Abstract
We analyzed the humoral immune response in 46 patients following structured treatment interruption (STI) to investigate the general potential of therapeutic vaccination in chronic HIV-1 infection. Evoked antibody titer increases to glycoprotein 120 (gp120) and p24 were low during 4 short-term STIs and only reached significance during a fifth long-term interruption. Although induction of binding antibodies to viral antigens was not associated with potent suppression of viremia, we observed that individuals with a rapid and high response to p24, and to a lesser extent also to gp120, lowered their viral set points significantly. Of note, the increase of the anti-p24 response correlated with specific CD4 T helper frequency to this antigen. Despite induction of binding antibody responses, which correlated with improved viral control, the increase in neutralizing activity was marginal and did not lead to this enhanced viral suppression. However, a subgroup of patients who potently suppressed viremia independently of STI had significantly higher pre-existing neutralization titers, suggesting a role of humoral immunity in conferring potent protection. In summary, measuring the kinetics of antibody responses provided a marker to validate the responsiveness and capacities of the immune system of HIV-1-infected individuals and reflected the patients' ability to decrease viral set points. (Blood. 2004;104:1784-1792)
Introduction
Vaccination strategies against HIV-1 developed thus far have failed to induce immune responses that are in breadth and potency comaparable with those elicited during natural infection.1-3 Moreover, it still remains unclear if solely cellular or humoral immune responses need to be evoked by an effective vaccine or if both arms of the immune system are required to procure protection.1-3
In this study we assessed humoral immunity in response to antigenic challenge in chronically HIV-1-infected patients, who have partially restored immune functions after prolonged antiretroviral therapy (ART), to explore the feasibility of therapeutic vaccination. Because immunogens that induce broad antibody responses are not available, we studied humoral immunity stimulated by autologous virus replication in patients undergoing short- and long-term structured treatment interruptions (STIs) during the Swiss Spanish Intermittent Therapy Trial (SSITT). Overall, only a modest improvement of viral set points compared with pretreatment time points was procured by the SSITT despite a general increase in HIV-1-specific CD4 and CD8 T-cell responses.4-7 Potent control of viremia observed in 17% of patients was not induced by STIs but reflected low pretreatment viral set points.4-6 Here we investigated antibody responses during this model vaccination trial to study the mechanisms underlying induction of humoral immune responses to HIV-1 and their relation to virus control in chronic infection.
Patients, materials, and methods
Patients
We studied humoral immune responses in 46 of the 133 chronically infected patients enrolled in the Swiss Spanish Intermittent Therapy Trial (SSITT) (Table 1). During the SSITT, patients underwent 4 consecutive STI cycles (2 weeks off, 8 weeks on treatment) followed by a fifth long treatment interruption (minimum of 12 weeks off treatment if no adverse effects occurred).4,5 Only patients who had at least one detectable rebound above more than 50 copies of RNA per milliliter during the 4 short STIs and who reached a viral load (VL) plateau during the fifth interruption before ART was resumed were included in the antibody analysis. For 22 patients enrolled at the University Hospital Zurich (Table 1, group A) more extensive sampling was performed that allowed a detailed analysis of viremia rebounds,5,7,8 T helper responses, and autologous neutralization activity. Written informed consent was obtained from all patients according to the guidelines of the participating clinical centers.
Patient characteristics
. | . | . | Before ART . | . | . | Baseline of STI trial . | . | . | After STI . | . | Autologous virus . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Months HIV-1 positive . | . | RNA copies per milliliter* . | . | Months of VL less than 50 copies per milliliter . | CD4 cells per cubic millimeter . | RNA copies per milliliter† . | CD4 cells per cubic millimeter . | Coreceptor usage . | . | . | . | . | |||||||
| Code . | Age, y . | Sex . | . | Clinical stage . | . | ART . | . | . | . | . | First cycle . | Fifth cycle . | HIV subtype . | Δlog VL‡ . | Virus control§ . | |||||||
| Group A: Zurich | ||||||||||||||||||||||
| 102 | 40 | M | >24 | C | 561 831 | AZT, 3TC, IDV | 32 | 723 | 76 805 | 491 | R5 | R5 | B | 0.86 | No | |||||||
| 106 | 40 | M | >24 | A | 402 | AZT, 3TC, NFV | 36 | 878 | 5 128 | 650 | ND | R5 | B | −1.11 | High | |||||||
| 107 | 45 | F | >24 | A | 5 216 | AZT, 3TC | 25 | 544 | 2 551 | 537 | ND | R5 | B | 0.31 | High | |||||||
| 109 | 37 | M | >24 | B | 34 752 | AZT, 3TC, RTV | 31 | 1 115 | 148 191 | 806 | R5 | R5 | B | −0.63 | No | |||||||
| 111 | 37 | M | >24 | A | 122 729 | ddI, d4T, NFV | 11 | 422 | 22 030 | 362 | R5 | R5 | B | 0.75 | No | |||||||
| 112 | 45 | M | 6-12 | A | 32 140 | AZT, 3TC, IDV | 25 | 347 | 6 866 | 431 | ND | R5 | B | 0.67 | High | |||||||
| 113 | 59 | M | 12-24 | A | 107 303 | AZT, 3TC, RTV | 20 | 995 | 34 110 | 792 | ND | R5 | B | 0.50 | No | |||||||
| 114 | 33 | M | >24 | A | 9 275 | AZT, 3TC, RTV | 29 | 907 | 28 478 | 784 | ND | R5 | B | −0.49 | No | |||||||
| 115 | 25 | M | 12-24 | A | 37 751 | d4T, 3TC, NFV | 21 | 570 | 9 368 | 558 | ND | R5 | B | 0.61 | No | |||||||
| 116 | 52 | M | >24 | C | 467 593 | AZT, 3TC, IDV | 32 | 350 | 31 500 | 202 | R5X4 | R5X4 | B | 1.17 | No | |||||||
| 117 | 34 | F | 12-24 | A | 29 344 | AZT, 3TC, RTV | 36 | 489 | 13 007 | 341 | ND | R5 | B | 0.35 | High | |||||||
| 118 | 32 | M | 12-24 | A | 16 927 | AZT, 3TC, IDV | 30 | 832 | 3 099 | 785 | R5 | R5 | B | 0.74 | High | |||||||
| 119 | 36 | M | 12-24 | A | 113 052 | d4T, 3TC, SQV, RTV | 12 | 440 | 99 274 | 394 | ND | R5 | B | 0.06 | No | |||||||
| 120 | 54 | M | 12-24 | A | 150 390 | AZT, 3TC, IDV | 28 | 766 | 38 252 | 511 | R5 | R5 | B | 0.59 | No | |||||||
| 121 | 37 | M | 3-6 | A | 164 772 | d4T, 3TC, NFV | 12 | 591 | 67 321 | 380 | R5 | R5 | B | 0.39 | No | |||||||
| 122 | 41 | M | >24 | A | 13 317 | AZT, 3TC, IDV | 30 | 669 | 24 982 | 481 | ND | R5 | B | −0.27 | No | |||||||
| 123 | 40 | F | >24 | A | 14 410 | AZT, 3TC, RTV | 25 | 1 335 | 118 | 529 | ND | R5 | B | 2.09 | High | |||||||
| 125 | 34 | F | >24 | A | 11 298 | ddI, d4T, NFV | 23 | 777 | 4 873 | 882 | R5 | R5 | E/CRF1 | 0.37 | High | |||||||
| 126 | 50 | M | >24 | A | 63 698 | AZT, 3TC, RTV | 34 | 842 | 19 782 | 441 | ND | R5 | B | 0.51 | No | |||||||
| 127 | 51 | F | >24 | A | 25 417 | d4T, 3TC, NFV | 22 | 839 | 106 923 | 614 | R5 | R5 | B | −0.62 | No | |||||||
| 128 | 42 | F | >24 | A | 9 404 | AZT, ddI, NFV | 25 | 749 | 20 236 | 949 | R5 | R5 | B | −0.33 | No | |||||||
| 130 | 67 | M | 6-12 | A | 537 | AZT, 3TC, NFV | 30 | 829 | 107 | 729 | ND | R5 | A | 0.70 | High | |||||||
| Group B: other centers | ||||||||||||||||||||||
| 43 | 51 | M | >24 | A | 34 493 | d4T, ddI | 41 | 666 | 8 490 | 466 | ND | ND | ND | 0.61 | No | |||||||
| 79 | 44 | M | >24 | A | 10 599 | AZT, 3TC, IDV | 33 | 697 | 5 847 | 465 | ND | ND | ND | 0.26 | High | |||||||
| 105 | 36 | F | >24 | A | 47 837 | d4T, ddI, NFV | 24 | 703 | 5 013 | 668 | ND | ND | ND | 0.98 | High | |||||||
| 140 | 56 | M | >24 | A | 20 964 | AZT, 3TC, RTV, SQV | 19 | 480 | 2 375 | 372 | ND | ND | ND | 0.95 | High | |||||||
| 164 | 33 | F | >24 | A | 13 322 | AZT, 3TC, NFV | 19 | 717 | 6 094 | 493 | ND | ND | ND | 0.34 | No | |||||||
| 208 | 45 | M | >24 | A | 13 322 | AZT, 3TC, NFV | 11 | 571 | 11 472 | 386 | ND | ND | ND | 0.06 | High | |||||||
| 260 | 32 | F | >24 | A | 16 000 | d4T, 3TC, IDV | 17 | 1 070 | 12 188 | 1 330 | ND | ND | ND | 0.12 | No | |||||||
| 275 | 35 | M | >24 | A | 33 709 | d4T, 3TC, NFV | 22 | 599 | 13 340 | 466 | ND | ND | ND | 0.40 | No | |||||||
| 288 | 40 | M | >24 | A | 14 027 | d4T, 3TC, NFV | 16 | 490 | 22 651 | 515 | ND | ND | ND | −0.21 | No | |||||||
| 351 | 39 | M | >24 | A | 510 | AZT, 3TC, RTV | 33 | 1 580 | 8 601 | 1 160 | ND | ND | ND | −1.23 | No | |||||||
| 388 | 48 | M | >24 | A | 36 976 | d4T, 3TC, NFV | 17 | 582 | 28 424 | 583 | ND | ND | ND | 0.11 | No | |||||||
| 401 | 68 | F | 12-24 | A | 54 210 | d4T, 3TC, NFV | 10 | 1 322 | 35 651 | 759 | ND | ND | ND | 0.18 | No | |||||||
| 410 | 33 | F | >24 | A | 513 | AZT, 3TC, RTV | 38 | 852 | 1 753 | 542 | ND | ND | ND | −0.53 | High | |||||||
| 455 | 36 | M | >24 | A | 52 849 | d4T, 3TC, NFV | 22 | 616 | 70 850 | 554 | ND | ND | ND | −0.13 | No | |||||||
| 580 | 55 | M | >24 | A | 21 965 | d4T, ddI | 32 | 1 143 | 14 458 | 971 | ND | ND | ND | 0.18 | No | |||||||
| 607 | 36 | M | 12-24 | A | 39 021 | d4T, 3TC, NFV | 21 | 565 | 19 713 | 437 | ND | ND | ND | 0.30 | No | |||||||
| 628 | 44 | F | >24 | A | 12 394 | d4T, 3TC, NFV | 26 | 605 | 6 862 | 489 | ND | ND | ND | 0.26 | High | |||||||
| 716 | 34 | M | 6-12 | A | 14 031 | AZT, 3TC, NFV | 9 | 722 | 12 888 | 462 | ND | ND | ND | 0.04 | No | |||||||
| 723 | 22 | F | 3-6 | A | 12 005 | d4T, 3TC, IDV | 16 | 1 056 | 3 105 | 964 | ND | ND | ND | 0.59 | High | |||||||
| 826 | 55 | M | >24 | A | 21 220 | ABC, AZT, 3TC | 31 | 952 | 10 487 | 717 | ND | ND | ND | 0.31 | High | |||||||
| 837 | 47 | M | >24 | A | 23 146 | AZT, 3TC, NFV | 32 | 1 025 | 7 828 | 878 | ND | ND | ND | 0.47 | No | |||||||
| 904 | 31 | M | >24 | A | 32 555 | AZT, 3TC, NFV | 19 | 804 | 4 345 | 1 158 | ND | ND | ND | 0.87 | High | |||||||
| 933 | 42 | M | 6-12 | B | 46 073 | d4T, RTV, SQV | 31 | 711 | 15 481 | 618 | ND | ND | ND | 0.47 | No | |||||||
| 957 | 37 | F | 12-24 | A | 4 919 | d4T, 3TC, NFV | 25 | 532 | 17 | 536 | ND | ND | ND | 2.46 | High | |||||||
. | . | . | Before ART . | . | . | Baseline of STI trial . | . | . | After STI . | . | Autologous virus . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Months HIV-1 positive . | . | RNA copies per milliliter* . | . | Months of VL less than 50 copies per milliliter . | CD4 cells per cubic millimeter . | RNA copies per milliliter† . | CD4 cells per cubic millimeter . | Coreceptor usage . | . | . | . | . | |||||||
| Code . | Age, y . | Sex . | . | Clinical stage . | . | ART . | . | . | . | . | First cycle . | Fifth cycle . | HIV subtype . | Δlog VL‡ . | Virus control§ . | |||||||
| Group A: Zurich | ||||||||||||||||||||||
| 102 | 40 | M | >24 | C | 561 831 | AZT, 3TC, IDV | 32 | 723 | 76 805 | 491 | R5 | R5 | B | 0.86 | No | |||||||
| 106 | 40 | M | >24 | A | 402 | AZT, 3TC, NFV | 36 | 878 | 5 128 | 650 | ND | R5 | B | −1.11 | High | |||||||
| 107 | 45 | F | >24 | A | 5 216 | AZT, 3TC | 25 | 544 | 2 551 | 537 | ND | R5 | B | 0.31 | High | |||||||
| 109 | 37 | M | >24 | B | 34 752 | AZT, 3TC, RTV | 31 | 1 115 | 148 191 | 806 | R5 | R5 | B | −0.63 | No | |||||||
| 111 | 37 | M | >24 | A | 122 729 | ddI, d4T, NFV | 11 | 422 | 22 030 | 362 | R5 | R5 | B | 0.75 | No | |||||||
| 112 | 45 | M | 6-12 | A | 32 140 | AZT, 3TC, IDV | 25 | 347 | 6 866 | 431 | ND | R5 | B | 0.67 | High | |||||||
| 113 | 59 | M | 12-24 | A | 107 303 | AZT, 3TC, RTV | 20 | 995 | 34 110 | 792 | ND | R5 | B | 0.50 | No | |||||||
| 114 | 33 | M | >24 | A | 9 275 | AZT, 3TC, RTV | 29 | 907 | 28 478 | 784 | ND | R5 | B | −0.49 | No | |||||||
| 115 | 25 | M | 12-24 | A | 37 751 | d4T, 3TC, NFV | 21 | 570 | 9 368 | 558 | ND | R5 | B | 0.61 | No | |||||||
| 116 | 52 | M | >24 | C | 467 593 | AZT, 3TC, IDV | 32 | 350 | 31 500 | 202 | R5X4 | R5X4 | B | 1.17 | No | |||||||
| 117 | 34 | F | 12-24 | A | 29 344 | AZT, 3TC, RTV | 36 | 489 | 13 007 | 341 | ND | R5 | B | 0.35 | High | |||||||
| 118 | 32 | M | 12-24 | A | 16 927 | AZT, 3TC, IDV | 30 | 832 | 3 099 | 785 | R5 | R5 | B | 0.74 | High | |||||||
| 119 | 36 | M | 12-24 | A | 113 052 | d4T, 3TC, SQV, RTV | 12 | 440 | 99 274 | 394 | ND | R5 | B | 0.06 | No | |||||||
| 120 | 54 | M | 12-24 | A | 150 390 | AZT, 3TC, IDV | 28 | 766 | 38 252 | 511 | R5 | R5 | B | 0.59 | No | |||||||
| 121 | 37 | M | 3-6 | A | 164 772 | d4T, 3TC, NFV | 12 | 591 | 67 321 | 380 | R5 | R5 | B | 0.39 | No | |||||||
| 122 | 41 | M | >24 | A | 13 317 | AZT, 3TC, IDV | 30 | 669 | 24 982 | 481 | ND | R5 | B | −0.27 | No | |||||||
| 123 | 40 | F | >24 | A | 14 410 | AZT, 3TC, RTV | 25 | 1 335 | 118 | 529 | ND | R5 | B | 2.09 | High | |||||||
| 125 | 34 | F | >24 | A | 11 298 | ddI, d4T, NFV | 23 | 777 | 4 873 | 882 | R5 | R5 | E/CRF1 | 0.37 | High | |||||||
| 126 | 50 | M | >24 | A | 63 698 | AZT, 3TC, RTV | 34 | 842 | 19 782 | 441 | ND | R5 | B | 0.51 | No | |||||||
| 127 | 51 | F | >24 | A | 25 417 | d4T, 3TC, NFV | 22 | 839 | 106 923 | 614 | R5 | R5 | B | −0.62 | No | |||||||
| 128 | 42 | F | >24 | A | 9 404 | AZT, ddI, NFV | 25 | 749 | 20 236 | 949 | R5 | R5 | B | −0.33 | No | |||||||
| 130 | 67 | M | 6-12 | A | 537 | AZT, 3TC, NFV | 30 | 829 | 107 | 729 | ND | R5 | A | 0.70 | High | |||||||
| Group B: other centers | ||||||||||||||||||||||
| 43 | 51 | M | >24 | A | 34 493 | d4T, ddI | 41 | 666 | 8 490 | 466 | ND | ND | ND | 0.61 | No | |||||||
| 79 | 44 | M | >24 | A | 10 599 | AZT, 3TC, IDV | 33 | 697 | 5 847 | 465 | ND | ND | ND | 0.26 | High | |||||||
| 105 | 36 | F | >24 | A | 47 837 | d4T, ddI, NFV | 24 | 703 | 5 013 | 668 | ND | ND | ND | 0.98 | High | |||||||
| 140 | 56 | M | >24 | A | 20 964 | AZT, 3TC, RTV, SQV | 19 | 480 | 2 375 | 372 | ND | ND | ND | 0.95 | High | |||||||
| 164 | 33 | F | >24 | A | 13 322 | AZT, 3TC, NFV | 19 | 717 | 6 094 | 493 | ND | ND | ND | 0.34 | No | |||||||
| 208 | 45 | M | >24 | A | 13 322 | AZT, 3TC, NFV | 11 | 571 | 11 472 | 386 | ND | ND | ND | 0.06 | High | |||||||
| 260 | 32 | F | >24 | A | 16 000 | d4T, 3TC, IDV | 17 | 1 070 | 12 188 | 1 330 | ND | ND | ND | 0.12 | No | |||||||
| 275 | 35 | M | >24 | A | 33 709 | d4T, 3TC, NFV | 22 | 599 | 13 340 | 466 | ND | ND | ND | 0.40 | No | |||||||
| 288 | 40 | M | >24 | A | 14 027 | d4T, 3TC, NFV | 16 | 490 | 22 651 | 515 | ND | ND | ND | −0.21 | No | |||||||
| 351 | 39 | M | >24 | A | 510 | AZT, 3TC, RTV | 33 | 1 580 | 8 601 | 1 160 | ND | ND | ND | −1.23 | No | |||||||
| 388 | 48 | M | >24 | A | 36 976 | d4T, 3TC, NFV | 17 | 582 | 28 424 | 583 | ND | ND | ND | 0.11 | No | |||||||
| 401 | 68 | F | 12-24 | A | 54 210 | d4T, 3TC, NFV | 10 | 1 322 | 35 651 | 759 | ND | ND | ND | 0.18 | No | |||||||
| 410 | 33 | F | >24 | A | 513 | AZT, 3TC, RTV | 38 | 852 | 1 753 | 542 | ND | ND | ND | −0.53 | High | |||||||
| 455 | 36 | M | >24 | A | 52 849 | d4T, 3TC, NFV | 22 | 616 | 70 850 | 554 | ND | ND | ND | −0.13 | No | |||||||
| 580 | 55 | M | >24 | A | 21 965 | d4T, ddI | 32 | 1 143 | 14 458 | 971 | ND | ND | ND | 0.18 | No | |||||||
| 607 | 36 | M | 12-24 | A | 39 021 | d4T, 3TC, NFV | 21 | 565 | 19 713 | 437 | ND | ND | ND | 0.30 | No | |||||||
| 628 | 44 | F | >24 | A | 12 394 | d4T, 3TC, NFV | 26 | 605 | 6 862 | 489 | ND | ND | ND | 0.26 | High | |||||||
| 716 | 34 | M | 6-12 | A | 14 031 | AZT, 3TC, NFV | 9 | 722 | 12 888 | 462 | ND | ND | ND | 0.04 | No | |||||||
| 723 | 22 | F | 3-6 | A | 12 005 | d4T, 3TC, IDV | 16 | 1 056 | 3 105 | 964 | ND | ND | ND | 0.59 | High | |||||||
| 826 | 55 | M | >24 | A | 21 220 | ABC, AZT, 3TC | 31 | 952 | 10 487 | 717 | ND | ND | ND | 0.31 | High | |||||||
| 837 | 47 | M | >24 | A | 23 146 | AZT, 3TC, NFV | 32 | 1 025 | 7 828 | 878 | ND | ND | ND | 0.47 | No | |||||||
| 904 | 31 | M | >24 | A | 32 555 | AZT, 3TC, NFV | 19 | 804 | 4 345 | 1 158 | ND | ND | ND | 0.87 | High | |||||||
| 933 | 42 | M | 6-12 | B | 46 073 | d4T, RTV, SQV | 31 | 711 | 15 481 | 618 | ND | ND | ND | 0.47 | No | |||||||
| 957 | 37 | F | 12-24 | A | 4 919 | d4T, 3TC, NFV | 25 | 532 | 17 | 536 | ND | ND | ND | 2.46 | High | |||||||
AZT indicates zidovudine; 3TC, lamivudine; IDV, indinavir; NFV, nelfinavir; ND, not done; RTV, ritonavir; ddI, didanosine; d4T, stavudine; and SQV, saquinavir.
Geometric mean if more than 1 value before initiation of antiretroviral therapy was available.
Post-STI viral load plateau: geometric mean of HIV RNA values between weeks 46 and 64. Four patients (patients 102, 109, 116, and 455) had plateau VLs calculated from the 2 or 3 time points just prior to restarting therapy. In 2 patients (patients 107 and 130), the week-46 data point was part of a peak and was therefore omitted from the estimation of the plateau. For the 42 patients who remained off therapy for extended periods, an average of 7.33 data points were used to calculate the plateau VL (range, 3-12 data points).
Improvement of viral set point: log difference between pre-ART VL and post-STI VL.
Control of viremia was defined as VL less than 5000 RNA copies per milliliter for at least 8 weeks between weeks 40 and 76.
Plasma antibody titers to p24 and gp120 antigen
Plasma immunoglobulin G (IgG) titers to recombinant glycoprotein 120 (gp120) from the JR-FL strain (provided by W. Olson, Progenics Pharmaceuticals, Tarrytown, NY) and recombinant p24 (Aalto BioReagents, Dublin, Ireland) were determined by enzyme-linked immunosorbent assay (ELISA) as described.9 Bound antibody was detected using alkaline phosphatase-conjugated anti-human IgG (Sigma, St Louis, MO) and the luminescence-generating CPD-Star system (Applied Biosystems, Rotkreuz, Switzerland). Midpoint titers were defined by linear regression analysis as the antibody dilutions giving half-maximal binding after background subtraction. Maximal binding was defined using the antibodies 2G12 and 37G12 (gifts from H. Katinger, Polymun, Vienna, Austria) as reference for anti-gp120 and anti-p24 antibody detection, respectively.
Plasma neutralization activity
Neutralization activity was evaluated as described with minor modifications.10,11 Buffy coats obtained from 3 healthy blood donors were depleted of CD8+ T cells using Rosette Sep cocktail (StemCell Technologies, Vancouver, BC, Canada), CD8- peripheral blood mononuclear cells (PBMCs) isolated by Ficoll-Hypaque centrifugation, and cells stimulated with phytohemagglutinin (PHA) and OKT3 as described.10 Autologous virus was isolated from patient PBMCs during the first (week 2 of the trial) and the beginning of the fifth cycle (weeks 42 to 50) as described.12
Virus inoculum was incubated with serial dilutions of heat-inactivated patient or control plasma for 1 hour at 37°C. Then, stimulated CD8- PBMCs were infected with aliquots of this pre-incubation mixture. After 72 hours, cultures were washed 3 times and then supplemented with medium and fresh stimulated PBMCs. Cultures were incubated for 6 to 10 days and assayed for p24 antigen.
Production of p24 antigen in absence of plasma was designated as 100%. Neutralization titers refer to the concentrations of the plasma in cultures on day 0. The reciprocal plasma dilutions causing 70% and 90% reduction in p24 production were determined by linear regression analysis. If the appropriate degree of inhibition was not achieved at the lowest serum dilution (1:40), a value of less than 1:40 (< 1:40) was recorded. Only plasma sampled during periods with no ART was used for neutralization activity analysis.
HIV-specific CD4+ T helper responses
Frequency of CD4+ T helper cells reactive with HIV-1 p24 peptides was measured by interferon-γ (IFN-γ) ELISPOT (enzyme-linked immunospot assay) as described.6
Data analysis
Statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Because in several cases multiple testing was performed, we analyzed significance on the individual and also after significance level adjustments using the Bonferroni adjustment. In Figures 1B and 6A, multiple comparisons were done using 1-way analysis of variance (ANOVA) (Kruskal-Wallis test with the Dunn multiple comparison post testing).
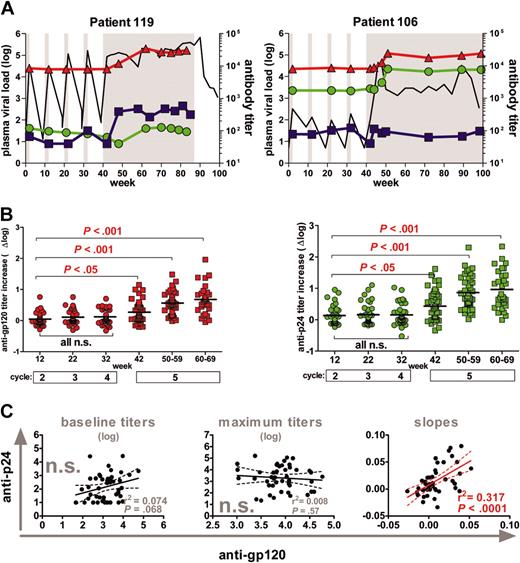
Induction of antibodies against gp120 and p24. (A) Representative profiles of one patient with high (119) and one patient with low (106) rebound. Longitudinal VL measurements and antibody responses are depicted. Gray shaded areas indicate drug-free treatment periods. Viral load (log plasma VL) depicts the log HIV-plasma RNA copies per milliliter and is indicated in black. Anti-gp120 (red) and anti-p24 (green) titers are shown as reciprocal serum dilution that yielded 50% saturation. Autologous neutralization titers against the patient isolates derived at the beginning of the fifth-cycle reciprocal serum dilution that yield 70% inhibition (blue) are shown. (B) Longitudinal anti-gp120 (red) and anti-p24 (green) titer increases (Δ log) over baseline titers (weeks 0 to 2; cycle 1) were compared using 1-way ANOVA (Kruskal-Wallis test with the Dunn multiple comparison post testing) (n = 46 patients); short STIs (circles); long-term STIs (squares). Solid bars indicate mean values. (C) Correlation analysis of anti-gp120 versus anti-p24 responses. (Left) Analysis of baseline titers; (middle) maximum titers; (right) correlation of slopes of antibody titers measured during cycles 1 to 4. All significant observations were significant on the individual level and also after Bonferroni correction. n.s. indicates not significant. The solid line indicates the regression line, and the dotted lines indicate 95% confidence intervals.
Induction of antibodies against gp120 and p24. (A) Representative profiles of one patient with high (119) and one patient with low (106) rebound. Longitudinal VL measurements and antibody responses are depicted. Gray shaded areas indicate drug-free treatment periods. Viral load (log plasma VL) depicts the log HIV-plasma RNA copies per milliliter and is indicated in black. Anti-gp120 (red) and anti-p24 (green) titers are shown as reciprocal serum dilution that yielded 50% saturation. Autologous neutralization titers against the patient isolates derived at the beginning of the fifth-cycle reciprocal serum dilution that yield 70% inhibition (blue) are shown. (B) Longitudinal anti-gp120 (red) and anti-p24 (green) titer increases (Δ log) over baseline titers (weeks 0 to 2; cycle 1) were compared using 1-way ANOVA (Kruskal-Wallis test with the Dunn multiple comparison post testing) (n = 46 patients); short STIs (circles); long-term STIs (squares). Solid bars indicate mean values. (C) Correlation analysis of anti-gp120 versus anti-p24 responses. (Left) Analysis of baseline titers; (middle) maximum titers; (right) correlation of slopes of antibody titers measured during cycles 1 to 4. All significant observations were significant on the individual level and also after Bonferroni correction. n.s. indicates not significant. The solid line indicates the regression line, and the dotted lines indicate 95% confidence intervals.
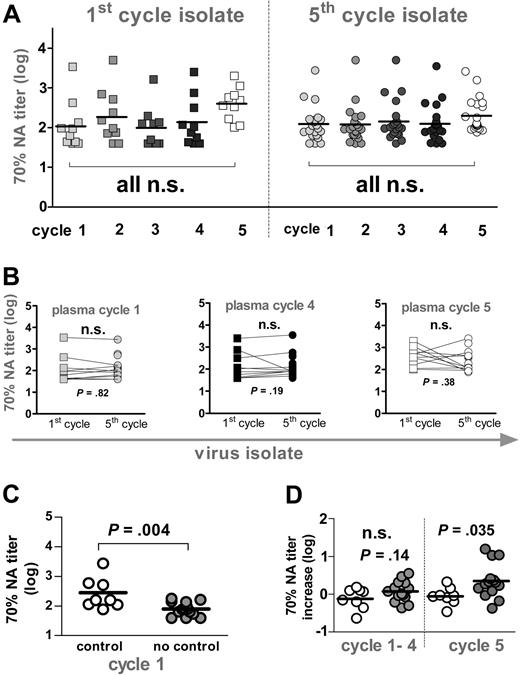
Neutralization activity. (A) Longitudinal analysis of autologous neutralization activity (NA) against patient isolates derived at the beginning of the first and the fifth cycle. Reciprocal titers achieving a 70% neutralizing activity are depicted. Squares indicate first-cycle isolate (n = 10); circles, fifth-cycle isolate (n = 22). Gray shaded symbols indicate plasma from cycle 1 (week 2), cycle 2 (week 12), cycle 3 (week 22), and cycle 4 (week 32). Open symbols depict plasma samples from cycle 5 (week 48). Titers at individual time points were compared using 1-way ANOVA (Kruskal-Wallis test with the Dunn multiple comparison post test). (B) Seventy percent neutralizing titers of sera from cycles 1 (week 2, gray), 4 (week 32, black), and 5 (week 48, open symbol) against first-(squares) and fifth-cycle isolates (circles) were compared using the Wilcoxon signed rank test. (C) Autologous neutralization activity at baseline (week 2) against fifth-cycle isolates from patients who potently control viremia (open symbol) or who do not control viremia (gray) were compared using the unpaired t test. (D) Mean increases in 70% neutralizing activity over baseline (week 2) activity in cycles 1 to 4 and cycle 5 against fifth-cycle isolates in controlling (open symbol) and noncontrolling (gray) patients were compared using the unpaired t test. (B-D) All significant observations were significant on the individual level and also after Bonferroni correction.
Neutralization activity. (A) Longitudinal analysis of autologous neutralization activity (NA) against patient isolates derived at the beginning of the first and the fifth cycle. Reciprocal titers achieving a 70% neutralizing activity are depicted. Squares indicate first-cycle isolate (n = 10); circles, fifth-cycle isolate (n = 22). Gray shaded symbols indicate plasma from cycle 1 (week 2), cycle 2 (week 12), cycle 3 (week 22), and cycle 4 (week 32). Open symbols depict plasma samples from cycle 5 (week 48). Titers at individual time points were compared using 1-way ANOVA (Kruskal-Wallis test with the Dunn multiple comparison post test). (B) Seventy percent neutralizing titers of sera from cycles 1 (week 2, gray), 4 (week 32, black), and 5 (week 48, open symbol) against first-(squares) and fifth-cycle isolates (circles) were compared using the Wilcoxon signed rank test. (C) Autologous neutralization activity at baseline (week 2) against fifth-cycle isolates from patients who potently control viremia (open symbol) or who do not control viremia (gray) were compared using the unpaired t test. (D) Mean increases in 70% neutralizing activity over baseline (week 2) activity in cycles 1 to 4 and cycle 5 against fifth-cycle isolates in controlling (open symbol) and noncontrolling (gray) patients were compared using the unpaired t test. (B-D) All significant observations were significant on the individual level and also after Bonferroni correction.
The pre-ART viral load corresponds to the last plasma HIV RNA value recorded before ART or, if 2 measurements within 6 months before initiation of ART were available, to the geometric mean of those values.
The post-STI viral load represents the plateau of viremia after STI (viral set point) and was determined as the geometric mean of plasma HIV RNA levels measured after week 40, when a steady state was reached (usually between weeks 46 and 64).
Control of viremia. Patients were grouped according to their ability to control viremia in the absence of ART between weeks 40 to 76 into a controlling and a noncontrolling group. Control of viremia was defined as a VL of fewer than 5000 RNA copies per milliliter for at least 8 weeks during this time period.
Improvement of viral set point: difference between pre-ART VL and post-STI VL. Positive values indicate improvement (decrease) of VL; negative values, increase. A decrease of VL by 0.5 logs is considered a significant change.
Viral antigen level. The total amount of virus produced during the individual cycles was estimated by calculating the AUC (area under the curve) of plasma viral load over time and used to demarcate viral antigen levels.13
Baseline titer. Plasma samples derived at the beginning of cycle 1 (weeks 0 to 2) were referred to as baseline (or pre-existing) levels and titer increases measured relative to these values.
Maximum titer. This is the highest titer measured during the whole observation period.
Slopes of antibody induction between weeks 0 to 40 were calculated as Δln(titer) per week by performing linear regression analysis.
Results
Repeated short-term exposure to viral antigen fails to boost humoral immunity
The primary intent of this study was to explore whether limited exposure to viral antigen, as present during vaccination or procured by brief viremic episodes during short-term STI, is capable of boosting humoral immunity in chronically HIV-1-infected patients who have widely restored immune functions as measured by elevated CD4 T-cell counts after successful antiretroviral therapy (ART). To this end we evaluated the induction of antibodies against HIV-1 envelope (Env) and Gag proteins in 46 patients in response to 4 consecutive 2-week and a fifth long-term treatment interruption during the SSITT (Table 1).4
Surprisingly, short-term STIs were highly inefficient in boosting antibody responses directed against viral antigens gp120 and p24 despite the repetitive and in most cases considerable exposure to viral antigen during the 4 short STIs (Figure 1A).4 Both anti-gp120 and anti-p24 titer increases were low and only reached statistical significance during the fifth interruption (Figure 1B). Of note, anti-p24 and anti-gp120 responses did not segregate together in terms of magnitude: We found no correlation between either baseline or maximum anti-gp120 and anti-p24 titers (Figure 1C). To compare the timing and the kinetics of the responses to gp120 and p24, we calculated the slopes of the antibody induction during the 4 STIs. The analysis of antibody slopes was restricted to the period of short STIs, because the length of the fifth interruption varied substantially between patients. We found that although absolute titers to gp120 and p24 were not linked, kinetics of the responses showed considerable similarity and correlated significantly (Figure 1C).
Humoral immunity as correlate of viremia control
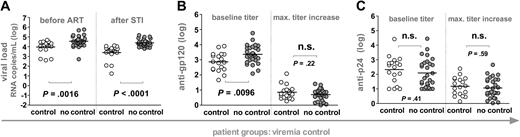
To explore whether pre-existing or novel induced antibody responses are correlates of viremia control, we stratified the 46 patients according to their ability to potently suppress viremia in the absence of ART below 5000 RNA copies per milliliter of plasma for at least 8 weeks (Figure 2A). Controlling (n = 18) and noncontrolling (n = 28) patients were indistinguishable in terms of their total CD4+ T-cell levels before and after STI, the length of their HIV infection before onset of ART, and also the duration of ART before entering the STI trial. As described previously, post-STI viral levels closely approximated pre-ART loads, indicating that pre-existing viral and immune properties lead to the potent containment of viremia observed in some individuals (Figure 2).4-7 When we analyzed whether responses to gp120 and p24 were associated with this potent viremia control, we found no difference between controlling and noncontrolling patient groups in respect to the magnitude of anti-gp120 and anti-p24 antibody induction (Figure 2B-C): The maximum titer increases over baseline anti-gp120 and anti-p24 titers reached in the controlling and the noncontrolling group were indistinguishable.
Influence of humoral immunity on potent viremia control. Patients were stratified according to their ability to potently suppress viremia upon STI to levels below 5000 RNA copies per milliliter of plasma for at least 8 weeks (n = 46). White and gray circles demarcate controlling and noncontrolling patients, respectively, and horizontal bars indicate mean values. All significant observations were significant on the individual level and also after Bonferroni correction. (A) Viral loads before ART and after STI are depicted. Anti-gp120 (B) and anti-p24 (C) titers at baseline (weeks 0 to 2) and maximum increases in titers reached during the trial are shown.Antibody reactivities and viral loads of the individual groups were compared using the unpaired t test.
Influence of humoral immunity on potent viremia control. Patients were stratified according to their ability to potently suppress viremia upon STI to levels below 5000 RNA copies per milliliter of plasma for at least 8 weeks (n = 46). White and gray circles demarcate controlling and noncontrolling patients, respectively, and horizontal bars indicate mean values. All significant observations were significant on the individual level and also after Bonferroni correction. (A) Viral loads before ART and after STI are depicted. Anti-gp120 (B) and anti-p24 (C) titers at baseline (weeks 0 to 2) and maximum increases in titers reached during the trial are shown.Antibody reactivities and viral loads of the individual groups were compared using the unpaired t test.
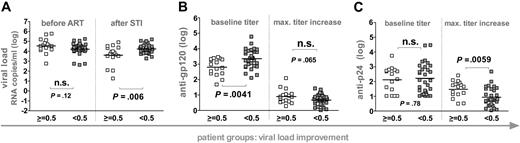
Potent control was not induced by the SSITT but reflected pre-existing conditions.4-7 Nonetheless, a moderate improvement of viral set points occurred in a subset of patients. We thus investigated if humoral immunity stimulated in response to STIs had any influence on this improved viremia control. To this end we grouped patients according to their success in decreasing pretreatment VLs by at least 0.5 logs (Figure 3). Using this stratification, patient groups were indistinguishable in terms of pre-ART VL levels but differed significantly in their post-STI VL levels (Figure 3). Again, no differences in CD4+ T-cell levels, length of infection, or duration of ART existed between patient groups. However, patients who improved viral set points (n = 16) mounted significantly higher anti-p24 antibody titer increases whereas anti-gp120 responses were equivalent in both groups.
Influence of humoral immunity on decreasing viral set points. Patients were stratified according to their ability to improve viremia control upon STI (n = 46). White squares depict patients who decreased viremia by more than 0.5 logs upon STI. Gray squares depict patients who did not reach a 0.5 log improvement of viremia. Horizontal bars indicate mean values. (A) Viral loads before ART and after STI are depicted. Anti-gp120 (B) and anti-p24 (C) titers at baseline (weeks 0 to 2) and maximum increases in titers reached during the trial are shown. Antibody reactivities and viral loads of the individual groups were compared using the unpaired t test. (A-C) All significant observations were significant on the individual level and also after Bonferroni correction.
Influence of humoral immunity on decreasing viral set points. Patients were stratified according to their ability to improve viremia control upon STI (n = 46). White squares depict patients who decreased viremia by more than 0.5 logs upon STI. Gray squares depict patients who did not reach a 0.5 log improvement of viremia. Horizontal bars indicate mean values. (A) Viral loads before ART and after STI are depicted. Anti-gp120 (B) and anti-p24 (C) titers at baseline (weeks 0 to 2) and maximum increases in titers reached during the trial are shown. Antibody reactivities and viral loads of the individual groups were compared using the unpaired t test. (A-C) All significant observations were significant on the individual level and also after Bonferroni correction.
Interestingly, patients who failed to control or improve viremia after final cessation of ART had significantly higher pre-existing anti-gp120 titers but did not differ in their baseline anti-p24 reactivity (Figures 2 and 3). In agreement with this, anti-gp120 baseline titers correlated positively with post-STI viral loads (r2 = 0.23, P = .0007). Thus, elevated pre-existing anti-gp120 titers were found at a higher frequency in patients who already had pre-existing high viral set points (Figure 2) and in patients who developed or maintained high viral loads upon STI (Figure 3). These findings could imply that development of anti-gp120 is strongly governed by the supply of viral antigen. In addition, high baseline gp120 titers may also to some extent be indicative of patient histories with higher viral set points and consequently of immune systems with an advanced degree of destruction less capable of inducing novel antibody responses.
Influence of pre-existing antibody response on induction of antibody titers during STI
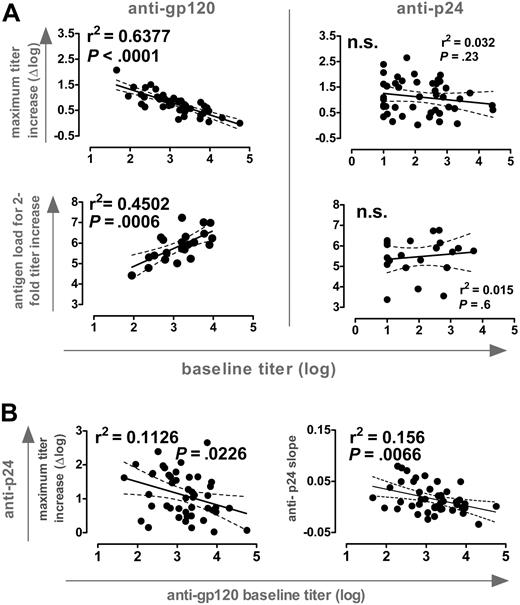
Anti-Env and anti-Gag antibody levels in plasma exceed HIV-1 antigen by several orders of magnitude.9 Consequently, antigen capture by pre-existing immunity could partially prevent presentation and antigenic stimulation and thus limit the success of therapeutic vaccination. This could be particularly of importance during STIs where autologous antigen is employed. When we probed for interdependencies between baseline titers and the novel induced responses, we found a striking disparity between reactivities to gp120 and p24: A strong inverse correlation between the magnitude of anti-gp120 titer increases and pre-existing responses existed whereas no such dependency was found for the induction of anti-p24 responses (Figure 4A). Moreover, antigen quantities needed to induce a 2-fold titer increase correlated strongly with baseline anti-gp120 but not anti-p24 levels. Therefore, anti-gp120 but not anti-p24 responses appeared to be governed by pre-existing responses to the respective antigen.
Influence of pre-existing antibody responses. (A) (Top) Correlation analysis of baseline titers versus maximum titer increases of antibodies directed to gp120 (left) and p24 (right); n = 46. (Bottom) The absolute amount of antigen to induce a 2-fold increase in titers against gp120 (left) and p24 (right) was estimated by calculating the total viremia load (AUC). Antigen levels were correlated with baseline activities against these antigens (n = 22, group A; Table 1). (B) Correlation analysis of baseline anti-gp120 titers versus maximum anti-p24 titer increases and slope of anti-p24 response (n = 46). For both panels, all significant observations were significant on the individual level and also after Bonferroni correction.
Influence of pre-existing antibody responses. (A) (Top) Correlation analysis of baseline titers versus maximum titer increases of antibodies directed to gp120 (left) and p24 (right); n = 46. (Bottom) The absolute amount of antigen to induce a 2-fold increase in titers against gp120 (left) and p24 (right) was estimated by calculating the total viremia load (AUC). Antigen levels were correlated with baseline activities against these antigens (n = 22, group A; Table 1). (B) Correlation analysis of baseline anti-gp120 titers versus maximum anti-p24 titer increases and slope of anti-p24 response (n = 46). For both panels, all significant observations were significant on the individual level and also after Bonferroni correction.
Remarkably, we also observed a significant inverse correlation between baseline anti-gp120 titers and the magnitude and slope of p24 antibody induction (Figure 4B). Thus, high pre-existing anti-gp120 titers were a negative correlate for induction of both anti-p24 and gp120 responses. No such associations were found between pre-existing and novel induced responses to p24. Although antigen capture by pre-existing anti-gp120 antibodies may well influence the efficacy of the anti-Env response, these data demonstrate that individuals with high pre-existing anti-gp120 titers could not react efficiently to new antigenic stimulation in general. This may well be the case, considering that high levels of baseline anti-gp120 antibodies were found in patients with high viral set points (Figures 2 and 3), which are likely to be associated with more severe impairment of the immune system. What the impairments in immune functions in these individuals are will require further investigations. Patients included in this study were mostly ranked as clinical stage A (Table 1) and thus had not suffered from severe immune defects before starting ART. No association between absolute CD4+ T-cell levels before or after STI and baseline gp120 titers existed. Equally, we did not note an association between length of HIV-1 infection or duration of ART and baseline anti-gp120 titers. Despite the lack of quantitative differences in CD4 T-cell numbers, qualitative differences may still occur, because particularly functional HIV-specific helper T cells are depleted by viral infection.14 Moreover, it has been shown that the rise in CD4 T-cell number upon successful treatment is not generally accompanied by a complete reconstitution of immune functions.15-17 Thus, absolute numbers of CD4 T cells cannot be envisioned as the sole parameter or the sole functional reason for immune defects.
Kinetics of antibody induction predicts improvement of viral set points
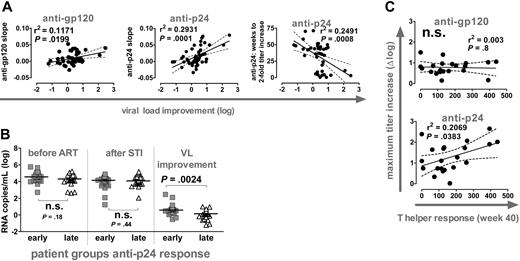
Anti-p24 and anti-gp120 titers increased during the 4 short-term STIs at a similar, low rate (Figure 1C). Most notably, the slopes of this elevation in titers reflected the extent of VL improvement (Figure 5A). Equally, when we calculated the weeks individuals needed to induce a 2-fold increase in anti-p24 titers, we found that a rapid induction of anti-p24 responses was associated with better control of VL upon STI.
Kinetics of anti-Gag responses predicts viral load improvement and T helper activity. (A) Correlation analysis of viral load improvement versus slopes of anti-gp120 (left) and anti-p24 responses (middle) during cycles 1 to 4 (n = 46). (Right) Correlation analysis of viral load improvement versus timing of anti-p24 response. Weeks required to achieve a 2-fold increase over baseline were calculated by linear regression analysis and plotted versus viral load improvement (n = 42). (B) Patients were stratified for early and late anti-p24 responses according to the slope of titers measured during cycles 1 to 4. Early response: slope more than 0.01; late response: slope less than 0.01. Early and late groups were compared with respect to viral set points before ART and after STI and for viral load improvement using the Mann-Whitney test. (A-B) All significant observations were significant on the individual level and also after Bonferroni correction. (C) Correlation analysis of CD4 T helper responses at week 40 with maximum titer increases of anti-gp120 and anti-p24 responses. CD4 T helper responses depict the number of IFN-γ-producing, spot-forming cells per million CD8-depleted PBMCs in response to p24 stimulation as measured by ELISPOT (n = 21). The observation was only significant on the individual level. Significance was lost after Bonferroni correction (P > .025).
Kinetics of anti-Gag responses predicts viral load improvement and T helper activity. (A) Correlation analysis of viral load improvement versus slopes of anti-gp120 (left) and anti-p24 responses (middle) during cycles 1 to 4 (n = 46). (Right) Correlation analysis of viral load improvement versus timing of anti-p24 response. Weeks required to achieve a 2-fold increase over baseline were calculated by linear regression analysis and plotted versus viral load improvement (n = 42). (B) Patients were stratified for early and late anti-p24 responses according to the slope of titers measured during cycles 1 to 4. Early response: slope more than 0.01; late response: slope less than 0.01. Early and late groups were compared with respect to viral set points before ART and after STI and for viral load improvement using the Mann-Whitney test. (A-B) All significant observations were significant on the individual level and also after Bonferroni correction. (C) Correlation analysis of CD4 T helper responses at week 40 with maximum titer increases of anti-gp120 and anti-p24 responses. CD4 T helper responses depict the number of IFN-γ-producing, spot-forming cells per million CD8-depleted PBMCs in response to p24 stimulation as measured by ELISPOT (n = 21). The observation was only significant on the individual level. Significance was lost after Bonferroni correction (P > .025).
By stratifying patients according to the anti-p24 response into an early and a late group, we confirmed that early responders had a significantly improved viremia control (Figure 5B). Of note, early and late responders were indistinguishable in regard to absolute VL levels before or after STI (data not shown).
In summary, anti-Gag responses were both with respect to magnitude and timing a powerful correlate of improvement of viremia control. A similar but less pronounced trend was evident for the kinetics but not the magnitude of the anti-gp120 response (Figures 2, 3, and 5A and data not shown). These results are very intriguing because they suggest that the ability of the immune system to mount a rapid antibody response during chronic infection may provide a measure for the ability of the immune system to react and to suppress viremia. Hence, measurements of antibody kinetics could provide a powerful tool in developing and assessing vaccines.
Anti-Gag responses are a potential surrogate measure for T helper activity
We previously reported that the viremic episodes during the 4 short STIs stimulated p24-specific T helper cell responses,6 but no direct and consistent correlation between these responses and potent viremia control was detected. Here we show that the increase in anti-p24 titers but not the anti-gp120 response gave a modest correlation with T helper activity measured after completion of the 4 STIs (Figure 5C). It has been suggested that anti-Env and anti-Gag humoral immune responses are regulated differently and that anti-Gag responses are more dependent on active T help.9,18 Our results could potentially suggest that anti-p24 responses are indeed linked to T helper activity. If such a link can be established and confirmed by larger surveys of T helper activity and humoral immune responses, the assessment of p24 antibody responses as shown here may provide a useful surrogate marker for T helper function.
Pre-existing neutralizing antibody responses are a correlate of potent viremia control
We have recently shown that neither CD8 nor CD4 T-cell responses were correlates of the potent viremia control observed in a fraction of SSITT patients.4-7 Here we investigated the influence of neutralizing activity on suppressing viremia. In agreement with the analysis of gp120 and p24 reactive antibodies, neutralization responses against autologous virus isolates derived during the first and the fifth interruption cycle were not significantly altered in most patients in response to the 4 short-term STIs (Figure 6A). Concordant with binding antibody responses, neutralization activity increased during the long interruption interval in most patients. However, this increase was with few exceptions small and did not reach statistical significance. Therefore, VL improvement upon STI was not associated with a boost in neutralization titers in these patients (data not shown). We observed no statistically significant difference in neutralization activity directed against the early (first cycle) and late (fifth cycle) isolates (Figure 6B). Consequently, failure to detect improved neutralization activity upon STI was not due to evolving neutralization escape viral variants.
Although levels of HIV-specific CD4+ and CD8+ T-cell responses maintained during ART have been described to be inversely related to viral set points before therapy,6 we found no association of potent viremia control with high cytotoxic T lymphocyte (CTL) and T helper levels in the 22 patients analyzed for neutralization activity (data not shown). Most notably, patients who potently suppressed viremia, both before ART and after STI, had significantly higher pre-existing neutralization titers than patients who were highly viremic (Figure 6C). Although it is difficult to define whether this higher neutralizing activity in the controlling patient group was cause or consequence of the enhanced viremia control, these results underscore the potential of the antiviral activity of antibodies in vivo. Of note, also in these controlling patients the increases in neutralization activity during the short and long STI cycles were negligible and were not associated with improved viremia control (Figure 6D). This is in contrast to STI after acute infection where induction of potent neutralizing activity in a subset of patients was reported.19 Despite the fact that during chronic infection STI failed to boost neutralization activity, our observations suggest a protective role of neutralizing antibodies in vivo and highlight the need to develop strategies that are capable of evoking such responses.
Discussion
The success of therapeutic vaccination will greatly depend on how capable immunogens are in eliciting broad immune responses, how these can be maintained and, most importantly, how effective the responses are in suppressing viremia. In this study, we performed a longitudinal analysis of binding and neutralizing antibody responses following STI to investigate the general potential of therapeutic vaccination in chronic infection. Exposure to autologous antigen during short-term treatment interruption reflects in many ways the situation of vaccination; however, unlike vaccination, treatment interruption bears the risk of damaging the immune system due to the ongoing virus replication.20 Hence, particularly during prolonged exposure to virus replication, immune functions might decline rapidly and fail to initiate novel responses. Nevertheless, because appropriate immunogens that potently evoke immune reactivities comparable with replicating virus are not available, studying immune responses to viral antigen in the tightly controlled setting of STI bears great potential in exploring modes and patterns of immune stimulation in HIV-1-infected patients upon antigenic challenge.
Although the evoked responses in our study were modest and did not lead to potent suppression of viremia, the kinetics of the elicited antibody responses gave a reliable measure of the ability of the immune system to react to antigenic challenge and to decrease viral set points. In particular, the kinetics of anti-p24 responses allowed a clear distinction of the patients' immune functions: Individuals who had a rapid and high response decreased viral set points significantly.
To date, our most reliable markers to monitor disease progression are measurements of plasma viremia and total CD4+ T-cell numbers in the periphery. However, although these measurements provide a clear reflection of the general clinical stage of the patients, subtle differences in immune functions cannot be defined by these parameters. After successful ART, absolute CD4 levels are at least in part restored, but this gain in absolute CD4+ T-cell numbers does not necessarily coincide with a complete restoration of immune functions.15-17 Our analysis of humoral immune responses in chronically HIV-1-infected patients illustrates that even individuals who have widely restored immune functions after successful antiretroviral therapy can differ substantially in their responses. In coming years it will remain a major challenge to define where exactly the restrictions in immune reconstitution are and how they can be overcome. Only when we are able to understand and reverse the deficiencies of the immune system that remain after successful ART can future therapies and vaccination be successful. A first step toward the understanding of these immune deficiencies is the identification of marker functions that reflect the functionality of the immune system. Definition of such surrogate markers would enable us to more accurately monitor and evaluate the success of immune reconstitution and vaccination strategies.
It has long been realized that anti-p24 antibody responses can bear prognostic value, and low, declining, or nonexisting anti-p24 responses are associated with disease progression.21-25 Here we show that even in individuals with partially restored immune functions after prolonged ART, anti-p24 responses may provide a reliable marker to validate the responsiveness and capacities of the immune system. We found that the magnitude of the anti-p24 response was correlated with specific CD4 T helper frequency in the subset of patients in whom T help could be measured. If this observation can be confirmed in larger patient cohorts, longitudinal assessment of p24 antibody responses may provide a sensitive and accurate surrogate measurement for HIV-specific CD4 helper activity. Notably, no indication exists in the literature that anti-p24 responses are protective per se. Concordantly, specific increase of solely anti-p24 responses as achieved in vaccination with diverse p24 antigen regimens has not shown clinical benefits.26-35 Hence, the boost in reactivites to p24 observed here likely reflects the capacity of the immune system to react to antigenic challenge and is not a direct correlate of protection. Previous studies on the prognostic value of antibodies to p24 measured absolute antibody levels at a specific time point and sought to derive associations with disease progression from these values.21-25 Here we show for the first time that the kinetics of the anti-p24 response but not the absolute titers gives a reflection of the immune system's activity. Our analysis does not give an estimate of disease progression as previous studies have attempted but focuses solely on the ability of the patient to react to antigenic challenge. Judging from the retrospective assessment in our study, it is feasible that measurement of p24 antibody kinetics may be particularly helpful when assessing immune functions of patients participating in future vaccine trials.
Conflicting reports exist on the prognostic value of gp120 responses. Reactivities with specific epitopes on gp120 have been associated with good or bad disease prognosis, but the potency of the predictive value of these responses remains unclear.23-25,36-38 Here we show that high pre-existing gp120 binding antibody titers were a strong negative correlate of immune functions and viremia control. This may seem paradoxical because anti-Env antibodies can harbor inhibitory activity and, thus, high titers of these antibodies should be of benefit. However, antibodies reactive with monomeric gp120 as measured here do not necessarily correlate with neutralizing antibody titers39 and could in principle also harbor enhancing activity.40,41 Although influence of enhancing antibodies cannot be ruled out, high anti-gp120 titers may well be consequence instead of cause of high viremia. Supporting the latter scenario, we provide evidence that patients with high anti-gp120 titers were subject to higher viral replication, which coincided with increased immune deficiency impairing novel antibody responses and viral defense mechanisms. Thus, anti-gp120 antibody titers might to some extent give a record of infection history and immune destruction. Which immune functions are impaired and how and why high anti-gp120 represents this unresponsive immune system will need to be further investigated, but it does not appear to be a simple function of length of infection before onset of ART, duration of ART, or absolute CD4 levels.
Although ample data exist demonstrating that neutralizing antibodies can protect against HIV-1 infection in vitro and in animal models in vivo, proof of their activity in infected humans remains circumstantial.19,41-51 Our analysis of neutralizing activity showed that high pre-existing neutralizing antibody titers correlated with potent viremia control, suggesting a role of humoral immunity in conferring protection in these individuals. Although STI induced binding antibody responses, which correlated with improved viral control, the increase in neutralizing activity was marginal and did not lead to this enhanced viral suppression. Antibodies directed against p24 are not known to bear substantial antiviral activity; nor do binding anti-gp120 responses necessarily reflect direct inhibitory activity.41 However, both responses could potentially contribute to viral control by activating complement or effector functions such as antibody-dependent cellular toxicity.41 Although the latter is in principle possible, it is likely that antibody responses by themselves were not the main correlate of the observed improved viremia control but reflect other immune parameters involved in HIV defense that were elicited alongside such as CD4 help.
Our studies provided us with general insights into the modes of antibody induction in chronic HIV-1 infection. We concluded the following: (1) anti-Env and anti-Gag responses are independently regulated in terms of magnitude as previously suggested9 ; and (2) anti-Env and anti-Gag responses have different requirements on antigen presentation and immune functions: Induction of anti-gp120 responses is steered by antigen supply and pre-existing immune responses, whereas stimulation of anti-p24 responses seems to depend on T helper activity. It has been recently suggested that in mice particulate or oligomeric antigen (eg, viral particles) but not monomeric antigen stimulates a memory B-cell response in the absence of T help.52 If the same applies for the human immune system, it is possible that some of the differences observed in our study are a result of the differential presentation of gp120 and p24 because the envelope proteins are accessible both in the oligomeric form on the virion and as a monomer in solution, whereas p24 antigen is presented to B cells only in a monomeric form.
Given the extended time periods of active virus replication required to mount changes in humoral immunity, it is unlikely that STI in its current form can be utilized to induce potent humoral immune responses. Exhaustion of the immune system and depletion of CD4 cells will occur, putting gains and losses of this approach in question.4-7,14,20 As a result, vaccination strategies capable of mounting responses similar to functional, replicating virus but without its drawbacks need to be designed. However, as we show here, the high and continuous exposure to antigen required to boost antibody responses in chronically infected patients will prove to be a challenge for therapeutic vaccination in general.
Appendix
Members of the Swiss HIV Cohort Study (SHCS) are M. Battegay, E. Bernasconi, H. Bucher, P. Bürgisser, M. Egger, P. Erb, W. Fierz, M. Fischer, M. Flepp (Chairman of the Clinical and Laboratory Committee), P. Francioli (President of the S.H.C.S., Centre Hospitalier Universitaire Vaudois, Lausanne), H. J. Furrer, M. Gorgievski, H. Günthard, P. Grob, B. Hirschel, L. Kaiser, C. Kind, T. Klimkait, B. Ledergerber, U. Lauper, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach (Head of data center), C. Rudin (Chairman of the Mother and Child Substudy), J. Schupbach, R. Speck, A. Telenti, A. Trkola, P. Vernazza (Chairman of the Scientific Board), T. Wagels, R. Weber, S. Yerly.
Prepublished online as Blood First Edition Paper, June 8, 2004; DOI 10.1182/blood-2004-01-0251.
Supported by the Swiss HIV Cohort Study (Swiss National Science Foundation grant 3345-062041), and Swiss National Science Foundation grant 3345-65168 (H.F.G., A.T.), the Swiss HIV Cohort Study project 290 (H.F.G.), a subcontract to A.T. from the National Institutes of Health grant R37 AI36082, and a grant from the Gebert-Rüf foundation (A.T.).
A complete list of the members of the Swiss HIV Cohort Study appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. P. Moore for helpful discussion, our patients for their commitment, C. Schneider and R. Hafner for excellent patient care, F. Burgener and E. Schlaepfer for laboratory support, and I. Nievergelt for administrative assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal