Abstract
Complete DiGeorge syndrome is a fatal congenital disorder characterized by athymia, hypoparathyroidism, and heart defects. Less than half of patients are 22q11 hemizygous. The goal of this study was to assess if immune suppression followed by postnatal thymus transplantation would lead to T-cell function in 6 infant patients who had host T cells at the time of transplantation. All infants had fewer than 50 recent thymic emigrants (CD3+CD45RA+CD62L+) per cubic millimeter (mm3) and all had some proliferative response to the mitogen phytohemagglutinin. Four infants had rash, lymphadenopathy, and oligoclonal populations of T cells in the periphery. Five of 6 patients are alive at the follow-up interval of 15 months to 30 months. The 5 surviving patients developed a mean of 983 host CD3+ T cells/mm3 (range, 536/mm3-1574/mm3), a mean of 437 recent thymic emigrants/mm3 (range, 196/mm3-785/mm3), and normal proliferative responses to phytohemaglutinin (follow-up from day 376 to day 873). The TCR repertoire became polyclonal in patients who presented with oligoclonal T cells. All patients had thymopoiesis on allograft biopsy. Postnatal thymus transplantation after treatment with Thymoglobulin shows promise as therapy for infants with complete DiGeorge syndrome who have significant proliferative responses to mitogens or who develop rash, lymphadenopathy, and oligoclonal T cells.
Introduction
DiGeorge syndrome is a congenital disorder characterized by defects in the third and fourth pharyngeal pouches, although abnormalities can be found extending from the first through sixth pharyngeal arches.1 Typically, defects are found in the heart, parathyroid gland, and thymus.2-6 The immune defect in DiGeorge syndrome usually is mild. In fewer than 1% of cases, there is profound immune deficiency secondary to athymia.7 Athymic patients are categorized as having complete DiGeorge syndrome.2,8 Patients with complete DiGeorge syndrome usually die within the first 2 years of life.2 Severe recurrent infections are a major problem.2
Peripheral blood testing can be used to identify those patients with DiGeorge syndrome who are athymic. Naive T cells (recent thymic emigrants) coexpress CD45RA and CD62L.9 We define athymic patients as those having less than 50 naive T cells/cubic millimeter (mm3).10 In patients having a sufficient blood volume for testing, we also quantitate the number of T-cell receptor rearrangement excision circles (TRECs) in circulating T cells.11-13 TRECs are episomal DNA circles that form when T-cell receptor (TCR) gene segments rearrange in the thymus. Athymic patients have less than 100 TRECs/100 000 T cells (normal value for a newborn is approximately 10 000 TRECs/100 000 T cells).10
Most athymic patients with DiGeorge syndrome have very low proliferative responses to mitogens and very low T-cell numbers. These patients can be treated with postnatal allogeneic thymus transplantation without immunosuppression.10 The mechanism of this experimental treatment is the migration of host bone marrow cells to slices of cultured donor thymus that are implanted into the patient's muscle. In the donor graft, the host bone marrow cells develop via thymopoiesis into host T cells that then emigrate out of the thymus into the peripheral blood.
We report the results of postnatal allogeneic thymus transplantation in 6 athymic patients with DiGeorge syndrome who had some T cells and T-cell proliferative responses to mitogens. Because of their T-cell function, these patients would likely have rejected allotransplants if no immunosuppression had been given. Thus, all patients were initially treated with Thymoglobulin (rabbit antithymocyte globulin, Imtix-SangStat, Lyon, France). This treatment was immediately followed by postnatal cultured allogeneic thymus transplantation. The excellent clinical and immunologic results in this group of patients supports the use of Thymoglobulin together with thymus transplantation to effect normal T-cell development in selected patients with complete DiGeorge syndrome.
Patients, materials, and methods
Patient inclusion criteria
All patients participated after their parents provided informed consent as approved by the Duke University Medical Center Institutional Review Board. All patients were enrolled in protocols reviewed by the Food and Drug Administration under an Investigational New Drug application. For inclusion in the study, patients had to have DiGeorge syndrome. This requirement was met by having one of the following: 22q11 hemizygosity, CHARGE association (coloboma, heart defect, choanal atresia, growth or developmental retardation, genital hypoplasia, ear defect including deafness),14-16 heart defect, or hypocalcemia. The patients also had to have evidence of athymia as indicated by less than 50 T cells/mm3 coexpressing CD45RA and CD62L. The patients' mothers, if multiparous, and/or their infants, were screened for the presence of antibodies against HLA. None were positive.
For descriptive purposes, patients were categorized as having “typical” or “atypical” complete DiGeorge syndrome. In both, there was evidence of athymia as defined in the preceding paragraph. Patients were categorized as having “atypical” complete DiGeorge syndrome if they had developed rash, lymphadenopathy, and oligoclonal T cells [more than 40% of T-cell receptor beta variable (TCRBV) families oligoclonal by immunoscope]10 prior to transplantation. The T cells did not coexpress CD45RA and CD62L. The pretransplantation evaluation of the patients with atypical complete DiGeorge syndrome has been described at length.17 The atypical patients did not have Ommen syndrome (by sequence evaluation of RAG1 and RAG2 in those patients who were normal at 22q11). The patients did not have engraftment of maternal T cells as determined by chromosomal or DNA studies prior to transplantation.
For inclusion in this protocol using immunosuppression, patients with typical complete DiGeorge syndrome had to have a response to phytohemaglutinin (PHA) of more than 5000 cpm or more than 20-fold above background (whichever was greater). Beginning with DIG102, all patients with atypical complete DiGeorge syndrome were treated under this protocol.
Screening for infections in allograft recipients
Because of the risk of lymphoproliferative disease after transplantation,18,19 patients (allograft recipients) were initially evaluated for evidence of cytomegalovirus (CMV) infection by urine culture and Epstein-Barr virus (EBV) infection by polymerase chain reaction (PCR) of peripheral blood. No patients were positive. Viral cultures of nasopharyngeal fluid, stool, and urine were obtained prior to transplantation. Electron microscopy of stool was also obtained to search for viral infections. All viral infections were treated promptly.
Thymus donor and tissue processing
Allogeneic postnatal thymus tissue was used after obtaining informed consent from the donor's parents under a protocol approved by the Duke Institutional Review Board. The donors were infants younger than 6 months of age undergoing cardiac surgery. No donors were related to the recipients. The thymus tissue was removed in order for the surgeon to perform the cardiac repair, not for this research protocol. Thymus tissue was processed and cultured as previously reported.10 The duration of culture averaged 17 days (range, 15-20 days), with the exact duration determined by logistical issues.
Thymus transplantation and Thymoglobulin use
Prior to thymus transplantation, the patient was given 2 doses (for patients DIG102 and DIG103) or 3 doses (for patients DIG104, DIG105, DIG106, and DIG107) of 2 mg/kg Thymoglobulin. These doses were given over approximately 12 hours once daily. The patients were pretreated with acetaminophen, methylprednisolone, and diphenhydramine. The methylprednisolone was given every 6 hours throughout the days of Thymoglobulin administration and was stopped prior to thymus transplantation. The exact scheduling of the Thymoglobulin and transplantation are given in Table 1. After the first 2 patients, the protocol was changed to give all patients 3 doses of Thymoglobulin. The variability in the schedule for the final 3 patients compared with the stated schedule was secondary to cardiac instability (DIG106), avoiding weekend surgery (DIG107), and using tissue prior to its expiration date (DIG105).
Treatment schedule
Patient ID no. . | Day 1 . | Day 2 . | Day 3 . | Day 4 . | Day 5 . | Day 6 . |
|---|---|---|---|---|---|---|
| DIG102 | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None | None |
| DIG103 | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None | None |
| DIG104 | Thymoglobulin | Thymoglobulin | Thymoglobulin | Rest | Rest | Transplantation |
| DIG105 | Thymoglobulin | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None |
| DIG106 | Thymoglobulin | Rest | Rest | Thymoglobulin | Thymoglobulin | Transplantation |
| DIG107 | Thymoglobulin | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None |
Patient ID no. . | Day 1 . | Day 2 . | Day 3 . | Day 4 . | Day 5 . | Day 6 . |
|---|---|---|---|---|---|---|
| DIG102 | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None | None |
| DIG103 | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None | None |
| DIG104 | Thymoglobulin | Thymoglobulin | Thymoglobulin | Rest | Rest | Transplantation |
| DIG105 | Thymoglobulin | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None |
| DIG106 | Thymoglobulin | Rest | Rest | Thymoglobulin | Thymoglobulin | Transplantation |
| DIG107 | Thymoglobulin | Thymoglobulin | Thymoglobulin | Rest | Transplantation | None |
Immune function
Routine immunologic evaluations by flow cytometry, proliferation assays, and the PCR-based immunoscope method for evaluating TCRBV repertoire were performed as described.10 There were 23 TCRBV families examined. Each immunoscope profile was identified as oligoclonal (≤ 4 peaks), polyclonal-skewed (> 4 peaks but not Gaussian-like), or polyclonal–Gaussian-like (> 4 peaks). The initial immunoscopes were done using RNA isolated from peripheral blood mononuclear cells (PBMCs); later immunoscopes were done using RNA isolated from CD4 T cells. TREC assays were conducted as described on purified CD3+ T cells.10
Immunohistochemistry evaluations of thymus graft biopsies
Biopsies of the thymus allografts were done approximately 3 months after transplantation (mean, 93 days; range, 75-118 days) as an open procedure in the operating room.10 The tissue was evaluated both using formalin-fixed, paraffin-embedded sections and frozen sections. The antibodies used10 included cytokeratin to detect epithelium; CD3, CD4, and CD8 to evaluate the T cells; CD1a and Ki-6720 to identify cortical thymocytes; CD20 to identify B cells; and TIA-121 in conjunction with CD8 to identify cytotoxic T cells.10 The microscope used for photomicrographs was an Olympus Vanox-S. The 10 × objective was a Planapo with a numerical aperture (N.A.) of 0.40; the 40 × objective was a Splan with an N.A. of 0.70. No imaging solution was used. The camera was an Olympus DP-11. Photoshop version 6.0 was used for acquisition and image processing.
Results
Clinical presentation
All 6 infants were diagnosed as having DiGeorge syndrome based on the typical congenital defects derived from the third and fourth pharyngeal pouches (thymus and parathyroid defects) and surrounding pharyngeal arches (heart defects). The clinical findings in these patients are shown in Table 2.
Syndromic and clinical findings
Syndrome and patient ID no. . | Age at transplantation, mos. . | Syndromic association . | Hypocalcemia . | Cardiac defect . | Other first to sixth pharyngeal pouch/arch abnormalities* . | Other clinical findings at presentation . |
|---|---|---|---|---|---|---|
| Typical complete | ||||||
| DiGeorge syndrome | ||||||
| DIG103 | 8.5 | CHARGE, 22q11 normal | No | PDA, right aortic arch, complete vascular ring, patent foramen ovale | Choanal atresia, hearing loss, poor swallow | Dermatitis, hiatal hernia on day 1 of life, hypogonadism, Dandy-Walker, mega cisternamagna, rotavirus, bilateral colobomas |
| DIG105 | 12.7 | Mother with gestational diabetes, 22q11 normal | Yes | VSD, small PDA, PFO | GER | 3 weeks of ventilation after birth, spina bifida occulta, seizure from multiple infections |
| Atypical complete | ||||||
| DiGeorge syndrome | ||||||
| DIG102 | 8.9 | 22q11 hemizygosity | Yes† | No | GER | Rash, FTT, PCP, Candida albicans in draining ear, lymphadenopathy |
| DIG104 | 4.0 | Mother with type I diabetes, 22q11 normal | Yes | PFO | Abnormal BAER on left, GER | Polyhydraminos, vertebral anomalies at T7 and T8, rash, lymphadenopathy, FTT, RSV on admission, enterovirus on admission |
| DIG106 | 10.8 | VCFS, 22q11 normal | Yes | VSD, large PDA, hypoplastic aortic arch with coarctation and subaortic stenosis | Cleft soft palate and notch of upper lip, malformed right ear, GER, right choanal atresia | Rash, FTT, severe developmental delay, ischemic brain injury, tracheostomy, infections, rash, lympadenopathy (biopsied) |
| DIG107 | 13.0 | CHARGE, 22q11 normal | Yes | Small ASD | Swallowing dysfunction, GER, left choanal atresia, abnormal BAER on left, seventh nerve palsy | Intermittent rash, lymphadenopathy (biopsied), multiple aspiration pneumonias, sepsis with Bacillus cereus, stools positive for Clostridium dificile toxin and rotavirus, PCP |
Syndrome and patient ID no. . | Age at transplantation, mos. . | Syndromic association . | Hypocalcemia . | Cardiac defect . | Other first to sixth pharyngeal pouch/arch abnormalities* . | Other clinical findings at presentation . |
|---|---|---|---|---|---|---|
| Typical complete | ||||||
| DiGeorge syndrome | ||||||
| DIG103 | 8.5 | CHARGE, 22q11 normal | No | PDA, right aortic arch, complete vascular ring, patent foramen ovale | Choanal atresia, hearing loss, poor swallow | Dermatitis, hiatal hernia on day 1 of life, hypogonadism, Dandy-Walker, mega cisternamagna, rotavirus, bilateral colobomas |
| DIG105 | 12.7 | Mother with gestational diabetes, 22q11 normal | Yes | VSD, small PDA, PFO | GER | 3 weeks of ventilation after birth, spina bifida occulta, seizure from multiple infections |
| Atypical complete | ||||||
| DiGeorge syndrome | ||||||
| DIG102 | 8.9 | 22q11 hemizygosity | Yes† | No | GER | Rash, FTT, PCP, Candida albicans in draining ear, lymphadenopathy |
| DIG104 | 4.0 | Mother with type I diabetes, 22q11 normal | Yes | PFO | Abnormal BAER on left, GER | Polyhydraminos, vertebral anomalies at T7 and T8, rash, lymphadenopathy, FTT, RSV on admission, enterovirus on admission |
| DIG106 | 10.8 | VCFS, 22q11 normal | Yes | VSD, large PDA, hypoplastic aortic arch with coarctation and subaortic stenosis | Cleft soft palate and notch of upper lip, malformed right ear, GER, right choanal atresia | Rash, FTT, severe developmental delay, ischemic brain injury, tracheostomy, infections, rash, lympadenopathy (biopsied) |
| DIG107 | 13.0 | CHARGE, 22q11 normal | Yes | Small ASD | Swallowing dysfunction, GER, left choanal atresia, abnormal BAER on left, seventh nerve palsy | Intermittent rash, lymphadenopathy (biopsied), multiple aspiration pneumonias, sepsis with Bacillus cereus, stools positive for Clostridium dificile toxin and rotavirus, PCP |
ASD indicates atrial septal defect; BAER, brainstem auditory evoked potentials; FTT, failure to thrive; GER, gastroesophageal reflux; PCP, Pneumocystis carnii pneumonia; PDA, patent ductus arteriosus; PFO, patent foramen ovale; RSV, respiratory syncytial virus; VSD, ventricular septal defect; VCFS, velocardiofacial syndrome.
The following are the postulated developmental sources of the anomalies1 : cleft soft palate and notch of upper lip, first arch; choanal atresia, first arch; ear anomalies, first and second arches; seventh nerve palsy, second arch; poor swallow (ninth cranial nerve), third arch; GER (related to ninth cranial nerve), third arch.
Hypocalcemia became clinically significant after thymus transplantation.
All of the patients studied were athymic based on lack of naive T cells in the peripheral blood. Specifically, all patients had less than 50 naive T cells (those coexpressing CD45RA and CD62L)/mm3. There were 2 patients with “typical complete DiGeorge syndrome” with no rash or adenopathy. They did have low levels of T-cell proliferative responses. There were 4 patients with the atypical form of complete DiGeorge syndrome, with rash, lymphadenopathy, and oligoclonal T-cell proliferations.17 The distinction between the typical and atypical patients is based on the presence of rash, lymphadenopathy, and oligoclonal T cells in the latter. Detailed case reports describing the history and evaluation of the atypical patients (DIG102, DIG104, DIG106, and DIG107) are found in Markert et al.17 Pretransplantation T cells in all patients tested (DIG102, DIG103, DIG104, DIG106, DIG107) were genetically recipient.
Transplantation and Thymoglobulin treatment
Beginning with patient DIG102, all patients who had developed the atypical complete DiGeorge syndrome phenotype (rash, lymphadenopathy, and oligoclonal T cells) and all typical complete DiGeorge syndrome patients with a PHA proliferative response of more than 5000 cpm (or more than 20-fold over background, whichever was higher) were treated with Thymoglobulin in addition to postnatal allogeneic thymus transplantation. Thus, 6 patients received Thymoglobulin prior to transplantation. Table 1 shows the timing of the Thymoglobulin treatment and thymus transplantation. The average age at transplantation was 9.7 months (range, 4-13 months; Table 2). This is older than the average age of previously reported patients who did not need immunosuppression at the time of transplantation (mean, 2.7 months; range, 1.1-4.4 months).10
Thymoglobulin administration was associated with some adverse events. Patient DIG102 had rash, fever, and wheezing, although these symptoms may have been related to a new onset adenoviral infection. The side effects resolved within 24 hours of the end of treatment. Patient DIG104 developed thrombocytopenia to 27 000/mm3 that was treated with one platelet transfusion prior to thymus transplantation. After transplantation, patient DIG104 developed elevated eosinophils to 6179/mm3 on day 4, elevated T cells to 11 997/mm3 on day 10 (the T-cell count had been 7942/mm3 prior to Thymoglobulin), and elevated liver enzymes with a peak ALT of 1712 U/L on day 15. A liver biopsy on day 17 was consistent with enteroviral infection. The eosinophil count, T-cell count, and liver enzyme abnormalities returned to baseline without treatment. Patient DIG104 had contracted respiratory syncytial virus (RSV) and enteroviral infections shortly before undergoing transplantation. It is likely that these infections contributed to the adverse events following Thymoglobulin administration.
Table 3 shows the HLA typing data for the patients. There was sharing with the donor of one HLA-DRB1 allele in DIG102, DIG103, and DIG104 (who also had sharing of one HLA-A allele). Patients DIG105 and DIG107 only shared one HLA-A allele with their donors; patient DIG106 shared one HLA-A allele and one HLA-B allele with his donor. There did not appear to be any relationship between matching and outcome. No infant had antibodies against HLA detected prior to transplantation.
HLA typing of donor and recipients
Recipient syndrome and patient ID no. . | HLA-A . | HLA-B . | HLA-DRB1 . | Donor . | HLA-A . | HLA-B . | HLA-DRB1 . |
|---|---|---|---|---|---|---|---|
| Typical complete DiGeorge syndrome | |||||||
| DIG103 | 1, 1 | 7, 57 | 15011, 03011 | MLM152 | 02xx* | 44xx, 5001/04 | 0301, 1101 |
| DIG105 | 1, 24 | 35, 14 | 13, 13 | MLM159 | 0101-09, 03xx | 07xx, 08xx | 0301, 1101 |
| Atypical complete DiGeorge syndrome | |||||||
| DIG102 | 1, 1 | 8, 57 | 0301, 0701 | MLM145 | 3601, 68xx | 15xx, 53xx | 0701, 1101 |
| DIG104 | 2, 74 | 58,71 | 11, 13 | MLM153 | 02xx | 15xx, 40xx | 0401, 1302 |
| DIG106 | 03xx, 31xx | 07xx, 35xx | 0802, 1501 | MLM159 | 0101-09, 03xx | 07xx, 08xx | 0301, 1101 |
| DIG107 | 2, 3 | 7, 60 | 15, 13 | MLM160 | 0101-09, 02xx | 08xx, 39xx | 0301, 0401 |
Recipient syndrome and patient ID no. . | HLA-A . | HLA-B . | HLA-DRB1 . | Donor . | HLA-A . | HLA-B . | HLA-DRB1 . |
|---|---|---|---|---|---|---|---|
| Typical complete DiGeorge syndrome | |||||||
| DIG103 | 1, 1 | 7, 57 | 15011, 03011 | MLM152 | 02xx* | 44xx, 5001/04 | 0301, 1101 |
| DIG105 | 1, 24 | 35, 14 | 13, 13 | MLM159 | 0101-09, 03xx | 07xx, 08xx | 0301, 1101 |
| Atypical complete DiGeorge syndrome | |||||||
| DIG102 | 1, 1 | 8, 57 | 0301, 0701 | MLM145 | 3601, 68xx | 15xx, 53xx | 0701, 1101 |
| DIG104 | 2, 74 | 58,71 | 11, 13 | MLM153 | 02xx | 15xx, 40xx | 0401, 1302 |
| DIG106 | 03xx, 31xx | 07xx, 35xx | 0802, 1501 | MLM159 | 0101-09, 03xx | 07xx, 08xx | 0301, 1101 |
| DIG107 | 2, 3 | 7, 60 | 15, 13 | MLM160 | 0101-09, 02xx | 08xx, 39xx | 0301, 0401 |
The “xx” designation was given if the typing was low resolution and did not yield the specific allele.
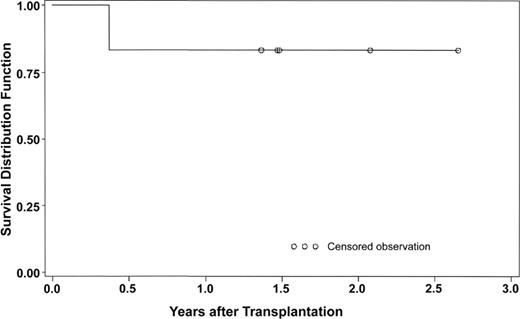
The Kaplan-Meier graph (Figure 1) shows the excellent survival with follow-up from 10 months to 26 months. This survival rate (5 of 6 patients) is similar to the survival of complete DiGeorge syndrome patients treated with thymus transplantation without immunosuppression.10
Survival of patients with complete DiGeorge syndrome treated with Thymoglobulin prior to thymus transplantation.
Survival of patients with complete DiGeorge syndrome treated with Thymoglobulin prior to thymus transplantation.
Immune reconstitution
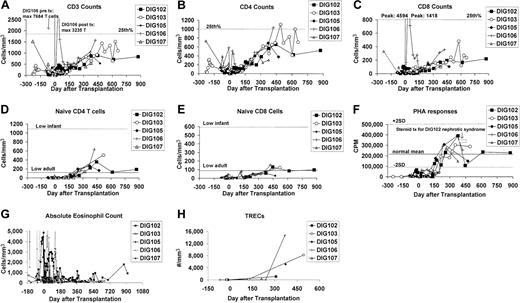
The results of immune reconstitution are encouraging (Figure 2). All surviving patients have more than 500 T cells/mm3 at the follow-up interval of 421 to 873 days after transplantation (Figure 2A). The CD4-to-CD8 ratio has normalized in all patients (Figure 2B-C). Although several patients presented with oligoclonal T-cell populations in the blood, naive T-cell numbers were less than 50/mm3 prior to transplantation. Naive T cells subsequently developed after transplantation, although the numbers at this early time are still in the low adult range (Figure 2D-E), far below the normal infant range. PHA responsiveness normalized in all patients by one year to be within 2 standard deviations (SD) of the mean of healthy adults (Figure 2F). All 5 surviving patients have been immunized to tetanus toxoid and all mount normal proliferative T-cell responses (data not shown). As expected, posttransplantation T cells were genetically recipient in all 5 survivors tested on day 312 (DIG102) to day 540 (DIG105) after transplantation. As T-cell function increased, eosinophil counts decreased, especially in DIG102 and DIG106 (Figure 2G).
Immune function. After transplantation, there is a variable change in (A) CD3, (B) CD4, and (C) CD8 T-cell numbers as clonal T-cell populations decrease in number. After transplantation there are increasing numbers of (D) naive CD4 T cells and (E) naive CD8 T cells and increasing (F) T-cell proliferative responses to PHA. Changes in the (G) absolute eosinophil counts and (H) TREC values are shown. For panels A to C, the 25th percentile for children ages one to 6 years22 is indicated by the dotted line. For panels D to E, the lowest value for 11 children aged one year23 is indicated by the dotted line; the lowest value for 9 adults23 is indicated by the solid line. In panel F, the mean for healthy adults is indicated by the solid line and the range of 2 standard deviations by the dotted lines. The patients' PBMC background proliferations (cells plus medium without mitogen) for the PHA assays (n=80) had a mean of 198 cpm with one standard deviation ranging from 74 cpm to 527 cpm. The normal control background had a mean of 173 cpm with one standard deviation ranging from 61 to 486 cpm.
Immune function. After transplantation, there is a variable change in (A) CD3, (B) CD4, and (C) CD8 T-cell numbers as clonal T-cell populations decrease in number. After transplantation there are increasing numbers of (D) naive CD4 T cells and (E) naive CD8 T cells and increasing (F) T-cell proliferative responses to PHA. Changes in the (G) absolute eosinophil counts and (H) TREC values are shown. For panels A to C, the 25th percentile for children ages one to 6 years22 is indicated by the dotted line. For panels D to E, the lowest value for 11 children aged one year23 is indicated by the dotted line; the lowest value for 9 adults23 is indicated by the solid line. In panel F, the mean for healthy adults is indicated by the solid line and the range of 2 standard deviations by the dotted lines. The patients' PBMC background proliferations (cells plus medium without mitogen) for the PHA assays (n=80) had a mean of 198 cpm with one standard deviation ranging from 74 cpm to 527 cpm. The normal control background had a mean of 173 cpm with one standard deviation ranging from 61 to 486 cpm.
TREC data are shown in Figure 2H. TREC numbers seem to increase in parallel with the increase in proliferative responses to PHA. Although the number of datapoints is low, the increases are similar to those seen in thymus transplantation patients who were not treated with Thymoglobulin.10
Immunoscope analyses have been informative in the patients with atypical complete DiGeorge syndrome who presented with oligoclonal T-cell populations. Figure 3 shows the immunoscopes before and after transplantation for all 3 surviving atypical patients. Using patient DIG102 as an example, there is an impressive improvement with 64% polyclonal Gaussian-like TCRBV families at 615 days compared with 0% prior to transplantation. These data suggest that the clones present prior to transplantation are suppressed as a polyclonal repertoire develops. The improvement in repertoire is impressive as this change is seen less than 1 year after transplantation.
Immunoscope evaluation. Evaluation of DIG102, DIG106, and DIG107 before and after thymus transplantation. The RT-PCR was done on CD4 RNA for the posttransplantation panels of DIG106 and DIG107; it was done on PBMC RNA for the other panels. The final panel includes the identification of the TCRBV families. Examples of oligoclonal (O), polyclonal skewed (S), and polyclonal Gaussian-like (G) panels are indicated in the DIG102 profiles. The pretransplantation profiles have been reported17 and are reprinted with permission from the American Academy of Allergy, Asthma, and Immunology. Copyright 2004.
Immunoscope evaluation. Evaluation of DIG102, DIG106, and DIG107 before and after thymus transplantation. The RT-PCR was done on CD4 RNA for the posttransplantation panels of DIG106 and DIG107; it was done on PBMC RNA for the other panels. The final panel includes the identification of the TCRBV families. Examples of oligoclonal (O), polyclonal skewed (S), and polyclonal Gaussian-like (G) panels are indicated in the DIG102 profiles. The pretransplantation profiles have been reported17 and are reprinted with permission from the American Academy of Allergy, Asthma, and Immunology. Copyright 2004.
The typical complete DiGeorge syndrome patients did not have rash, lymphadenopathy, nor clonal T cells prior to transplantation. In DIG105, the T-cell numbers were too low for testing of repertoire prior to transplantation. In DIG103, 67% of the T-cell families were polyclonal-skewed and 29% were oligoclonal. Patient DIG103 has been tested since undergoing transplantation and his repertoire has improved, as has been previously reported for other patients with typical complete DiGeorge syndrome after thymus transplantation.10
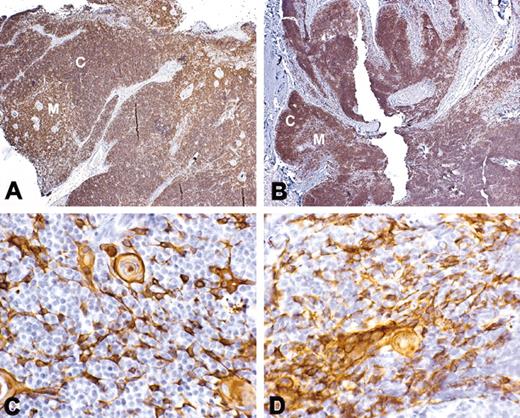
Graft biopsies in all patients showed evidence of thymopoiesis. Thymopoiesis was defined as presence of lacy cytokeratin staining and presence of thymocytes with the CD3+, CD1a+, and Ki-67+ phenotype of typical cortical thymocytes. The density of T cells in the graft varied from patient to patient. Figure 4 shows the biopsy from patient DIG107 that had a high density of T cells. Patients DIG105 and DIG106 received thymus tissue from the same donor. The biopsy from DIG105 resembled that in Figure 4, whereas the biopsy from DIG106 had the least-developed thymus with scattered cortical thymocytes without development of medulla (data not shown). The clinical condition of the patients varied significantly with DIG105 being very stable and DIG106 being very ill; this may have affected the rate of development of thymopoiesis in the grafts.
Thymus histology following transplantation. (A,C) Normal thymus; (B,D) biopsy from DIG107 conducted at 3 months after thymus transplantation. Panels A and B show staining with CD3 antibody (original magnification ×10). Panels C and D show staining with cytokeratin. Positive reaction with antibody is indicated by the brown color. Both cortex (C) and medulla (M) containing light lacy cytokeratin-positive epithelial cells and Hassall bodies are evident in the transplant biopsy from this patient.
Thymus histology following transplantation. (A,C) Normal thymus; (B,D) biopsy from DIG107 conducted at 3 months after thymus transplantation. Panels A and B show staining with CD3 antibody (original magnification ×10). Panels C and D show staining with cytokeratin. Positive reaction with antibody is indicated by the brown color. Both cortex (C) and medulla (M) containing light lacy cytokeratin-positive epithelial cells and Hassall bodies are evident in the transplant biopsy from this patient.
As shown in Table 4, the patients have done remarkably well after transplantation with few adverse events after day 120. Infections, which were frequent in the peritransplantation period, have dramatically lessened in frequency and severity (Table 4). All patients are living at home. The remaining clinical issues are feeding (DIG103, DIG106, DIG107), hearing deficits (DIG103, DIG107), and developmental delay (DIG103, DIG106), which are unrelated to thymus transplantation.
Adverse events and outcomes
Syndrome and patient ID no. . | Infectious adverse events . | Immune adverse events . | Other adverse events . | Status . |
|---|---|---|---|---|
| Typical complete | ||||
| DiGeorge syndrome | ||||
| DIG103 | Central line infections with Enterococcus faecalis, day + 4, with Candida parapsilosis, day + 48, and Staphylococcus epidermitis, day + 53; fever and coagulopathy for 3 weeks starting day + 89 (cultures negative) | None | Feeding intolerance, days + 89 to + 114, with diarrhea, coagulopathy, and fever; clot around central line, day + 67, associated with elevated platelets (701 000/mm3), treated with aspirin until patient had bleeding via rectum | At home on tube feedings 2.1 years after thymus transplantation |
| DIG105 | Stool positive for C difficile toxin, days - 5, + 13, + 56, + 77; stool positive for enterovirus, day + 13; wound infection from suture removal, day + 39; fever, day + 161—treated 2 days in hospital (culture negative) | Thrombocytopenia (to 30 000/mm3) with elevated antiplatelet antibodies, day + 19, that responded to IVIG; ANC dropped to 315/mm3 but responded to discontinuation of trimethoprim/sulfamethoxazole | Poor oral intake | At home on oral feedings 1.5 years after transplantation |
| Atypical complete | ||||
| DiGeorge syndrome patients | ||||
| DIG102 | Adenovirus in stool, days + 8, + 28, + 53, + 98, + 99, + 119; central line infections, days + 340, + 432, + 580 | Nephrotic syndrome, days + 261 to + 416; urticaria started day + 286, resolved; mediastinal lymphadenopathy with biopsy day + 913 showing normal nodes | Hypoxemia at night, day + 603 from VCFS, resolved without therapy; sucrase deficiency (feeding intolerance) since birth | At home on sacrosidase in oral feedings 2.7 years after transplantation |
| DIG104 | Respiratory syncytial virus (RSV) in lungs, day - 6 to death at day + 137; enterovirus in stool, days + 1 to + 27; urine with yeast, days + 33, + 42; blood positive for S epidermidis, days + 56, + 76, + 94; C difficile toxin in stool, days + 115, + 127; respiratory arrest, day + 120 from RSV; intubated, day + 130 for RSV | Rash and lymphadenopathy, day - 6 to death | Infantile spasms, day + 104 to death; bladder retention, day + 16 to death, secondary to fatty filum of spinal cord; developmental delay from birth to death; decubitus ulcer, day + 115 | Death from RSV on day + 137 after transplantation |
| DIG106 | Blood with S epidermidis, days - 8, 0, + 93; urine with enterococcus, days + 7, + 23, + 142; stool positive for C difficile toxin, days + 36, + 182; rhinovirus in tracheal aspirate, days + 126, + 133, + 142, + 147, then negative after treatment | Absolute eosinophil count to 10 804/mm3 with rash on day + 39, treated with steroids | Swallow study showed aspiration, so patient was placed on all tube feedings; pulmonary hypertension, moderate; severe developmental delay | At home on tube feedings 1.5 years after transplantation |
| DIG107 | Stool positive for adenovirus, day - 10; stool positive for rotavirus, days - 7, + 30, + 41, + 56, + 80, + 89; urine positive for Klebsiella pneumoniae, day - 7 | None | Frank aspiration on swallowing, study day - 9; deafness documented day + 40; diarrhea secondary to rotavirus until 6 months after transplantation | At home on tube feedings 1.4 years after transplantation |
Syndrome and patient ID no. . | Infectious adverse events . | Immune adverse events . | Other adverse events . | Status . |
|---|---|---|---|---|
| Typical complete | ||||
| DiGeorge syndrome | ||||
| DIG103 | Central line infections with Enterococcus faecalis, day + 4, with Candida parapsilosis, day + 48, and Staphylococcus epidermitis, day + 53; fever and coagulopathy for 3 weeks starting day + 89 (cultures negative) | None | Feeding intolerance, days + 89 to + 114, with diarrhea, coagulopathy, and fever; clot around central line, day + 67, associated with elevated platelets (701 000/mm3), treated with aspirin until patient had bleeding via rectum | At home on tube feedings 2.1 years after thymus transplantation |
| DIG105 | Stool positive for C difficile toxin, days - 5, + 13, + 56, + 77; stool positive for enterovirus, day + 13; wound infection from suture removal, day + 39; fever, day + 161—treated 2 days in hospital (culture negative) | Thrombocytopenia (to 30 000/mm3) with elevated antiplatelet antibodies, day + 19, that responded to IVIG; ANC dropped to 315/mm3 but responded to discontinuation of trimethoprim/sulfamethoxazole | Poor oral intake | At home on oral feedings 1.5 years after transplantation |
| Atypical complete | ||||
| DiGeorge syndrome patients | ||||
| DIG102 | Adenovirus in stool, days + 8, + 28, + 53, + 98, + 99, + 119; central line infections, days + 340, + 432, + 580 | Nephrotic syndrome, days + 261 to + 416; urticaria started day + 286, resolved; mediastinal lymphadenopathy with biopsy day + 913 showing normal nodes | Hypoxemia at night, day + 603 from VCFS, resolved without therapy; sucrase deficiency (feeding intolerance) since birth | At home on sacrosidase in oral feedings 2.7 years after transplantation |
| DIG104 | Respiratory syncytial virus (RSV) in lungs, day - 6 to death at day + 137; enterovirus in stool, days + 1 to + 27; urine with yeast, days + 33, + 42; blood positive for S epidermidis, days + 56, + 76, + 94; C difficile toxin in stool, days + 115, + 127; respiratory arrest, day + 120 from RSV; intubated, day + 130 for RSV | Rash and lymphadenopathy, day - 6 to death | Infantile spasms, day + 104 to death; bladder retention, day + 16 to death, secondary to fatty filum of spinal cord; developmental delay from birth to death; decubitus ulcer, day + 115 | Death from RSV on day + 137 after transplantation |
| DIG106 | Blood with S epidermidis, days - 8, 0, + 93; urine with enterococcus, days + 7, + 23, + 142; stool positive for C difficile toxin, days + 36, + 182; rhinovirus in tracheal aspirate, days + 126, + 133, + 142, + 147, then negative after treatment | Absolute eosinophil count to 10 804/mm3 with rash on day + 39, treated with steroids | Swallow study showed aspiration, so patient was placed on all tube feedings; pulmonary hypertension, moderate; severe developmental delay | At home on tube feedings 1.5 years after transplantation |
| DIG107 | Stool positive for adenovirus, day - 10; stool positive for rotavirus, days - 7, + 30, + 41, + 56, + 80, + 89; urine positive for Klebsiella pneumoniae, day - 7 | None | Frank aspiration on swallowing, study day - 9; deafness documented day + 40; diarrhea secondary to rotavirus until 6 months after transplantation | At home on tube feedings 1.4 years after transplantation |
Discussion
The 6 patients in this report all had complete DiGeorge syndrome. All had multiple defects centering on derivatives of the third and fourth pharyngeal pouches. Included in these defects was at least one of the following: heart defect, hypocalcemia, 22q11 hemizygosity, or CHARGE association. The patients had no evidence of thymic function based on low naive T-cell numbers (< 50/mm3) and, in those cases tested, TREC values of less than 100/100 000 T cells.
There were 2 infants with typical complete DiGeorge syndrome in this report and 4 with atypical complete DiGeorge syndrome.17 The patients with typical complete DiGeorge syndrome had higher-than-expected numbers of T cells and T-cell proliferative responses to PHA. Because of the concern of graft rejection, Thymoglobulin was used to immunosuppress these T cells.
The patients with atypical complete DiGeorge syndrome presented with histories of rash and lymphadenopathy. They had oligoclonal populations of T cells. The presence of the T cells with a proliferative response to PHA made diagnosis of athymia very difficult. Only by assessing naive T-cell numbers by flow cytometry with CD45RA and CD62L antibodies and by TREC assays was the underlying athymia appreciated. The immunoscope assessment of the T cells also showed the very restricted repertoire of these patients. These last 2 tests are not routinely available in hospital clinical laboratories. Access to research laboratories is helpful to confirm the diagnosis of atypical complete DiGeorge syndrome, which can be made initially based on clinical findings and absence of naive T cells on flow cytometry.
The syndromic associations in this small group of patients with complete DiGeorge syndrome are similar to what the authors have seen in patients treated without immunosuppression.10 One of the 6 patients was hemizygous for chromosome 22q1124 (DIG102). There were 2 patients with CHARGE association14-16,25 (DIG003, DIG007), and 2 patients were infants of diabetic mothers (DIG104, insulin-dependent; DIG105, gestational).26,27 One carried the diagnosis of velocardiofacial syndrome (VCFS).28 It is important to realize that T-cell numbers should be measured in infants with the characteristic findings of DiGeorge syndrome whether or not they have 22q11 hemizygosity. By combining these patients with the previously reported patients with complete DiGeorge syndrome who have received thymus transplants10,17 and an additional 5 patients who underwent transplantation subsequent to these publications, we have found that of 24 infants with complete DiGeorge syndrome, 42% have 22q11 hemizygosity, 29% have CHARGE association, 21% are infants of diabetic mothers, and 8% have no syndromic associations. In all groups, the diagnosis of DiGeorge syndrome is based on the recognition of anomalies caused by defects in the third and fourth pharyngeal pouches.
We previously reported good results of thymus transplantation in patients with typical complete DiGeorge syndrome.10 The patients in that report all had less than a 20-fold response or less than 5000 cpm in response to PHA (whichever was higher). It was not known whether thymus transplantation would be effective in patients with typical or atypical complete DiGeorge syndrome who had more proliferative function than the previously reported patients. Because of the proliferative function of the 6 patients in this report, graft rejection was thought to be likely. Thus, Thymoglobulin was used for immunosuppression for 2 to 3 days prior to transplantation. This report documents the success with this approach.
The kinetics of T-cell depletion and return after Thymoglobulin were different than in past reports reviewing use of this drug. Renal transplant patients have depressed T-cell counts for one year after a 3-day course of Thymoglobulin.29 In this study, the oligoclonal T cells returned to baseline levels at 2 to 4 weeks after Thymoglobulin (Figure 2). Normal naive T cells started appearing in the peripheral blood at approximately 4 months after transplantation (Figure 2), similar to what is observed in DiGeorge syndrome patients given thymus transplants without prior immunosuppression.10
In summary, the outcomes are very encouraging. The survival rate is 5 of 6, with the single death being from RSV infection that was present prior to transplantation. All allograft biopsies showed evidence of thymopoiesis (Figure 4) and all patients developed normal PHA responses. The TCRBV repertoires have become polyclonal in the 3 surviving patients with atypical complete DiGeorge syndrome who presented with oligoclonal T-cell proliferations. The T cells that developed in the 2 patients with typical complete DiGeorge syndrome are also polyclonal. The data to date indicate that transplantation of postnatal thymus tissue may be a successful therapy to treat the immune deficiency of patients with atypical complete DiGeorge syndrome and those with typical complete DiGeorge syndrome who have slightly elevated proliferative responses to mitogens. We will continue to follow these patients and monitor their immune function parameters.
Prepublished online as Blood First Edition Paper, April 20, 2004; DOI 10.1182/blood-2003-08-2984.
Supported by National Institutes of Health (NIH) grants R01-AI47040, M03-RR30 (NCRR, Clinical Research), and the American Association of Allergy, Asthma, and Immunology Women Physicians in Allergy Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff of the Duke General Clinical Research Center for the excellent care of patients DIG102, DIG103, DIG105, and DIG107. We thank Dr Elizabeth R. Hauser for assistance with statistical issues. We acknowledge the contributions of Dr James Jaggers in thymus procurement and the expert anesthesiology assistance of Drs Allison K. Ross, John B. Eck, Guy D. Dear, D. Ryan Cook, and Robert L. Coleman. We thank Drs Scott Langdon and Michael Cook of the Duke Comprehensive Cancer Center core facilities for immunoscope and flow cytometry analysis and Maria E. Rhein for assistance with the TREC assay. We thank Drs David T. Price (Johnson City, TN), Stacey M. Jones (Little Rock, AR), Jane M. El-Dahr (New Orleans, LA), and John Bohnsack (Salt Lake City, UT) for referring the patients. We thank Drs Rebecca H. Buckley, Larry W. Williams, Joseph L. Roberts, Laurie A. Myers, Rebecca Uram, Joseph M. Wisniewski, Ariana D. Buchanan, Philip N. Rancitelli, and Amy M. Scurlock for care of the patients. We thank Dr Barton F. Haynes for his continued encouragement. M.L.M. and L.P.H. are members of the Duke Comprehensive Cancer Center.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal