Comment on Gunzer et al, page 2801
The paper by Gunzer and colleagues in this issue identifies a new mode of interaction for T cells and B cells using 3-D tissue culture methods and intravital microscopy of T-B encounters in living mouse lymph nodes.
The typical way that an immunologist assesses the function of isolated lymphocytes is in suspension cultures where T-cell activation is accompanied by formation of cellular clusters that may incorporate dozens or hundreds of T cells and many antigen-presenting cells (APCs). These systems are 3-D in that cells in the clusters have many neighbors, but the systems lack the physical scaffold offered by solid tissues. Gunzer et al1 have adopted a different in vitro model based on collagen gels, which they feel better mimics the reticular fiber scaffold of lymph nodes. While this point is controversial, the results from these studies have nonetheless been interesting in identifying novel cell-cell interaction modes. For example, Gunzer et al identified “serial encounters” between T cells and antigen-bearing dendritic cells (DCs). Serial encounters are brief cell-cell interactions of a few minutes each, separated by periods of migration in the collagen gel, that are sufficient to induce T-cell proliferation. These contrast with the long-lived “immunologic synapses” that are a prominent feature in suspension culture models.2 While stable, long-lived T-cell-DC interactions are also a prominent feature of in vivo immune responses, periods of serial encounters were observed prior to and after the stable interactions,3 suggesting that this interaction mode is important in vivo and that the collagen gel model has predictive power.FIG1
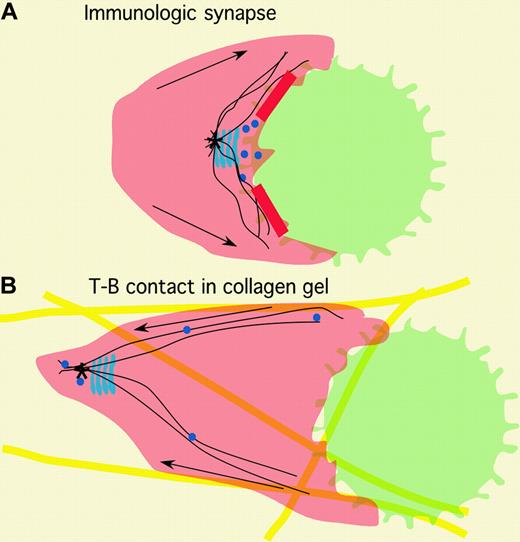
Comparison of immunologic synapse to model of new contact described by Gunzer and colleagues. The T cells (red) and B cells (green) form an immunologic synapse (A) in which the secretory apparatus (blue) and microtubule organizing center (MTOC; *) are positioned close to the interface, and molecule transport (arrows) is toward the interface, characterized by a ring or adhesion molecules (solid red). Both the T and B cells are relatively sessile. In a collagen gel (yellow), the same cells form a different structure in which the T cell continues to migrate (B). The migratory behavior of the T cell is consistent with maintenance of the MTOC and secretory apparatus in the uropod and the movement of the molecule linked to surface receptor away from the interface to the back of the cell (arrows). The B cell appears to attach to the leading lamellipodia and to be pushed forward. The nature of the adhesive structure is not known, but overall cytoskeletal organization is likely to be very different from an immunologic synapse.
Comparison of immunologic synapse to model of new contact described by Gunzer and colleagues. The T cells (red) and B cells (green) form an immunologic synapse (A) in which the secretory apparatus (blue) and microtubule organizing center (MTOC; *) are positioned close to the interface, and molecule transport (arrows) is toward the interface, characterized by a ring or adhesion molecules (solid red). Both the T and B cells are relatively sessile. In a collagen gel (yellow), the same cells form a different structure in which the T cell continues to migrate (B). The migratory behavior of the T cell is consistent with maintenance of the MTOC and secretory apparatus in the uropod and the movement of the molecule linked to surface receptor away from the interface to the back of the cell (arrows). The B cell appears to attach to the leading lamellipodia and to be pushed forward. The nature of the adhesive structure is not known, but overall cytoskeletal organization is likely to be very different from an immunologic synapse.
In this issue of Blood, Gunzer and colleagues use the collagen gel model to examine interactions of naive T and B cells. The interactions observed in the collagen gel were again novel and completely unanticipated. The T cells engage the B cells, paralyze the B-cell locomotion process, and then push the B cells around in the collagen gel for long periods. This interaction is long-lived and can lead to T-cell activation but is dramatically different than the immunologic synapse observed in suspension culture and again suggests that the 3-D environment has a powerful effect on interactions. Figure 1 shows the model for the cytoskeletal organization of a T cell forming an immunologic synapse and the bulldozer-like pushing interaction observed in the collagen gel model. In the immunologic synapse, the T-cell motility machinery is focused on the interface and both cells are typically sessile.4 In the collagen gel, the T cell apparently engages the B cells with its leading edge but maintains motile function and with a uropod-like trailing edge that it uses to push the B cells. The closest data to this model are observations by Nieto et al,5 who showed that migrating T cells drag other cells through attachment at the uropod. (The observation of the pushing action is unprecedented.)
Gunzer et al visualized similar interactions in vivo using intravital confocal microscopy and also saw T-B pairs migrating with the B cell in front and the T cell in back. In this case, the B cell is not paralyzed and maintains polarized morphology, so it is possible that the B cell may be pulling the T cells. However, the simplest interpretation of the in vivo data is that it is similar to the collagen gel system in which the T cell clearly appears to push the B cells. It should be pointed out that interactions of naive T and B cells may not be physiologically relevant in vivo since the most potent APC for a naive T cell is a dendritic cell, and T-B collaboration would typically take place between a recently divided activated T cell, and a recently antigen receptor-stimulated B cell.6 Gunzer et al examine the interaction of naive T cells and activated B cells and find that they interact through serial encounters similar to naive T cells and dendritic cells in the collagen gel. The interaction of activated helper T cells and recently antigen receptor-stimulated B cells was not examined.
These studies add a new interaction mode to the repertoire of immune cells, which is neither an immunologic synapse nor a classical serial encounter, although it may be more related to the latter than the former, based on the polarization of the T cell. The physiologic relevance of this interaction mode for T-B collaboration remains to be established, but it is likely that this type of interaction will play a role in vivo if the record for predictive power of the collagen gel model holds.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal