Abstract
The multidrug resistance-1 (MDR1) gene product, P-glycoprotein (P-gp), and the multidrug resistance–related proteins (MRPs) are members of the adenosine triphosphate (ATP)–binding cassette (ABC) transporter gene superfamily that regulates the trafficking of drugs, peptides, ions, and xenobiotics across cell membrane barriers. Three-dimensional modeling of human MDR1/P-gp indicates that these glycoproteins function as efficient, ATP-dependent gate-keepers, which scan the plasma membrane and its inner leaflet to flip lipophilic substrates to the outer membrane leaflet. Delineation of the adverse prognostic power of MDR1 in adult acute myeloid leukemia (AML) raised hopes that pharmacologic blockade of P-gp would improve the outcome of conventional cytotoxic therapy, perhaps more so than in any other human malignancy. Phase 3 clinical trials investigating first- and second-generation P-gp antagonists have yielded conflicting results, emphasizing the importance of applying preclinical principals to realistically appraise expectations for clinical benefit. Structure-based design strategies and the delineation of transcriptional regulators of survival gene cassettes promise to yield novel, more-effective strategies to overcome drug resistance. Lessons learned from investigations of these and other mechanisms of cellular defense hold promise for a renaissance in the development of targeted therapeutics in acute leukemia.
Introduction
Over the past 2 decades, information garnered from cell biology has strengthened our understanding of how malignant cells survive toxic insults and become resistant to antineoplastics. Anthracycline resistance in particular may arise from the actions of one or more membrane transport proteins that promote the expulsion of xenobiotics, the best characterized of which is P-glycoprotein (P-gp), a 170-kDa adenosine triphosphate (ATP)–dependent multispecific drug transporter.1 Recent investigations have shown that P-gp's substrate specificity and mechanism of export are more sophisticated than first realized. P-gp contributes to antineoplastic resistance in at least 2 ways: active drug extrusion and elevation of cellular apoptotic threshold. Reports linking overexpression of the MDR1 gene and P-gp to adverse treatment outcome in adult acute myeloid leukemia (AML) provided the evidence necessary to implicate this multidrug resistance (MDR) phenotype as an important biologic target for pharmacologic modulation.2-5 Following mixed results of clinical trials testing early generations of competitive P-gp antagonists, perceptions that the strategy of disabling P-gp may yet yield significant therapeutic advancement inAML have now been challenged. In this overview, we highlight key aspects of P-gp biology as a platform to critically appraise the results of clinical trials performed to date. Emphasis is placed on the analysis of phase 3 studies with comparison of differences in methodology and patient selection. A comprehensive overview of alternate mechanisms of resistance is beyond the scope of this report.
The MDR superfamily
The MDR ATP-binding cassette (ABC) transporters comprise one of the largest gene families encoding structurally related transmembrane proteins, which include over 50 known members. These genes share a signature DNA coding sequence for an ATP-binding domain necessary for the transport function of the proteins. Initial evidence linking membrane transporters to the phenomenon of MDR derived from investigations from Victor Ling's laboratory in which a glycoprotein of 170 kDa, referred to as P-glycoprotein (P-gp), was shown to limit cellular accumulation and cytotoxicity of structurally and functionally diverse antineoplastics in chinese hamster ovary cells.1,6 P-gp's role in this MDR phenotype was confirmed by gene transfection studies demonstrating recapitulation of the resistant phenotype in previously sensitive cells.7 The gene encoding P-gp, termed “MDR1,” encodes a 1280-residue polypeptide chain8 organized into 2 homologous halves.9 The protein's putative function as an ATP-dependent export pump that expels small molecules from the cell interior was surmised from sequence homology to bacterial ABC transporters.10 Mammalian ABC transporters act largely as substrate exporters and include the MDR1 gene product, P-gp, the cystic fibrosis transmembrane conductance regulator (CFTR), and the transporter associated with antigen processing (TAP).11 To date, 49 human ABC transporter genes have been sequenced and classified into 8 subfamilies, ABCA through ABCG and ANSA (arsenite and antimonite transporter), on the basis of their sequence homology scores (Table 1). In this recently adopted nomenclature, MDR1 is defined as ABCB1,12 which includes 2 family members, MDR3 (ABCB7) and Sister P-gp (ABCB11) that share 75% and 50% sequence identity to MDR1, respectively.
Human ATP binding cassette transporters
Name . | Functional description . |
|---|---|
| ABC1: subfamily A | |
| ABC1 | ABCA1 is a major regulator of cellular cholesterol and phospholipid homeostasis. It mediates, for example, the efflux of phospholipids (PS) and cholesterol from macrophages to apoA-1, reversing foam cell formation. Likely not involved in hepatic cholesterol secretion and intestinal apical cholesterol transport. |
| ABCA2 | ABCA2 is a regulator of steroid and lipid transport, primarily in neural membranes. |
| ABCA3 | ABCA3 is expressed almost exclusively in the lung tissue and is most likely involved in the formation of surfactant protein (SP) in lamellar bodies in the lungs. |
| ABCR | This protein is a retina-specific ABC transporter with N-retinylidene-PE (phosphatidylethanolamine) as a substrate. It is expressed exclusively in retina photoreceptor cells, indicating the gene product mediates transport of an essential molecule across the photoreceptor cell membrane. |
| ABCA5 | No information available about this protein. |
| ABCA6 | Actual function and substrate are unknown, though it is speculated, based on certain structural features and responses in regulation to cholesterol, that it is involved in the homeostasis of macrophage lipids. |
| ABCA7 | This full transporter has been detected predominantly in myelo-lymphatic tissues with the highest expression in peripheral leukocytes, thymus, spleen, and bone marrow. The function of this protein is not yet known; however, the expression pattern suggests a role in lipid homeostasis in cells of the immune system. Alternative splicing of this gene results in 2 transcript variants. |
| ABCA8 | This gene is clustered among 4 other ABC1 family members on 17q24, but neither the substrate nor the function of this gene is known. |
| ABCA9 | This gene is clustered among 4 other ABC1 family members on 17q24 and may play a role in monocyte differentiation and macrophage lipid homeostasis. |
| ABCA10 | This gene is clustered among 4 other ABC1 family members on 17q24, but neither the substrate nor the function of this gene is known. ABCA10 expression is suppressed by cholesterol import into macrophages, indicating that it is a cholesterol-responsive gene. |
| ABCA12 | No information available about this protein. |
| ABCA13 | The predicted ABCA13 protein consists of 5058 amino acid residues, making it the largest ABC protein described to date. ABCA13 contains a hydrophobic, predicted transmembrane segment at the N-terminus, followed by a large hydrophilic region. |
| MDR/TAP: subfamily B | |
| ABCB1 | The protein (also called P-glycoprotein) is an ATP-dependent drug efflux pump for xenobiotic compounds with broad substrate specificity. It is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. |
| ABCB2 | The protein is a half-ABC transporter functioning as a peptide transporter involved in the pumping of degraded cytosolic peptides across the endoplasmic reticulum into the membrane-bound compartment where class I molecules assemble. |
| ABCB3 | The protein is a half-abc transporter functioning as a peptide transporter involved in antigen presentation. It forms a heterodimer with TAP1/ABCB2 in order to transport peptides from the cytoplasm to the endoplasmic reticulum. Alternative splicing of this gene produces 2 products which differ in peptide selectivity and level of restoration of surface expression of MHC class I molecules. |
| ABCB4 | Most likely involved in biliary phosphatidylcholine secretion from hepatocytes in a bile salt-dependent manner. Biliary phosphatidylcholine (PC) is necessary to keep the bile “nontoxic”. |
| ABCB5 | No information available about this protein. |
| ABCB6 | This half-transporter likely plays a role in mitochondrial function and possibly transports iron. |
| ABCB7 | This gene encodes a half-transporter involved in the transport of heme from the mitochondria to the cytosol. With iron/sulfur cluster precursors as its substrates, this protein may play a role in metal homeostasis. |
| ABCB8 | The function of this half-transporter has not yet been determined; however, it may involve the compartmentalization and transport of heme, as well as peptides, from the mitochondria to the nucleus and cytosol. This protein may also play a role in the transport of phospholipids into mitochondrial membranes. |
| ABCB9 | The function of this half-transporter has not yet been determined; however, this protein may play a role in lysosomes. Alternative splicing of this gene results in 2 known products, which are likely to have different substrate specifications. |
| ABCB10 | No information available about this protein. |
| BSEP | BSEP is the major canalicular bile salt export pump in humans, and is responsible for active transport of bile salts across the hepatocyte canalicular membrane into bile. It represents the molecular basis of the bile salt—dependent bileflow. BSEP activity is necessary for PC secretion via PGY3/ABCB4. |
| CFTR/MPR: subfamily C | |
| MRP1 | MRP1 functions as a multispecific organic anion transporter, with (oxidized) glutathione, cysteinyl leukotrienes, and activated aflatoxin B1 as substrates. This protein also transports glucuronides and sulfate conjugates of steroid hormones and bile salts. It also transports drugs and other hydrophobic compounds in the presence of glutathione. |
| MRP2 | MRP2 is expressed in the canalicular (apical) part of the hepatocyte and functions in biliary transport of mainly anionic conjugates with glutathione, sulfate, or glucuronosyl (eg, glucuronosyl bilirubin). Other substrates include anticancer drugs such as vinblastine (similar specificity as MRP1/ABCC1); appears to contribute to drug resistance. |
| MRP3 | The specific function of this protein has not yet been determined; however, this protein may play a role in the transport of biliary and intestinal excretion of organic anions including bile salts. |
| MRP4 | The human multidrug resistance protein MRP4 is an organic anion transporter that transports cyclic nucleotides and some nucleoside monophosphate analogs including nucleoside-based antiviral drugs (specificity similar to MRP5). MRP4 also transports prostaglandins. |
| MRP5 | The human multidrug resistance protein MRP5 is an organic anion transporter that transports cyclic nucleotides and some nucleoside monophosphate analogs including nucleoside-based antiviral drugs (specificity similar to MRP4). |
| MRP6 | In humans, MRP6 is highly expressed in the liver and kidney. Lower expression was found in tissues affected by pseudoxanthoma elasticum, including skin, retina, and vessel walls. Functional studies suggest that small peptides (BQ123) are transported by rat Mrp6. Recent studies show transport of glutathione conjugates. |
| CFTR/MPR: subfamily C | |
| CFTR | This protein functions as a chloride channel and controls the regulation of other transport pathways. |
| SUR1 | This protein functions as a modulator of ATP-sensitive potassium channels and insulin release. |
| SUR2 | This protein is thought to form ATP-sensitive potassium channels in cardiac, skeletal, and vascular and nonvascular smooth muscle. Protein structure suggests a role as the drug-binding channel-modulating subunit of the extrapancreatic ATP-sensitive potassium channels. |
| MRP7 | MRP7/ABCC7 has been shown to transport estradiol(2)17beta glucuronide and also LTC4, although not as well. E(2)17betaG transport was saturable, with Km and Vmax values of 57.8 μM and 53.1 pmol/mg/min, respectively. |
| ABCC11 | MRP8/ABCC11 is expressed at low levels in all tissues, except kidney, spleen, and colon. This gene and family member ABCC12 are determined to be derived by duplication and are both localized to chromosome 16q12.1. Their chromosomal localization, potential function, and expression patterns identify them as candidates for paroxysmal kinesigenic choreoathetosis, a disorder characterized by attacks of involuntary movements and postures, chorea, and dystonia. Multiple alternatively spliced transcript variants have been described for this gene. |
| ABCC12 | ABCC12 is expressed at low levels in testes, ovary, and prostate tissues. This gene and family member ABCC11 are determined to be derived by duplication and are both localized to chromosome 16q12.1. Multiple alternatively spliced transcript variants encoding different isoforms have been described for this gene but some of their full-length sequences are not available. |
| ABCC13 | ABCC13 is highly expressed in human fetal liver cells. Levels in cells that were induced to differentiate by TPA decreased markedly. This evidence suggests that ABCC13 may be involved in hematopoiesis. |
| ALD: subfamily D | |
| ALD | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs (coenzyme As) in the organelle. All known peroxisomal ABC transporters are half-transporters, which require a partner half-transporter molecule to form a functional homodimeric or heterodimeric transporter. This peroxisomal membrane protein is likely involved in the peroxisomal transport or catabolism of very long chain fatty acids. |
| ALDL1 | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs in the organelle. All known peroxisomal ABC transporters are half-transporters, which require a partner half-transporter molecule to form a functional homodimeric or heterodimeric transporter. The function of this peroxisomal membrane protein is unknown; however, this protein is speculated to function as a dimerization partner of ABCD1 and/or other peroxisomal ABC transporters. |
| PXMP1 | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs in the organelle. All known peroxisomal ABC transporters are half-transporters, which require a partner half-transporter molecule to form a functional homodimeric or heterodimeric transporter. |
| PXMPIL | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs in the organelle. The function of this peroxisomal membrane protein is unknown. However, it is speculated that it may function as a heterodimer for another peroxisomal ABC transporter and, therefore, may modify the adrenoleukodystrophy phenotype. It may also play a role in the process of peroxisome biogenesis. |
| OABP: subfamily E | |
| RNASEL1 | This protein is a member of the OABP subfamily. Alternatively referred to as the RNase L inhibitor, this protein functions to block the activity of ribonuclease L. Activation of ribonuclease L leads to inhibition of protein synthesis in the 2-5A/RNase L system, the central pathway for viral interferon action. |
| GNC20: subfamily F | |
| ABC50 | Unlike other members of the superfamily, this protein lacks the transmembrane domains that are characteristic of most ABC transporters. This protein may be regulated by tumor necrosis factor-α and play a role in enhancement of protein synthesis and the inflammation process. |
| ABCF2 | No information available about this protein. |
| ABCF3 | No information available about this protein. |
| White: subfamily G | |
| ABCG1 | ABCG1 is involved in macrophage cholesterol efflux and may regulate cellular lipid homeostasis in other cell types. |
| ABCG2 | This protein functions as a xenobiotic transporter, which may play a major role in multidrug resistance. It likely serves as a cellular defense mechanism in response to mitoxantrone and anthracycline exposure. Recently it has been shown to transport organic anions but also steroids (cholesterol, estradiol, progesterone, testosterone) and certain chlorophyll metabolites. |
| ABCG4 | Given ABCG4's sequential similarity to ABCG1 and its similarity to other members of the ABCG family, ABCG4 is most likely involved in cholesterol transport in the brain. |
| ABCG5 | ABCG5 functions as a half-transporter to limit intestinal absorption and promote biliary excretion of sterols. It is expressed in a tissue-specific manner in the liver, colon, and intestine. This gene is tandemly arranged on chromosome 2, in a head-to-head orientation with family member ABCG8. |
| ABCG8 | ABCG8 functions as a half-transporter to limit intestinal absorption and promote biliary excretion of sterols. It is expressed in a tissue-specific manner in the liver, colon, and intestine. This gene is tandemly arranged on chromosome 2, in a head-to-head orientation with family member ABCG5. |
| ANSA: subfamily H | |
| ANSAI | Human arsenite transporter ANSAI is a dimeric ABC ATPase with a nucleotide-binding domain and a transmembrane domain. Although its function in humans is not very well known, it is known that this protein functions as an arsenite and antimonite exporter pump in E coli. |
| ANSAII | The function of ANSAII is not completely understood, but its 98% identity with ANSAI, and homology with ABC proteins in other species whose functions are well understood, suggests that its function is the same as that of ANSAI. |
Name . | Functional description . |
|---|---|
| ABC1: subfamily A | |
| ABC1 | ABCA1 is a major regulator of cellular cholesterol and phospholipid homeostasis. It mediates, for example, the efflux of phospholipids (PS) and cholesterol from macrophages to apoA-1, reversing foam cell formation. Likely not involved in hepatic cholesterol secretion and intestinal apical cholesterol transport. |
| ABCA2 | ABCA2 is a regulator of steroid and lipid transport, primarily in neural membranes. |
| ABCA3 | ABCA3 is expressed almost exclusively in the lung tissue and is most likely involved in the formation of surfactant protein (SP) in lamellar bodies in the lungs. |
| ABCR | This protein is a retina-specific ABC transporter with N-retinylidene-PE (phosphatidylethanolamine) as a substrate. It is expressed exclusively in retina photoreceptor cells, indicating the gene product mediates transport of an essential molecule across the photoreceptor cell membrane. |
| ABCA5 | No information available about this protein. |
| ABCA6 | Actual function and substrate are unknown, though it is speculated, based on certain structural features and responses in regulation to cholesterol, that it is involved in the homeostasis of macrophage lipids. |
| ABCA7 | This full transporter has been detected predominantly in myelo-lymphatic tissues with the highest expression in peripheral leukocytes, thymus, spleen, and bone marrow. The function of this protein is not yet known; however, the expression pattern suggests a role in lipid homeostasis in cells of the immune system. Alternative splicing of this gene results in 2 transcript variants. |
| ABCA8 | This gene is clustered among 4 other ABC1 family members on 17q24, but neither the substrate nor the function of this gene is known. |
| ABCA9 | This gene is clustered among 4 other ABC1 family members on 17q24 and may play a role in monocyte differentiation and macrophage lipid homeostasis. |
| ABCA10 | This gene is clustered among 4 other ABC1 family members on 17q24, but neither the substrate nor the function of this gene is known. ABCA10 expression is suppressed by cholesterol import into macrophages, indicating that it is a cholesterol-responsive gene. |
| ABCA12 | No information available about this protein. |
| ABCA13 | The predicted ABCA13 protein consists of 5058 amino acid residues, making it the largest ABC protein described to date. ABCA13 contains a hydrophobic, predicted transmembrane segment at the N-terminus, followed by a large hydrophilic region. |
| MDR/TAP: subfamily B | |
| ABCB1 | The protein (also called P-glycoprotein) is an ATP-dependent drug efflux pump for xenobiotic compounds with broad substrate specificity. It is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. |
| ABCB2 | The protein is a half-ABC transporter functioning as a peptide transporter involved in the pumping of degraded cytosolic peptides across the endoplasmic reticulum into the membrane-bound compartment where class I molecules assemble. |
| ABCB3 | The protein is a half-abc transporter functioning as a peptide transporter involved in antigen presentation. It forms a heterodimer with TAP1/ABCB2 in order to transport peptides from the cytoplasm to the endoplasmic reticulum. Alternative splicing of this gene produces 2 products which differ in peptide selectivity and level of restoration of surface expression of MHC class I molecules. |
| ABCB4 | Most likely involved in biliary phosphatidylcholine secretion from hepatocytes in a bile salt-dependent manner. Biliary phosphatidylcholine (PC) is necessary to keep the bile “nontoxic”. |
| ABCB5 | No information available about this protein. |
| ABCB6 | This half-transporter likely plays a role in mitochondrial function and possibly transports iron. |
| ABCB7 | This gene encodes a half-transporter involved in the transport of heme from the mitochondria to the cytosol. With iron/sulfur cluster precursors as its substrates, this protein may play a role in metal homeostasis. |
| ABCB8 | The function of this half-transporter has not yet been determined; however, it may involve the compartmentalization and transport of heme, as well as peptides, from the mitochondria to the nucleus and cytosol. This protein may also play a role in the transport of phospholipids into mitochondrial membranes. |
| ABCB9 | The function of this half-transporter has not yet been determined; however, this protein may play a role in lysosomes. Alternative splicing of this gene results in 2 known products, which are likely to have different substrate specifications. |
| ABCB10 | No information available about this protein. |
| BSEP | BSEP is the major canalicular bile salt export pump in humans, and is responsible for active transport of bile salts across the hepatocyte canalicular membrane into bile. It represents the molecular basis of the bile salt—dependent bileflow. BSEP activity is necessary for PC secretion via PGY3/ABCB4. |
| CFTR/MPR: subfamily C | |
| MRP1 | MRP1 functions as a multispecific organic anion transporter, with (oxidized) glutathione, cysteinyl leukotrienes, and activated aflatoxin B1 as substrates. This protein also transports glucuronides and sulfate conjugates of steroid hormones and bile salts. It also transports drugs and other hydrophobic compounds in the presence of glutathione. |
| MRP2 | MRP2 is expressed in the canalicular (apical) part of the hepatocyte and functions in biliary transport of mainly anionic conjugates with glutathione, sulfate, or glucuronosyl (eg, glucuronosyl bilirubin). Other substrates include anticancer drugs such as vinblastine (similar specificity as MRP1/ABCC1); appears to contribute to drug resistance. |
| MRP3 | The specific function of this protein has not yet been determined; however, this protein may play a role in the transport of biliary and intestinal excretion of organic anions including bile salts. |
| MRP4 | The human multidrug resistance protein MRP4 is an organic anion transporter that transports cyclic nucleotides and some nucleoside monophosphate analogs including nucleoside-based antiviral drugs (specificity similar to MRP5). MRP4 also transports prostaglandins. |
| MRP5 | The human multidrug resistance protein MRP5 is an organic anion transporter that transports cyclic nucleotides and some nucleoside monophosphate analogs including nucleoside-based antiviral drugs (specificity similar to MRP4). |
| MRP6 | In humans, MRP6 is highly expressed in the liver and kidney. Lower expression was found in tissues affected by pseudoxanthoma elasticum, including skin, retina, and vessel walls. Functional studies suggest that small peptides (BQ123) are transported by rat Mrp6. Recent studies show transport of glutathione conjugates. |
| CFTR/MPR: subfamily C | |
| CFTR | This protein functions as a chloride channel and controls the regulation of other transport pathways. |
| SUR1 | This protein functions as a modulator of ATP-sensitive potassium channels and insulin release. |
| SUR2 | This protein is thought to form ATP-sensitive potassium channels in cardiac, skeletal, and vascular and nonvascular smooth muscle. Protein structure suggests a role as the drug-binding channel-modulating subunit of the extrapancreatic ATP-sensitive potassium channels. |
| MRP7 | MRP7/ABCC7 has been shown to transport estradiol(2)17beta glucuronide and also LTC4, although not as well. E(2)17betaG transport was saturable, with Km and Vmax values of 57.8 μM and 53.1 pmol/mg/min, respectively. |
| ABCC11 | MRP8/ABCC11 is expressed at low levels in all tissues, except kidney, spleen, and colon. This gene and family member ABCC12 are determined to be derived by duplication and are both localized to chromosome 16q12.1. Their chromosomal localization, potential function, and expression patterns identify them as candidates for paroxysmal kinesigenic choreoathetosis, a disorder characterized by attacks of involuntary movements and postures, chorea, and dystonia. Multiple alternatively spliced transcript variants have been described for this gene. |
| ABCC12 | ABCC12 is expressed at low levels in testes, ovary, and prostate tissues. This gene and family member ABCC11 are determined to be derived by duplication and are both localized to chromosome 16q12.1. Multiple alternatively spliced transcript variants encoding different isoforms have been described for this gene but some of their full-length sequences are not available. |
| ABCC13 | ABCC13 is highly expressed in human fetal liver cells. Levels in cells that were induced to differentiate by TPA decreased markedly. This evidence suggests that ABCC13 may be involved in hematopoiesis. |
| ALD: subfamily D | |
| ALD | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs (coenzyme As) in the organelle. All known peroxisomal ABC transporters are half-transporters, which require a partner half-transporter molecule to form a functional homodimeric or heterodimeric transporter. This peroxisomal membrane protein is likely involved in the peroxisomal transport or catabolism of very long chain fatty acids. |
| ALDL1 | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs in the organelle. All known peroxisomal ABC transporters are half-transporters, which require a partner half-transporter molecule to form a functional homodimeric or heterodimeric transporter. The function of this peroxisomal membrane protein is unknown; however, this protein is speculated to function as a dimerization partner of ABCD1 and/or other peroxisomal ABC transporters. |
| PXMP1 | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs in the organelle. All known peroxisomal ABC transporters are half-transporters, which require a partner half-transporter molecule to form a functional homodimeric or heterodimeric transporter. |
| PXMPIL | This protein is a member of the ALD subfamily, which is involved in peroxisomal import of fatty acids and/or fatty acyl-CoAs in the organelle. The function of this peroxisomal membrane protein is unknown. However, it is speculated that it may function as a heterodimer for another peroxisomal ABC transporter and, therefore, may modify the adrenoleukodystrophy phenotype. It may also play a role in the process of peroxisome biogenesis. |
| OABP: subfamily E | |
| RNASEL1 | This protein is a member of the OABP subfamily. Alternatively referred to as the RNase L inhibitor, this protein functions to block the activity of ribonuclease L. Activation of ribonuclease L leads to inhibition of protein synthesis in the 2-5A/RNase L system, the central pathway for viral interferon action. |
| GNC20: subfamily F | |
| ABC50 | Unlike other members of the superfamily, this protein lacks the transmembrane domains that are characteristic of most ABC transporters. This protein may be regulated by tumor necrosis factor-α and play a role in enhancement of protein synthesis and the inflammation process. |
| ABCF2 | No information available about this protein. |
| ABCF3 | No information available about this protein. |
| White: subfamily G | |
| ABCG1 | ABCG1 is involved in macrophage cholesterol efflux and may regulate cellular lipid homeostasis in other cell types. |
| ABCG2 | This protein functions as a xenobiotic transporter, which may play a major role in multidrug resistance. It likely serves as a cellular defense mechanism in response to mitoxantrone and anthracycline exposure. Recently it has been shown to transport organic anions but also steroids (cholesterol, estradiol, progesterone, testosterone) and certain chlorophyll metabolites. |
| ABCG4 | Given ABCG4's sequential similarity to ABCG1 and its similarity to other members of the ABCG family, ABCG4 is most likely involved in cholesterol transport in the brain. |
| ABCG5 | ABCG5 functions as a half-transporter to limit intestinal absorption and promote biliary excretion of sterols. It is expressed in a tissue-specific manner in the liver, colon, and intestine. This gene is tandemly arranged on chromosome 2, in a head-to-head orientation with family member ABCG8. |
| ABCG8 | ABCG8 functions as a half-transporter to limit intestinal absorption and promote biliary excretion of sterols. It is expressed in a tissue-specific manner in the liver, colon, and intestine. This gene is tandemly arranged on chromosome 2, in a head-to-head orientation with family member ABCG5. |
| ANSA: subfamily H | |
| ANSAI | Human arsenite transporter ANSAI is a dimeric ABC ATPase with a nucleotide-binding domain and a transmembrane domain. Although its function in humans is not very well known, it is known that this protein functions as an arsenite and antimonite exporter pump in E coli. |
| ANSAII | The function of ANSAII is not completely understood, but its 98% identity with ANSAI, and homology with ABC proteins in other species whose functions are well understood, suggests that its function is the same as that of ANSAI. |
ABC transporters are evolutionarily conserved from bacteria to man with broad functional diversity. Human family members participate in physiologic functions ranging from trans-membrane trafficking of small molecules to cell signaling.13 Certain human ABC transporters, such as MDR1, are conserved from bacteria to man. The LmrA multidrug transporter from Lactococcus lactis, for example, displays similar antineoplastic specificity when transfected into human lung fibroblasts and can functionally substitute for P-gp.14 Pathogenic microorganisms utilize ABC transporters to exclude antibiotics from the cell interior and have been implicated in clinical resistance to selected antibiotics.
Molecular architecture of MDR1
ABC transporters have a 4-domain organization comprising 2 membrane-spanning domains (MSDs), which create a translocation channel, and 2 nucleotide-binding domains (NBDs), which catalyze substrate export. Orientation of the 4 domains varies according to host organism, histogenic tissue of residence, and the function performed. In prokaryotes, all 4 domains are encoded on separate polypeptide chains, whereas in eukaryotes a single polypeptide chain encodes all 4 domains.13,15
The crystal structure of the MDR1 gene homologs, Escherichia coli MsbA16 and BtuCD,17 provides a model of ABC transporter topology and insight into the mechanism underlying export function. MsbA transports lipid A, a major component of the bacterial outer cell membrane.18 Although the crystal structure of the human MDR1 gene product P-gp has not been characterized, extrapolation from bacterial ABC transporter topology16 allows construction of a homology model (Figure 1). The ATP binding domain is the most highly conserved domain within the transporter family, and contains the Walker A and B motifs as well as the ABC signature motif.19 From the crystal structure of the Eco-MsbA bacterial homolog and the molecular model of human MDR1, it can be surmised that these transporters act not simply as transmembrane channels traversing the lipid bilayer, but rather as flipases that scan the lower leaflet of the bilayer for lipophilic substrates, accepting them from either the cytoplasmic interface or within the lipid bilayer to flip substrates to the outer membrane leaflet. This entropically driven “flip-flop” mechanism accounts for the unusually broad range of hydrophobic substrates exported by members of the MDR ABC transporters.
The 3-dimensional structure of the human MDR1 P-gp membrane transporter. Structure-based sequence alignment of human MDR1 on the E coli MsbA ABC transporter (1JSQ) was performed using the Clustal W program (European Bioinformatics Institute, Hamburg, Germany). When the MDR1 sequence was analyzed using the tertiary structure prediction program 3D-PSSM (3-dimensional position-sensitive scoring matrix), it demonstrated the greatest concordance with 1JSQ, confirming that these transporters are structurally conserved. A structural model of human P-gp was created using 1JSQ as the template structure in Internal Coordinate Mechanism (ICM) software (Molsoft, San Diego, CA). The overall topologic architecture of the human MDR1 ABC transporter is predicted to have a similar tertiary structure to that of Eco-msbA, which is a homodimer. Each monomer is composed of 3 domains: a membrane-spanning domain (MSD), an intracellular domain (ICD), and a nucleotide binding domain (NBD). The transporter is approximately 1200 nm in length with the MSD being about 520 nm. The trans-membrane α-helices are tilted between 30° and 40° from the plane of the membrane, forming a cone-shaped structure with a substantial opening of about 250 nm on either side facing the lipid bilayer. The outer membrane leaflet half of the trans-membrane domain forms intermolecular contacts that hold the 2 monomers together by burying a solvent accessible area of about 8500 nm2. The base of the chamber facing the cytoplasm is about 600 nm in the widest dimension (D.M., B. George, A.F.L., unpublished data, July 2003).
The 3-dimensional structure of the human MDR1 P-gp membrane transporter. Structure-based sequence alignment of human MDR1 on the E coli MsbA ABC transporter (1JSQ) was performed using the Clustal W program (European Bioinformatics Institute, Hamburg, Germany). When the MDR1 sequence was analyzed using the tertiary structure prediction program 3D-PSSM (3-dimensional position-sensitive scoring matrix), it demonstrated the greatest concordance with 1JSQ, confirming that these transporters are structurally conserved. A structural model of human P-gp was created using 1JSQ as the template structure in Internal Coordinate Mechanism (ICM) software (Molsoft, San Diego, CA). The overall topologic architecture of the human MDR1 ABC transporter is predicted to have a similar tertiary structure to that of Eco-msbA, which is a homodimer. Each monomer is composed of 3 domains: a membrane-spanning domain (MSD), an intracellular domain (ICD), and a nucleotide binding domain (NBD). The transporter is approximately 1200 nm in length with the MSD being about 520 nm. The trans-membrane α-helices are tilted between 30° and 40° from the plane of the membrane, forming a cone-shaped structure with a substantial opening of about 250 nm on either side facing the lipid bilayer. The outer membrane leaflet half of the trans-membrane domain forms intermolecular contacts that hold the 2 monomers together by burying a solvent accessible area of about 8500 nm2. The base of the chamber facing the cytoplasm is about 600 nm in the widest dimension (D.M., B. George, A.F.L., unpublished data, July 2003).
Regulation of MDR1 gene expression
P-gp is constitutively expressed in many normal tissues including hematopoietic stem cells, natural killer cells, liver, kidney, intestinal mucosa, muscle, brain, and testis.20 The human MDR1 gene is located on chromosome 7, band p21-21.1, and extends over more than 100 kb containing 28 exons.21 Inducible MDR1 gene expression in normal and transformed cells is initiated by signals from widely divergent stimuli that converge on a common region of the MDR1 promoter referred to as the “MDR1 enhanceosome.”22 The MDR1 promoter lacks a consensus TATA box found on many protein-encoding genes, and instead contains an inverted CCAAT and a GC-rich element, which serve as binding sites for the trimeric NF-Y and Sp family of transcription factors. Upon DNA binding, these transcription proteins recruit histone-acetyl transferases to promote acetylation of surrounding histones and consequent remodeling of chromatin and MDR1 promoter activation.
Activation of MDR1 occurs rapidly in response to varied cellular stresses ranging from DNA injury to serum starvation, linking MDR1 induction to a more global antiapoptotic response. Generation of reactive oxygen species appears integral to activation signals triggered by epidermal growth factor (EGF),23 tumor necrosis factor-α (TNFα),24 doxorubicin,25 and nuclear factor κB (NF-κB).26 Alternatively, mutant ras isoforms converge on c-raf to interact directly with Sp-1–binding elements and to up-regulate MDR1 expression.27 Similarly, the orphan nuclear receptor (SXR/PXR), steroid receptor, and the xenobiotic receptor may directly bind to and activate the MDR1 promoter.28 Molecular events intrinsic to neoplastic transformation may coordinately regulate MDR1 in chemotherapy-naive malignancies. Mutant p53 proteins transactivate the MDR1 promoter, whereas wild-type p53 acts as a repressor.29 Similarly, hypoxia, acting through the oxygen-sensitive transcription factor hypoxia inducible factor-1 (HIF-1α), activates vascular endothelial growth factor (VEGF) and MDR1 gene transcription, and also augments P-gp transport capacity.30 Although constitutive c-jun-N-terminal kinase (JNK) activity is associated with early induction failure in AML and overexpression of MRP1, its potential role in coregulation of MDR1 is not clear.31
MDR1 expression promotes cell survival
Signaling pathways constitutively active in AML such as PI-3K/Akt/Rac-1,32 PLCγ/Raf/Erk,33,34 and PKCα35,36 each have been implicated in transcriptional activation of MDR1. AML cells that express P-gp display a reproducible survival advantage ex vivo.37 Although the precise mechanism is not clear, 2 explanations are supported by laboratory investigations: (1) coregulation of P-gp and antiapoptotic Bcl-2 family members, and (2) suppression of caspase activation.38,39 Evidence for the latter was provided by a recent 5760-gene cDNA microarray analysis of the human multiple myeloma cell line RPMI8226 and its doxorubicin-selected P-gp–expressing subline. The study identified a cluster of 29 genes that participates in an apoptotic response mediated by ceramide and mitochondrial permeability transition.40 Although ceramide may initiate programmed cell death by direct activation of caspases, its proapoptotic activity is inhibited through glycosylation by glucosylceramide synthase (GCS). In multidrug-resistant carcinoma cells, glucosylceramide accumulation enhances P-gp function,41 whereas in HL-60 cells, GCS overexpression suppresses doxorubicin-induced ceramide generation and consequent apoptotic response. Moreover, in leukemic specimens the ceramide content is significantly lower in patients with chemotherapy-resistant disease compared with chemotherapy-sensitive disease, owing to elevations in GCS and sphingomyelin synthase (SMS) activity.42 KG1a cells, which constitutively express P-gp, undergo prolonged cell-cycle arrest and apoptosis when exposed to a P-gp inhibitor in vitro and demonstrate reduced engraftment in animal models.43 Recapitulation of this effect in Fas-activated, P-gp–transfected cells indicates that P-gp exerts a protective effect on AML cell survival by inhibiting caspase activation by the death-inducing signal complex (DISC) that is dependent on ATP hydrolysis.44
MDR1 detection
Numerous methods have been applied to evaluate MDR1 expression in clinical specimens. Despite efforts to establish standardized methodology, development of consensus recommendations has been difficult, owing to differences in assay sensitivity or specificity, the need to distinguish between normal and malignant cells, and controversy as to the minimum MDR1 threshold relevant to treatment outcome.45,46 Molecular techniques such as reverse transcriptase–polymerase chain reaction (RT-PCR) provide sensitive and often quantitative measures of MDR1 gene message, but with compromised specificity owing to nonneoplastic cell contamination. Immunodetection methods such as flow cytometry and immunohistochemistry offer the advantage of P-gp assessment according to phenotype or morphology, and transport capacity.
Functional assays employing fluorophore substrates such as rhodamine123 and Di(OC)2, incorporate P-gp modulators to enhance specificity. The latter approach was initially met with enthusiasm and expectations for greater relevance to therapeutic strategies of P-gp blockade. Indeed, functional assays that assess modulator-induced changes in fluorophore retention and efflux demonstrate strong correlation with flow cytometric assessment of MDR1 protein expression in acute leukemias.45,47 Despite this, functional assays have yielded no greater prognostic discrimination in larger studies, implying that the fluorescent dyes used in these assays lack sufficient specificity for antineoplastic resistance, perhaps as a result of disparity in sites of pharmacophore interaction compared with antineoplastics.45,47 A consensus conference reinforced the notion that P-gp expression in AML and other hematologic malignancies is heterogeneous, admonishing the application of arbitrary thresholds for outcome analysis.46
Rationale for therapeutic targeting
Establishment of MDR1/P-gp as an independent prognostic variable for induction failure in adult AML provided convincing evidence for the importance of this biologic feature. P-gp displays close linkage with well-recognized prognostic variables including advanced age, unfavorable karyotype, treatment-induced or secondary leukemia, and expression of the human progenitor cell antigen, CD34.2-5,48-50 AML specimens that express P-gp display reduced cellular accumulation and relative in vitro resistance to lipophilic or positively charged antineoplastics such as the anthracyclines and calicheamicin that can be overcome by P-gp antagonists.51-53 In the vast majority of reports investigating de novo or secondary adult AML, P-gp/MDR1 is an independent prognostic variable associated with reduced probability for remission with conventional anthracene containing induction regimens or gemtuzumab ozogamicin, and in some reports, inferior leukemia-free and overall survival.2-5,45,52,53 Predictive power for induction outcome increases proportionate to surface density of P-gp expression.50 In 2 sequential reports from the Southwest Oncology Group (SWOG), which included more than 560 patients, P-gp expression was detected in only 17% of adults younger than 35 years, compared with 39% between 35 and 50 years and 71% of patients older than 65 years.4,47 Not surprisingly, the age-related propensity for P-gp expression in AML corresponds to the rising prevalence of the CD34+ surface phenotype with advancing age. Like in normal hematopoietic progenitors, P-gp export function is governed by maturation phenotype and is largely restricted to more primitive blast populations coexpressing CD34.45,54 Whether CD34 directly influences P-gp function remains unknown. Nevertheless, P-gp's functional linkage to CD34 may account at least in part for the variance both in the reported predictive value of P-gp and its relation to French-American-British (FAB) type. Indeed, only acute promyelocytic leukemia (APL), which displays a mature myeloid phenotype and exquisite sensitivity to anthracycline monotherapy, consistently lacks P-gp expression, and thereby serves as a model for clinical strategies intended to reverse drug resistance in non-APL subtypes.5,45,50 Questions remain as to the threshold of P-gp+ expression necessary to impact clinical outcome, with estimates from larger trials ranging from 1% to 5% to as high as 35%.2-4,49,55,56
Given the importance of MDR1's prognostic value for induction outcome, its up-regulation at relapse may reflect selection of pre-existing MDR clones and/or treatment-induced acquired resistance. Through an analysis of allele-specific MDR1 gene polymorphisms in 30 paired specimens, Van den Heuvel-Eibrink et al57 provided convincing evidence that P-gp is not up-regulated at relapse, and in fact, MDR1 gene-related clonal selection does not occur after failure of standard therapy. This finding supports the notion that MDR1 is an independent prognostic variable for the induction resistance, but interacts with other variables such as karyotype and disease type to impact remission duration and survival.4,5 Moreover, leukemia therapy would be expected to select for more than one mechanism of resistance, thereby lowering the prognostic importance of P-gp in the relapsed setting. Alternate transporters such as MRP1, MRP2, and the half-transporter breast cancer– or mitoxantrone-resistance protein (BCRP, MXR, ABCG2) emerge following initial treatment failure.58-63 Given these factors, P-gp would be expected to portend greatest prognostic discrimination in previously untreated populations with high prevalence of constitutive expression, such as elderly patients with secondary AML. Indeed, an analysis of 221 patients with AML enrolled on European Organization for Research and Treatment of Cancer (EORTC) adult trials showed that P-gp's predictive value for induction failure reached 90% in chemotherapy-naive adults, compared with 73% and 44% for patients with late or early relapsed disease, respectively,64 indicating that it is the former population that should be expected to benefit most from P-gp inhibition strategies.
Chemosensitizer pharmacology
Numerous anticancer drugs of diverse structure and mechanism of action are P-gp substrates, including the anthracyclines, vinca alkaloids, taxanes, camptothecins, and epipodophyllotoxins.53,65,66 Most, if not all of the modulators selected for clinical investigation act as competitive inhibitors by binding to P-gp within its substrate channel, and either displacing or sterically interfering with the binding of antineoplastics. Like antineoplastic substrates, pharmacologic modulators of P-gp are structurally diverse, but share the feature of lipophilicity necessary for plasma membrane penetration before passive diffusion into the cytosol. These include calcium channel blockers (verapamil, nifedipine, diltiazem), immunosuppressants (cyclosporines), steroid hormones (progesterone, tamoxifen), antibiotics (ceftriaxone), protein kinase inhibitors (staurosporine, H-87, imatinib mesylate),67 and monoclonal antibodies (MRK16, UIC-2).68 Not surprisingly, these compounds differ in their capacity to modulate P-gp function in vivo based on extent of protein binding, site of P-gp interaction, and binding affinity. The latter is impacted by topologic differences in P-gp arising from DNA polymorphisms, mutations, and prior drug selection pressures.2,3,69 Conversely, the capacity for a given modulator to sensitize resistant cells is equally dependent on the binding affinity of the antineoplastic to be modulated and prior antineoplastic drug exposure.
Preclinical studies
Tsuruo and colleagues reported in 198170 that verapamil (6.6 μM) completely reversed vincristine resistance in a P-gp–expressing lymphocytic leukemia cell line selected for resistance to vinca alkaloids. Doxorubicin sensitivity was only partially restored, providing the first evidence that verapamil interacts with a P-gp binding site preferentially occupied by vinca alkaloids.71 Although evidence for clinical reversal of chemotherapy resistance by verapamil was later demonstrated in selected cases,4 cardiac toxicity limited dose escalation to concentrations necessary for sustained P-gp blockade.72,73
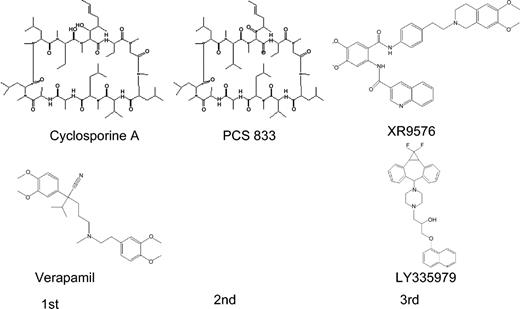
Subsequent studies identified alternate modulator candidates with wide ranging potency (Figure 2). Animal models confirmed these differences in potency with a rank order: cyclosporin-D analog PSC833 (1 μM) greater than cyclosporin-A (CsA; 1-5 μM) greater than dexniguldipine (DNG; 5 μM) greater than verapamil/dexverapamil (10 μM) greater than tamoxifen (10 μM) equals quinidine (10 μM).74,75 Third-generation P-gp antagonists display greater specificity for the MDR1 transporter, higher binding affinity, and reduced pharmacokinetic interactions. XR9576 (tariquidar; Xenova Slough, United Kingdom) is a novel anthranilic acid derivative that potentiates the cytotoxicity of a wide range of antineoplastics at submicromolar concentrations.76 By virtue of its high-affinity binding to P-gp and the ABCG2-encoded BCRP, export blockade is sustained more than 20 hours after XR9576 removal from the medium. A second member of the third-generation class of modulators, LY335979 (zosuquidar; Eli Lilly, IN), displays a similar pharmacologic profile with activity at low micromolar concentrations and specificity restricted to the MDR1 gene product.77,78
The chemical structures of select first-, second-, and third-generation P-gp inhibitors. First-generation drugs are cyclosporin-A and verapamil. Second-generation drugs are analogs of first-generation drugs, of which PSC833 is a cyclosporine analogue. Examples of 2 third-generation drugs are tariquidar (XR9576) and zosuquidar (LY335979), which are currently under evaluation in phase 1/2 clinical trials.
The chemical structures of select first-, second-, and third-generation P-gp inhibitors. First-generation drugs are cyclosporin-A and verapamil. Second-generation drugs are analogs of first-generation drugs, of which PSC833 is a cyclosporine analogue. Examples of 2 third-generation drugs are tariquidar (XR9576) and zosuquidar (LY335979), which are currently under evaluation in phase 1/2 clinical trials.
Concerns that successful in vivo blockade of P-gp may give rise to unacceptable toxicities in organs that natively express the protein gained support from murine gene knock-out models. Mice deficient in both the mdr1a and mdr1b genes (mdr1a/1b–/–), the mrp1 gene (mrp1–/–), or combined mdr1a/1b and mrp1 knock-outs (mdr1a/1b–/–, mrp1–/–) display greater sensitivity to vincristine or etoposide toxicity. Vincristine, which is normally limited by neurotoxicity, gave rise to severe myelosuppression and gastrointestinal toxicity when administered at conventional doses.79 These studies indicate that ABC transporters are dispensable for normal development, but serve a protective function against possible exogenous toxins.
Clinical trials in AML
The clinical development of P-gp antagonists in AML was based on the premise that pharmacologic blockade of P-gp should restore the dose-dependent cytotoxicity of antineoplastics, analogous to the clinical model of APL. Critical aspects influencing P-gp modulation as an exploitable therapeutic strategy are summarized in Table 2. First, MDR1 must represent a dominant biologic mechanism limiting anticancer drug response, or at least, P-gp should have high predictive power for treatment outcome in the population to be studied, such as the chemotherapy-naive patient. Second, the targeted antineoplastic must possess high avidity for P-gp and have overlapping interactions with the modulator pharmacophore. Third, blood concentrations of the modulator sufficient for P-gp export inhibition must be achieved with minimal added toxicity.
Variables impacting effectiveness of competitive P-gp antagonists
P-gp prognostic power in study population |
| Frequency MDR1 phenotype |
| Functional P-gp |
| Effective modulator blood concentration |
| Duration of modulator and anticancer drug (ACD) exposure |
| ACD substrate avidity for P-gp |
| Overlapping pharmacophore interaction (ACD vs modulator) |
| Change in ACD elimination |
| Non—P-gp mechanisms of resistance |
P-gp prognostic power in study population |
| Frequency MDR1 phenotype |
| Functional P-gp |
| Effective modulator blood concentration |
| Duration of modulator and anticancer drug (ACD) exposure |
| ACD substrate avidity for P-gp |
| Overlapping pharmacophore interaction (ACD vs modulator) |
| Change in ACD elimination |
| Non—P-gp mechanisms of resistance |
For many first-generation inhibitors such as tamoxifen and verapamil, the pharmacologic profile was inadequate. Variability in drug absorption, excessive protein binding, unpredictable plasma levels, or unacceptable toxicity limited their clinical development.80 The most promising compounds to emerge from phase 1/2 studies were quinine and CsA.81-83 These agents could be administered at doses sufficient to yield pharmacologically active serum concentrations as confirmed in ex vivo assays,84,85 with acceptable toxicity when combined with antineoplastic(s). Dose-dependent prolongation of myelosuppression and hyperbilirubinemia occurred with both agents, although it was more evident with CsA. As anticipated from animal models, delayed elimination of the targeted antineoplastics(s) occurred almost uniformly and was accompanied by wide interpatient variation. Despite this, administration of conventional doses of the targeted cytotoxic was not compromised, assuring adequate drug exposure in all individuals. Evidence from sequential specimen analyses showing that MDR1-expressing clones were eliminated in responding patients provided proof-of-principle needed to proceed with comparative studies.83
First-generation modulators
Five randomized phase 3 trials were performed in adult patients with AML using either quinine or CsA as MDR1 modulators, however, only 2 of these trials by the Groupe Francais des Myelodysplasies (GFM) and SWOG targeted sufficient numbers of chemotherapy-naive patients with expected high penetrance of P-gp expression (Table 3). The initial quinine trial performed by the Groupe Ouest Est Leucemies Aigues Myeloblastiques (GOELAM) study group involved more than 300 patients of varied disease types and relapse status.86 Overall, 53% of patients randomized to the addition of quinine to mitoxantrone and intermediate dose cytarabine (Ara-C) achieved a complete remission (CR) compared with 45% of controls (P = .19). Although an improved remission rate was not observed, induction resistance was significantly reduced in the experimental arm (28% versus 40%; P = .04) at the expense of an increase number of neutropenic deaths (12% versus 5%; P = .01). Despite the diagnostic heterogeneity and increased toxicity, the investigators noted a nonsignificant improvement in remission rate in P-gp+ cases with the addition of quinine (60% versus 35%; P > .05), whereas no difference was observed in the P-gp– cohort.
Randomized phase 3 trials investigating P-gp modulators in AML
Study group . | Reference no. . | Modulator (dose) . | Diagnosis . | No. of patients . | Chemotherapy . | Outcome; P < .05 . |
|---|---|---|---|---|---|---|
| GOELAMS-MAQ2 | Solary et al 86 | Quinine (30 mg/kg/d) | AL | 315 | ID Ara-C + mitox (induction only) | Lower resistance; increased induction deaths |
| GFM and GOELAMS | Wattell et al87 | Quinine (30 mg/kg/d) | RAEB+t, MDS-AML | 131 | ID Ara-C + mitox (induction only) | Increased CR and OS in P-gp* patients |
| MRC | Liu Yin et al89 | CsA (2.5-5 mg/kg) | AML (RoR) | 235 | ADE vs timed sequential HiDAC-DE (induction only) | Negative |
| SWOG 9126 | List et al84 | CsA (16 mg/kg/d) | RAEB-t, AML (RoR, 2°) | 231 | HiDAC + DNR (induction and consolidation) | Lower resistance; increased RFS and OS |
Study group . | Reference no. . | Modulator (dose) . | Diagnosis . | No. of patients . | Chemotherapy . | Outcome; P < .05 . |
|---|---|---|---|---|---|---|
| GOELAMS-MAQ2 | Solary et al 86 | Quinine (30 mg/kg/d) | AL | 315 | ID Ara-C + mitox (induction only) | Lower resistance; increased induction deaths |
| GFM and GOELAMS | Wattell et al87 | Quinine (30 mg/kg/d) | RAEB+t, MDS-AML | 131 | ID Ara-C + mitox (induction only) | Increased CR and OS in P-gp* patients |
| MRC | Liu Yin et al89 | CsA (2.5-5 mg/kg) | AML (RoR) | 235 | ADE vs timed sequential HiDAC-DE (induction only) | Negative |
| SWOG 9126 | List et al84 | CsA (16 mg/kg/d) | RAEB-t, AML (RoR, 2°) | 231 | HiDAC + DNR (induction and consolidation) | Lower resistance; increased RFS and OS |
GFM denotes Groupe Francais des Myelodysplasies; CsA, cyclosporin-A; AL, acute leukemia; RoR, relapsed or refractory; 2°, secondary AML; 1°, primary AML; ID, intermediate-dose cytarabine; Ara-C, cytarabine; HiDAC, high-dose cytarabine; mitox, mitoxantrone; DNR, daunorubicin; VAD, infusional vincristine, doxorubicin, and dexamethasone; 6TG, 6-thioguanine; RFS, relapse-free survival; OS, overall survival.
More convincing results were reported from a second phase 3 trial performed by GOELAM and GFM in which the same regimen was investigated in chemotherapy-naive patients with high-risk myelodysplastic syndrome (MDS) or related leukemia.87 Among the 131 patients registered, 46% expressed the MDR1 protein. Quinine selectively improved induction response (CR: 52% versus 18%; P = .02) and extended median survival (13 months versus 8 months; P = .01) in P-gp+ patients, whereas no benefit was observed in P-gp– cases. Mucositis was more frequent in the quinine-treated cohort, whereas tinnitus and vertigo necessitated infrequent treatment interruption but without any added risk for induction death. A third, well-controlled comparative study performed by the GOELAM study group in younger (< 60 years) patients with de novo AML demonstrated no benefit with the addition of quinine.88 Nonetheless, fewer than one third of the 425 patients treated in the study had an MDR1 phenotype. When analyzed according to blast rhodamine efflux capacity, the addition of quinine significantly improved the remission rate (48% versus 83%; P = .01), and extended event-free (12 months versus 24 months) and 4-year (32% versus 47%) survival rates, whereas no differences were evident in patients lacking export function. The selective benefit of quinine in P-gp+ patients in these studies implies that the improvement in outcome with the addition of quinine derives solely from a targeted interaction with the MDR1 transporter, rather than a significant change in systemic mitoxantrone exposure.
The encouraging results of pilot studies and the proclivity for blood cell binding made CsA a promising first-generation modulator for clinical investigation. Two randomized controlled trials have been completed in adults with high-risk AML (Table 3). In the Medical Research Council (MRC) trial,89 patients with relapsed or refractory AML were randomized to receive standard versus timed sequential ADE induction with or without CsA. Most patients enrolled in this study received a CsAdosage (5 mg/kg/d) inadequate for P-gp blockade, and not surprisingly, experienced no clinical benefit.
In the SWOG 9126 trial,84 patients received treatment with 16 mg/kg/d CsA, which yielded blood concentrations exceeding 1500 ng/mL, sufficient for P-gp blockade in a surrogate biologic assay. Patients with refractory anemia with excess blasts in transformation (RAEB-t), secondary AML, or relapsed/refractory AML received sequential treatment with high-dose Ara-C and daunorubicin (DNR) during induction and consolidation, with the latter administered as a 72-hour continuous infusion. Among the 226 patients evaluable for induction outcome, treatment with CsA significantly reduced the frequency of resistant disease (47% versus 31%; P = .0077) and the need for second induction (4% versus 20%; P = .024), while marginally improving the rate of CR (45% versus 36%; P = .065). Relapse-free survival (RFS) at 2 years was significantly improved with the addition of CsA (34% versus 9%; P = .035) as was overall survival (22% versus 12%; P = .046). When analyzed according to P-gp phenotype, CsA extended median survival 3-fold in patients with P-gp+ leukemia (12 months versus 4 months) with a corresponding increase in RFS (17 months versus 7 months), whereas no difference was observed in patients lacking P-gp.
Although nausea was more common with CsA, there was no increase in mucositis or induction mortality. Delayed elimination of bilirubin and DNR occurred routinely, with CsA increasing steady-state blood concentrations of DNR and DNR-ol (daunorubicinol) 2- and 4-fold, respectively. The increase in anthracycline systemic exposure in the SWOG trial raised concern that the benefit with CsA derived solely from augmented DNR dose intensity. Importantly, the CR rate increased and the frequency of resistance to induction therapy decreased in proportion to the magnitude of rise in DNR steady-state serum concentration only for CsA-treated patients. No such relationship was apparent in the control group. An analogous treatment effect was also demonstrable for RFS and overall survival (OS). These findings provide convincing evidence that the CsA-associated improved outcome resulted from a targeted pharmacodynamic interaction with P-gp to restore DNR sensitivity. Moreover, as predicted by P-gp prognostic models, the benefit of modulation was greatest in chemotherapy-naive individuals (2-year OS, 26% with CsA versus 5%; RFS, 60% versus 5%) compared with relapsed or refractory patients (OS, 21% versus 14%; RFS, 28% versus 10%).
Second-generation modulators
First-generation P-gp antagonists represented a chemically diverse group with varied pharmacologic profiles developed for alternate indications. The promising results of these initial trials fostered interest in the development of modulators with greater potency, specificity, and P-gp–binding affinity. Among the second-generation modulators to enter clinical development, only PSC833 (valspodar; Novartis) has completed phase 3 trials in high-risk AML. PSC833, a cyclosporin-D analog with 10-fold-greater potency for P-gp blockade than CsA, lacks the intrinsic renal toxicity of the parent compound and is devoid of immunosuppressive properties. Phase 1/2 trials revealed that PSC833, like CsA, delayed the hepatic elimination of bilirubin and natural product-derived antineoplastics.90-93 For this reason, considerable effort was invested during phase 1 development to estimate mean reductions in chemotherapy dosage when coadministered with PSC833 necessary to approximate conventional drug exposure.
Four phase 3 trials were performed either in older patients with AML or patients with relapsed or refractory disease. Of greater importance, however, is that the dose of the antineoplastic(s) to be modulated was empirically reduced in patients receiving PSC833, thereby precluding expectations to exploit dose-dependent cytotoxicity with successful P-gp inhibition (Table 4). The Eastern Cooperative Oncology Group (ECOG) trial was suspended prematurely after an interim analysis revealed no improvement in rate of CR in the experimental arm.94 In the Cancer and Leukemia Group B (CALGB) study, induction mortality was excessive with PSC833 (20% versus 44%; P = .008), necessitating closure of the study with less than a third of the planned accrual.95 Both of the remaining trials failed to reach their intended endpoints of improved RFS or CR rate (Table 4).
Phase 3 trials investigating PSC833 (valspodar) in AML
Study group . | Reference no. . | Diagnosis . | No. of patients . | Chemotherapy . | Outcome, P < .05 . |
|---|---|---|---|---|---|
| ECOG 2995 | Advani et al94 | Relapse or refractory | 127 | MEC (induction and consolidation) | Premature closure (no increase in CR) |
| CALGB 9720 | Baer et al96 | Age > 60 years | 120 | D-E-Ara-C (induction and consolidation) | Premature closure (toxicity) |
| HOVON, MRC (C302) | Sonneveld and List80 | Age > 60 years | 428 | DNR + Ara-C (induction) | Negative (RFS) |
| Novartis C301 | Sonneveld and List80 | Relapse or refractory | 256 | MEC (induction) | Negative (CR) |
| GOELAM | Solary et al88 | de novo AML age < 60 years | 425 | Ara-C + IDA (induction and consolidation); quinine | Increased CR (P-gp+) |
Study group . | Reference no. . | Diagnosis . | No. of patients . | Chemotherapy . | Outcome, P < .05 . |
|---|---|---|---|---|---|
| ECOG 2995 | Advani et al94 | Relapse or refractory | 127 | MEC (induction and consolidation) | Premature closure (no increase in CR) |
| CALGB 9720 | Baer et al96 | Age > 60 years | 120 | D-E-Ara-C (induction and consolidation) | Premature closure (toxicity) |
| HOVON, MRC (C302) | Sonneveld and List80 | Age > 60 years | 428 | DNR + Ara-C (induction) | Negative (RFS) |
| Novartis C301 | Sonneveld and List80 | Relapse or refractory | 256 | MEC (induction) | Negative (CR) |
| GOELAM | Solary et al88 | de novo AML age < 60 years | 425 | Ara-C + IDA (induction and consolidation); quinine | Increased CR (P-gp+) |
MEC indicates mitoxantrone (M), etoposide (E), cytarabine (Ara-C); D, daunorubicin; CR, complete remission rate; RFS, relapse-free survival; and IDA, idarubicin.
These unexpected and disappointing results raised serious concern that the strategy of P-gp modulation may be untenable. After all, resistance to chemotherapy is multifactorial, and therefore interrupting only one mechanism of cell defense may be insufficient.96 Given the encouraging results of first-generation studies, however, closer scrutiny is in order before abandoning a previously promising line of investigation. The failed studies had design characteristics that differ significantly from the first-generation phase 3 studies which reported clinical benefit. In each of the PSC833 trials, the dosage of the targeted antineoplastic(s) was reduced 30% to 50% to accommodate expected elevations in systemic drug exposure in the experimental arm. However, earlier studies showed that a pharmacokinetic interaction did not occur in up to a third of patients,92 indicating that treatment outcome in the PSC833 arm might be undermined by chemotherapy underdosing in a significant proportion of patients. In the SWOG trial,85 DNR was administered by continuous infusion to optimize potentiation of anthracycline activity in the presence of CsA. In vitro studies have shown that modulator sensitization of multidrug resistant cells is both concentration- and time-dependent, and is maximized by prolonged exposure to chemotherapy.97-99 Extending the duration of DNR infusion in and of itself represents one means to overcome drug resistance; however, by attenuating peak serum concentration (Cmax), it also limits toxicity arising from unpredictable changes in anthracycline elimination while preserving the potential to exploit dose response with P-gp blockade. To what extent, if any, schedule disparity contributed to the success of the SWOG trial is unknown. Moreover, the GOELAM and GFM trials produced benefit in P-gp+ patients using rapid infusions of conventional doses of mitoxantrone.87,88 Skepticism remains as to whether etoposide is a high-affinity substrate for P-gp, which may have exacerbated toxicity in 3 of the PSC833 phase 3 trials with little expectation to potentiate cytotoxicity.100,101 Last it is plausible that CsA is a more effective resistance modifier by virtue of its actions on alternate targets of cell defense. CsA weakly augments anthracycline retention and cytotoxicity in multidrug-resistant cells that overexpress the ABC transporters MRP-1 or the half-transporter BCRP (ABCG2).102-104 In addition, CsA suppresses angiogenic response to VEGF, an autocrine growth factor with adverse prognostic relevance in AML, and also lowers apoptotic threshold by an as-yet-undefined mechanism in leukemic cells.105-107
PSC833, like its predecessor CsA, is a potent inhibitor of the cytochrome P450 isozyme 3A4 (CYP3A4), which contributes to its highly variable pharmacokinetic interaction with antineoplastic substrates.108 Given the lessons learned from the failed trials, further clinical testing of new generations of P-gp modulators remains reasonable in appropriate study populations. Phase 3 trials remain in progress in the U.S. cooperative groups. The CALGB continues investigation of PSC833 in the setting of de novo AML, whereas the SWOG is exploring the potential of CsA in older patients with AML using a continuous DNR infusion schedule.
Third-generation modulators
Highly specific third-generation modulators were developed using quantitative structure-activity relationships (QSARs) and combinatorial chemistry techniques. As a consequence, these agents display minimal activity against other members of the ABC transporter family, lack interaction with CYP 3A4, and unlike their predecessors act as noncompetitive P-gp antagonists. As a consequence, when coadministered with chemotherapy, pharmacokinetic interactions are nominal in the trials performed to date.109,110 Third-generation modulators in clinical development include tariquidar (XR9576; Xenova), zosuquidar (LY335979; Eli Lilly, Indianapolis, IN), laniquidar (R101933), and ONT-093.110 The advantage of these nontransported P-gp antagonists derives from their greater affinity for P-gp than transported substrates, and their capacity for sustained inhibition. The preferred site for P-gp interaction is unclear, and it remains to be seen whether these agents bind at the NDB to inhibit ATPase activity.
Fourth-generation MDR1 inhibitors
The next generation of drug resistance modifiers are emerging from efforts to delineate structural interactions with P-gp and transcriptional regulators of MDR1. One class of compounds has already entered the clinical arena, the farnesyl protein transferase inhibitors (FTIs). In a cell line engineered to overexpresses human MDR1, the FTI SCH66336 (lonafarnib; Sarasar; Schering-Plough, Kenilworth, NJ) abrogated DNR and rhodamine123 export with a potency comparable to CsA. SCH66336 impedes ATP utilization by P-gp by interacting directly with the substrate binding site (MSD), a feature shared with another FTI, R115777 (tipifarnib; Zarnestra; Johnson & Johnson, New Brunswick, NJ).112 Ideally, compounds that inhibit ATPase activity of ABC transporters or the utilization of ATP by drug-resistant cells offer the prospect to disrupt multiple mechanisms of cell defense while enhancing selectivity for malignant cells. One such approach conjugates conventional antineoplastics to polyethylene-derived block copolymers and has shown initial success with its ability to circumvent multiple ABC transporters in resistance cells.113
The concept of pharmacologic inhibition of P-gp represents only the initial attempt to develop therapeutic strategies to counter drug resistance. Immunotherapy, for example, using P-gp–derived synthetic peptides in animal models elicits a serologic response that is not only P-gp–specific, but impedes transport function.114 Similarly, MDR1 gene expression is coordinately regulated with the Wilm tumor suppressor WT-1, raising expectation that promotion of WT-1–specific immune response may extend to multidrug-resistant clones.22,115 Novel antineoplastics that target transcriptional regulators of MDR1 and related stress-response genes offer the advantages of intrinsic cytotoxicity coupled with suppression of survival gene cassettes relevant to the resistant phenotype. Indeed, the NF-Y transcription factor regulates not only MDR1, but also the HOXB4 and telomerase genes to influence maturation and replication potential of hematopoietic cells.22,116,117 There are 2 agents, ecteinascidin (ET)–743 and HMN-176, that interact with the DNA minor groove to interfere with binding of the Y-box protein. Moreover, this same transcription factor facilitates adenovirus replication and host cell cytolysis, raising expectation that replication-defective oncolytic adenoviruses may selectively eliminate multidrug-resistant leukemic cells.118
Conclusions
The concept of reversing chemotherapy resistance mediated by MDR1/P-gp to improve treatment outcome in AML remains a reality yet to be proven. The notion that this strategy is now an anachronism, however, is unfounded, and dismisses the therapeutic implications of one of the most powerful prognostic markers ever identified in this disease. Clinical testing of drug resistance modulators like other modern day targeted therapeutics must be rationally developed with a firm basis in the lessons learned in the laboratory and with proper selection of patient populations in which the predictive power and the potential for benefit is greatest. More important, from the insight gained from investigations of core binding factor leukemias, delineation of critical transcriptional control elements in MDR1 regulation is essential to advancing treatment outcome for the majority of adults with AML.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2003-07-2490.
We are grateful to Ben George for his expertise in the design of the figures, including computer-generated images, and Melissa Strey for her excellent assistance with the preparation of the manuscript. We also thank Dr Benjamin Djulbegovic at the H. Lee Moffit Cancer Center for invaluable help in reviewing the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal