Abstract
Tetraspanins are thought to facilitate the formation of multiprotein complexes at cell surfaces, but evidence illuminating the biologic importance of this role is sparse. Tetraspanin CD151 forms very stable laminin-binding complexes with integrins α3β1 and α6β1 in kidney and α3β1 and α6β4 in skin. It is encoded by a gene at the same position on chromosome 11p15.5 as the MER2 blood group gene. We show that CD151 expresses the MER2 blood group antigen and is located on erythrocytes. We examined CD151 in 3 MER2-negative patients (2 are sibs) of Indian Jewish origin with end-stage kidney disease. In addition to hereditary nephritis the sibs have sensorineural deafness, pretibial epidermolysis bullosa, and β-thalassemia minor. The 3 patients are homozygous for a single nucleotide insertion (G383) in exon 5 of CD151, causing a frameshift and premature stop signal at codon 140. The resultant truncated protein would lack its integrin-binding domain. We conclude that CD151 is essential for the proper assembly of the glomerular and tubular basement membrane in kidney, has functional significance in the skin, is probably a component of the inner ear, and could play a role in erythropoiesis.
Introduction
The 4-transmembrane domain superfamily (TM4SF) of integral proteins (tetraspanins) comprises at least 28 members in mammals.1,2 Tetraspanins are widely distributed on animal cells, and several are known to be expressed on human hematopoietic cells, including CD9, CD37, CD53, CD63, CD81, CD82, CD151, and CD231.1,3,4 To our knowledge, members of this protein family have not previously been described on mature erythrocytes. In addition to the characteristic 4 membrane-spanning domains, tetraspanins have short, cytosolic N- and C-termini and one small (EC1) and one large (EC2) extracellular loop. EC2 comprises 78 to 150 amino acids and contains 4 conserved cysteine residues, 2 of which are in a CCG motif. EC2 of most tetraspanins also contains a PxxCC motif, 1 or 2 disulfide bonds, and at least 1 N-glycan.
Tetraspanins in the cell membrane aggregate with each other and with a variety of other transmembrane proteins, in particular integrins, to form clusters, known as the tetraspanin web or tetraspanin-enriched microdomains.5,6 Integrins are heterodimeric, transmembrane receptors, consisting of noncovalently linked α and β subunits. They are 2-way signaling molecules, which dynamically link the extracellular matrix (ECM) with the cytoskeleton.7,8
The functions of tetraspanins are not clearly defined and might be multifarious. They appear to be involved in cell motility, possibly by regulating integrin-dependent cell migration, metastasis, and cell polarization, proliferation, and differentiation.1,2,4,9 They could function as molecular facilitators and transmembrane linkers, by recruiting signaling enzymes and tethering them to integrins, and by engaging other transmembrane proteins in specific lateral associations.1,2
CD151 (PETA-3/SFA-1) has the characteristic structure of a tetraspanin.10 It is a 253-amino acid protein with a single N-glycosylation site in EC211,12 and is palmitoylated on several cysteine residues.6,13,14 Immunoblotting reveals a doublet of bands of apparent kDa 28 and 32, representing unglycosylated and glycosylated forms.10 The human CD151 gene is located on chromosome 11p15.5 and comprises 8 exons, with exons 2 to 8 encoding CD151 polypeptide.13,15 CD151 has a wide cell and tissue distribution, including epithelium, endothelium, muscle, renal glomeruli and proximal and distal tubules, Schwann cells, and dendritic cells, with a single RNA species observed in most human adult tissues.3,11 CD151 is expressed at high levels on platelets and megakaryocytes.3 As with other tetraspanins, CD151 is associated in cell membranes with several integrins.1,2,4,9,10 Unlike other tetraspanin-integrin associations, CD151 remains associated with α3β1, α6β1, and α7β1in stringent detergents, such as Triton X-100.16-18 These integrins bind preferentially to laminin. It is proposed that CD151 is always associated with integrins, and CD151 association, occurring early in biosynthesis, might be a requirement for α3 integrin maturation and cell surface expression.16,19
MER2 (RAPH1), the only antigen of the RAPH blood group system, was initially recognized by 2 murine monoclonal antibodies, produced following immunization of mice with a human small cell carcinoma line.20 They defined a polymorphism on red cells in which 92% of English blood donors were MER2+ and 8% were apparently MER2–.20 MER2 was assigned to chromosome 11p15 by somatic cell hybridization studies.20 Subsequently, 3 examples of human alloanti-MER2 were identified, all made by Jews originating from India and living in Israel.21 Two were sibs and the third was unrelated. All had end-stage kidney disease. In addition to nephrotic syndrome resulting from hereditary nephritis, which deteriorated to end-stage renal failure, the sibs had pretibial bullous skin lesions, neurosensory deafness, bilateral lacrimal duct stenosis, nail dystrophy, and β-thalassemia minor.22 The male sib also had only one kidney and dystrophic teeth, whereas the female sib had agenesis of the distal vagina and bilateral cervical ribs. Two additional siblings had β-thalassemia minor without other clinical problems. Fewer clinical details are available for the unrelated Israeli patient, but he had end-stage renal failure and was on dialysis for 3 years before death. One other example of alloanti-MER2 was identified in a Turkish woman, a healthy blood donor.23
The combination of familial progressive renal failure and sensorineural deafness is well recognized and often called Alport syndrome. Most cases of classic Alport syndrome are inherited in an X-linked fashion, but up to 15% demonstrate autosomal inheritance, which is usually recessive.24 The 3 Israeli MER2– cases have a broader phenotype than would be consistent with a diagnosis of pure Alport syndrome, but we included DNA from 2 patients with a clinical diagnosis of autosomal recessive Alport syndrome in our study for comparison.
We have demonstrated that the MER2 antigen is carried on tetraspanin CD151, the first evidence for a member of the tetraspanin superfamily on erythrocytes, and that absence of CD151 is associated with renal failure. We conclude that CD151 is essential for the proper assembly of the glomerular and tubular basement membranes in kidney, has functional significance in the skin and inner ear, and may also play a role in erythropoiesis.
Patients and methods
Patients
Blood samples were obtained from the Israeli sibs (Pt1 and Pt2), the unrelated Israeli patient (Pt3), the Turkish blood donor (D1), and 2 patients with Alport syndrome with informed consent.
The 2 patients with autosomal recessively inherited Alport syndrome were as follows. Pt4 is 1 of 7 children born to healthy, consanguineous (1st cousin) parents. The others, including 3 brothers, are all healthy. The patient presented with hematuria and proteinuria and then developed chronic renal failure and high-tone sensorineural deafness in his 30s. He had classic changes of Alport syndrome on electron microscopic examination of his renal biopsy. His 3 sons all have microscopic hematuria. Mutation screening in COL4A5 (the gene involved in X-linked Alport syndrome) was negative. Pt5 is the fifth of 5 children born to consanguineous (1st cousin) Moroccan parents. His mother has hematuria, proteinuria, and a biopsy “consistent with the diagnosis of Alport syndrome.” Four of the 5 children, including Pt5, have hematuria, 1 is deaf, and 2 also have congenital cataracts. Mutation screening in COL4A5 was negative.
Serologic analyses
Serologic studies involved standard indirect agglutination tests with anti–human immunoglobulin G (IgG; BPL, Elstree, United Kingdom) or anti–mouse IgG (Dako Cytomation, Glostrop, Denmark), read in a capillary. For blocking tests, 5 μL packed erythrocytes were incubated first with 10 μL human serum for 60 minutes at 37° C, then washed 4 times, incubated with mouse monoclonal antibody for 60 minutes at room temperature, then subjected to an antiglobulin test. Methods for treatment with proteases and thiol-reducing agents were as described.25
Molecular genetic analyses
Genomic DNA was isolated from whole blood taken from Pt2 and from 3 MER2-positive control samples with a genomic DNA purification kit (Promega, Madison, WI). Genomic DNA was isolated from the plasma of Pt1, Pt3, and D1 (cell samples were not available) with the QIAamp DNA purification kit (Qiagen, Crawley, United Kingdom). Genomic DNA from 2 cases of Alport syndrome (Pt4 and Pt5) was also analyzed.
All exons, intron-exon borders, and some short introns of CD151 were amplified by polymerase chain reaction (PCR). Most of the primers used for amplification were described by Whittock and McLean.15 The primer set for amplification of exon 1 was designed with assistance of primer analysis software (Oligo; Molecular Biology Insights, Plymouth, PA): forward primer 5′-CTGGGGGAAGGTATGAGGTC-3′ and reverse primer 5′-GGGTGGGGAGAATGGAG-3′. The PCR assays were carried out in a thermal cycler (GeneAmp PCR System 9700; Applied Biosystems, Foster City, CA) on 100 to 200 ng genomic DNA as a template in a total reaction volume of 50 μL. The reactions contained 2.5 mM Mg2+ (final concentration) in a buffer provided by polymerase manufacturers, 0.2 mM deoxynucleoside triphosphate (dNTPs; 100 mM dNTP mix set; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), 0.5 μM each forward and reverse primer (MWG Biotech AG, Ebesberg, Germany), and 1 U AmpliTaq Gold DNA polymerase (Applied Biosystems). The primer annealing temperatures varied from 60° C for exons 2 and 3; 65° C for exons 1, 4, 5 and 6; and 68° C for exons 7 and 8. Following the initial denaturing at 94° C for 10 minutes, 35 cycles of PCR were performed at 94° C for 30 seconds followed by the annealing temperature for 30 seconds and then 72° C for 1 minute. All reactions were terminated after a 10-minute extension at 72° C.
The sequences of 8 CD151 exons were determined by direct sequencing of PCR products following the electrophoresis of amplification products in 0.8% agarose gel and detection by ethidium bromide staining. PCR products were excised and purified from agarose with a QIAEX II gel extraction kit (Qiagen, Crawley, United Kingdom) and directly sequenced on both DNA strands (ABI-PRISM 3100 Applied Biosystems automated DNA sequencer; Applied Biosystems). The reference sequence used for comparison was a Homo sapiens chromosome 11 genomic contingent (GenBank accession NT_035113). The DNA and translational changes are numbered from the codon for the translation initiating methionine.
DNA fragments containing CD151 exons 5 and 6 with point mutations that altered recognition sites for restriction enzymes were amplified by the same PCR conditions as described earlier. The restriction fragment length polymorphism (RFLP) test was used to digest PCR products directly in a total volume of 20 μL. The restriction enzymes used were obtained from the New England Biolabs (United Kingdom) Ltd (Hitchin, United Kingdom) and were used in a concentration of 10 U per sample. Restriction fragments were analyzed on 1.5% agarose gels.
Furthermore, mRNA was isolated from 4 to 5 × 106 cells from Epstein-Barr virus (EBV)–transformed lymphoblastoma cell lines derived from Pt2 and a control individual (Oligotex; Qiagen). First-strand cDNA for PCR amplification was prepared with Omniscript reverse transcriptase (Qiagen) and oligo (dT)12-18 as the primer. Whole CD151 cDNA was amplified with the following primers: forward 5′-ATGGGTGAGTTCAACGAGAAGAAG-3′ and reverse 5′-CTAGTAGTGCTCCAGCTTGAGACT-3′ (MWG Biotech AG, Ebesberg, Germany) and Expand High Fidelity PCR system (Roche Diagnostic GmbH, Mannheim, Germany). Amplification was achieved over 30 cycles at 94° C for 1 minute, 65° C for 1 minute, and 72° C for 2 minutes, followed by a final extension time of 10 minutes. The 762-bp product was isolated by gel purification and directly sequenced as described.
Erythroid cell culture
Buffy coats were prepared from whole blood units of 1 MER2– individual lacking anti-MER2 and 2 MER2+ individuals. The buffy coats were diluted 1:1 in Hanks buffered salt solution to which 5% tri-sodium citrate had been added (HBSS-C; Sigma Biochemicals, Poole, United Kingdom) layered onto a Histopaque gradient and centrifuged at 400g for 30 minutes at 20° C. Mononuclear cells at the interface were collected, incubated in red cell lysis buffer, and washed 3 times in HBSS-C, followed by a final wash in degassed phosphate-buffered saline supplemented with 0.5% bovine serum albumen (BSA) and 0.6% citrate-phosphate-dextrose (Sigma) pH 7.2 (MACS buffer). CD34+ cells were isolated from the washed mononuclear cells by positive selection with the MiniMACS magnetic bead system (Miltenyi Biotec, Bisley, United Kingdom). CD34+ cells were labeled by using the direct CD34 progenitor cell isolation kit containing FcR blocking reagent to prevent nonspecific binding to Fc receptors and anti-CD34 (QBEND/10) conjugated to superparamagnetic microbeads. Washed mononuclear cells were suspended in MACS buffer at 300 μL/108 cells and incubated with 100 μL/108 FcR block and 100 μL/108 anti-CD34 at 4° C for 30 minutes. Cells were washed once and resuspended in MACS buffer (500 μL/108) then passed over a 30-μm sieve placed on the top of a primed MS column held in a magnet. Cells were passed through the sieve and onto the column and washed 4 times with MACS buffer (500 μL). Labeled cells were retained in the column, the column was removed from the magnet, and cells were eluted into 1 mL MACS buffer. The isolation procedure was repeated to increase the purity of the isolated cells.
According to the method of Sposi et al26 isolated CD34+ cells were cultured in Stem Span serum-free expansion medium (Stem Cell Technologies, Vancouver, Canada) containing Iscoves modified Dulbecco medium (IMDM), 1% bovine serum albumen, 200 μg/mL (iron saturated) human transferrin, 10–4M 2-mercaptoethanol, and 2 mL L-glutamine. The medium was supplemented with the following cytokines: 10 μg/mL recombinant human (rH) stem cell factor, 1 μg/mL rH interleukin 3 (IL-3; R&D Systems Europe, Abingdon, United Kingdom), 3 IU/mL erythropoietin (Roche Products, Welwyn Garden City, United Kingdom), 40 μg/mL low-density lipoprotein (Calbiochem/Merck Biosciences, Nottingham, United Kingdom), and 0.1 ng/mL Prograf (Fujisawa, Killorglin, Ireland). Cells were cultured at 0.5 to 1 × 105/mL in vented T25 Falcon flasks (Becton Dickinson, Oxford, United Kingdom) in 5% CO2 at 37° C. Cell passaging was carried out as necessary.
Flow cytometry
Cultured erythroid progenitor cells, platelets, EBV-transformed lymphocytes, and erythrocytes were analyzed by flow cytometry after labeling with a variety of antibodies: mouse monoclonal anti-CD151 (clone 11G5a; Serotec, Kidlington, Oxford, United Kingdom), anti-MER2 (2F7), anti-GPA (glycophorin A) (BRIC256), pooled antibodies to the Lutheran glycoprotein (BRIC221, BRIC224), anti-Rh polypeptide (BRIC69) (all IBGRL, Bristol, United Kingdom), anti-β1 (TS2/16), anti-α5 (SNAK52), and anti-CD61 (PM6/13; Serotec) were used as required. An eluate of human anti-M, prepared by adsorption and elution from antigen-positive cells (Elu-Kit II; Gamma Biologicals, Houston, TX), was used in the dual-labeling studies. For single labeling, cells were incubated with primary antibody at room temperature for 30 minutes and washed once. Bound antibody was detected by adding R phycoerythrin (RPE)–conjugated F(ab′)2 anti–mouse globulin (Dako) and incubating at 4° C in the dark for 30 minutes. Cells were washed once, and the geometric mean (FL2) was recorded for each test. Cells were dual labeled by incubating the cells consecutively with monoclonal anti-CD151 or anti-MER2 and with an eluate of human anti-M for 60 minutes at room temperature. Cells were washed once, and RPE-conjugated F(ab′)2 anti–mouse globulin (Dako) and fluorescein isothiocyanate (FITC)–conjugated F(ab′)2 fragment anti–human globulin (Dako) were added. After incubation at 4° C in the dark the cells were washed once, and FL1 versus FL2 fluorescence was recorded. Flow cytometry was performed on a FacsCalibur flow cytometer (Becton Dickinson, Oxford, United Kingdom) and analyzed with CellQuest software. Isotype controls (Dako) were tested in parallel to ascertain background fluorescence as required; for dual-labeling compensation was set by using single-labeled positive cells prior to acquisition of data.
Protein model
A homology model for CD151 EC2 was constructed on the basis of the crystal structure of CD81 (coordinates 1G8Q in the PDB27 ). The amino acid sequence of CD151 EC2 was initially aligned with CD81 (25.3% sequence identity) using the ClustalW routine within Megalign 5.06 (DNASTAR, Madison WI). Manual adjustment produced 3 nonaligned loop insert regions in CD151 of 11, 6, and 2 residues. With the use of SYBYL6.9 (Tripos, St Louis, MO) the CD81 sequence was mutated to that of CD151, and conformations of nonaligned loop regions were predicted by using the Loop Search routine. The overall model was iteratively minimized by the Kollman all-atom force-field implemented in SYBYL6.9, combined with manual adjustment of Ramachandran outlier regions identified by PROCHECK.28
Histologic analyses
The open biopsy of single right kidney (Pt1) and skin biopsy from involved skin lesions (Pt2) were performed in 1976 and 1986, respectively.
For light microscopy, tissues were fixed in 4% buffered formaldehyde, dehydrated, embedded in paraffin, sectioned, and stained with hematoxylineosin, Masson-trichrome, methanamine silver, and periodic-acid Schiff reagents. For immunofluorescence, tissues were frozen fresh and sectioned at approximately 4 μm on cryostat.
For ultrastructural analysis, fresh samples of kidney and skin tissues were fixed in 3% glutaraldehyde and then postfixed in 1% osmium tetroxide, followed by dehydration and embedding in epoxy resin by using standard procedures. Sections (50 nm thick) were used for transmission electron microscopy (Philips EM300, cut film 3 1/4 × 4 inch and Philips EM201, 80 kV, camera 35-mm Philips, Eindhoven, The Netherlands).
Results
MER2 is a marker for CD151
Location of CD151, like MER2, on chromosome 11p15 and apparent similarities between MER2 and CD151 regarding susceptibility to thiols and proteases led us to examine the possibility that MER2 is a marker for CD151. One example of monoclonal anti-CD151 (11G5a; Serotec) reacted with human erythrocytes by an antiglobulin hemagglutination test. Erythrocytes from 40 blood donors gave comparable results by an antiglobulin hemagglutination test with the monoclonal anti-CD151 and 2 monoclonal anti-MER2 (1D12 and 2F7). Incubation of MER2+ erythrocytes with human alloanti-MER2 blocked binding of murine anti-CD151 and murine anti-MER2 but not binding of an unrelated murine monoclonal antibody (BRIC 108, anti-Lub); whereas incubation of MER2+ erythrocytes with human alloanti-Fyb, an unrelated antibody, did not block binding of murine anti-CD151, -MER2, or -Lub (data not shown).
Treatment of erythrocytes with papain enhanced indirect hemagglutination with monoclonal anti-MER2 and anti-CD151, whereas treatment with trypsin, α-chymotrypsin, pronase, or with the disulphide bond reducing agent 2-aminoethylisothiouronium bromide destroyed reactivity with those antibodies (data not shown).
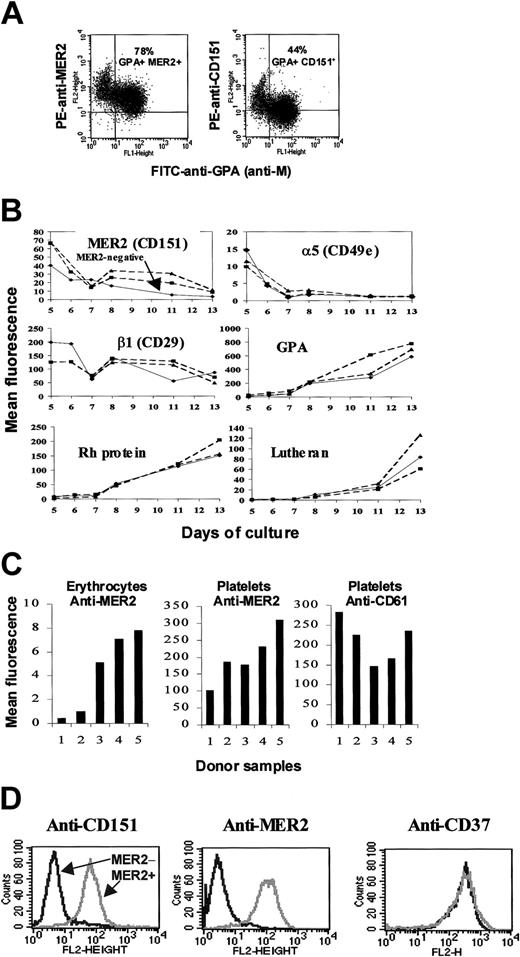
It has been suggested that most people with MER2– erythrocytes express MER2 on other cells and tissues and, therefore, do not produce anti-MER2.29 An analogous situation occurs with individuals who have the Fy(a–b–) phenotype resulting from an inactivating mutation in the GATA1 binding site upstream of the FY gene.30 On this basis only 4 individuals are known who have made alloanti-MER2 and are therefore true MER2-negatives. When erythroid cells (predominantly proerythroblasts) derived from adult MER2+ CD34+ cells were cultured in the presence of IL-3, stem cell factor (SCF), and erythropoietin (EPO) and examined for expression of MER2 and CD151 by dual staining with PE-conjugated murine monoclonal anti-MER2 or anti-CD151 and FITC-conjugated human alloanti-GPA (anti-M) the same pattern of staining was observed with anti-MER2 and anti-CD151 (Figure 1A). These results clearly demonstrate that CD151 and MER2 are expressed on GPA-positive erythroid cells. Anti-CD151 (11G5a) consistently gave a weaker signal than anti-MER2 (2F7), providing an explanation for the difference in number of positive erythroid cells. MER2 expression decreased over time with increasing maturation of the erythroid cells, from proerythroblasts (day 5) to polychromatophilic erythroblasts (day 13) in a manner comparable to that of β1 integrin (Figure 1B). In contrast, the erythroid markers RhAG, Lutheran, and GPA progressively increase in expression over the time of the cultures, whereas expression of α5 integrin was rapidly lost (Figure 1B). Culture of CD34+ cells derived from an individual with MER2– erythrocytes, but who had not made anti-MER2, expressed MER2 antigen at day 5, but at a lower level than MER2+ donors (Figure 1B). These results are consistent with the suggestion that MER2– red cells from individuals lacking anti-MER2 result from reduced expression of MER2 rather than inherited absence of the antigen. Levels of expression of MER2 on erythrocytes and platelets from 5 donors, 2 MER2– (as determined by an erythrocyte indirect antiglobulin test) and 3 MER2+ showed MER2 was variably expressed on the platelets of all 5 donors (Figure 1C).
Expression of MER2. (A) Flow cytometric studies of erythroid precursors (proerythroblasts) cultured from CD34+ cells. Dual staining with FITC-conjugated anti-GPA (anti-M) and PE-conjugated anti-MER2 (2F7) or anti-CD151 (11G5a) revealed a population of GPA+ erythroid cells that express CD151 (top right quadrant). (B) Changes in expression of MER2 (CD151) and other cell surface markers during ex vivo erythropoiesis. Continuous line indicates serologically MER2– (without anti-MER2); dotted lines, MER2+. Days of culture are days after isolation of CD34+ cells. The dip in expression around day 7 is characteristic of these cultures. (C) Comparative levels of expression of MER2 (CD151) on erythrocytes and platelets of 5 donors. Tests with CD61 on platelets are included as a control. Samples 1 and 2 are MER2– (without anti-MER2), and samples 3 to 5 are MER2+ as determined by an indirect antiglobulin test on erythrocytes. (D) Expression of MER2 (CD151) on lymphoblastoid cells. Flow cytometry histograms for lymphoblastoid cell lines derived from a MER2+ individual (gray peak) and from Pt2, the MER2– patient with anti-MER2 (black peak), incubated with PE-conjugated anti-MER2, anti-CD151, and anti-CD37.
Expression of MER2. (A) Flow cytometric studies of erythroid precursors (proerythroblasts) cultured from CD34+ cells. Dual staining with FITC-conjugated anti-GPA (anti-M) and PE-conjugated anti-MER2 (2F7) or anti-CD151 (11G5a) revealed a population of GPA+ erythroid cells that express CD151 (top right quadrant). (B) Changes in expression of MER2 (CD151) and other cell surface markers during ex vivo erythropoiesis. Continuous line indicates serologically MER2– (without anti-MER2); dotted lines, MER2+. Days of culture are days after isolation of CD34+ cells. The dip in expression around day 7 is characteristic of these cultures. (C) Comparative levels of expression of MER2 (CD151) on erythrocytes and platelets of 5 donors. Tests with CD61 on platelets are included as a control. Samples 1 and 2 are MER2– (without anti-MER2), and samples 3 to 5 are MER2+ as determined by an indirect antiglobulin test on erythrocytes. (D) Expression of MER2 (CD151) on lymphoblastoid cells. Flow cytometry histograms for lymphoblastoid cell lines derived from a MER2+ individual (gray peak) and from Pt2, the MER2– patient with anti-MER2 (black peak), incubated with PE-conjugated anti-MER2, anti-CD151, and anti-CD37.
CD34+ cells were not available from any of the MER2– individuals with anti-MER2. However, comparative analysis of lymphoblastoid cell lines derived from a MER2+ individual and from 1 of the Israeli sibs with anti-MER2 (Pt2) demonstrated strong reactivity with 2 monoclonal anti-CD151 and 2 monoclonal anti-MER2 with the lymphoid cells from the MER2+ donor and complete absence of reactivity with the lymphoblastoid cell line from Pt2 (Figure 1D). Collectively these results establish that MER2 is a marker for CD151 and show that CD151 is expressed on erythroid cells, at a higher level in erythroid precursors than on mature erythrocytes.
Analysis of CD151 in individuals who have made alloanti-MER2
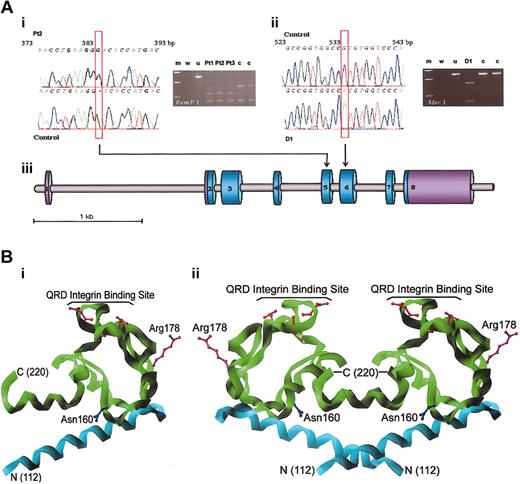
Direct sequencing of all CD151 exons from Pt1, Pt2, and Pt3, the Israeli patients with anti-MER2 and renal failure, revealed a single nucleotide insertion G383 in exon 5 of the gene (Figure 2A). The mutation was homozygous and caused a frameshift after Lys127, introducing a premature stop signal at codon 140. The mutation in Pt2 was confirmed by cDNA sequencing. The nucleotide insertion would result in a translated protein lacking most of the large extracellular loop (EC2) between transmembrane domains 3 and 4 and transmembrane domain 4. It is doubtful whether this truncated protein would reach the plasma membrane32 or that the severely truncated EC2 region would fold to a functional protein domain. D1, the Turkish blood donor with anti-MER2, was homozygous for a G533A mutation in exon 6 (Figure 2A), encoding Arg178His. No other mutations were detected in the 8 exons and flanking intronic sequences of CD151 in the 4 individuals. Analysis of CD151 in 2 patients with recessive inheritance of Alport syndrome in which the causative mutation is unknown did not reveal any abnormalities (data not shown).
Sequence and RFLP analyses of CD151 and structure of CD151 large extracellular region. (A) Sequence and RFLP analyses of CD151. (i) DNA sequence of the MER2– patient with a hereditary nephritis (Pt2) exhibiting a G383 insertion in CD151 exon 5. The G383 insert introduced a novel recognition site 381GG-ACN10 for BsmF. PCR-RFLP gel analysis: wild-type allele was cut into restriction fragments of 86 and 401 bp and the mutation in 3 MER2-negative patients with end-stage renal disease (Pt1, Pt2, Pt3) caused further reduction of the 401-bp fragment to 2 additional 135- and 266-bp fragments. Lanes are identified as a standard 10-kb DNA ladder (m), blank control (w), uncut allele (u), and control samples (c). (ii) DNA sequence of the MER2– healthy blood donor D1 with the G533A mutation in CD151 exon 6. The PCR product from D1 was cut at a novel restriction site 528TGG CCA for McsI to fragments of 160 and 326 bp, whereas the wild-type allele was left intact. PCR-RFLP gel analysis lanes labeled as in (i). (iii) Schematic representation of CD151 gene and the relative placement of the 2 mutations. (B) Structure of CD151 large extracellular region. (i, left) Homology model of a monomer of the CD151 large extracellular loop (EC2) is shown as a Cα-ribbon cartoon. The amino terminus (residue 112) is labeled N, and the carboxy terminus (residue 220) is labeled C. Because of the incorporation of a stop codon at position 140 in the MER2– patients, all residues represented by the green ribbon are not expressed. The side chain is shown for Arg 178 (purple), which is mutated to His in D1. Residues constituting the QRD proposed integrin binding site are shown in red, and the single N-linked glycosylation site (Asn 160) in blue. (ii, right) Tetraspanins readily form homodimers.31 Proposed homodimeric assembly of CD151, based on the observed oligomerization in the crystal structure of CD81. Coloring and labels as described for panel i.
Sequence and RFLP analyses of CD151 and structure of CD151 large extracellular region. (A) Sequence and RFLP analyses of CD151. (i) DNA sequence of the MER2– patient with a hereditary nephritis (Pt2) exhibiting a G383 insertion in CD151 exon 5. The G383 insert introduced a novel recognition site 381GG-ACN10 for BsmF. PCR-RFLP gel analysis: wild-type allele was cut into restriction fragments of 86 and 401 bp and the mutation in 3 MER2-negative patients with end-stage renal disease (Pt1, Pt2, Pt3) caused further reduction of the 401-bp fragment to 2 additional 135- and 266-bp fragments. Lanes are identified as a standard 10-kb DNA ladder (m), blank control (w), uncut allele (u), and control samples (c). (ii) DNA sequence of the MER2– healthy blood donor D1 with the G533A mutation in CD151 exon 6. The PCR product from D1 was cut at a novel restriction site 528TGG CCA for McsI to fragments of 160 and 326 bp, whereas the wild-type allele was left intact. PCR-RFLP gel analysis lanes labeled as in (i). (iii) Schematic representation of CD151 gene and the relative placement of the 2 mutations. (B) Structure of CD151 large extracellular region. (i, left) Homology model of a monomer of the CD151 large extracellular loop (EC2) is shown as a Cα-ribbon cartoon. The amino terminus (residue 112) is labeled N, and the carboxy terminus (residue 220) is labeled C. Because of the incorporation of a stop codon at position 140 in the MER2– patients, all residues represented by the green ribbon are not expressed. The side chain is shown for Arg 178 (purple), which is mutated to His in D1. Residues constituting the QRD proposed integrin binding site are shown in red, and the single N-linked glycosylation site (Asn 160) in blue. (ii, right) Tetraspanins readily form homodimers.31 Proposed homodimeric assembly of CD151, based on the observed oligomerization in the crystal structure of CD81. Coloring and labels as described for panel i.
A model of the CD151 protein
We modeled the structure of CD151 EC2 by using the coordinates of the crystal structure of CD81 (Figure 2B).27 The absence of most of EC2 would prohibit association with integrin subunits α3 and α6, which bind with CD151 through the same site (Q194RD) on EC2 (Figure 2B).33 The G533A mutation in D1 results in a change at codon 178 in EC2 spatially distinct from the Q194RD motif, consistent with the lack of clinical phenotype (Figure 2B). The model suggests the change from arginine to histidine at this site is likely to be tolerated within the protein fold, implying this exchange produces a functional tetraspanin with an altered surface not recognized by human alloanti-MER2. We have, however, no immunochemical evidence that CD151 was present in this individual.
Histologic examination of kidney biopsy material
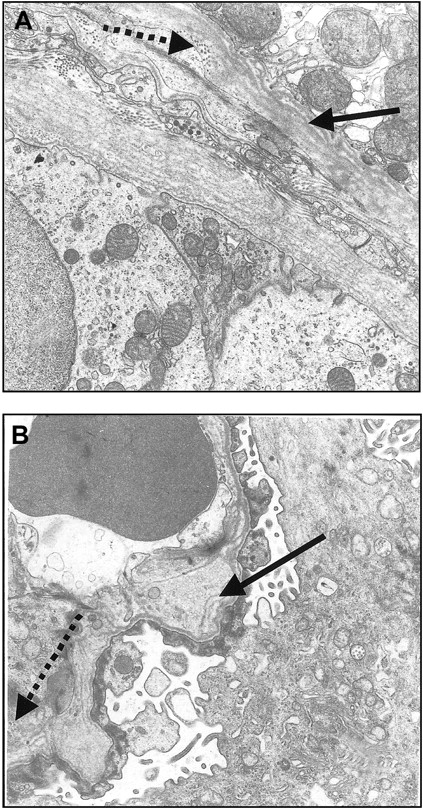
Kidney biopsy from Pt1, one of the Israeli patients with renal failure, was obtained. Under light microscopy, except for focal thickening of the basement membrane in occasional glomeruli, no apparent abnormalities were seen (not shown). Similarly, immunofluorescence failed to reveal evidence of fibrinogen, immunoglobulin, or complement deposition (not shown). Electron microscopy, however, showed splitting of the tubular basement membrane and thickening, reticulation and fragmentation of the lamina densa of the glomerular basement membrane with inclusion of electrondense particles (Figure 3). Clusters of foam cells in the interstitium were also noted.
Electron micrographs of kidney biopsy specimens showing glomerular and tubular basement membrane of Pt1. (A) Thickening and splitting of the tubular basement membrane (arrow) and granular inclusion of electron-dense particles (dotted arrow). Magnification × 17 000. (B) Focal thickening and irregularity of the glomerular basement membrane with splitting of the lamina densa (arrow) and inclusion of electron-dense particles (dotted arrow). Magnification × 17 000.
Electron micrographs of kidney biopsy specimens showing glomerular and tubular basement membrane of Pt1. (A) Thickening and splitting of the tubular basement membrane (arrow) and granular inclusion of electron-dense particles (dotted arrow). Magnification × 17 000. (B) Focal thickening and irregularity of the glomerular basement membrane with splitting of the lamina densa (arrow) and inclusion of electron-dense particles (dotted arrow). Magnification × 17 000.
Pretibial epidermolysis bullosa in the Israeli patients
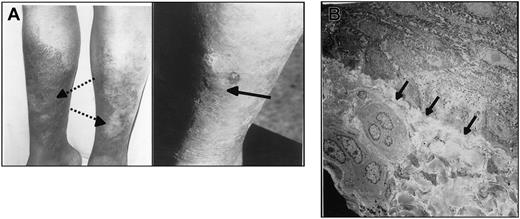
Pt1 and Pt2, the 2 Israeli sibs with hereditary nephritis, also had pretibial epidermolysis bullosa. Figure 4A shows bullous skin lesions with adjacent atrophy and scarring in patient Pt2.
Pretibial epidermolysis bullosa in Pt2. (A) Photograph showing bullous skin lesion (arrow) with adjacent atrophy and scarring (dotted arrows). (B) Slitlike separation of epidermis from dermis (arrows). Magnification × 20 000.
Pretibial epidermolysis bullosa in Pt2. (A) Photograph showing bullous skin lesion (arrow) with adjacent atrophy and scarring (dotted arrows). (B) Slitlike separation of epidermis from dermis (arrows). Magnification × 20 000.
Under light microscopy skin specimens from Pt2 demonstrated complete separation of the epidermis from the dermis with moderate focal epidermal edema. The upper dermis showed mild mononuclear perivascular infiltration (not shown). Immunofluorescence studies revealed essentially negligible deposition of C3 and immunoglobulin A (not shown). Electron microscopy showed slitlike separation of epidermis from dermis (Figure 4B).
Findings by light microscopy and immunofluorescence on skin specimens from Pt1 were similar with those of Pt2. Repeated attempts to identify the basal membrane by means of electron microscopy performed at the time of active bullous disease were unsuccessful.
Discussion
The study of human blood groups has led to advances in our understanding of many of the integral proteins of the human erythrocyte membrane. Most of the 29 blood group systems have null phenotypes in which homozygosity for inactivating mutations in the blood group gene leads to an absence of the blood group protein from the erythrocytes and any other cells in which it is usually expressed.29 These null phenotypes, natural human knockouts, are generally rare but are often disclosed by the presence of alloantibodies, produced in response to transfusion or pregnancy.
MER2, the only antigen of the RAPH blood group system, is apparently absent from erythrocytes of 8% of Caucasians.20 However, these probably represent the low end of a spectrum of erythrocyte expression, with a site density below the threshold for detection by indirect agglutination techniques. We have shown that expression of MER2 on erythroid cells decreases throughout ex vivo erythropoiesis and is detectable on erythroid progenitors of apparently MER2– individuals (confirming studies by Lucien et al34 ) (Figure 1B). We have also shown that MER2 is abundant on platelets of individuals with both MER2+ and MER2– erythrocyte phenotypes, although there may be some degree of correlation between strength of antigen expression on erythrocytes and platelets (Figure 1C). The reason for the individual variation in the expression of MER2 on erythrocytes and platelets is presently unknown.
We obtained blood samples from the 4 known individuals who lack the MER2 antigen and have made anti-MER2. Three of these are Indian Jews living in Israel and all have end-stage kidney disease.21,22 All 3 had a frameshift mutation in exon 5 of CD151, a nucleotide insertion that would result in a translated protein lacking most of the large extracellular loop (EC2) between transmembrane domains 3 and 4. It is doubtful whether this truncated protein would reach the plasma membrane32 or that the severely truncated EC2 region would fold to a functional protein domain. In addition to nephrotic syndrome, which deteriorated to end-stage renal failure, other symptoms associated with this mutation are pretibial bullous skin lesions, neurosensory deafness, bilateral lacrimal duct stenosis, nail dystrophy, and β-thalassemia minor.22 The fourth individual with anti-MER2, a blood donor with no apparent clinical phenotype,23 was homozygous for a missense mutation encoding an amino acid substitution at a site in EC2 of CD151 that, according to our model, is likely to be tolerated within the protein fold.
The glomerular basement membrane (GBM) of the kidney contains the ECM proteins laminin 11 and type-IV collagen. In humans, mutations in type IV collagen genes are frequently associated with hereditary glomerulonephritis (Alport syndrome).24 Mutation of the β2 chain of laminin 11 in mice results in a severe congenital nephrotic syndrome.35 A kidney biopsy from one of the Israeli patients with renal failure (Pt1) revealed thickening and splitting of the tubular basement membrane and thickening, reticulation, and fragmentation of the lamina densa of GBM with inclusion of electron-dense particles (Figure 3). Clusters of foam cells in the interstitium were also apparent. In human kidney CD151 is strongly expressed in glomeruli and colocalizes with α3β1 at the GBM side of cells.17 Mice with an inactive α3 integrin gene also have abnormal kidneys with thickening and fragmentation of the glomerular basement membrane.36 In renal tubular cells CD151 and α6β1 are concentrated at the tubular basement membrane.17 CD151 associates with α3 and α6 during biosynthesis.33 Abnormal basement membranes in our patients may result from the absence of this association and consequent failure to transport α3β1 and α6β1 to the plasma membrane.
The 2 Israeli sibs (Pt1 and Pt2) exhibited sensorineural deafness.22 This is also found in Alport syndrome. Type IV collagen was absent from the inner ear in a canine model of X-linked Alport syndrome,37 and sensorineural hearing loss has also been linked to a defect in the laminin α2 chain in mice.38 Laminin 11 is found in the basilar membrane in humans.39 To our knowledge CD151 has not been described in the inner ear, but our results suggest it may be important for ECM assembly in this organ.
The 2 Israeli sibs (Pt1 and Pt2) also had pretibial epidermolysis bullosa (Figure 4A). Skin biopsy (Pt2) revealed complete separation of the epidermis from the dermis with moderate focal epidermal edema. The upper dermis showed mild mononuclear perivascular infiltration. In humans, CD151 is co-distributed with integrins α3β1 and α6β4 at the basolateral surface of basal keratinocytes where it is concentrated in hemidesmosomes.40 CD151 is the only tetraspanin identified in hemidesmosomes and its recruitment is regulated by α6β4.40 Integrin α6β4 binds the basement membrane protein laminin 5 and tightens the dermal-epidermal junction by also binding the amino-terminal NC-1 domain of type VII collagen.41 Mutations in the type VII collagen gene (COL7A1) are associated with epidermolysis bullosa,42 as are mutations in the genes encoding laminin 5 and integrins β4 and α6.43-45 CD151-deficiency may cause epidermolysis bullosa in our patients by reducing the expression of α6β4 in hemidesmosomes and thereby compromising the stability of the dermal-epidermal junction.
Analysis of DNA from 2 patients with recessive inheritance of Alport syndrome of unknown origin did not reveal any mutations in CD151. Because the MER2– patients we studied also have pretibial epidermolysis bullosa, a condition not associated with Alport syndrome, these observations suggest that mutations in CD151 are unlikely to represent a previously unrecognized genetic mechanism giving rise to autosomal recessive Alport syndrome.
CD151 is a common link between laminin binding complexes in kidney and skin, and its absence provides the most likely explanation for the concomitant occurrence of nephritis, sensorineural deafness, epidermolysis bullosa, and the other abnormalities, apart from thalassemia minor, seen in the Israeli patients. Complexes between CD151 and α3β1 and CD151 and α6β1 are exceptionally strong when compared with other tetraspaninintegrin interactions.18 The CD151-deficient patients we studied show that these interactions are critical for the correct assembly of kidney basement membranes and play an important role in the integrity of basement membranes in skin, inner ear, and other tissues.
Severe anemia observed in the CD151-deficient patients studied here can be attributed, in part at least, to the occurrence of β-thalassemia minor. The endogenous blood levels of erythropoietin were 129 and 135 mU/mL in Pt1 and Pt2, respectively (normal range, 10-30 mU/mL).46 However, these patients require much higher doses of recombinant erythropoietin to maintain their hemoglobin levels between 8 and 10 g/dL than other patients with β-thalassemia trait undergoing chronic hemodialysis,46 suggesting their marrow response to erythropoietin is impaired. Integrins and growth factor receptors act through common signaling pathways to regulate cell cycle progression in mammalian cells.47 The impaired marrow response to erythropoietin found in CD151-deficient patients may result from defects in the membrane assembly of integrin(s) in erythroid progenitors that affect signaling pathways also used by the erythropoietin receptor.
Prepublished online as Blood First Edition Paper, July 20, 2004; DOI 10.1182/blood-2004-04-1512.
Supported by grants from DiaMed AC (Switzerland) (V.K.C.) and from the United Kingdom NHS R&D Directorate (D.J.A., G.D., and C.A.G.) and by a studentship from the United Kingdom Medical Research Council (N.B.).
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 7, 2003.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The electron micrographs of kidney were performed by Prof Ben-Bassat (RIP), Department of Pathology, Beilinson Medical Center, Petach Tikva, Israel. We thank Prof Yuri Kopolovic, Director of Department of Pathology, Tel-Hashomer Hospital, Sackler School of Medical, Tel-Aviv University, Israel, for help with the analysis of electron micrographs of skin; Dr Y Schechter for providing information on patient Pt3; and Professor Adrian S Woolf, Institute of Child Health, London, United Kingdom, for helpful discussions regarding hereditary nephritis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal