ||||
| 12 | 0 | 0 | 5 | 1 | 2 | 3 | ||||
| 13 | 0 | 0 | 1 | 14 | 0 | 6 | ||||
| 14 | 0 | 0 | 2 | 4 | 0 | 2 | ||||
| 15 | 7 | 0 | 4 | 0 | 0 | 0 | ||||
| 16 | 1 | 3 | 1 | 3 | 0 | 6 | ||||
| 17 | 2 | 1 | 3 | 0 | 4 | 1 | ||||
| 18 | 0 | 1 | 1 | 0 | 1 | 1 | ||||
| 19 | 7 | 2 | 2 | 0 | 1 | 0 | ||||
| 20 | 0 | 0 | 1 | 0 | 0 | 3 | ||||
| 21 | 2 | 0 | 0 | 0 | 0 | 0 | ||||
| 22 | 5 | 0 | 0 | 1 | 0 | 1 | ||||
| X | 0 | 0 | 5 | 1 | 0 | 0 | ||||
Values indicate number of cases that have the different chromosomal imbalances.
Summary of genomic changes detected by CGH in the most frequently affected chromosomes. Lines on the right side of the ideograms indicate gains of chromosomal material. Lines on the left side indicate losses of chromosomal material.
Summary of genomic changes detected by CGH in the most frequently affected chromosomes. Lines on the right side of the ideograms indicate gains of chromosomal material. Lines on the left side indicate losses of chromosomal material.
Correlation between CGH and FISH studies
Translocations of IGH were observed in 24 (47%) of the 51 cases evaluated by FISH, and IGH/CCND1 rearrangement was detected in 12 cases (23%). Remarkably, the 6 patients with gains on 11q had IGH translocations (P = .006). Only 3 of these 6 cases had t(11;14)(q13;q32) and none of them had an extra copy of t(11;14). There was no association between IGH translocations and other genomic imbalances of individual chromosomes. IGH translocations were similarly distributed between the groups of patients with abnormal CGH (49%) and those with normal CGH (44%). Likewise, we also failed to find associations between IGH translocations and genomic losses. In contrast, ploidy status evaluated by flow cytometry was closely related to the presence of IGH translocations. Thus, hyperdiploid MM patients (DNA index > 1.08) were significantly less likely to have IGH translocations than the nonhyperdiploid (DNA index < 0.9 for hypodiploid cases and between 0.9 and 1.08 for diploid ones) MM patients (17% vs 83% respectively, P = .03).
All 20 cases with loss on 13q detected by CGH showed deletion of the RB gene by FISH analysis. In addition, FISH analysis detected RB deletion in 10 more cases where CGH showed a normal profile of chromosome 13.
Association between genomic changes and clinical-biologic parameters
Patients with chromosomal imbalances as well as those with chromosomal losses were more likely to have advanced stages (P = .005) and high serum levels of β2-microglobulin (P = .04). When we focused on changes of individual chromosomes, losses on 13q were associated with advanced stage (P = .02), while gains on 11q had low hemoglobin levels (P = .03) and high β2-microglobulin levels (P = .04). There was no association between percentage of S-phase PCs and any genomic changes.
Prognostic impact of genomic changes and other biologic parameters
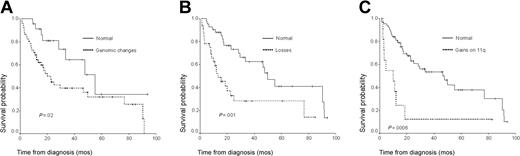
In univariate analysis, the presence of genomic changes was associated with adverse prognosis in comparison with patients without chromosomal imbalances (Figure 2A). When we analyzed the prognostic influence of gains and losses separately, losses were associated with short survival (Figure 2B), while the 19 cases with genomic changes consisting of only gains, without concomitant losses, displayed a similar outcome to that of healthy patients (median OS: 49.8 months vs 55 months). Upon analyzing individual genomic changes, it was observed that loss on 13q as well as gains on 3q, 5q, 9q, and 11q were associated with short survival (Table 3; Figure 2C). By contrast, gains of 1q did not influence disease outcome. Since FISH studies showed IGH translocations and RB deletions in some of the cases with normal CGH, we also compared the survival in those patients with normal CGH but abnormal FISH with that of patients with both normal CGH and FISH. No significant differences were observed between these 2 groups (median OS: 55.0 vs 55.3 months). Additional clinical and biologic factors with a significant negative influence on survival (P < .05) were as follows: age older than 60, ECOG performance status of 2 or more, number of plasma cells in S phase more than 2%, β2-microglobulin more than 4.5, and treatment with conventional chemotherapy versus ASCT.
Overall survival of patients according to chromosomal imbalances. (A) Presence of genomic changes. (B) Presence of genomic losses. (C) Presence of gains on 11q.
Overall survival of patients according to chromosomal imbalances. (A) Presence of genomic changes. (B) Presence of genomic losses. (C) Presence of gains on 11q.
Univariate analysis of the most frequent genomic changes
Genomic imbalance . | No. of cases . | Median OS, mo . | P* . |
|---|---|---|---|
| Genomic changes | |||
| Yes | 51 | 20.1 | .02 |
| No | 23 | 55.0 | |
| Loss of chromosomal material | |||
| Yes | 32 | 13.3 | .001 |
| No | 42 | 49.8 | |
| Gains of chromosomal material | |||
| Yes | 48 | 23.0 | .1 |
| No | 26 | 47.5 | |
| Chromosome 1q | |||
| Gain | 23 | 29.5 | .4 |
| Normal | 51 | 33.5 | |
| Chromosome 3q | |||
| Gain | 8 | 4.7 | <.001 |
| Normal | 66 | 46.4 | |
| Chromosome 5q | |||
| Gain | 12 | 12.1 | .004 |
| Normal | 62 | 46.4 | |
| Chromosome 9q | |||
| Gain | 12 | 12.1 | .046 |
| Normal | 62 | 46.4 | |
| Chromosome 11q | |||
| Gain | 11 | 10.0 | <.001 |
| Normal | 63 | 46.4 | |
| Chromosome 13q | |||
| Loss | 20 | 15.6 | .048 |
| Normal | 54 | 47.5 |
Genomic imbalance . | No. of cases . | Median OS, mo . | P* . |
|---|---|---|---|
| Genomic changes | |||
| Yes | 51 | 20.1 | .02 |
| No | 23 | 55.0 | |
| Loss of chromosomal material | |||
| Yes | 32 | 13.3 | .001 |
| No | 42 | 49.8 | |
| Gains of chromosomal material | |||
| Yes | 48 | 23.0 | .1 |
| No | 26 | 47.5 | |
| Chromosome 1q | |||
| Gain | 23 | 29.5 | .4 |
| Normal | 51 | 33.5 | |
| Chromosome 3q | |||
| Gain | 8 | 4.7 | <.001 |
| Normal | 66 | 46.4 | |
| Chromosome 5q | |||
| Gain | 12 | 12.1 | .004 |
| Normal | 62 | 46.4 | |
| Chromosome 9q | |||
| Gain | 12 | 12.1 | .046 |
| Normal | 62 | 46.4 | |
| Chromosome 11q | |||
| Gain | 11 | 10.0 | <.001 |
| Normal | 63 | 46.4 | |
| Chromosome 13q | |||
| Loss | 20 | 15.6 | .048 |
| Normal | 54 | 47.5 |
Log-rank test.
In multivariate analysis, only 4 variables retained their independent prognostic influence: presence of genomic losses, gains on 11q, age older than 60, and treatment without ASCT (Table 4). Additional multivariate analysis was separately performed for patients treated with conventional chemotherapy and those undergoing ASCT. In patients who underwent transplantation, the only 2 variables with independent influence on survival were genomic losses and a high number of S-phase PCs. In patients treated with conventional chemotherapy, 2 variables retained independent prognostic value: genomic losses and gains on 11q.
Multivariate analysis
. | Global series . | . | Conventional chemotherapy . | . | ASCT . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Losses | 3.1 (1.4-6.6) | .003 | 2.7 (1.2-6.8) | .028 | 7 (1.1-42.9) | .021 | |||
| Gains on 11q | 2.7 (1.1-6.7) | .045 | 2.8 (0.9-8.1) | .050 | NA | NS | |||
| Older than 60 y | 2.1 (0.9-4.7) | .046 | NA | NS | NA | NS | |||
| S-phase PCs more than 2% | NA | NS | NA | NS | 9.9 (1.5-63.4) | .01 | |||
| ASCT | 2.9 (1.2-7.1) | .009 | |||||||
. | Global series . | . | Conventional chemotherapy . | . | ASCT . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Losses | 3.1 (1.4-6.6) | .003 | 2.7 (1.2-6.8) | .028 | 7 (1.1-42.9) | .021 | |||
| Gains on 11q | 2.7 (1.1-6.7) | .045 | 2.8 (0.9-8.1) | .050 | NA | NS | |||
| Older than 60 y | 2.1 (0.9-4.7) | .046 | NA | NS | NA | NS | |||
| S-phase PCs more than 2% | NA | NS | NA | NS | 9.9 (1.5-63.4) | .01 | |||
| ASCT | 2.9 (1.2-7.1) | .009 | |||||||
ASCT indicates autologous stem cell transplantation; HR, hazard rate; NA, not applicable; and NS, not significant.
Discussion
Comparative genomic hybridization (CGH) has been previously used in a descriptive fashion in order to investigate in a single experiment the overall incidence of gains and losses of chromosomal material in MM patients. In fact, CGH has proved to be the most robust technique for identification of the total number of chromosome imbalances.19-22 This is the first report that shows a prognostic impact of genomic imbalances detected by CGH in MM. Both the presence of genomic changes and the detection of genetic losses were associated with a significant decrease in survival compared with patients without such characteristics. When cases with genomic changes consisting of only gains, excluding losses, were compared with healthy patients no difference in survival was found, supporting the notion that loss of chromosomal material has a negative impact on survival. Nevertheless, when the analysis was focused on specific abnormalities, gains on 3q, 5q, 9q, and 11q were associated with poor prognosis. It should be noted that gains of the q arm of chromosomes 3, 5, 9, and 11 are not synonymous with trisomy since most of these cases just gain genetic material from the q arm. Therefore, CGH demonstrates a high incidence of partial gains that are underestimated in cytogenetic studies and that could be misinterpreted by FISH analysis, since the presence of 3 signals is considered to indicate complete trisomies when centromeric probes are used; but as our CGH studies show this is not completely correct, because some of these cases do not consist of gains of the whole chromosome but of gains on the long arm, including the centromere, but not the p arm. Moreover this study shows the adverse prognostic value of these partial trisomies, which may be related to the different nature of these gains since they reflect structural and not numeric abnormalities. Gains on 1q, the most frequent abnormality detected by CGH, did not confer adverse prognosis to MM patients. These results are in accordance with those previously reported concerning the lack of prognostic influence of structural abnormalities of chromosome 1,14 although recently an association between chromosome 1 abnormalities and a shortened survival in MM has been described.24
In multivariate analysis, losses of chromosomal material and gains on 11q were the only significant chromosomal parameters. Furthermore, these variables retained the prognostic value when the analysis was restricted to those patients treated with conventional chemotherapy. However, for patients who underwent ASCT, the proliferative activity of plasma cells (S-phase numbers), which is a well-known prognostic factor in MM, appeared to be more significant than gains on 11q and together with genomic losses were the 2 independent factors predicting survival. Chromosome 11 abnormalities detected either by cytogenetics or by FISH have been previously associated with unfavorable outcome.6,25-27 However, different types of chromosome 11 abnormalities (monosomies, trisomies, structural abnormalities of 11q) were always grouped under the same variable. The present study demonstrates for the first time that gain of chromosomal material on the 11q arm is one of the most powerful prognostic factors in MM. These prognostic findings should be considered as a preliminary observation, and further analyses in a larger group of patients need to be carried out in order to confirm these data.
Detection of monosomy and deletions of 13q has been documented as a powerful adverse indicator in MM patients, whether assessed by traditional karyotype analysis or by FISH.4,6,11,13,25,28 This study confirms the same dismal prognostic impact for this abnormality as detected by CGH. Nevertheless, losses on 13q were not selected as an independent prognostic factor in this series of patients, and in fact, it was a less potent survival predictor than losses of any chromosome when grouped and considered as a single variable. This suggests that the negative impact on prognosis would not be limited to loss on chromosome 13, but that other losses can also have this adverse impact. This finding should be considered in further studies designed to investigate the prognostic impact of cytogenetic changes. Notably, the incidence of deletions of 13q detected by FISH was higher than that found by CGH because of the sensitivity limits of the latter technique. Therefore, although the power of CGH to detect deletions of 13q is substantially higher than that of cytogenetics, the more effective technique for specifically analyzing RB deletion is FISH. Nevertheless, some of these cases with RB deletion detected only by FISH showed an abnormal CGH profile for other chromosomes, which underlines the high clonal heterogeneity that makes it difficult to tackle all the chromosomal abnormalities potentially present in MM using a single genetic technology.
The high incidence of chromosomal imbalances detected by CGH compared with cytogenetics supports the increasingly accepted notion that almost all myeloma cases harbor cytogenetic abnormalities. However, the limited resolution of metaphase-CGH (5-10 megabases [Mb]) could explain the lack of imbalances in some normal cases as well as how additional genomic changes in abnormal cases were overlooked. The recent development of microarray-based CGH (array-CGH) will help to circumvent this drawback since it allows the high-resolution detection of DNA copy number aberrations.29,30 Moreover, this study shows the effectiveness of CGH to delineate novel critical genomic regions, which have so far been neglected and that could be the starting point for further studies using high-resolution genome analysis with the aim of identifying new putative oncogenes or tumor suppressor genes. The combination of genomic array results with gene expression profiling data could provide important insights into the genesis and hopefully treatment of MM patients.
Recently, a strong association between IGH rearrangements and nonhyperdiploid variant MM has been demonstrated.14,16,17 However, we did not find a different prevalence of IGH translocation according to the presence of genomic losses evaluated by CGH. By contrast, in line with a previous study, a significant relationship between IGH translocations and ploidy categories as detected by DNA content was found.16 The explanation for that could be the different significance of ploidy status depending on the different approaches of ploidy detection. Karyotype has been the classical tool used for ploidy categorization, but it is limited by the small number of informative cases. DNA index assessed by flow cytometry can reliably discriminate only between hyperdiploid and nonhyperdiploid cases, since hypodiploid patients may be included within the diploid group. Although CGH offers the advantage of identifying chromosomal imbalances in a higher proportion of patients than cytogenetics, the global genomic imbalance or aneuploidy cannot be precisely calculated since gains and losses involve regions that are very different in size. Nevertheless, this study also failed to find a lower prevalence of IGH translocations in the group of patients with only gains as might be expected. This would imply a more complex explanation for the association between IGH translocations and nonhyperdiploid MM than the notion that speculates that the proliferative advantage provided by IGH translocations is permissive to chromosomal losses.16 Interestingly, IGH translocations were significantly associated with gains on 11q and were not caused by extra copies of t(11;14). Further analysis based on a large series of patients will be needed to better define this striking association.
In summary, this study demonstrates for the first time the prognostic value of genomic changes as detected by CGH in MM and illustrates a previously undescribed relationship between IGH rearrangements and gains on 11q.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-04-1319.
Partially supported by Spanish Fondo de Investigación Sanitaria (FIS) grants (98/1161, 01/1161, and G03/136) and “Junta de Castilla y León” grants (SA113/01, SA25/02, and SA032/04).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Mark Anderson from the University Technology Transfer Office, and Pilar Fernández, M. Ángeles Hernández, Amador Crego, and Ana Simón for technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal