Abstract
We investigated the effects of the iron regulatory peptide hepcidin on iron transport by the human intestinal epithelial Caco-2 cell line. Caco-2 cells were exposed to hepcidin for 24 hours prior to the measurement of both iron transport and transporter protein and mRNA expression. Incubation with hepcidin significantly decreased apical iron uptake by Caco-2 cells. This was accompanied by a decrease in both the protein and the mRNA expression of the iron-response element containing variant of the divalent metal transporter (DMT1[+IRE]). In contrast, iron efflux and iron-regulated gene1 (IREG1) expression were unaffected by hepcidin. Hepcidin interacts directly with a model intestinal epithelium. The primary effect of this regulatory peptide is to modulate the apical membrane uptake machinery, thereby controlling the amount of iron absorbed from the diet. (Blood. 2004;104:2178-2180)
Introduction
An adequate supply of dietary iron is essential to normal human physiology. However, only recently have many of the molecular mechanisms involved in its absorption been elucidated.1 The regulation of intestinal iron absorption relies on signals generated by the main sites of iron storage (the liver) and utilization (the bone marrow)—often called the stores regulator and the erythroid regulator, respectively—which together coordinate intestinal uptake with the body's requirement. The nature of these signals has been the subject of much speculation but recently hepcidin, a 20-25 amino acid peptide first isolated from blood and urine,2,3 which is differentially expressed in the liver in response to changes in hepatic iron content,4 has been proposed to act as the stores regulator. Recent work has shown that hepcidin is inappropriately expressed in models of inflammation and in the anemia of chronic disease.5,6 Furthermore, hepcidin is tightly regulated by phenylhydrazine-induced hemolytic anemia and by phlebotomy,5 adding credence to the hypothesis that this peptide might also act as the erythroid regulator.
We have recently reported that injection of hepcidin into mice decreased duodenal iron uptake.7 However, it was unclear whether this was due to hepcidin acting within the intestinal epithelium or at a remote site. To address this problem, we have utilized the Caco-2 TC7 cell model of human intestinal epithelial cells to determine the effects of hepcidin on iron absorption and the levels of intestinal iron transporters divalent metal transporter 1 (DMT1) and iron-regulated gene 1 (IREG1), which are abundantly expressed in these cells.8-10
Study design
Experiments employed Caco-2 TC7 cells, grown for 21 days on Transwell inserts (Corning-Costar, High Wycombe, United Kingdom), stimulated with hepcidin (10 μM), synthesized and analyzed as described previously,7 for 24 hours prior to experimentation. Iron transport assays were performed as described previously.8-10 Uptake was initiated by adding 10 μM Fe2+ ascorbate (1:100 molar ratio), containing 10 μCi/mL (37 kBq/mL) 55Fe, to the apical chamber and terminated after 60 minutes. Cellular iron uptake and efflux into the basolateral medium were determined by scintillation counting.
Total plasma membranes were prepared from Caco-2 TC7 cells11 and subjected to Western blotting using commercially available antibodies to DMT1 (+iron-responsive element [IRE]), DMT1 (non-IRE), and IREG1 (1:500 dilution; Alpha Diagnostics International, San Antonio, TX). Cross reactivity was visualized using ECL Plus (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) according to the manufacturer's instructions. Band densities were semiquantified by densitometric analysis using Scion Image software (Scion, Frederick, MD).
Iron transporter transcripts were analyzed by real-time polymerase chain reaction (PCR) using LightCycler Fast Start DNA Master SYBR Green 1 (Roche Diagnostics, Mannheim, Germany) on a LightCycler real-time PCR instrument (version 3.5; Roche Diagnostics), according to the manufacturer's protocol and using the primer sequences shown in Table 1.
Primer sequences used for real-time PCR analysis of iron transporter transcripts
. | Forward primer (5′ to 3′) . | Reverse primer (5′ to 3′) . |
|---|---|---|
| HPRT | TTGTAGCCCTCTGTGTGCTCAAG | GCCTGACCAAGGAAAGCAAAGTC |
| DMT1 | ||
| (non-IRE) | GGACCTAGGGCATGTGGCAT | ACACAAGTGAGTCAGCGTGG |
| DMT1 | ||
| (+ IRE) | AGTGGTTTATGTCCGGGACC | TTTAACGTAGCCACGGGTGG |
| IREG1 | CGTCATTGCTGCTAGAATCG | AGACTGAAATCAATACGAGC |
. | Forward primer (5′ to 3′) . | Reverse primer (5′ to 3′) . |
|---|---|---|
| HPRT | TTGTAGCCCTCTGTGTGCTCAAG | GCCTGACCAAGGAAAGCAAAGTC |
| DMT1 | ||
| (non-IRE) | GGACCTAGGGCATGTGGCAT | ACACAAGTGAGTCAGCGTGG |
| DMT1 | ||
| (+ IRE) | AGTGGTTTATGTCCGGGACC | TTTAACGTAGCCACGGGTGG |
| IREG1 | CGTCATTGCTGCTAGAATCG | AGACTGAAATCAATACGAGC |
Levels of transporter mRNA were normalized to the housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) using LightCycler Relative Quantification Software version 1.0.
Data are presented as mean plus or minus standard error of the mean (SEM). Statistical differences (P < .05) between groups were determined using the Student unpaired t test.
Results and discussion
Hepcidin, added to the basolateral chamber of the Transwell culture system 24 hours prior to experimentation, significantly decreased (P < .04) iron uptake across the apical membrane of Caco-2 TC7 cells (control: 453.3 ± 47.3 pmol/cm2 per hour; hepcidin: 268.3 ± 63.9 pmol/cm2 per hour; n = 6). Total iron efflux was decreased in direct proportion to the reduced apical uptake (control: 5.8 ± 0.7 pmol/cm2 per hour; hepcidin: 2.9 ± 0.4 pmol/cm2 per hour; n = 6). Therefore, the transfer of absorbed iron into the basolateral medium (measured as a percentage of apical uptake) was not different in the control and hepcidin-treated groups (control: 1.3% ± 0.2%; hepcidin: 1.1% ± 0.2%).
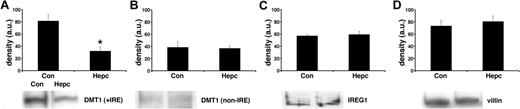
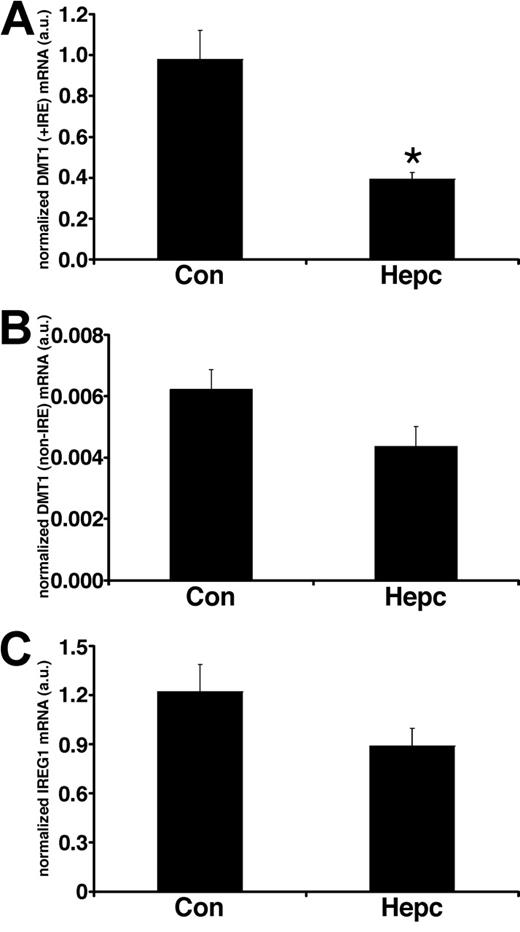
In parallel sets of cells we studied the effects of hepcidin on iron transporter expression. Following hepcidin treatment, there was a significant decrease (P < .01) in the membrane expression of DMT1 (+IRE) protein (Figure 1) but no effect on the non-IRE isoform. Protein expression of the efflux transporter IREG1 was also unaffected by hepcidin. Similarly, there was no effect of hepcidin on the expression of the housekeeper protein, villin. The observed changes in iron transporter protein expression were paralleled by alterations in transporter mRNA levels following hepcidin treatment. DMT1 (+IRE) mRNA was significantly decreased (P < .05), whereas DMT1 (non-IRE) and IREG1 expression were unaltered by hepcidin (Figure 2).
Effects of hepcidin on iron transporter protein expression. Iron transporter protein expression was determined by Western blotting of total plasma membrane fractions isolated from Caco-2 TC7 cells. Following exposure to hepcidin, DMT1 (+IRE) protein was significantly decreased (*P < .01, Student unpaired t test), whereas DMT1 (non-IRE) (P > .2) and IREG1 (P > .2) protein expression was unaltered. Densitometry data are means ± SEM of 3 to 4 observations in each group and are accompanied by representative bands for each protein studied.
Effects of hepcidin on iron transporter protein expression. Iron transporter protein expression was determined by Western blotting of total plasma membrane fractions isolated from Caco-2 TC7 cells. Following exposure to hepcidin, DMT1 (+IRE) protein was significantly decreased (*P < .01, Student unpaired t test), whereas DMT1 (non-IRE) (P > .2) and IREG1 (P > .2) protein expression was unaltered. Densitometry data are means ± SEM of 3 to 4 observations in each group and are accompanied by representative bands for each protein studied.
Real-time PCR analysis of iron transporter transcripts following hepcidin treatment. Real-time PCR analysis of total RNA isolated from Caco-2 TC7 cells demonstrated that DMT1 (+IRE) mRNA levels were significantly decreased following exposure to hepcidin for 24 hours (*P < .005, Student unpaired t test), whereas DMT1 (non-IRE) (P > .08) and IREG1 (P > .1) mRNA levels were unaffected by hepcidin treatment. Quantitative measurements for each transporter mRNA were derived from a standard curve constructed from known amounts of PCR product. Data have been normalized to the expression of the housekeeper gene HPRT and are expressed as means ± SEM from 6 separate experiments.
Real-time PCR analysis of iron transporter transcripts following hepcidin treatment. Real-time PCR analysis of total RNA isolated from Caco-2 TC7 cells demonstrated that DMT1 (+IRE) mRNA levels were significantly decreased following exposure to hepcidin for 24 hours (*P < .005, Student unpaired t test), whereas DMT1 (non-IRE) (P > .08) and IREG1 (P > .1) mRNA levels were unaffected by hepcidin treatment. Quantitative measurements for each transporter mRNA were derived from a standard curve constructed from known amounts of PCR product. Data have been normalized to the expression of the housekeeper gene HPRT and are expressed as means ± SEM from 6 separate experiments.
A major role for hepcidin in the regulation of iron metabolism was established in studies with knockout mice,12,13 in which deletion of the hepcidin gene led to the development of a severe iron overload similar to that observed in human haemochromatosis and in the Hfe-/- mouse. Recently, transgenic mice overexpressing hepcidin have been generated13 that exhibited severe body iron deficiency and microcytic hypochromic anemia, suggesting a reciprocal relationship exists between hepcidin expression and iron accumulation. Hepcidin expression has been shown to be elevated in inflammation and in chronic disease.5,6 In these pathologic disorders, the ensuing anemia is thought to occur as a consequence of both iron retention within the reticuloendothelial cells and inappropriately low intestinal iron absorption.14,15 Recent studies by Frazer et al16 demonstrated that liver hepcidin expression was decreased in response to dietary iron deficiency. Furthermore, the reduced liver hepcidin mRNA levels correlated with increased intestinal iron absorption and elevated expression of the intestinal DMT1 (+IRE) transporter. In our own studies, direct injection of hepcidin into mice decreased specifically the apical uptake step of duodenal iron absorption.7 In light of these findings, the aim of our present study was to determine whether hepcidin elicited its effects by interacting directly with intestinal epithelial cells. The data presented here demonstrate that hepcidin specifically decreases iron uptake across the apical surface of the Caco-2 epithelial layer, which is consistent with our previous findings.7 At the molecular level these changes in iron transport are explained by a reduction in DMT1(+IRE) transporter expression following hepcidin treatment. Interestingly, Frazer et al16 also showed a correlation between decreased hepcidin and elevated IREG1 expression. In our present study we did not observe any effect of hepcidin on IREG1 expression or function and this is supported by our previous work in which injection of hepcidin into mice had no effect on mucosal iron transfer.7 Thus it is possible that other humoral mediators in addition to hepcidin are required to regulate IREG1 expression.
The prevailing serum hepcidin concentration is unclear. Extrapolations from urinary measurements suggested that circulating hepcidin was within the nanomolar range3 and was positively correlated with serum ferritin levels.17 However, a recent study indicated that serum levels could be much higher, in the mid- to high-micromolar range.18 The concentration of human synthetic hepcidin used in our study (10 μM) sits between these 2 extremes. As noted previously, our synthetic hepcidin contains a mixture of species including one or more active forms.7 At present the nature of the circulating active form of hepcidin remains unclear. The effects of hepcidin observed in the present study are unlikely to be due to toxicity since there was no effect of peptide incubation on monolayer transepithelial resistance or paracellular permeability (data not shown).
In conclusion, our data provide the first direct evidence that hepcidin can regulate intestinal iron absorption by interacting with a model intestinal epithelial cell line. The primary effect of hepcidin is to modulate the apical membrane uptake machinery, thereby controlling the amount of iron absorbed from the diet.
Prepublished online as Blood First Edition Paper, June 3, 2004; DOI 10.1182/blood-2004-03-0829.
Supported by Biotechnology and Biological Sciences Research Council (BBSRC; grant no. 90/D13 400) and The Wellcome Trust.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal