Abstract
On activation, human neutrophils release microparticles, called ectosomes, directly from the cell surface membrane. Microparticles from platelets, endothelial cells, and monocytes were reported to support coagulation or to modulate vascular homeostasis by activating monocytes as well as endothelial cells. We find that neutrophil ectosomes have no proinflammatory activity on human macrophages as assessed by the release of interleukin 8 (IL-8) and tumor necrosis factor α (TNFα). On the contrary, ectosomes increase the release of transforming growth factor β1 (TGFβ1), suggesting that ectosomes down-modulate cellular activation in macrophages. Polymorphonuclear neutrophil (PMN) ectosomes are able to block inflammatory response of macrophages to zymosan and lipopolysaccharide (LPS). We show that an early-phase TGFβ1 secretion and the exposure of phosphatidylserine on the surface of ectosomes independently contribute to this effect. Ectosome-cell contact was sufficient for their immunomodulatory function as shown by blocking phagocytosis with cytochalasin D. Thus, neutrophils release potent anti-inflammatory effectors, in the form of ectosomes, at the earliest stage of inflammation, already providing a drive to its resolution.
Introduction
Polymorphonuclear neutrophils (PMNs) constitute the bulk of the leukocytes found in our blood. PMNs ingest and eventually kill invading pathogens by means of potent antimicrobial agents released during the process of degranulation. Because this microbicidal weaponry largely lacks specificity, it can lead to severe tissue damage if not controlled or secluded adequately from surrounding tissue.1 At the time of degranulation, activated PMNs release small microvesicles directly from the cell surface membrane. Many eukaryotic cells, including tumor cells, release vesicles by ectocytosis (ectosomes), either spontaneously or in response to various stimuli.2-14 Data on the function(s) of ectosomes have accumulated.6,7,9,11,15-17 So far, ectosomes have been associated with procoagulant and proinflammatory effects. Ectosomes derived from endothelial cells have been described to induce procoagulant activity in monocytic cells,18 whereas ectosomes from platelets and monocytes were shown to directly promote hemostasis and induce inflammation by activating endothelial cells.9,11 MacKenzie et al6 described monocyte-derived ectosomes as being important proinflammatory agents by mediating the rapid secretion of interleukin 1β (IL-1β).
We recently described characteristics and molecular properties of ectosomes released by human PMNs.19,20 Importantly for the work presented herein, PMN ectosomes expose phosphatidylserine on their outer membrane leaflet, and all ectosome preparations used in our assays are devoid of any cellular debris or apoptotic cells/bodies, as shown by electron microscopy.20 Here, we show that PMN-derived ectosomes, unexpectedly, feature immunosuppressive/anti-inflammatory functions. Challenging a linear model of induction and resolution of inflammation, our data suggest a more complex balance of proinflammatory and anti-inflammatory events initiated in a parallel manner.
Material and methods
Antibodies and reagents
Zymosan A from Saccharomyces cerevisiae, cytochalasin D, actinomycin D, formyl-methionine-leucine-phenylalanine (fMLP), and C5a were from Sigma (St Louis, MO), and lipopolysaccharide (LPS) was from Calbiochem (La Jolla, CA). Human transforming growth factor β1 (TGFβ1) Duoset enzyme-linked immunosorbent assay (ELISA) development system as well as neutralizing monoclonal mouse anti–human-TGFβ antibodies (αTGF-Abs) were from R&D Systems (Minneapolis, MN). OptEIA ELISA kits for IL-8, IL-10, and tumor necrosis factor α (TNFα) were from Becton Dickinson (San Diego, CA).
Isolation and stimulation of PMNs, collection of ectosomes
PMNs were isolated from fresh buffy coats according to the technique described previously.19 Briefly, a fresh buffy coat was diluted 1/1 (vol/vol) with phosphate-buffered saline–ethylenediaminetetraacetic acid (PBS-EDTA; 2 mM), mixed gently with 0.25 vol 4% Dextran T500 (Amersham Pharmacia Biotech, Dübendorf, Switzerland), and left for 30 minutes for erythrocyte sedimentation. The leukocyte-rich supernatant was aspirated and centrifuged for 10 minutes at 200g. The pellet was resuspended in 9 mL ultrapure water to lyze erythrocytes. Isotonicity was restored by addition of 3 mL KCl (0.6 M) and 40 mL NaCl (0.15 M). Cells were then centrifuged 10 minutes at 350g and resuspended in 20 mL PBS-EDTA. This suspension was layered over 20 mL Ficoll-Hypaque (Sigma) and centrifuged for 30 minutes at 350g. The PMN-rich pellet was recovered and washed twice in PBS-EDTA. All manipulations were performed at 4° C, thus minimizing PMN activation.
For stimulation, PMNs (107 cells/mL) pooled from 4 different healthy blood donors were diluted 1/1 (vol/vol) in prewarmed (37° C) RPMI 1640 (Life Technologies, Basel, Switzerland) with 1 μM fMLP or 100 ng/mL C5a (final concentrations; Sigma), and incubated for 20 minutes at 37° C. PMNs were removed by centrifugation (4000g at 4° C), and the supernatants were stored overnight at –80° C. The ectosomes contained in the supernatant were then concentrated with Centriprep centrifugal filter devices (10 000 MW cut-off; Millipore, Bedford, MA) and stored in aliquots at –80° C until use. Freeze/thawing or 18 hours of incubation at 37° C did not alter the activity of ectosomes (data not shown).
Isolation and culture of human monocyte-derived macrophages (HMDMs)
Monocytes were isolated from fresh buffy coats as previously described.21 Briefly, a buffy coat was diluted 1/1 (vol/vol) with Hanks balanced saline solution (HBSS), layered over Ficoll-Hypaque (Sigma), and centrifuged for 30 minutes at 350g. Monocytes were recovered, washed twice in HBSS, and layered over a Percoll gradient. Percoll was prepared by mixing 1 volume NaCl 1.5 M with 9 volumes of Percoll (Sigma). The Percoll gradient was done by mixing 1.5:1 (vol/vol) isosmotic Percoll with PBS/Citrate (NaH2PO4 1.49 mM; Na2HPO4 9.15 mM; NaCl 139.97 mM; C6H5Na3O7.2H2O 13 mM; pH 7.2). Isolated monocytes were resuspended at 2 × 106 cells/mL in Dulbecco modified essential medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (DMEM+). Monocytes were then allowed to adhere for 1 hour at 37° C on culture plates. Adherent monocytes were washed 3 times with prewarmed DMEM+ and finally incubated for 7 days in DMEM+ supplemented with 10% normal human serum (pooled from 40 healthy donors). The culture was maintained in 5% CO2 at 37° C, and the medium was changed at days 3 and 7. Macrophages were used between days 7 and 10.
Activation of HMDMs
HMDMs were washed several times with prewarmed DMEM+ without serum. Subsequently, each well was filled with 200 μL(final volume) fresh DMEM+ without serum. Zymosan (5 μg/mL final concentration), LPS (10 ng/mL final concentration), and/or ectosomes were added, and supernatants were collected 24 hours later (if not stated otherwise). For kinetic experiments, supernatants were collected at the time points 1, 2, 4, 8, and 24 hours. For some experiments, HMDMs were pretreated with 0.5 μM cytochalasin D or 0.5 μg/mL actinomycin D in DMEM+ for 60 minutes at 37° C prior to washes and incubation with zymosan and/or ectosomes. Assays were performed in triplicate. Results are representative of 3 independent experiments. Data represent mean ± standard deviation. Alternatively, assays were performed with macrophages obtained from 5 different healthy donors. Results were then separately plotted for each donor to allow better assessment of individual variations. The amount of ectosome-protein used in our experiments was quantified by using a Bradford protein assay (Pierce, Rockford, IL). If not otherwise stated, the final ectosome concentration in our assays was approximately 5μg/mL. The amount of C5a-derived ectosomes, however, corresponds to the amount released by equal numbers of PMNs when compared with fMLP-derived ectosomes. Ectosomal membranes were collected by hypotonic lysis of ectosomes with ultrapure water. Lysed ectosomes were then ultracentrifuged (45 minutes, 250 000g at 4° C), and the pellet containing the membranes was recovered. To investigate the role of phosphatidylserine in the activity of ectosomes, annexin V was used at a final concentration of 50 μg/mL.
Collection of supernatants and analysis of cytokines
HMDM supernatants were collected, put on ice, and spun for 10 minutes at 2000 rpm at 4° C (Mikro 24-48R centrifuge; Hettich, Bäch, Switzerland) to remove cellular debris. Finally, supernatants were collected and stored at –80° C until use. Cytokine concentrations were determined by ELISA according to manufacturers' instructions.
Membrane labeling of ectosomes
An amphiphilic cell linker dye kit (PKH67; Sigma) was used, following the labeling procedure provided by the manufacturer. Briefly, ectosomes resuspended in 200 μL Diluent C were incubated with 200 μL diluent C/dye solution (dye diluted 1/200) for a minute at room temperature, with gentle shaking. RPMI 1640 (1 mL; without phenol red) was added to stop the reaction. Labeled ectosomes were separated from the remaining unbound dye by ultracentrifugation (20 minutes, 160 000g at 4° C) and washed with 0.9% NaCl.
Confocal fluorescence microscopy
HMDMs were generated as described in “Activation of HMDMs” on 8-well culture slides (Falcon; Becton Dickinson). After 7 to 10 days of culture, macrophages were washed several times with serum free DMEM+ medium and incubated with fluorescently labeled ectosomes (as described in “Membrane labeling of ectosomes”). Analysis was performed on an Axiovert Confocal Laser Scanning Microscope (LSM 510) from Zeiss AG (Feldbach, Switzerland).
Results
PMN ectosomes do not induce proinflammatory activity in macrophages
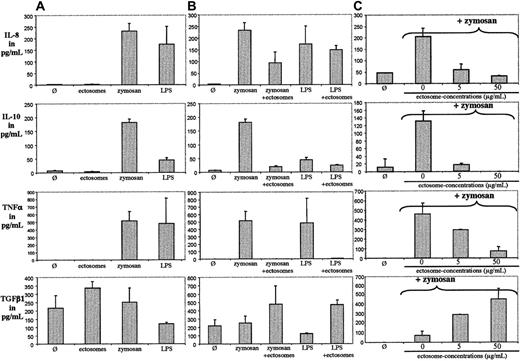
To investigate whether ectosomes derived from neutrophils have a similar proinflammatory effect as has been reported for ectosomes of different cellular origin, we coincubated PMN ectosomes with human monocyte-derived macrophages (HMDMs) for 24 hours. Supernatants were analyzed for IL-8, TNFα, IL-10, and TGFβ1. No stimulating or activating effect of ectosomes on macrophages was observed (Figure 1A), whereas zymosan and LPS induced the release of IL-8, TNFα, and IL-10. Surprisingly, ectosomes increased the release of the anti-inflammatory cytokine TGFβ1.
PMN ectosomes are not proinflammatory but anti-inflammatory. (A) HMDMs were incubated with medium alone (Ø), with medium supplemented with ectosomes, zymosan, or LPS for 24 hours. Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. (B) HMDMs were incubated with medium alone (Ø), with medium supplemented with zymosan or LPS, with or without ectosomes for 24 hours. Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. (C) HMDMs were incubated with medium alone (Ø), with medium supplemented with zymosan with or without ectosomes for 24 hours. Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. Error bar indicates standard deviation of the mean.
PMN ectosomes are not proinflammatory but anti-inflammatory. (A) HMDMs were incubated with medium alone (Ø), with medium supplemented with ectosomes, zymosan, or LPS for 24 hours. Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. (B) HMDMs were incubated with medium alone (Ø), with medium supplemented with zymosan or LPS, with or without ectosomes for 24 hours. Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. (C) HMDMs were incubated with medium alone (Ø), with medium supplemented with zymosan with or without ectosomes for 24 hours. Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. Error bar indicates standard deviation of the mean.
PMN ectosomes down-regulate the inflammatory response of HMDMs to zymosan
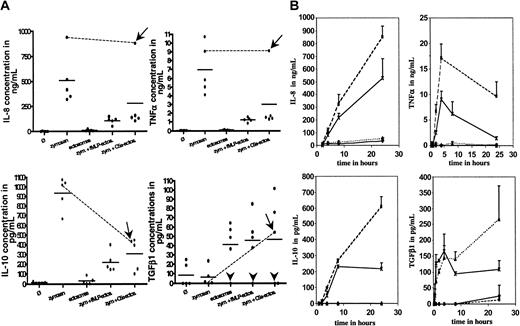
Through coincubation of zymosan or LPS with ectosomes we assessed whether ectosomes, besides being intrinsically noninflammatory, alter the response of HMDMs to proinflammatory stimuli. Zymosan and LPS induced the release of IL-8, IL-10, and TNFα but not of TGFβ1. The release of TGFβ1 induced by ectosomes was not modified by zymosan or LPS. Interestingly, the release of TNFα induced by zymosan and LPS could be blocked by ectosomes, and the release of IL-8 and IL-10 was reduced. Interestingly, the overall anti-inflammatory response of activated macrophages to ectosomes could not be blocked by preincubating ectosomes in normal human serum (ie, in the presence of serum proteins) for 30 minutes at 37° C. Furthermore, as shown in Figure 1C, ectosome-induced down-regulation of IL-8, IL-10, TNFα secretion and induction of the secretion of TGFβ1 was dose dependent. In the highest amount used in our assay, ectosomes completely blocked the inflammatory response of HMDMs. Of note, the release of cytokines by macrophages and its inhibition/promotion by ectosomes after activation with stimuli has an intrinsic variability because nearly each experiment being carried out with cells and ectosomes was isolated from different blood donors. This variability is reflected in Figure 1A-C by the different background releases of TGFβ1 in resting macrophages, as well as in Figure 2A, where we compared the results from 5 different healthy blood donors by using, in addition, ectosomes produced from fMLP- and C5a-activated PMNs. Both ectosome preparations had globally a significant anti-inflammatory activity on macrophages. However, we noticed variability between individuals, illustrated by 2 donors in particular. The first donor's macrophages were unable to produce detectable amounts of TGFβ1 in all tested conditions (Figure 2A, arrowheads), suggesting that the cells were defective in TGFβ1 production and/or secretion. These macrophages, however, featured a pronounced anti-inflammatory response to ectosomes, suggesting that, at least in this individual, TGFβ1 is not involved in the release of pro-inflammatory cytokines. The second donor's macrophages were unable to significantly down-modulate their IL-8 and TNFα responses to zymosan in the presence of C5a-ectosomes but, in these same conditions, responded very well to fMLP-ectosomes (Figure 2A, arrows and lines to link the cytokine concentrations in the presence of zymosan with and without C5a-ectosomes). The IL-10 responses of this individual were very similar to all other donors, in all tested conditions, suggesting that ectosomes produced with different stimuli might have different effects, which are only seen in given individuals.
Variability of ectosome activity among a group of 5 donors/kinetic of cytokine release by HMDMs dependent on zymosan and/or ectosomes. (A) HMDMs isolated from 5 different healthy blood donors were tested separately for their response to zymosan (zym., 5 μg/mL) in the presence or absence of ectosomes (ectos.) derived from PMN activated with either fMLP or C5a. fMLP-derived ectosomes were used at 5 μg/mL, and ectosome amounts released from equal numbers of PMN were used for fMLP- and C5a-ectosomes. Each dot represents one donor. Bars indicate the mean cytokine concentrations. Arrowheads indicate TGFβ1 “nonproducers”; arrows, donor who reacted strangely to C5a-ectosomes; lines link the cytokine response of this donor to zymosan in the presence and absence of C5a-ectosomes. (B) HMDMs were incubated with medium alone (Ø) with or without ectosomes and with medium supplemented with zymosan with or without ectosomes for up to 24 hours. Supernatants were harvested after 1, 2, 4, 8, and 24 hours and analyzed for IL-8, IL-10, TNFα and TGFβ1. HMDM alone (♦); HMDM + zymosan (▪); HMDM + ectosomes (▴); HMDM + ectosomes + zymosan (×). Error bars equal standard deviation of the mean.
Variability of ectosome activity among a group of 5 donors/kinetic of cytokine release by HMDMs dependent on zymosan and/or ectosomes. (A) HMDMs isolated from 5 different healthy blood donors were tested separately for their response to zymosan (zym., 5 μg/mL) in the presence or absence of ectosomes (ectos.) derived from PMN activated with either fMLP or C5a. fMLP-derived ectosomes were used at 5 μg/mL, and ectosome amounts released from equal numbers of PMN were used for fMLP- and C5a-ectosomes. Each dot represents one donor. Bars indicate the mean cytokine concentrations. Arrowheads indicate TGFβ1 “nonproducers”; arrows, donor who reacted strangely to C5a-ectosomes; lines link the cytokine response of this donor to zymosan in the presence and absence of C5a-ectosomes. (B) HMDMs were incubated with medium alone (Ø) with or without ectosomes and with medium supplemented with zymosan with or without ectosomes for up to 24 hours. Supernatants were harvested after 1, 2, 4, 8, and 24 hours and analyzed for IL-8, IL-10, TNFα and TGFβ1. HMDM alone (♦); HMDM + zymosan (▪); HMDM + ectosomes (▴); HMDM + ectosomes + zymosan (×). Error bars equal standard deviation of the mean.
Kinetic response of HMDMs to ectosomes and/or zymosan
Next, we assessed whether the diminished release of pro-inflammatory cytokines in response to ectosomes was due to a true blockade of intracellular proinflammatory pathways or rather a delayed response. We, therefore, investigated the time-dependent effect of ectosomes on the cytokine secretion of both, resting and zymosan-stimulated HMDMs (Figure 2B). Ectosomes induced a rapid and sustained release of TGFβ1 in resting and stimulated HMDMs. In the presence of zymosan, ectosomes had a profound anti-inflammatory activity on HMDMs detectable at all time points. The kinetics of IL-8, IL-10, and TNFα release by activated macrophages were similar in the absence and presence of ectosomes, although in the latter the amplitudes were significantly decreased. This finding indicates that the immunosuppressive activity of ectosomes is not due to a delay in the response of macrophages to proinflammatory stimuli, but rather to an active suppression of cell activation.
Ectosomes are phagocytozed by HMDMs
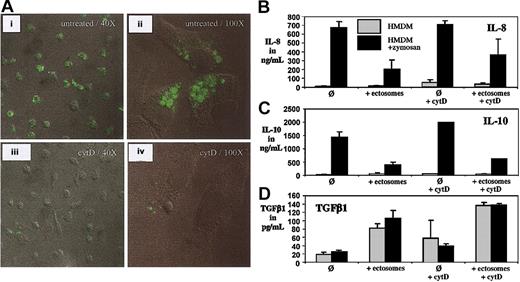
We previously observed that ectosomes bind specifically to macrophage-like cells differentiated from THP-1 cells.20 Here, we addressed the question whether ectosomes are phagocytozed by macrophages and whether the process of phagocytosis is involved in the immunosuppressive function of ectosome on macrophages.
We performed confocal fluorescence microscopy of HMDMs after incubation with fluorescently labeled ectosomes. As shown, ectosomes do not only bind to macrophages but also are internalized (Figure 3A). We performed assays with HMDMs that were kept in medium alone and others that were preincubated with cytochalasin D (cytD), a potent inhibitor of phagocytosis. In the absence of cytD, most macrophages have phagocytozed ectosomes after 30 minutes, as reflected by the intense fluorescent staining of most of the cells (Figure 3Ai). In contrast, cytD-preincubated macrophages show a marked decrease in fluorescence and hence uptake of ectosomes (Figure 3Aiii). At higher magnification, cells that have ingested ectosomes accumulated fluorescence in intracellular compartments (Figure 3Aii), which were not visible after pretreatment with cytD (Figure 3Aiv).
Confocal microscopy of phagocytosis and phagocytosis independent anti-inflammatory activity of ectosomes. (A) HMDMs were incubated with fluorescently labeled ectosomes for 30 minutes, fixed, and analyzed by confocal laser microscopy (i-ii). Alternatively, macrophages were preincubated cytochalasin D (cytD) prior to the addition of ectosomes (iii-iv). The lens used was a Zeiss Plan-Neofluar 40× and 100× from Zeiss AG (Feldbach, Switzerland). The imaging medium was Vectashield fluorescence mounting medium (Vector Laboratories, Burlingame, CA). The camera was a Zeiss Axiovert Laser Scanning Microscope (LSM510) from Zeiss AG (Feldbach, Switzerland). The acquisition software was LSM510 Software (Zeiss). (B-D) HMDMs were incubated with medium alone or with medium supplemented with zymosan for 24 hours. Alternatively, ectosomes were added to the incubation medium with or without preincubating the macrophages with cytD. Error bars indicate standard deviation of the mean.
Confocal microscopy of phagocytosis and phagocytosis independent anti-inflammatory activity of ectosomes. (A) HMDMs were incubated with fluorescently labeled ectosomes for 30 minutes, fixed, and analyzed by confocal laser microscopy (i-ii). Alternatively, macrophages were preincubated cytochalasin D (cytD) prior to the addition of ectosomes (iii-iv). The lens used was a Zeiss Plan-Neofluar 40× and 100× from Zeiss AG (Feldbach, Switzerland). The imaging medium was Vectashield fluorescence mounting medium (Vector Laboratories, Burlingame, CA). The camera was a Zeiss Axiovert Laser Scanning Microscope (LSM510) from Zeiss AG (Feldbach, Switzerland). The acquisition software was LSM510 Software (Zeiss). (B-D) HMDMs were incubated with medium alone or with medium supplemented with zymosan for 24 hours. Alternatively, ectosomes were added to the incubation medium with or without preincubating the macrophages with cytD. Error bars indicate standard deviation of the mean.
We then used again cytD to determine whether phagocytosis is required for ectosomes to fulfill their anti-inflammatory effects on macrophages. Because CytD significantly influenced the TNFα response of HMDMs in the presence or absence of zymosan, we excluded TNFα results from our analysis. As shown in Figure 3B-C, inhibiting ectosome-phagocytosis could not block the anti-inflammatory effect of ectosomes on HMDMs.
Role of ectosomal membranes in anti-inflammatory activity of ectosomes
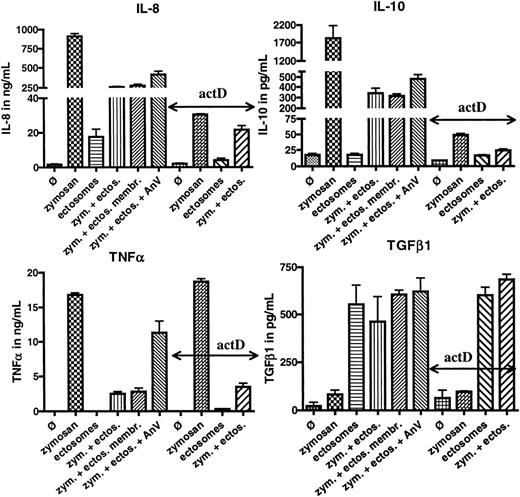
The binding of ectosomes to macrophages being sufficient to mediate their activity, we tested whether the membranes of ectosomes can produce the same effects than whole ectosomes. As depicted in Figure 4, ectosomal membranes were indeed able to mediate very similar effects than whole ectosomes, suggesting that ligand(s) on the surface of ectosomes mediate the anti-inflammatory signal. The concentrated ectosomal content did not inhibit the release of TNFα and IL-8 induced by zymosan but reduced the secretion of IL-10 (data not shown).
Role of ectosomal membranes and phosphatidylserine in ectosomal activity/post-transcriptional control of ectosomes on macrophages. HMDMs were incubated with medium alone (Ø), medium with ectosomes (ectos., 5 μg/mL), or medium supplemented with zymosan (zym., 5 μg/mL) for 24 hours. In some experiments, zymosan medium was supplemented with ectosomes, ectosomes with 50 μg/mL annexin V (AnV), or ectosomal membranes (ectos. membr.). Alternatively, macrophages were preincubated for 1 hour with actinomycin D (actD). Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. Error bars equal standard deviation of the mean.
Role of ectosomal membranes and phosphatidylserine in ectosomal activity/post-transcriptional control of ectosomes on macrophages. HMDMs were incubated with medium alone (Ø), medium with ectosomes (ectos., 5 μg/mL), or medium supplemented with zymosan (zym., 5 μg/mL) for 24 hours. In some experiments, zymosan medium was supplemented with ectosomes, ectosomes with 50 μg/mL annexin V (AnV), or ectosomal membranes (ectos. membr.). Alternatively, macrophages were preincubated for 1 hour with actinomycin D (actD). Supernatants were analyzed for IL-8, IL-10, TNFα, and TGFβ1. Error bars equal standard deviation of the mean.
Because ectosomes and apoptotic cells share the exposure of phosphatidylserine (PS) on their outer membrane leaflet and PS has been implicated in the immunomodulatory functions of apoptotic cells, we investigated the role of PS in the activity of ectosomes. We used the calcium-dependent ligand of PS, annexin V, to block the binding of PS to its binding sites on the surface of macrophages.22 As shown in Figure 4, annexin V inhibited the activity of ectosomes on TNFα only, whereas the anti-inflammatory activity of ectosomes with regard to IL-8, IL-10, and TGFβ1 remained unchanged on addition of annexin V. These data indicate that, although PS is implicated in the anti-inflammatory response mediated by ectosomes, other interactions appear to be necessary for the full functional activity of ectosomes.
Ectosomes modulate macrophages' response at the post-transcriptional level
Next, we sought to obtain more information about the mechanism by which ectosomes “reprogram” macrophages. We used actinomycin D, an inhibitor of gene transcription, to dissect the level(s) at which ectosomes modulate the cytokine response of macrophages. As shown in Figure 4, actinomycin D had no effect on the release of TNFα and TGFβ1 in the presence of zymosan and/or ectosomes, suggesting that the synthesis of both of these cytokines, as well as their modulation by ectosomes, are regulated after transcription. These results are consistent with the kinetics previously illustrated in Figure 2. Indeed, both TNFα and TGFβ1 were secreted already 30 minutes after activation of the macrophages with zymosan, whereas IL-8 and IL-10 levels rose at much slower rates. Consistently, IL-8 and IL-10 responses were highly affected by the preincubation of macrophages with actinomycin D, indicating that in contrast to TNFα and TGFβ1, macrophages respond to zymosan by the de novo transcription and synthesis of IL-8 and IL-10. The very low levels of both of these cytokines measured after 24 hours were further reduced in the presence of ectosomes (Figure 4). Thus, it is likely that ectosomes control at least in part the production of IL-8 and IL-10 after transcription as well.
Effect of neutralizing anti-TGFβ antibodies on anti-inflammatory activity of ectosomes
The kinetic data presented earlier suggested that the first event occurring during co-incubation of macrophages with zymosan and ectosomes is the ectosome-induced release of TGFβ1. We tested whether the rapid rise in local TGFβ1 concentration as shown in Figure 2B is responsible for the subsequent down-regulation of IL-8, IL-10, and/or TNFα release in activated macrophages. As shown in Figure 5, neutralizing αTGF-Abs had a lowering effect on the anti-inflammatory activity of ectosomes. This effect ranged from complete blockade of ectosome function with respect to down-regulation of IL-8 to only partial inhibition of the down-regulatory activity of ectosomes in the case of IL-10 and TNFα, suggesting a contribution of TGFβ1 in the immunomodulatory activity of ectosomes.
Effect of neutralizing anti–human-TGFβ antibodies on anti-inflammatory activity of ectosomes. HMDMs were incubated with medium alone (Ø), medium with ectosomes, or medium supplemented with zymosan (5 μg/mL) for 24 hours. Alternatively, zymosan medium was supplemented with ectosomes alone (no antibodies) or ectosomes with 5 μg/mL or 50 μg/mL αTGFβ-Abs. Supernatants were analyzed for IL-8, IL-10, and TNFα.
Effect of neutralizing anti–human-TGFβ antibodies on anti-inflammatory activity of ectosomes. HMDMs were incubated with medium alone (Ø), medium with ectosomes, or medium supplemented with zymosan (5 μg/mL) for 24 hours. Alternatively, zymosan medium was supplemented with ectosomes alone (no antibodies) or ectosomes with 5 μg/mL or 50 μg/mL αTGFβ-Abs. Supernatants were analyzed for IL-8, IL-10, and TNFα.
Discussion
This study associates ectosomes to an active role in the resolution of inflammation. This function of PMN-derived ectosomes is striking, because it counterbalances the immediate proinflammatory effects of many of the substances released by activated PMNs. Our data challenge the sequential model of induction and resolution of inflammation and suggest a balanced occurrence of proinflammatory and anti-inflammatory events, orchestrated in a parallel manner.
To assess the variability of this phenomenon, we studied the anti-inflammatory effect of ectosomes on macrophages from different donors as well as ectosomes obtained from PMNs activated with different physiologic stimuli (fMLP and C5a). Although we observed some differences in the responses of macrophages to fMLP-derived as compared with C5a-derived ectosomes, the overall effects of ectosomes were very similar. However, there were some striking individual variations as, for instance, seen in macrophages derived from one donor, which did not produce any TGFβ1 in response to ectosomes. That such individual differences are relevant in vivo during inflammation is an attractive hypothesis.
Ectosomes share with apoptotic bodies the expression of PS in the outer membrane leaflet.20 Similarly to apoptotic cells, the uptake of ectosomes by macrophages might depend on the exposure of PS in the outer membrane leaflet.23 In addition, and in analogy to apoptotic cells, the binding of ectosomes by macrophages is accompanied by a reprogramming of the macrophages, featuring an anti-inflammatory phenotype, and this biologic effect does not require phagocytosis.
The receptor for PS has recently been shown to be directly involved in the resolution of inflammation by inducing the release of TGFβ1 in macrophages that have ingested apoptotic cells.24,25 Here, we show that ectosomes, in further analogy to apoptotic bodies, promote the dose-dependent release of TGFβ1 in activated as well as resting macrophages and an inhibition of IL-8, IL-10, and TNFα secretion in activated macrophages. Anti-TGFβ-Abs counteracted the inhibition of IL-8 release induced by ectosomes, suggesting that TGFβ1 contributes, in an autocrine and/or paracrine manner, to the anti-inflammatory potential of ectosomes; similar observation has been made with apoptotic cells. However, and in distinction to what happened with apoptotic cells, αTGF-Abs had little effect on the inhibition of IL-10 and TNFα release.26
Furthermore, PS seems not to be solely responsible for the production of TGFβ1 induced by ectosomes, because incubation with an excess of annexin V, which binds to PS in a calcium-dependent manner and prevents its binding to other ligands/receptors, could not block its release. In addition, annexin V could only decrease the anti-inflammatory activity of ectosomes with regard to TNFα but not to IL-8, IL-10 and, as mentioned, TGFβ1.
It, thus, seems likely that ectosomes and apoptotic cells engage distinct but overlapping pools of receptors on the surface of macrophages. From the data obtained with actinomycin D we conclude that ectosomes modulate the response of macrophages to proinflammatory stimuli mainly at the post-transcriptional level, in contrast to apoptotic cells, which induce the de novo synthesis of TGFβ1.24
Our report follows a series of papers on functions of ectosomes, alternatively called microparticles or microvesicles.6,7,9,11,15,16 In most cases, ectosomes were described as having proinflammatory properties. Plasma counts for microparticles of various cellular origin were analyzed in a number of pathophysiologic situations, and such microparticles were found to induce endothelial dysfunction.8,14,27 This contrasts our data for PMNs, suggesting that the cellular origin of ectosomes as well as the target cells may determine their biologic response. This fact is illustrated by the work of Mesri et al15,28 that described activating properties of PMN microparticles on endothelial cells. However, the microparticles used in those experiments were isolated after several hours. Microparticles were not selected for early-released ectosomes, as used in this work, and may have contained early apoptotic components and microparticle membranes modified by hydrolysis, conferring to them very different properties.29
In conclusion, ectocytosis of PMNs early during activation might produce microparticles with immunosuppressive function and create an immediate counterweight for simultaneously unfolding proinflammatory mechanisms. Fascinating is that PMNs, best known for their powerful and immediate phlogistic properties, release particles/ectosomes that seem to down-regulate the inflammatory activity of the second wave of cells (macrophages) that will be attracted to the site of initial damage.
Prepublished online as Blood First Edition Paper, June 22, 2004; DOI 10.1182/blood-2004-01-0361.
Supported by the Swiss National Foundation (SNF; grant 3200-066708), a grant from ZLF Bioplasma AG (Bern, Switzerland), the Stiftung für Medizinische und Biologische Forschung, and a personal grant from the Fundazione per la ricerca sulla trasfusione e sui trapianti (Lugano, Switzerland) (O.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christoph Hess and Jameel Inal for reviewing the manuscript and Peter Henson for providing useful comments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal