Abstract
Differentiating embryonic stem (ES) cells are an increasingly important source of hematopoietic progenitors, useful for both basic research and clinical applications. Besides their characterization in colony assays, protocols exist for the cultivation of lymphoid, myeloid, and erythroid cells. With the possible exception of mast cells, however, long-term expansion of pure hematopoietic progenitors from ES cells has not been possible without immortalization caused by overexpression of exogenous genes. Here, we describe for the first time an efficient yet easy strategy to generate mass cultures of pure, immature erythroid progenitors from mouse ES cells (ES-EPs), using serum-free medium plus recombinant cytokines and hormones. ES-EPs represent long-lived, adult, definitive erythroid progenitors that resemble immature erythroid cells expanding in vivo during stress erythropoiesis. When exposed to terminal differentiation conditions, ES-EPs differentiated into mature, enucleated erythrocytes. Importantly, ES-EPs injected into mice did not exhibit tumorigenic potential but differentiated into normal erythrocytes. Both the virtually unlimited supply of cells and the defined culture conditions render our system a valuable tool for the analysis of factors influencing proliferation and maturation of erythroid progenitors. In addition, the system allows detailed characterization of processes during erythroid proliferation and differentiation using wild-type (wt) and genetically modified ES cells.
Introduction
In humans, about 2 × 1011 new erythrocytes are produced every day.1 In healthy individuals, erythrocyte numbers are kept strictly constant, but under stress conditions like hypoxia or severe blood loss, erythrocyte numbers are rapidly restored by increased production of mature red cells. This requires a flexible pool of erythroid progenitors responsive to external signals as well as tight regulation of erythroid progenitor proliferation, differentiation, and survival. Although many genes have been shown to be involved in erythroid development, little is known about the genes and processes involved in maintaining homeostasis.
Recently established culture conditions allow the expansion of primary erythroid progenitors from mice and humans.2-4 We have shown that the cooperative action of erythropoietin (Epo), Kit ligand (KL), and glucocorticoid hormones induces expansion of proerythrobast-like erythroid progenitors.3,4 However, due to the limited life span of primary cells (15-20 days)3,4 analyses were often hindered by cell number limitation. In addition, early embryonic lethality of mice deficient in the gene of interest frequently makes examination impossible.
In vitro differentiation of mouse embryonic stem cells has been shown to be a powerful tool to overcome cell number and lethality problems.5-7 Protocols have been established to generate different hematopoietic lineages from differentiating embryonic stem (ES) cells either by embryoid body formation or cocultivation on stromal cell lines (eg, OP9 feeder cells).8-21 For many experimental applications, however, it is necessary to direct ES cell differentiation to generate pure, homogeneous cultures that can be further cultivated and analyzed. With the possible exception of mast cells,15 long-term expansion of pure populations of hematopoietic cells from ES cells has not been possible without immortalization caused by overexpression of exogenous genes.20-23
To direct ES cell differentiation into the erythroid lineage, we established a 3-step protocol combining the embryoid body (EB) differentiation technique with erythroid culture conditions. The use of defined culture conditions allowed us to direct differentiation into the erythroid lineage without contamination of other cell types. Mass cultures of pure erythroid progenitors and, upon induction of terminal differentiation, mature red blood cells were efficiently generated following this protocol. In contrast to fetal liver–derived erythroid progenitors, ES cell–derived erythroid progenitors exhibit a remarkably longer life span. Starting from 20 000 ES cells, more than 1011 erythroid progenitors can be produced within 10 weeks. Using this system, the analysis of processes occurring during erythroid expansion and differentiation can be easily performed without limitation in cell numbers or progenitor heterogeneity. To demonstrate the applicability for the analysis of erythroid cells derived from gene-targeted ES cells we generated erythroid progenitors from Flk-1–/– ES cells and indicate for the first time a role of Flk-1 during erythropoiesis.
Materials and methods
ES cell culture
The wild-type ES cell line CCE and Flk-1–/– ES cell line24 (kind gift of E. F. Wagner, IMP, Vienna, Austria) were propagated without feeders in ES cell proliferation medium (Dulbecco modified Eagle medium [DMEM]; 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin [all from Gibco/BRL, Carlsbad, CA], 15% fetal calf serum [FCS; Euroclone, Milano, Italy], 1% leukemia inhibitory factor [LIF]–supernatant, 1.5 × 10–4 M monothioglycerol [MTG; Sigma, St Louis, MO]) on gelatinized plates.5 The basic medium was changed from DMEM to Iscove modified Dulbecco medium (IMDM; Gibco/BRL) 2 days prior to in vitro differentiation.
Embryoid body differentiation
To induce embryoid body formation,7 1000 ES cells/mL were plated into nontreated petri dishes (Greiner Bio-One, Kremsmünster, Austria) containing EB differentiation medium. EB differentiation medium consisted of IMDM, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco/BRL), 15% FCS (Euroclone), 50% methylcellulose, 50 μg/mL ascorbic acid, 300 μg/mL iron-saturated human transferrin (all from Sigma), 5% protein-free hybridoma medium (PFHM-II; Gibco/BRL) and 4 × 10–4 M MTG. At day 5 of differentiation, embryoid bodies were harvested by centrifugation and replated into fresh EB differentiation medium and further cultivated for 1 to 4 days. Before dissociation, EBs were harvested by centrifugation and were incubated in 1 × Trypsin (Gibco/BRL) at 37° C for 2 to 7 minutes. FCS (2 mL) was added and EB dissociated by repeated pipetting. Dead and differentiated cells were removed by centrifugation over a Ficoll cushion (lymphocyte separation medium; 1.078 g/cm3; Eurobio, Paris, France).
Generation of ES cell–derived erythroid cultures (ES-EPs)
EB-derived cells were seeded at a concentration of 2 × 106/mL into serum-free medium (StemPro34 plus nutrient supplement; Gibco/BRL) plus human recombinant erythropoietin (Epo; 2 U/mL; Erypo; Janssen-Cilag AG, Baar, Switzerland), murine recombinant Kit-ligand (KL or stem cell factor [SCF]; 100 ng/mL; R&D Systems, Minneapolis, MN), 10–6 M dexamethasone (Sigma), and 40 ng/mL insulin-like growth factor 1 (IGF-1; Promega, Madison, WI).At day 1 and day 3 of cultivation, suspension cells were harvested from adherent cells, cell aggregates were removed by filtration through a 70-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ) and replated into a new dish.
Cell number and size was determined daily using an electronic cell counter (CASY-1; Schärfe-System, Reutlingen, Germany). Cell density was maintained between 2 and 4 × 106 cells/mL. Cumulative cell number was calculated as described in Chakravarthy et al.25
In vitro differentiation of ES-EPs into erythrocytes
ES-EPs were washed twice with phosphate-buffered saline (PBS) and cultivated at 2 × 106 cells/mL in serum-free medium (StemPro34 plus nutrient supplement) supplemented with 10 U/mL Epo, insulin (10 ng/mL; Actrapid HM; Novo Nordisk), 3 × 10–6 M of the glucocorticoid receptor antagonist ZK112.993,3 and one mg/mL iron-saturated human transferrin. Differentiating cells were analyzed for cell size, hemoglobin content, and morphology as previously described.3 Hemoglobin values obtained from triplicate determinations were averaged and normalized to cell number and cell volume, as described in von Lindern et al.3
Colony assays
ES-EPs derived from 6-day-old embryoid bodies were cultivated for 16 days under erythroid conditions, washed twice in PBS, and resuspended in IMDM. For each sample, 1 × 104 cells were used. Diluted cell suspension and recombinant cytokines specific for each assay (see below in this paragraph) were mixed with either MethoCult M3234 (Stem Cell Technologies, Vancouver, British Columbia, Canada; for erythroid colony-forming unit [CFU-E] and erythroid blast-forming unit [BFU-E] assay) or MethoCult M3434 (Stem Cell Technologies; complete medium for BFU-E, granulocyte erythroid macrophage mixed–colony-forming unit [CFU-GEMM], granulocyte macrophage–colony-forming unit [CFU-GM], granulocyte colony-forming unit [CFU-G], macrophage colonyforming unit [CFU-M] assay) following manufacturer's instructions (duplicates). For the CFU-E assay, cells were cultured in the presence of 0.2 U/mL Epo. Colonies were scored at day 2 and day 3. For BFU-E assay, 3 U/mL Epo, 100 ng/mL KL, and 10 ng/mL murine interleukin 3 (mIL-3; R&D Systems, Tustin, CA) were added to the medium. BFU-E colonies were counted at day 8. Myeloid colonies were assayed between day 8 and day 12.
RNA extraction and reverse transcriptase (RT)–PCR analysis
Total RNA was prepared using Trizol (Gibco/BRL). cDNA synthesis was performed using Superscript II and oligo(dT)12-18 primers (Invitrogen, Carlsbad, CA) following manufacturer's instructions. The same cDNAsample was used for polymerase chain reaction (PCR) amplification with different primer sets, using standard protocols using Taq polymerase (Invitrogen). Cycling parameters were as follows: denaturation at 94° C for 30 seconds; annealing at various temperatures (Table 1) for one minute; elongation at 72° C for one minute. The number of cycles varied between 25 and 35 cycles, depending on the particular mRNA abundance. The primers that were used are listed in Table 1.
Sequence and annealing temperature of RT-PCR primers used
Name . | Forward primer . | Reverse primer . | Temperature, °C . |
|---|---|---|---|
| β-actin | 5′ ATGAAGATCCTGACCGAGCG 3′ | 5′ TACTTGCGCTCAGGAGGAGC 3′ | 58 |
| Rex-1 | 5′ CGTGTAACATACACCATCCG 3′ | 5′ GAAATCCTCTTCCAGAATGG 3′ | 55 |
| Gata-1 | 5′ TAAGGTGGCTGAATCCTCTGCATC 3′ | 5′ ACGTTCTTGGACACCTTGAAGACGG 3′ | 64 |
| SCL | 5′ ATTGCACACACGGGATTCTG 3′ | 5′ GAATTCAGGGTCTTCCTTAG 3′ | 50 |
| EKLF | 5′ ACCACCCTGGGACAGTTTCT 3′ | 5′ GAAGGGTCCTCCGATTTCAG 3′ | 60 |
| Epo-R | 5′ TTCTCCTCGCTATCACCGCATC 3′ | 5′ CCTCAAACTCGCTCTCTGGGCT 3′ | 64 |
| α-globin | 5′ CTCTCTGGGGAAGACAAAAGCAAC 3′ | 5′ GGTGGCTAGCCAAGGTCACCAGCA 3′ | 55 |
| βmajor-globin | 5′ CACAAACCCCAGAAACAGACA 3′ | 5′ CTGACAGATGCTCTCTTGGG 3′ | 55 |
| βH1-globin | 5′ CTCAAGGAGACCTTTGCTCA 3′ | 5′ AGTCCCCATGGAGTCAAAGA 3′ | 55 |
| ϵ-globin | 5′ GGAGAGTCCATTAAGAACCTAGACAA 3′ | 5′ CTGTGAATTCATTGCCGAAGTAC 3′ | 55 |
| ζ-globin | 5′ GCTCAGGCCGAGCCCATTGG 3′ | 5′ TAGCGGTACTTCTCAGTCAG 3′ | 55 |
| CEBPα | 5′ GGTGGACAAGAACAGCAACGAG 3′ | 5′ TAGAGATCCAGCGACCCGAAAC 3′ | 55 |
| CEBPϵ | 5′ AGCCCCCGACACCCTTGATGA 3′ | 5′ GTCCCCTTCTCAAGGCACCCT 3′ | 58 |
| G-CSFR | 5′ CCCCTCAAACCTATCCTGCCTC 3′ | 5′ TCCAGGCAGAGATGAGCGAATG 3′ | 58 |
| GM-CSF | 5′ TGAGGAGGATGTGGCTGCAGAA 3′ | 5′ TGTGCCACATCTCTTGGTCCCT 3′ | 65 |
| MPO | 5′ ATGCAGTGGGGACAGTTTCTG 3′ | 5′ GTCGTTGTAGGATCGGTACTG 3′ | 55 |
| c-fms | 5′ GCGATGTGTGAGCAATGGCAGT 3′ | 5′ AGACCGTTTTGCGTAAGACCTG 3′ | 60 |
| ICSBP | 5′ GTCCCAACTGGACATTTCCG 3′ | 5′ CATTCACGCAGCCAGCAG 3′ | 58 |
| IL-7Rα | 5′ TTACTTCAAAGGCTTCTGGAG 3′ | 5′ CTGGCTTCAACGCCTTTCACCTCA 3′ | 58 |
| Pax5 | 5′ CGGGTCAGCCATGGTTGTG 3′ | 5′ GTGCTGTCTCTCAAACACG 3′ | 52 |
| Gata-3 | 5′ TATGTGCCCGAGTACAGCTC 3′ | 5′ TGTAGTACAGCCCACAGGCA 3′ | 57 |
| Ets-1 | 5′ CTACGGTATCGAGCATGCTCAGTG 3′ | 5′ AAGGTGTCTGTCTGGAGAGGGTCC 3′ | 58 |
| Flt3 | 5′ TCTTGAGACCGTTACAAACC 3′ | 5′ ATGTCTGTTCCGAACAACTC 3′ | 54 |
| CXCR4 | 5′ AAATTTTTGTGTAAGGCTGTC 3′ | 5′ CCCAAAAGGATGAAGGAGTCG 3′ | 52 |
Name . | Forward primer . | Reverse primer . | Temperature, °C . |
|---|---|---|---|
| β-actin | 5′ ATGAAGATCCTGACCGAGCG 3′ | 5′ TACTTGCGCTCAGGAGGAGC 3′ | 58 |
| Rex-1 | 5′ CGTGTAACATACACCATCCG 3′ | 5′ GAAATCCTCTTCCAGAATGG 3′ | 55 |
| Gata-1 | 5′ TAAGGTGGCTGAATCCTCTGCATC 3′ | 5′ ACGTTCTTGGACACCTTGAAGACGG 3′ | 64 |
| SCL | 5′ ATTGCACACACGGGATTCTG 3′ | 5′ GAATTCAGGGTCTTCCTTAG 3′ | 50 |
| EKLF | 5′ ACCACCCTGGGACAGTTTCT 3′ | 5′ GAAGGGTCCTCCGATTTCAG 3′ | 60 |
| Epo-R | 5′ TTCTCCTCGCTATCACCGCATC 3′ | 5′ CCTCAAACTCGCTCTCTGGGCT 3′ | 64 |
| α-globin | 5′ CTCTCTGGGGAAGACAAAAGCAAC 3′ | 5′ GGTGGCTAGCCAAGGTCACCAGCA 3′ | 55 |
| βmajor-globin | 5′ CACAAACCCCAGAAACAGACA 3′ | 5′ CTGACAGATGCTCTCTTGGG 3′ | 55 |
| βH1-globin | 5′ CTCAAGGAGACCTTTGCTCA 3′ | 5′ AGTCCCCATGGAGTCAAAGA 3′ | 55 |
| ϵ-globin | 5′ GGAGAGTCCATTAAGAACCTAGACAA 3′ | 5′ CTGTGAATTCATTGCCGAAGTAC 3′ | 55 |
| ζ-globin | 5′ GCTCAGGCCGAGCCCATTGG 3′ | 5′ TAGCGGTACTTCTCAGTCAG 3′ | 55 |
| CEBPα | 5′ GGTGGACAAGAACAGCAACGAG 3′ | 5′ TAGAGATCCAGCGACCCGAAAC 3′ | 55 |
| CEBPϵ | 5′ AGCCCCCGACACCCTTGATGA 3′ | 5′ GTCCCCTTCTCAAGGCACCCT 3′ | 58 |
| G-CSFR | 5′ CCCCTCAAACCTATCCTGCCTC 3′ | 5′ TCCAGGCAGAGATGAGCGAATG 3′ | 58 |
| GM-CSF | 5′ TGAGGAGGATGTGGCTGCAGAA 3′ | 5′ TGTGCCACATCTCTTGGTCCCT 3′ | 65 |
| MPO | 5′ ATGCAGTGGGGACAGTTTCTG 3′ | 5′ GTCGTTGTAGGATCGGTACTG 3′ | 55 |
| c-fms | 5′ GCGATGTGTGAGCAATGGCAGT 3′ | 5′ AGACCGTTTTGCGTAAGACCTG 3′ | 60 |
| ICSBP | 5′ GTCCCAACTGGACATTTCCG 3′ | 5′ CATTCACGCAGCCAGCAG 3′ | 58 |
| IL-7Rα | 5′ TTACTTCAAAGGCTTCTGGAG 3′ | 5′ CTGGCTTCAACGCCTTTCACCTCA 3′ | 58 |
| Pax5 | 5′ CGGGTCAGCCATGGTTGTG 3′ | 5′ GTGCTGTCTCTCAAACACG 3′ | 52 |
| Gata-3 | 5′ TATGTGCCCGAGTACAGCTC 3′ | 5′ TGTAGTACAGCCCACAGGCA 3′ | 57 |
| Ets-1 | 5′ CTACGGTATCGAGCATGCTCAGTG 3′ | 5′ AAGGTGTCTGTCTGGAGAGGGTCC 3′ | 58 |
| Flt3 | 5′ TCTTGAGACCGTTACAAACC 3′ | 5′ ATGTCTGTTCCGAACAACTC 3′ | 54 |
| CXCR4 | 5′ AAATTTTTGTGTAAGGCTGTC 3′ | 5′ CCCAAAAGGATGAAGGAGTCG 3′ | 52 |
Flow cytometry
All monoclonal antibodies used were purchased in fluorochrome-coupled form from Pharmingen (BD Biosciences, San Diego, CA). Cells (5 × 105 per sample) were washed with PBS, preincubated with PBS/2% FCS for 10 minutes at 4° C, incubated with the appropriate antibodies for 30 minutes at 4° C, washed twice, and analyzed on a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Retroviral transduction of ES-EP
The FMEV-GFP retroviral vector was a kind gift of Hannes Klump (Medical School Hannover [MHH], Germany). Retroviral transduction of ES-EPs was carried out as previously published with minor modifications.26 Briefly, cell-free supernatant of retroviral particles (kind gift of H. Klump) with a multiplicity of infection (MOI) of one particle per cell26 was added to 2 × 106 cells/mL ES-EP (cultivated for 33 days) in a 24-well plate. Polybrene (Sigma) was added to a final concentration of 4 μg/mL and cells were centrifuged for 45 minutes at 150g at room temperature (day one). At day 2 and day 3, respectively, medium was replaced with fresh medium containing retroviral particles and polybrene as described. At day 4, green fluorescent protein–positive (GFP+) cells were sorted using a FACS-Vantage SE (Becton Dickinson Immunocytometry Systems). Transduction efficiency was between 1% and 4% as determined by fluorescence activated cell sorting (FACS) analysis.
In vivo differentiation
GFP-expressing ES-EPs (1.5 × 107) in a volume of 300 μL PBS were injected into the tail vein of sublethally irradiated 129SV mice. Mice were killed between day one and day 14 after injection. Cells were isolated from spleen, bone marrow, and peripheral blood and analyzed for GFP, CD117, Ter119, B220, Mac-1, and Sca-1 expression by flow cytometry. GFP+ cells from spleen and bone marrow were FACS-sorted, centrifuged onto glass slides, and subjected to hematologic staining. For each time point, 3 mice were analyzed.
Hematologic staining
ES-EPs were cytocentrifuged onto glass slides and stained with histologic dyes (Diff-Quick I-II; Dade Behring AG, Marburg, Germany) and neutral benzidine (O-Dianisidine; Sigma) for hemoglobin,3,4 and embedded in Entellan (Merck, Darmstadt, Germany). Images were collected on an Axioplan 2 microscope (Carl Zeiss, Gottingen, Germany) using Plan-Apochromad objective lenses (100 × magnification, 1.4 numerical aperture, or 63 × magnification and 1.4 numerical aperture). Pictures were acquired using a Leica DFC 320 camera (Leica Microsystems Imaging Solutions, Cambridge, United Kingdom). Image Access software (Imagic Bildverarbeitungs, Glattbrugg, Switzerland) was used for image processing.
Preparation of chromosome spreads
ES-EP cells (1 × 107) were incubated with 0.05 μg/mL to 0.2 μg/mL colchicine for 2 hours at 37° C, then 0.6% KCl (both obtained from Sigma) was added for 8 minutes at 37° C. Thereafter, cells were fixed in 75% MeOH/25% acetic acid. Chromosomes were stained with 5% Giemsa (Sigma). More than 270 metaphases of 5 independent long-term cultures were counted.
Sequencing of p53
Sequencing of the p53 gene was carried out as previously described.27
Results
Directing differentiation toward the erythroid lineage
ES cell differentiation is believed to be a stochastic process resulting in the generation of various different cell lineages.6,7 To direct ES cell differentiation, generate a homogeneous mass culture of pure immature erythroid progenitors, and subsequently differentiate the progenitors into mature erythrocytes, we developed the following 3-step protocol. Step I: ES cells were differentiated into EBs. Step II: EBs were dissociated into single cells at the correct stage and expanded in serum-free medium containing erythropoietin (Epo), Kit-ligand (KL), and dexamethasone (Dex). This cytokine-hormone combination has been shown to sustain stress erythropoiesis in vivo28 and to allow expansion of fetal liver erythroid progenitors (FL-EPs) in culture.3,4 Step III: Erythroid progenitors could be differentiated in vitro into mature erythrocytes at various time points of cultivation by addition of high concentrations of Epo, transferrin, insulin, and withdrawal of KL and dexamethasone.
Determination of erythroid/hematopoietic potential in developing embryoid bodies
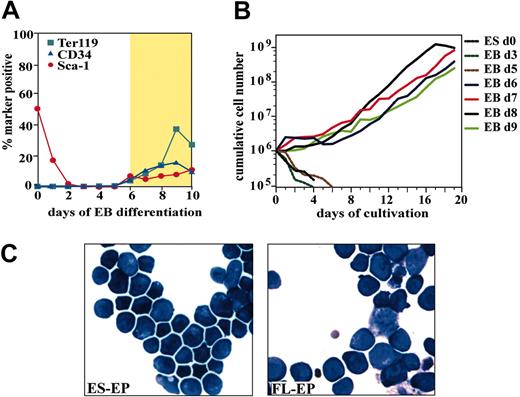
First, it was necessary to determine at which stage of EB differentiation outgrowth of definitive erythroid precursors can occur. It has been shown that 4-day-old EBs can form primitive erythrocytes, whereas definitive erythroid cells develop shortly afterward.7 We detected early hematopoietic (CD34, Sca-1) and erythroid (Ter119) markers on dissociated EB cells from day 6 onwards (Figure 1A).
Development, expansion, and characterization of erythroblasts derived from differentiated ES cells. (A) Flow cytometry analysis of CD34, Sca-1, and Ter119 expression during embryoid body differentiation. The gold section marks EB from which erythroid outgrowth could be observed. (B) To determine which stage of EB differentiation sustains expansion of erythroid progenitors, cells from EBs differentiated for 0 to 9 days were dissociated, cultivated under erythroid conditions, and cumulative cell numbers were determined for 20 days. (C) Morphology of ES-EPs and FL-EPs cultivated for 12 days. Cytospin preparations were stained with benzidine and hematologic dyes. Original magnification, × 63.
Development, expansion, and characterization of erythroblasts derived from differentiated ES cells. (A) Flow cytometry analysis of CD34, Sca-1, and Ter119 expression during embryoid body differentiation. The gold section marks EB from which erythroid outgrowth could be observed. (B) To determine which stage of EB differentiation sustains expansion of erythroid progenitors, cells from EBs differentiated for 0 to 9 days were dissociated, cultivated under erythroid conditions, and cumulative cell numbers were determined for 20 days. (C) Morphology of ES-EPs and FL-EPs cultivated for 12 days. Cytospin preparations were stained with benzidine and hematologic dyes. Original magnification, × 63.
EBs were harvested at the indicated time of differentiation and dissociated into single-cell suspension. To induce erythroid outgrowth, EB-derived single cells were then cultivated under erythroid specific conditions (“Materials and methods”). Sustained expansion of erythroid progenitors (ES-EPs) was obtained from 6- to 9-day-old EBs, while cells from earlier stages did not proliferate (Figure 1B). Outgrowth of ES-EPs was observed after 5 to 6 days of cultivation under erythroid conditions; thereafter, cultures showed an average population doubling time of 26 hours.
Generation of pure and homogeneous erythroid populations
To determine the homogeneity and maturity of the ES cell–derived erythroid population, marker expression of ES-EPs was analyzed by flow cytometry over time and compared with that of fetal liver–derived erythroid progenitors (FL-EPs) (Table 2). After 2 to 3 weeks of cultivation, ES-EP cultures did not contain detectable amounts of cells expressing myeloid and lymphoid markers and exclusively exhibited a proerythroblast morphology highly comparable to that of FL-EPs (Figure 1C). Pure ES-EPs expressed a marker combination characteristic for proerythroblasts (CD71/CD117/Ter119).3 In contrast, due to their short in vitro life span, FL-EPs of similar purity could never be obtained (Table 2). Interestingly, ES-EPs cultured for up to 60 days coexpressed CD117 and high levels of Ter119 (Table 2, MFI) whereas FL-EPs cultured for 7 days and 14 days typically showed a much weaker Ter119 signal and lower numbers of Ter119/CD117 coexpressing cells. ES-EP cultures contained higher numbers of CD34+-expressing cells and lower numbers of Sca1+-expressing cells compared with wild-type FL-EPs (Table 2) and p53–/– FL-EPs.3,4 Thus, CD117hi/Ter119hi/CD34lo ES-EPs represent a distinct, immature erythroid progenitor that closely resembles the progenitors selectively enhanced in spleen during stress erythropoiesis.28
Marker expression of ES-EP cells at different time points of expansion from EBs
. | EB . | . | ES-EP . | . | . | . | FL-EP . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker . | Day 6, % . | Day 7, % . | Day 14, % . | Day 21, % . | Day 40, % . | Day 82, % . | Day 7, % . | Day 14, % . | |||||
| Multipotent | |||||||||||||
| Sca-1 | 8.6 ± 1.9 | 2.0 ± 0.7 | 1.1 ± 0.6 | < 1 | < 1 | < 0.1 | 4.8 ± 2.9 | 2.3 ± 2.4 | |||||
| CD34 | 5.5 ± 2.3 | 25.3 ± 7.7 | 15.9 ± 1.3 | 13.9 ± 0.1 | 8.8 ± 1.6 | < 1 | 11.6 ± 6.1 | 6.5 ± 2.1 | |||||
| CD117 | 61.2 ± 5 | 50.1 ± 10.0 | 45.6 ± 11 | 38.4 ± 2.3 | 64.0 ± 9.1 | 62.0 ± 7.7 | 59.5 ± 6.4 | 57.5 ± 3.5 | |||||
| Erythroid | |||||||||||||
| Ter119 | 2.9 ± 1.4 | 40.0 ± 4.4 | 75.6 ± 7.9 | 94.3 ± 2.4 | 88.5 ± 5.7 | 69.6 ± 14.8 | 42.2 ± 3.0 | 31.5 ± 5.5 | |||||
| [MFI of Ter119] | [13.9 ± 8.3] | [185.4 ± 81.5] | [191.7 ± 64.8] | [425.5 ± 120.9] | [175.0 ± 42.44] | [42.0 ± 1.4] | [28.6 ± 1.5] | [91.5 ± 33.4] | |||||
| Ter119/CD117 | 0.9 ± 0.3 | 35.0 ± 15.2 | 47.0 ± 5.7 | 37.9 ± 3.2 | 65.0 ± 23.5 | 13.9 ± 2.0 | 11.0 ± 2.8 | 13.0 ± 0.7 | |||||
| Myeloid | |||||||||||||
| Mac1 | < 0.1 | 3.7 ± 2.1 | < 1 | < 1 | < 0.1 | < 0.1 | 7.3 ± 4.0 | < 1 | |||||
| Lymphoid | |||||||||||||
| B220 | 1.0 ± 0.4 | < 1 | < 1 | < 0.1 | < 0.1 | < 0.1 | 1.2 ± 0.1 | < 1 | |||||
| CD19 | < 0.1 | < 1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 2.2 ± 0.8 | < 1 | |||||
| CD4 | < 0.1 | < 1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 2.4 ± 1.5 | < 1 | |||||
| Endothelial | |||||||||||||
| Flk-1 | 41.6 ± 8.7 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | ND | 1.6 ± 0.6 | 1.1 ± 1.6 | |||||
| Proliferating cell marker | |||||||||||||
| CD71 | 91.0 | 98 ± 1.2 | 99.0 ± 0.8 | 99.8 ± 0.2 | 99.7 ± 0.3 | ND | 99.0 ± 1.4 | 99.2 ± 0.4 | |||||
. | EB . | . | ES-EP . | . | . | . | FL-EP . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker . | Day 6, % . | Day 7, % . | Day 14, % . | Day 21, % . | Day 40, % . | Day 82, % . | Day 7, % . | Day 14, % . | |||||
| Multipotent | |||||||||||||
| Sca-1 | 8.6 ± 1.9 | 2.0 ± 0.7 | 1.1 ± 0.6 | < 1 | < 1 | < 0.1 | 4.8 ± 2.9 | 2.3 ± 2.4 | |||||
| CD34 | 5.5 ± 2.3 | 25.3 ± 7.7 | 15.9 ± 1.3 | 13.9 ± 0.1 | 8.8 ± 1.6 | < 1 | 11.6 ± 6.1 | 6.5 ± 2.1 | |||||
| CD117 | 61.2 ± 5 | 50.1 ± 10.0 | 45.6 ± 11 | 38.4 ± 2.3 | 64.0 ± 9.1 | 62.0 ± 7.7 | 59.5 ± 6.4 | 57.5 ± 3.5 | |||||
| Erythroid | |||||||||||||
| Ter119 | 2.9 ± 1.4 | 40.0 ± 4.4 | 75.6 ± 7.9 | 94.3 ± 2.4 | 88.5 ± 5.7 | 69.6 ± 14.8 | 42.2 ± 3.0 | 31.5 ± 5.5 | |||||
| [MFI of Ter119] | [13.9 ± 8.3] | [185.4 ± 81.5] | [191.7 ± 64.8] | [425.5 ± 120.9] | [175.0 ± 42.44] | [42.0 ± 1.4] | [28.6 ± 1.5] | [91.5 ± 33.4] | |||||
| Ter119/CD117 | 0.9 ± 0.3 | 35.0 ± 15.2 | 47.0 ± 5.7 | 37.9 ± 3.2 | 65.0 ± 23.5 | 13.9 ± 2.0 | 11.0 ± 2.8 | 13.0 ± 0.7 | |||||
| Myeloid | |||||||||||||
| Mac1 | < 0.1 | 3.7 ± 2.1 | < 1 | < 1 | < 0.1 | < 0.1 | 7.3 ± 4.0 | < 1 | |||||
| Lymphoid | |||||||||||||
| B220 | 1.0 ± 0.4 | < 1 | < 1 | < 0.1 | < 0.1 | < 0.1 | 1.2 ± 0.1 | < 1 | |||||
| CD19 | < 0.1 | < 1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 2.2 ± 0.8 | < 1 | |||||
| CD4 | < 0.1 | < 1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 2.4 ± 1.5 | < 1 | |||||
| Endothelial | |||||||||||||
| Flk-1 | 41.6 ± 8.7 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | ND | 1.6 ± 0.6 | 1.1 ± 1.6 | |||||
| Proliferating cell marker | |||||||||||||
| CD71 | 91.0 | 98 ± 1.2 | 99.0 ± 0.8 | 99.8 ± 0.2 | 99.7 ± 0.3 | ND | 99.0 ± 1.4 | 99.2 ± 0.4 | |||||
Freshly dissociated cells from embryoid bodies (EBs) day 6, erythroid progenitors from mouse ES cells (ES-EPs) cultured between 7 days and 82 days and as a control, fetal liver-derived erythroid progenitors (FL-EPs) after 7 days and 14 days of cultivation were analyzed by flow cytometry for the expression of a panel of hematopoietic and endothelial lineage markers. For Ter119, the mean fluorescent intensity (MFI) is also plotted. Mean values and standard deviation were calculated from at least 3 independent determinations.
ND indicates not determined.
To further demonstrate the purity of ES-EP cultures, semiquantitative RT-PCR was performed to analyze the expression of erythroid-, myeloid-, and lymphoid-specific genes. ES-EPs expressed the erythroid-specific genes SCL, Epo receptor, EKLF, and Gata-1 (for review see Cantor and Orkin29 ) but were negative for marker genes of the other lineages (Figure 2A). Besides RT-PCR and FACS analysis, colony assay experiments were performed to analyze the myelo-erythroid potential of ES-EP cultures. ES-EPs were cultivated for 16 days under erythroid conditions and were subsequently plated for colony assay formation with either Epo alone (CFU-E); Epo, KL, and IL-3 (BFU-E); or Epo, IL-3, IL-6, and KL (BFU-E, CFU-GEMM, CFU-GM, CFU-M, CFU-G). ES-EPs generated almost exclusively CFU-E colonies, very few BFU-E colonies, and no myeloid colonies, confirming the absence of myeloid potential and their proerythroblast nature (Figure 2C).
Analysis of gene expression and colony formation of ES-EPs. (A) Semiquantitative RT-PCR analysis of hematopoietic-specific genes expressed in undifferentiated ES cells (ES), 20-day-old ES-EP cultures from day 7 EBs, and freshly prepared bone marrow (BM) is shown. ES-EPs express erythroid-specific genes but none of the tested genes characteristic of myeloid or B-lymphoid cells. (B) ES-EPs express adult but not fetal globins, as shown by semiquantitative RT-PCR analysis of the globin chains indicated. (C) Colony formation ability of ES-EPs was measured. Mean values of 3 independent experiments plus or minus the standard deviation (SD) are shown.
Analysis of gene expression and colony formation of ES-EPs. (A) Semiquantitative RT-PCR analysis of hematopoietic-specific genes expressed in undifferentiated ES cells (ES), 20-day-old ES-EP cultures from day 7 EBs, and freshly prepared bone marrow (BM) is shown. ES-EPs express erythroid-specific genes but none of the tested genes characteristic of myeloid or B-lymphoid cells. (B) ES-EPs express adult but not fetal globins, as shown by semiquantitative RT-PCR analysis of the globin chains indicated. (C) Colony formation ability of ES-EPs was measured. Mean values of 3 independent experiments plus or minus the standard deviation (SD) are shown.
ES-EPs represent adult, definitive erythroid cells
During vertebrate development the different types of erythroid cells generated can be distinguished by expression of different globin forms: besides the ubiquitously expressed α-globin, embryonic (yolk sac) erythroid cells express high levels of ζ- and ϵ-globin, fetal cells βH1-globin, and adult, definitive erythroid cells express βmajor-globin (for review see Palis and Segel30 and Brotherton et al31 ). To determine the developmental stage of ES-EPs we analyzed globin expression by RT-PCR. α-globin was expressed in all samples analyzed. Cells freshly dissociated from day-6 and day-7 EBs showed expression of all, embryonal (ϵ- and ζ-), fetal (βH1-), and adult (βmajor-) forms of globin. After cultivation for 14 days, ES-EPs exclusively expressed the adult βmajor-globin gene (Figure 2B). Thus, ES-EP cells represent an essentially pure population of definitive erythroid progenitors.
ES-EPs exhibit a longer life span than primary murine erythroblasts
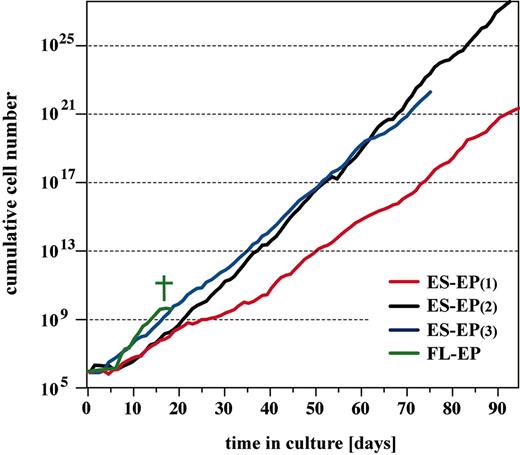
Primary mouse erythroblasts can be expanded for only 15 to 20 days in culture. Thereafter, cells cease growth and either differentiate or undergo apoptosis.2-4 In contrast, ES-EPs proliferated exponentially for more than 70 days (Figure 3). Thus, the ES-EP approach was highly efficient, yielding at least a 1011-fold amplification within 10 weeks of cultivation (103-fold amplification within one week of EB differentiation [data not shown]; 108-to1012-fold amplification in 9 weeks of ES-EP proliferation [Figure 3]). Importantly, the long life span was not a unique feature of the CCE ES cell line but was also obtained from 2 other ES cell lines tested (WT26 and WT41, C57BL/6 × 129SV, first generation [F1]; data not shown).
Long-term expansion of erythroid progenitors. Dissociated cells from day-6 and day-7 EBs and primary fetal liver cells derived from embryonic day 12.5 syngeneic mouse embryos were cultivated in serum-free erythroid medium. Cumulative cell number is plotted. † indicates senescence of FL-EP.
Long-term expansion of erythroid progenitors. Dissociated cells from day-6 and day-7 EBs and primary fetal liver cells derived from embryonic day 12.5 syngeneic mouse embryos were cultivated in serum-free erythroid medium. Cumulative cell number is plotted. † indicates senescence of FL-EP.
Immortalized p53–/– fetal liver erythroblasts3 tend to become aneuploid and factor independent during long-term cultivation (H.B., personal communication, 2002-2003). In contrast, ES-EP cells remained diploid (Table 3) and strictly factor dependent throughout cultivation time. Withdrawal of either Epo, SCF, or dexamethasone resulted in cell death or terminal differentiation within 24 to 48 hours (data not shown). Importantly, no mutations were detectable in the p53 gene in long-lived ES-EPs (determined by sequencing, data not shown). In conclusion, ES-EPs represent long-lived, genetically stable, definitive erythroid progenitors that are clearly distinct from FL-EPs.
Long-term cultivated ES-EPs exhibit a stable karyotype
. | No. of ES-EP cells . | . | |
|---|---|---|---|
| No. of chromosomes/cell . | 62 d . | 96 d . | |
| 38 | 1 | 1 | |
| 39 | 3 | 4 | |
| 40 | 140 | 126 | |
| 41 | 1 | 0 | |
| 42 | 0 | 0 | |
| 78 | 0 | 0 | |
| 79 | 0 | 0 | |
| 80 | 0 | 0 | |
| 81 | 0 | 0 | |
| 82 | 0 | 0 | |
| Total no. of counted cells | 144 | 133 | |
. | No. of ES-EP cells . | . | |
|---|---|---|---|
| No. of chromosomes/cell . | 62 d . | 96 d . | |
| 38 | 1 | 1 | |
| 39 | 3 | 4 | |
| 40 | 140 | 126 | |
| 41 | 1 | 0 | |
| 42 | 0 | 0 | |
| 78 | 0 | 0 | |
| 79 | 0 | 0 | |
| 80 | 0 | 0 | |
| 81 | 0 | 0 | |
| 82 | 0 | 0 | |
| Total no. of counted cells | 144 | 133 | |
Chromosome spreads of ES-EPs cultured for 62 days (n = 3) or 96 days (n = 2) reveal a diploid set of chromosomes in essentially all metaphases analyzed.
ES-EPs differentiate into hemoglobinized, enucleated red blood cells in vitro
The next question to be addressed was whether ES-EPs could be induced to terminally differentiate into mature red blood cells, even after prolonged in vitro cultivation. To induce differentiation, ES-EPs from passage 16 and passage 94 were transferred to differentiation culture conditions containing high concentrations of Epo, transferrin, and insulin. In both cases, cells formed enucleated erythrocytes within 72 hours (Figure 4A-B). They underwent 3 to 4 “differentiation divisions,” reduced their cell size, and accumulated hemoglobin with similar kinetics as primary bone marrow or fetal liver erythroblasts (Figure 4B-D and Dolznig et al4 ). Thus, the extraordinarily long life span of ES-EP cells did not impair their ability to differentiate into mature erythrocytes in vitro.
In vitro differentiation of erythroid cells derived from ES cells, fetal liver, and bone marrow. (A) Morphology of ES-EPs before and after 3 days of differentiation, as analyzed on cytospins by hematologic and benzidine staining. Original magnification, × 63. Differentiation kinetics of ES-EPs expanded for 16 days and 94 days, and erythroblasts derived from bone marrow and fetal liver were analyzed by measuring (B) accumulation of hemoglobin, (C) cell size, and (D) cell proliferation.
In vitro differentiation of erythroid cells derived from ES cells, fetal liver, and bone marrow. (A) Morphology of ES-EPs before and after 3 days of differentiation, as analyzed on cytospins by hematologic and benzidine staining. Original magnification, × 63. Differentiation kinetics of ES-EPs expanded for 16 days and 94 days, and erythroblasts derived from bone marrow and fetal liver were analyzed by measuring (B) accumulation of hemoglobin, (C) cell size, and (D) cell proliferation.
ES-EPs are not tumorigenic
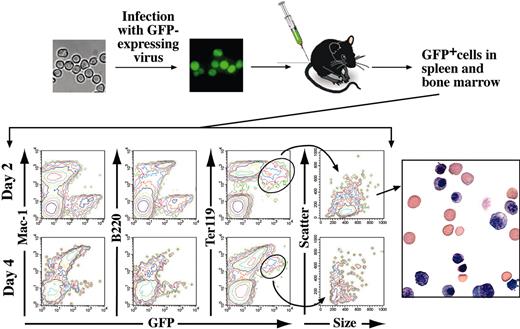
Injection of mouse embryonic stem cells or embryoid body cells subcutaneously leads to the formation of teratocarcinomas.32 Therefore, a very important question was whether ES-EPs possess tumorigenic or leukemogenic potential. ES-EPs cultivated for 31 days under erythroid conditions were transduced with a Friend-mink cell focus-forming (FMEV) retroviral vector expressing GFP.33,34 This vector combines the long terminal repeat of Friend mink cell focus-forming viruses with the 5′ untranslated leader region of the murine embryonic stem cell virus and was reported to confer high expression of GFP in hematopoietic cells.33 Sorted GFP+ ES-EPs behaved like their parental counterparts with respect to proliferation and differentiation in vitro (∼26 hours population doubling time and normal terminal differentiation within 3 days; data not shown). However, GFP fluorescence was abruptly diminished in terminally differentiated cells (data not shown). After 3 days of differentiation, 80% of the cells were highly positive for GFP, whereas after 7 days of differentiation only 15% of the population was positive for GFP and fluorescence intensity of GFP was very low. In addition, after 3 days of differentiation, the mean fluorescence intensity of the cells dramatically drops by at least 30% per day. This temporally coincides with enucleation. The gradual loss of GFP expression in mature erythrocytes might be explained by retroviral silencing of the gene (for review see Lund et al35 ) or by transcriptional or translational inactivation36,37 of GFP. Because the vast majority of the ES-EPs that lost GFP were found in the enucleated erythrocyte fraction (determined by FACS, data not shown) the loss of GFP expression is most likely due to transcriptional or translational inactivation. Therefore, terminally differentiated erythrocytes may fail to express detectable levels of GFP. To test tumorigenicity, GFP+ ES-EPs were either injected intravenously into the tail vein or injected subcutaneously into 10 sublethally irradiated, syngeneic mice each. No leukemia was observed in intravenously injected mice within an observation time of 22 months. GFP-expressing cells could be detected in the bone marrow, spleen, and peripheral blood. Two days after injection, 1% to 2% of all Ter119+ cells were positive for GFP in bone marrow and spleen of 3 mice analyzed. As expected, the number of GFP+ cells in peripheral blood was lower (0.1%-0.2%). The percentage of GFP-expressing cells continuously decreased until day 8, when the number of GFP+ cells fell below detection limit (not shown). GFP+ cells were FACS-sorted from spleen and bone marrow 2 days after injection. Cytospin preparations of the GFP+ cells contained erythroblasts as well as terminally differentiated erythrocytes (Figure 5).
In vivo, ES-EP terminally differentiate and do not induce tumor formation. GFP-expressing ES-EPs (1.5 × 107) were intravenously injected into sublethally irradiated syngeneic mice. FACS analysis for GFP and erythroid (Ter119), lymphoid (B220), and myeloid (Mac-1) markers of cells derived from spleens 2 days and 4 days after injection is shown. Sorting of GFP+ cells from spleen and bone marrow by FACS at day 2 reveals the presence of erythroblasts and enucleated erythrocytes in the GFP-transduced population. Original magnification, × 100.
In vivo, ES-EP terminally differentiate and do not induce tumor formation. GFP-expressing ES-EPs (1.5 × 107) were intravenously injected into sublethally irradiated syngeneic mice. FACS analysis for GFP and erythroid (Ter119), lymphoid (B220), and myeloid (Mac-1) markers of cells derived from spleens 2 days and 4 days after injection is shown. Sorting of GFP+ cells from spleen and bone marrow by FACS at day 2 reveals the presence of erythroblasts and enucleated erythrocytes in the GFP-transduced population. Original magnification, × 100.
Furthermore, unlike ES cells, which formed rapidly growing teratocarcinomas after subcutaneous injection of 7.5 × 106 cells into mice, ES-EPs failed to form tumors upon subcutaneous injection of 2 × 107 cells within 22 months. In summary, ES-EPs do not exhibit any detectable tumorigenic or leukemogenic potential but differentiate into mature erythrocytes in vivo.
Analysis of gene-targeted ES cells
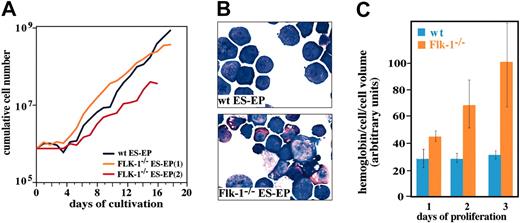
A particular advantage of the ES-EP technique is the possibility of a detailed analysis of genes and mechanisms involved in erythropoiesis bypassing the generation of chimeric or homozygous animals. Very often, alterations of these genes lead to early embryonic lethality, thus prohibiting in vivo analysis. We decided to analyze ES cells lacking the receptor tyrosine kinase Flk-1 (KDR) to demonstrate the applicability of our strategy to genetically modified ES cells. Flk-1–/– mice die around embryonic day 8.5 due to defects in endothelial and hematopoietic development.38 Yet, in vitro, Flk-1–/– EBs were reported to contain definitive erythrocytes.39,40 In our studies, initial outgrowth of wild-type (wt) and Flk-1–/– ES-EP occurred with similar kinetics (Figure 6A), confirming previously published data.39,40 However, Flk-1–deficient ES-EPs showed massive spontaneous terminal differentiation under proliferation conditions. Figure 6B shows that proliferating wt cultures predominantly consisted of immature erythroblasts while Flk-1–/– ES-EPs accumulated terminally differentiated erythrocytes. This spontaneous differentiation is further demonstrated by the strong accumulation of hemoglobin in Flk-1–/– but not in wt cultures during proliferation (Figure 6C).
Increased spontaneous differentiation in Flk-1–/– ES-EP cultures. (A) Outgrowth kinetics of wt and Flk-1–/– ES-EPs derived from day 6 EBs are shown. (B) Hematologic and hemoglobin staining of wt and Flk-1–/– ES-EPs under proliferation conditions (12 days in culture) is shown. Flk-1–/– ES-EP cultures contain more differentiated erythroid cells compared with wt ES-EP cultures. Original magnification, × 100. (C) Under proliferation conditions, Flk-1–/– ES-EP cultures produce more hemoglobin than wt ES-EPs. Error bars indicate standard deviation (mean ± SD).
Increased spontaneous differentiation in Flk-1–/– ES-EP cultures. (A) Outgrowth kinetics of wt and Flk-1–/– ES-EPs derived from day 6 EBs are shown. (B) Hematologic and hemoglobin staining of wt and Flk-1–/– ES-EPs under proliferation conditions (12 days in culture) is shown. Flk-1–/– ES-EP cultures contain more differentiated erythroid cells compared with wt ES-EP cultures. Original magnification, × 100. (C) Under proliferation conditions, Flk-1–/– ES-EP cultures produce more hemoglobin than wt ES-EPs. Error bars indicate standard deviation (mean ± SD).
Discussion
The generation of genetically modified mice from gene-targeted ES cells is a powerful tool to investigate the function of a certain gene during mouse development. However, analysis is often not feasible due to early embryonic lethality of mice deficient in this gene or due to difficulties in obtaining sufficient amounts of material for research. In vitro differentiation of ES cells has become an attractive alternative to rapidly analyze the function of genes during development.5,7 To analyze erythroid development, previous studies used cocultivation systems,9,18 colony assays,5,7 or immortalizing agents20,21,23 to generate erythroid cells from ES cells. While colony assays result in the terminal differentiation of cells, thus preventing the study of erythroid proliferation, cocultivation of differentiating ES cells on stromal cell lines suffer from contamination of other lineages, poor proliferation potential, and also high levels of spontaneous differentiation. On the other hand, immortalized cell lines tend to be genetically unstable, unresponsive to environmental changes, or unable to terminally differentiate.
In this report, we describe for the first time the generation and mass cultivation of pure erythroid progenitor cultures derived from mouse ES cells allowing detailed analyses on the cellular and biochemical levels. To direct ES cell differentiation toward the erythroid lineage and to maintain erythroid cells in an immature state we used a serum-free culture system previously developed for the in vitro expansion of primary, definitive erythroid cells from hematopoietic organs.3,4
Initial experiments showed that embryoid bodies need to differentiate for at least 6 days to obtain outgrowth of definitive erythroid cells. This is consistent with previously published data.6
The outgrowth of erythroid progenitors from embryoid bodies using the described strategy was very efficient. Estimations made from outgrowth kinetics imply erythroid potential in about 25% to 30% of EB-derived cells.
ES cell–derived erythroid progenitors resemble primary, definitive erythroid progenitors in cell morphology, molecular markers, growth, and differentiation kinetics. Even though primitive erythroid potential has been described as early as day 47 and remains detectable until day 7 of EB differentiation, under our conditions only definitive erythroblasts were able to proliferate. Like primary erythroblasts,3,4 ES-EPs possess a proerythroblast morphology and have an average population doubling time of 26 hours. As for primary erythroblasts, sustained proliferation is strictly dependent on the cooperative action of Epo, KL, and Dex.2,41 The dependence on a glucocorticoid for proliferation implies a close similarity of ES-EPs with erythroid progenitors described to play a role during stress erythropoiesis.2,28,42 This suggestion is also supported by the coexpression of Ter119, CD117, and CD34 on ES-EPs, since this marker combination was found to be indicative for stress-induced erythroblasts present in the spleen. Differentiation into mature erythrocytes could be induced by the addition of high amounts of Epo, transferrin, insulin, and the withdrawal of KL and Dex. Upon induction of differentiation, ES-EPs reduced their cell size, enucleated, and accumulated hemoglobin similar to differentiating primary erythroblasts.
Interestingly, ES-EPs exhibit a much longer life span than primary erythroid cells derived from fetal liver. While fetal liver–derived cells can be expanded for about 15 to 20 days, ES-EPs are able to grow for more than 70 days. Importantly, cells remain factor dependent and genetically stable during the long cultivation time and retain their ability to terminally differentiate into mature erythrocytes. The reason for this unusually long in vitro life span is not understood but may be explained by the ES cell origin. However, this extended life span does not seem to be indicative of immortalization or transformation, as the cells did not induce tumor formation. ES-EPs injected into mice terminally differentiated into mature erythrocytes within 3 to 4 days (the same time ES-EPs need for terminal differentiation in vitro). We hypothesize that in vivo, terminal differentiation is the default fate of erythroid progenitors. In contrast to our very-well-defined in vitro conditions that promote growth but not differentiation of the cells, in vivo, ES-EPs are exposed to differentiation factors present in the blood-forming system, inducing terminal differentiation. An example for such a differentiation factor could be transforming growth factor β1 (TGFβ1). TGFβ1 has been shown to inhibit erythroid proliferation and to induce differentiation of erythroid progenitor cells.43-45 The addition of relatively small amounts of TGFβ1 (0.5 ng/mL) to proliferating ES-EPs leads to the inhibition of proliferation and induction of differentiation under proliferation conditions (Gabi Litos and H.B., unpublished results, March 2004). Thus, our system of well-defined medium conditions together with the virtually unlimited supply of immature cells may very well be useful to identify positive as well as negative regulators of terminal erythroid maturation.
Given the rapidly growing number of genes shown to be essential for hematopoiesis, systems permitting a rapid and detailed analysis of the desired lineage become very important. To demonstrate the usefulness of our approach for the analysis of genetically modified ES cells, ES-EPs from Flk-1–/– ES cells were generated. Mouse embryos deficient for the Flk-1 gene die in utero between days 8.5 and 9.5 after coitus due to defects in vascular and hematopoietic development.38 Additionally, Flk-1–/– cells do not contribute to primitive and definitive hematopoiesis in adult chimeras and chimeric fetal livers.46 In previous reports,39,40 Flk-1–/– ES cells were differentiated into embryoid bodies and the erythroid potential of wt and Flk-1–deficient cells was analyzed. Here, no difference in erythroid colony formation was found between wt and knock-out cells.
Using our system, we found that the erythroid outgrowth from Flk-1–/– embryoid bodies was comparable to the outgrowth from wt EBs, confirming previously published data.39,40 However, after a cultivation time of one week, the time when a homogeneous ES-EP population is generated, Flk-1–/– ES-EP cultures show massive spontaneous differentiation resulting in a strong accumulation of mature, hemoglobinized erythrocytes in Flk-1–/– cultures (Figure 6; S.C. et al, manuscript in preparation). These data indicate for the first time a role of Flk-1 during late stages of erythropoiesis. Support for a role of Flk-1 in erythropoiesis comes from recent publications reporting a positive regulatory role of vascular endothelial growth factor A (VEGF-A), the ligand of Flk-1. Upon addition of VEGF-A to differentiating human or murine embryoid bodies, the amount of primitive erythroid colonies was increased several fold47 and the presence of VEGF-A in developing mouse embryos was shown to be essential for the survival of primitive erythrocytes.48 VEGF-A also enhanced the production of definitive erythroid colonies from differentiating monkey ES cells.49 Finally, human definitive erythroblasts were shown to express and secrete VEGF-A during terminal maturation.50 Since the amount of secreted VEGF is known to be elevated in stress situations like hypoxia,51 it is possible that VEGF signaling via Flk-1 is a mechanism to induce proliferation of erythroblasts resulting in a rapid expansion of the erythroid pool. Although it is important to mention that VEGF-A not only binds to Flk-1 but also to Flt-1, a direct link between VEGF and Flk-1 in erythropoiesis has to be established to be able to connect the above-mentioned data with our observations (S.C. et al, manuscript in preparation).
In addition to the use of gene-targeted ES cells, retroviral gene transfer (shown here by overexpression of the reporter gene GFP) also offers the possibility to perform gain-of-function experiments.
Prepublished online as Blood First Edition Paper, May 27, 2004; DOI 10.1182/blood-2004-02-0570.
Supported by grant 808 714 of the Austrian Industrial Research Promotion Fund (FFF).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Uta Möhle-Steinlein for help with ES cell differentiation, Erwin Wagner for providing us with the Flk-1–/– ES cell line, and Andrea Kolbus, Manuela Baccarini, and Meinrad Busslinger for critically reading the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal