Abstract
Endothelial cell leakiness is regulated by mediators such as thrombin, which promotes endothelial permeability, and anti-inflammatory agents, such as angiopoietin-1. Here we define a new pathway involved in thrombin-induced permeability that involves the atypical protein kinase C isoform, PKCζ. Chemical inhibitor studies implicated the involvement of an atypical PKC isoform in thrombin-induced permeability changes in human umbilical vein endothelial cells. Thrombin stimulation resulted in PKCζ, but not the other atypical PKC isoform, PKCλ, translocating to the membrane, an event known to be critical to enzyme activation. The involvement of PKCζ was confirmed by overexpression of constitutively active PKCζ, resulting in enhanced basal permeability. Dominant-negative PKCζ prevented the thrombin-mediated effects on endothelial cell permeability and inhibited thrombin-induced activation of PKCζ. Rho activation does not appear to play a role, either upstream or downstream of PKCζ, as C3 transferase does not block thrombin-induced PKCζ activation and dominant-negative PKCζ does not block thrombin-induced Rho activation. Finally, we show that angiopoietin-1 inhibits thrombin-induced PKCζ activation, Rho activation, and Ca++ flux, thus demonstrating that the powerful antipermeability action of angiopoietin-1 is mediated by its action on a number of signaling pathways induced by thrombin and implicated in permeability changes. (Blood. 2004; 104:1716-1724)
Introduction
One of the essential functions of the endothelial cell (EC) lining is to maintain the essentially impermeable nature of the blood vessel, controlling the passage of solutes and inflammatory cells from the circulation to the tissues. EC hyperpermeability is a characteristic of blood vessels in many pathologies, for example, newly formed microvessels in tumors are highly permeable. Indeed, such hyperpermeability allows the deposition of fibrin in tumors, which supports and promotes cell adhesion and migration, essential steps in the angiogenic response.1,2 In chronic inflammatory states such as in rheumatoid arthritis and atherosclerosis, increased transmigration of inflammatory cells across the activated endothelium is seen. A number of factors have previously been described that promote alterations in vessel integrity, for example, thrombin,3,4 which produces plasma leakage; tumor necrosis factor,5,6 which induces adhesion and transmigration of leukocytes; and vascular EC growth factor (VEGF),2,7 which induces cell migration and vascular hyperpermeability.
Thrombin is a potent activator of ECs and in vitro studies show that it induces phosphorylation changes in platelet endothelial cell adhesion molecule (PECAM)-1 and vascular endothelial (VE)-cadherin,8 and an associated increase in the permeability of confluent EC monolayers.3,4 Thrombin signaling in vascular ECs is mediated by the protease-activated receptor PAR-1, a G-protein-coupled receptor that can potentially activate multiple downstream signaling effectors,9 including phospholipases, with subsequent changes in intracellular Ca++ concentration and Ca++/calmodulin-dependent myosin light chain kinase (MLCK) activity. Thrombin also activates the small GTPase, Rho, via PAR-1-mediated effects on the G protein, G12/13. The activation of Rho can also take place through a protein tyrosine kinase-dependent pathway.10,11 Rho has multiple downstream targets, including myosin light chain (MLC) phosphatase, which regulates MLC, and which is involved in actin reorganization.12,13 Thrombin activation of protein kinase C (PKC) has also been linked to permeability changes in EC monolayers.14-22
The PKC family of serine/threonine kinases are involved in signal transduction and are dependent on lipids for their activity. The isoforms of PKC are classified according to their structure, activation, and substrate requirements.23 The classic PKCs (α, βI, βII, γ) are Ca++-dependent and regulated by diacyglycerol (DAG) or phosphotidylserine (PS); the novel PKCs (δ, ϵ, η, θ) are also activated by DAG or PS but are Ca++-independent; whereas the atypical PKCs (ζ, λ) are regulated by PS and are independent of both DAG and Ca++.24 The activity of PKCs are controlled by their relocalization and phosphorylation status, such that enzyme localization to the plasma membrane and enzyme phosphorylation are indicative of enzyme activation.23-26 In terms of regulation of endothelial permeability, the specific isoform of PKC involved appears to be highly dependent on species, organ, stimulant, and culture conditions. For thrombin-induced permeability changes, there have been reports dismissing the involvement of PKCs,10 or implicating PKCα,19,21,27,28 PKCϵ,21 or PKCζ.29
Angiopoietin-1 (Ang-1) is the ligand for the EC-specific tyrosine kinase receptor Tie2,30 and both Ang-1 and Tie2 expression are essential for successful angiogenesis. Knockout of ligand or receptor leads to disrupted angiogenesis, which results from defects in the interactions between ECs and adjacent mesenchymal cells and extracellular matrix.31,32 The Ang-1/Tie2 axis also appears to be involved in maintaining endothelial integrity in adult vessels. Transgenic expression of Ang-1 results in leakage-resistant blood vessels,33 whereas systemic administration of Ang-1, using adenovirus-mediated gene delivery, inhibits edema and vessel leakage following challenge with mustard oil or VEGF.34 These results are complemented by in vitro studies that have shown that Ang-1 stabilizes EC networks and promotes EC survival.35 Recent studies in our laboratory have shown that Ang-1 is both an antipermeability and anti-inflammatory agent as it reduces basal EC permeability, prevents VEGF and thrombin-stimulated EC permeability increases, and inhibits tumor necrosis factor α-mediated transendothelial neutrophil migration.8 Thus, Ang-1 and Tie2 signaling have an essential role, not only in vascular development, but also in vascular maintenance.
The results described herein add to our understanding of thrombin-induced permeability changes. We define another signaling pathway critical in mediating thrombin-induced permeability. Thus, together with previously reported effects of thrombin on RhoA (Rho kinase), Ca++ flux (Ca/calmodulin), and protein tyrosine kinases, we show here that thrombin also activates PKCζ to regulate EC permeability. Interference with any of these pathways can severely compromise EC permeability. Importantly, we demonstrate that Ang-1 inhibits EC permeability through its action on these pathways. We have shown previously that Ang-1 inhibits the potential downstream targets of the protein tyrosine kinases, the junctional proteins PECAM and VE-cadherin.8 As we show here, Ang-1 also inhibits thrombin-induced PKCζ activation, Rho activation, and Ca++ flux.
Materials and methods
Reagents
Ang-1, obtained from Regeneron (Tarrytown, NJ), was used as described.8,36 Bisindolylmaleimide I hydrochloride and calphostin C were from Calbiochem Novabiochem (Croydon, Vic, Australia). Chelerythrine chloride and H-89 were from Biomol Research Laboratories (Plymouth Meeting, PA). Thrombin (from human plasma), protease inhibitor cocktail, and fluorescein isothiocyanate (FITC)-conjugated dextran (40 000 kDa) were from Sigma-Aldrich (St Louis, MO). Fura 2 acetoxymethyl ester (fura 2/am) and pluronic F-127 were from Molecular Probes (Eugene, OR).
Cells and cell culture
Human umbilical vein endothelial (HUVE) cells were grown in M119 medium with 20% fetal calf serum (FCS) as described.36 Cells were used at passage 4 or less. For infection with adenoviral constructs, cells were grown to 80% confluence and exposed to 2.5 × 106 plaque forming units/25 cm2 culture area for 2 hours in M119 medium with 2% FCS and a further 22 hours with medium containing 20% FCS.
Recombinant adenoviral constructs
DNA constructs encoding FLAG-PKCζ and FLAG-PKCζ T410A, in pCMV5, and Myr-PKCζ-FLAG in pCMV6 were a generous gift from Dr Alex Toker (Biomedical Research Institute, Boston, MA). Recombinant adenoviruses were constructed by subcloning EcoRI fragments from the pCMV5 constructs and HindIII-EcoRI fragment from the pCMV6 construct into the pAdEasy-1 vector (Qbiogene, Carlsbad, CA). Virus was amplified in HEK293 cells and purified by CsCl gradient ultracentrifugation. Virus titers were determined using the tissue culture infectious dose 50 method, as recommended by Qbiogene.
Endothelial permeability assays
Assays were performed as described.8 HUVE cells (105) were cultured in transwells (3 μm; Corning Costar, Cambridge, MA) for 24 hours in complete medium and then in 2% FCS medium for an additional 24 hours. Cells were pretreated with either bisindolylmaleimide I (100 nM or 6 μM), calphostin C (100 nM), chelerythrine chloride (1 μM), or Ang-1 (0.1 μg/mL), as required. FITC-conjugated dextran (2 μg) was added to the upper chamber of all wells and cells were then treated with thrombin (0.2 U/mL). The amount of FITC-dextran in the lower chambers of the transwells was determined using a LS 50B Luminescence Spectrometer (Perkin Elmer, Beaconsfield, United Kingdom; excitation wavelength, 485 nm; emission wavelength, 530 nm). Permeability is given as the amount of FITC-dextran passing from the upper chamber to the lower chamber. Adenovirus-infected ECs were plated on to transwells 24 hours after the infection procedure and treated in the same manner as uninfected cells.
Immunoblotting
HUVE cells were plated into fibronectin-coated flasks before infection as described in “Cells and cell culture.” Following infection, cells were serum-starved overnight. Cells were then treated with Ang-1, thrombin, and/or bisindolylmaleimide I, as required, lysed in ice-cold lysis buffer (50 mM Tris.HCl, pH 7.4, with 1% NP-40, 150 mM NaCl, 2 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 1 mM NaVO4, 100 mM NaF, 10 mM Na4P2O7, and protease inhibitor cocktail). Protein concentrations were assayed using Bradford Reagent (BioRad, Hercules, CA). Equal amounts of protein were loaded onto 10% acrylamide gels, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membrane, blocked with 5% skim milk powder and 0.1% Triton-X100 in phosphate-buffered saline (PBS), and probed with a polyclonal rabbit antibody directed against phosphorylated Thr410 of the activation loop of PKCζ.37 This antibody was a generous gift from Dr Alex Toker. After washing, membranes were incubated with antirabbit secondary antibody and reactive bands were detected by chemiluminescence (ECL Western Blotting Detection Reagents; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). Membranes were stripped using stripping buffer (Re-Blot Plus Western Blot Recycling Kit; Chemicon, Temecula, CA) and reprobed with rabbit anti-PKCζ antibody (Upstate Biotechnology, Lake Placid, NY).
Immunofluorescence
HUVE cells (6 × 104) were cultured in fibronectin-coated glass LabTek chamber slides (Nalge Nunc International, Naperville, IL) and incubated for 3 days prior to staining. Cells were then treated with Ang-1, thrombin, and/or bisindolylmaleimide I, as required. Cells were washed once with serum-free M119 medium, fixed in 4% paraformaldehyde/PBS for 5 minutes, permeabilized with acetone for 5 minutes at -20 °C, and washed twice with PBS. The fixed cells were incubated with anti-PKCζ, anti-PKCλ, anti-PKCα (BD Transduction Laboratories, San Diego, CA), or anti-PKCϵ (Upstate Biotechnology), and a mouse monoclonal anti-VE-cadherin36 overnight at 4°C, followed by Alexa Fluor 488 goat antirabbit immunoglobulin G (IgG) and Alexa Fluor 594 goat antimouse IgG (Molecular Probes), respectively. Coverslips were mounted using fluorescence microscopy mounting medium (Dako, Carpinteria, CA). Cells were imaged by epifluorescence microscopy on an Olympus BX-51 microscope (Olympus, Hamburg, Germany) equipped with excitation filters for fluorescein and acquired to a Photometrics Cool Snap FX charge-coupled device camera (Roper Scientific, Munich Germany). All images were captured using Uplan FI lenses (40×/0.75) and were adjusted for brightness and contrast using V++ software (Digital Optics, Auckland, New Zealand).
Cell fractionation
Following infection of HUVE cells with wild-type PKCζ as described in “Cells and cell culture,” cells were incubated with or without 0.2 U/mL thrombin for 15 minutes, washed with PBS, and harvested by scraping into 1 mL homogenization buffer (20 mM Tris.HCl, pH 7.4, with 0.5 mM EDTA [ethylenediaminetetraacetic acid], 0.5 mM EGTA, 10 mM β-mercaptoethanol, 5% glycerol, 2 mM NaF, 1 mM NaVO4, and protease inhibitor cocktail). Cells were mechanically homogenized and centrifuged for 15 minutes at 1200g at 4°C. The supernatant was further centrifuged for 1 hour at 125 000g at 4 °C. The resultant supernatant containing the cytosolic components was removed, and the pellet containing the membrane components was resuspended in 0.5 mL homogenization buffer supplemented with 0.5% Triton X100 and 100 mM NaCl. Proteins in 20 μL of each fraction were separated by SDS-PAGE, transferred to PVDF membranes, and probed with the anti-PKCζ antibody, as described in “Immunoblotting.”
Measurement of Ca++ release from intracellular stores
HUVE cells were cultured to confluence on fibronectin-coated coverslips. Cells were loaded with fura 2/am by incubating the coverslips in 500 μL Hanks medium (137 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4.7H2O, 0.8 mM KH2PO4, 4.2 mM NaHCO3, 5.5 mM glucose, and 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4) containing 5 μM fura 2/am and 0.0075% pluronic F-127, for 1 hour at 37°C in humidified air supplemented with 5% CO2, in the presence or absence of 0.1 μg/mL Ang-1 as required. Coverslips were washed twice in Hanks medium, with or without 0.1 μg/mL Ang-1 as required, and transferred to an incubation chamber at 34°C. Cells were treated with thrombin (0.2 U/mL) and fura 2 fluorescence was measured using a Nikon Diaphot epifluorescence microscope (Nikon, Melville, NY), intensified CCD camera (Photonic Science ISIS-3/S20; Robertsbridge, East Sussex, United Kingdom) and Axon Imaging Workbench Software (Molecular Devices, Union City, CA).38 The fluorescence ratio (F340/F380) was measured for 100 cells in each of the individual experiments. In order to pool experiments, the basal Ca++ flux in each experiment has been normalized.
Actin staining
HUVE cells were cultured to confluence in fibronectin-coated glass LabTek chamber slides and changed to 2% FCS medium overnight. Cells were treated with Ang-1 and thrombin as required. Cells were washed once with PBS then fixed with 4% paraformaldehyde/PBS for 10 minutes, permeabilized with acetone for 5 minutes at -20°C, and then washed twice with PBS. The cells were incubated with rhodamine phalloidin (Molecular Probes) for 20 minutes and washed several times with PBS before being mounted. Images were captured as described in “Immunofluorescence.”
Rho activity assay
The activation of Rho was assayed using the EZ-detect Rho Activation Kit (Pierce Biotechnology, Rockford, IL). HUVE cells were grown to confluence in fibronectin-coated flasks, and then starved overnight in serum-free medium. Cells were treated with Ang-1 or thrombin as required. One milligram of protein was used for each pull-down assay, following the manufacturer's protocol.
Statistics were determined using the Student t test.
Results
Identification of PKC isoform involved in thrombin-induced permeability
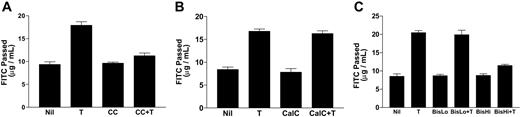
PKC activation has been linked to thrombin-induced changes in EC permeability,14-22 although the results from many laboratories are far from consistent. It would appear that species, organ, stimulant, and culture conditions can influence whether PKCs are involved, and if so, the relevant isoform. To clarify the role of PKC in our system and to determine the specificity of isoforms involved in thrombin-induced permeability, we took advantage of the varying specificities of PKC inhibitors. We used chelerythrine chloride, which inhibits all PKC isoforms,39 calphostin C (an inhibitor of classic and novel PKC40 ), and bisindolylmaleimide I (a concentration-dependent inhibitor of PKCs41 ). At 100 nM it inhibits classic and novel PKC isoforms whereas at 6 μM it inhibits all PKC isoforms.41,42 Using the passage of FITC-labeled dextran through monolayers of HUVE cells as a measure of permeability, we found that chelerythrine chloride at 1 μM (Figure 1A) inhibited thrombin-stimulated EC permeability, supporting the involvement of PKCs. Calphostin C (Figure 1B) and bisindolylmaleimide I at 100 nM (BisLo, Figure 1C) had no effect on the thrombin-induced EC permeability. However, bisindolylmaleimide I at 6 μM (BisHi, Figure 1C) inhibited the permeability change. The effects observed with both calphostin C and low-dose bisindolylmaleimide suggested that classic and novel PKCs are not involved, and the high-dose bisindolylmaleimide result together with chelerythrine chloride inhibitor suggests that atypical PKC isoforms may play a role in thrombin-induced EC permeability increases seen under these experimental conditions.
PKC inhibitors selectively block thrombin-stimulated permeability increases in ECs. Cells were untreated (Nil), treated with thrombin (0.2 U/mL; T) for 15 minutes, pretreated with inhibitor for 15 minutes, or pretreated with inhibitor followed by thrombin. FITC-dextran passage (μg/mL) during 30 minutes is shown. (A) Chelerythrine chloride (CC; 1 μM) blocks thrombin-stimulated permeability increases in ECs. (B) Calphostin C (CalC; 100 nM) does not block thrombin-stimulated permeability increases in ECs. (C) Bisindolylmaleimide I blocks thrombin-stimulated permeability increases in ECs at high concentrations (BisHi; 6 μM) but not at low concentrations (BisLo; 100 nM). Error bars indicate SEM.
PKC inhibitors selectively block thrombin-stimulated permeability increases in ECs. Cells were untreated (Nil), treated with thrombin (0.2 U/mL; T) for 15 minutes, pretreated with inhibitor for 15 minutes, or pretreated with inhibitor followed by thrombin. FITC-dextran passage (μg/mL) during 30 minutes is shown. (A) Chelerythrine chloride (CC; 1 μM) blocks thrombin-stimulated permeability increases in ECs. (B) Calphostin C (CalC; 100 nM) does not block thrombin-stimulated permeability increases in ECs. (C) Bisindolylmaleimide I blocks thrombin-stimulated permeability increases in ECs at high concentrations (BisHi; 6 μM) but not at low concentrations (BisLo; 100 nM). Error bars indicate SEM.
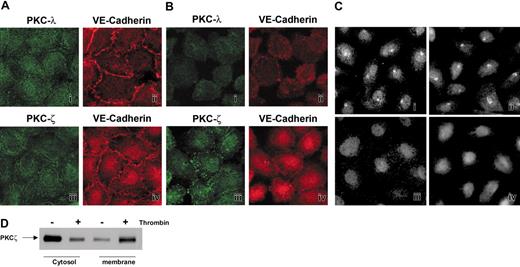
The 2 atypical PKC isoforms, PKCζ and PKCλ, are present in ECs.43 Since PKCs typically translocate to the membrane upon activation, we used translocation as an indicator as to whether these isoforms are involved in thrombin-mediated permeability changes.44,45 Confluent quiescent ECs were stained with anti-PKCζ and anti-PKCλ antibodies. Both PKCζ and PKCλ were distributed throughout the cytoplasm (Figure 2Ai,iii). The confluence of the ECs was confirmed by counter-staining for the junctional protein VE-cadherin (Figure 2Aii,iv). When these cells were treated with thrombin, PKCζ underwent redistribution (Figure 2Biii), whereas PKCλ (Figure 2Bi) remained evenly distributed despite significant contraction of individual cells, VE-cadherin, staining is shown in Figure 2Bii,iv. The cell contraction and the formation of gaps became evident with extended treatment with thrombin, as has been reported previously.10,12 Cells with and without thrombin treatment were also stained for PKCα and PKCϵ, since these 2 isoforms have been linked to thrombin-induced activation.19,21,22,27,28 PKCα displayed a similar diffuse staining pattern to PKCζ, and thrombin did not cause any relocalization (Figure 2Ci-ii). PKCϵ displayed nuclear staining, as previously reported,29 and there was no clear relocalization with thrombin treatment, even though there was obvious cell retraction (Figure 2Ciii-iv). These results support our inhibitor studies, suggesting that classic and novel PKC isoforms do not play a major role in thrombin-induced permeability changes in our system. Targeting of PKC isoforms to the membrane typically occurs upon enzyme activation,23 and the translocation of PKCζ in thrombin-induced permeability was confirmed by cell fractionation studies. The results shown in Figure 2D indicate enrichment of PKCζ in the membrane fraction after thrombin treatment.
Thrombin causes PKCζ relocalization in ECs. (A) Cells were stained for the 2 atypical isoforms of PKC, ζ and λ (i,iii), and for the junctional protein, VE-cadherin (ii,iv). (B) EC monolayers were stained to demonstrate changes in PKCλ (i) and PKCζ (iii) localization following treatment with thrombin (0.2 U/mL). Cells also were stained for VE-cadherin (ii,iv) to demonstrate changes in cell-cell interactions following thrombin stimulation. (C) ECs were stained for PKCα (i-ii) and PKCϵ (iii-iv). Treatment of cells with thrombin does not alter PKCα (ii) and PKCϵ (iv) localization. (D) Thrombin causes PKCζ redistribution to membranes. PKCζ immunoblot of cytosolic and membrane fractions of PKCζ-infected HUVE cells, fractioned following incubation with (+) or without (-) thrombin (0.2 U/mL) for 15 minutes.
Thrombin causes PKCζ relocalization in ECs. (A) Cells were stained for the 2 atypical isoforms of PKC, ζ and λ (i,iii), and for the junctional protein, VE-cadherin (ii,iv). (B) EC monolayers were stained to demonstrate changes in PKCλ (i) and PKCζ (iii) localization following treatment with thrombin (0.2 U/mL). Cells also were stained for VE-cadherin (ii,iv) to demonstrate changes in cell-cell interactions following thrombin stimulation. (C) ECs were stained for PKCα (i-ii) and PKCϵ (iii-iv). Treatment of cells with thrombin does not alter PKCα (ii) and PKCϵ (iv) localization. (D) Thrombin causes PKCζ redistribution to membranes. PKCζ immunoblot of cytosolic and membrane fractions of PKCζ-infected HUVE cells, fractioned following incubation with (+) or without (-) thrombin (0.2 U/mL) for 15 minutes.
Activation of PKCζ increases the permeability of ECs
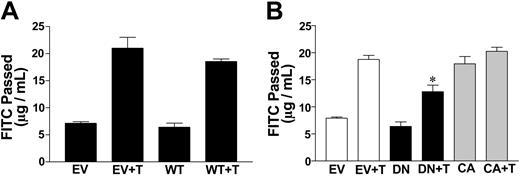
To confirm that PKCζ is a mediator of thrombin-stimulated increases in EC permeability, wild-type, dominant-negative, and constitutively active PKCζ were overexpressed in ECs by infection with adenoviruses carrying these constructs. Dominant-negative PKCζ has the critical threonine of the activation loop, at position 410, mutated to alanine.37 Constitutively active PKCζ results from fusion of the amino-terminal myristoylation sequence of p60 c-Src to the amino terminus of PKCζ, thereby constitutively targeting the resultant protein to the cell membrane.37 When cells overexpressing wild-type PKCζ were tested for permeability, they responded to thrombin in a similar way to cells infected with the empty vector (Figure 3A), indicating that overexpression of this isoform per se does not alter the permeability of the cells or their response to thrombin. Infection of ECs with adenovirus carrying dominant-negative PKCζ resulted in a slight decrease in the basal permeability in those lines tested, which may indicate that the mutant is able to inhibit some of the basal activity attributed to PKCζ. The dominant-negative PKCζ mutant was able to significantly inhibit the thrombin-stimulated increase in EC permeability when compared with cells infected with empty vector (Figure 3B). Conversely, ECs infected with adenovirus carrying constitutively active PKCζ were highly permeable, even in the absence of thrombin. When cells infected with the constitutively active PKCζ construct were stimulated with a maximal dose of thrombin (approximately 1 U/mL for 15 minutes), there was a 40% increase in permeability compared with the basal permeability (data not shown), indicating that thrombin is able to promote permeability over and above that seen in the presence of constitutively active PKCζ expression.
Activation of PKCζ increases the permeability of ECs. (A) Wild-type PKCζ does not affect basal- or thrombin-induced permeability. ECs were infected with pAdEasy-1 adenovirus empty vector (EV) or pAdEasy-1 constructs encoding wild-type PKCζ (WT), 2 days prior to assay. Cells were either untreated or treated with thrombin at 0.2 U/mL (T). FITC-dextran passage (μg/mL) during 30 minutes is shown. Data shown are means ± standard error of the mean (SEM) of the pool of 3 experiments where each experiment was performed in triplicate. (B) Dominant-negative PKCζ blocks thrombin-stimulated permeability increases in ECs. ECs were infected with pAdEasy-1 adenovirus empty vector (EV) or dominant-negative PKCζ (DN) or constitutively active PKCζ (CA) 2 days prior to assay. Cells were either untreated or treated with thrombin at 0.2 U/mL (T). FITC-dextran passage (measured in μg/mL) during 30 minutes is shown. Data are the means ± SEM of a pool of 2 experiments where each experiment was performed in triplicate for each group. *P < .005 (compared with EV and T).
Activation of PKCζ increases the permeability of ECs. (A) Wild-type PKCζ does not affect basal- or thrombin-induced permeability. ECs were infected with pAdEasy-1 adenovirus empty vector (EV) or pAdEasy-1 constructs encoding wild-type PKCζ (WT), 2 days prior to assay. Cells were either untreated or treated with thrombin at 0.2 U/mL (T). FITC-dextran passage (μg/mL) during 30 minutes is shown. Data shown are means ± standard error of the mean (SEM) of the pool of 3 experiments where each experiment was performed in triplicate. (B) Dominant-negative PKCζ blocks thrombin-stimulated permeability increases in ECs. ECs were infected with pAdEasy-1 adenovirus empty vector (EV) or dominant-negative PKCζ (DN) or constitutively active PKCζ (CA) 2 days prior to assay. Cells were either untreated or treated with thrombin at 0.2 U/mL (T). FITC-dextran passage (measured in μg/mL) during 30 minutes is shown. Data are the means ± SEM of a pool of 2 experiments where each experiment was performed in triplicate for each group. *P < .005 (compared with EV and T).
Angiopoietin-1 inhibits thrombin-induced relocalization and phosphorylation of PKCζ in ECs
As we have reported previously, Ang-1 is a potent inhibitor of thrombin-induced permeability8 (Figure 4A). It is also able to inhibit basal permeability, which is consistent with Ang-1 inhibiting basal EC activation. We therefore investigated whether the Ang-1 had an effect on PKCζ activation.
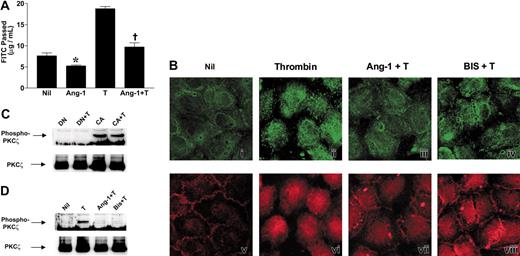
Angiopoietin-1 inhibits thrombin-induced PKCζ activation. (A) Ang-1 inhibits thrombin-stimulated EC permeability increases. Cells were untreated (Nil), treated with Ang-1 (Ang-1; 0.1 μg/mL) for 30 minutes, treated with thrombin (T; 0.2 U/mL) for 15 minutes, or pretreated with Ang-1 (0.1 μg/mL) for 30 minutes followed by thrombin (Ang-1 + T). FITC-dextran passage (measured in μg/mL) during 30 minutes is shown. Data shown are means ± SEM of a pool of 3 experiments where each group from each experiment was performed in triplicate. *P < .02 compared with Nil; †P < .0001 compared with thrombin alone. (B) EC monolayers were stained to demonstrate changes in PKCζ localization following: (i) no treatment (Nil); treatment with thrombin (0.2 U/mL) either alone (ii; Thrombin), after pretreatment with Ang-1 (iii; 0.1 μg/mL; Ang-1+ T), or after pretreatment with PKC inhibitor bisindolylmaleimide I (iv; 6 μM; BIS + T). Counterstaining for VE-cadherin is shown in panels v-viii. (C) Dominant-negative PKCζ inhibits thrombin-stimulated PKC phosphorylation. ECs were infected with pAdEasy-1 constructs encoding dominant-negative PKCζ (DN) or constitutively active PKCζ (CA). DN indicates cells overexpressing dominant-negative PKCζ with no treatment; DN + T, cells overexpressing dominant-negative PKCζ and treated with thrombin; CA, cells overexpressing constitutively active PKCζ with no treatment; CA + T, cells overexpressing constitutively active PKCζ treated with thrombin. Proteins were separated by SDS-PAGE before transfer to PVDF membrane and probing with an antibody directed against phosphorylated Thr410 of the activation loop of PKCζ (top panel). Membranes were stripped and reprobed with an anti-PKCζ antibody (bottom panel). (D) Ang-1 blocks thrombin-stimulated PKCζ phosphorylation. ECs were infected with pAdEasy-1 constructs encoding wild-type PKCζ. Cells were lysed after no treatment (Nil), treatment with thrombin (0.2 U/mL) either alone (T) or after pretreatment with Ang-1 (Ang-1 + T; 0.1 μg/mL) or bisindolylmaleimide I (Bis + T; 6 μM). Proteins were separated by SDS-PAGE before transfer to PVDF membrane and probing with an antibody directed against phosphorylated Thr410 of the activation loop of PKCζ (top panel). Membranes were stripped and reprobed with an anti-PKCζ antibody (bottom panel).
Angiopoietin-1 inhibits thrombin-induced PKCζ activation. (A) Ang-1 inhibits thrombin-stimulated EC permeability increases. Cells were untreated (Nil), treated with Ang-1 (Ang-1; 0.1 μg/mL) for 30 minutes, treated with thrombin (T; 0.2 U/mL) for 15 minutes, or pretreated with Ang-1 (0.1 μg/mL) for 30 minutes followed by thrombin (Ang-1 + T). FITC-dextran passage (measured in μg/mL) during 30 minutes is shown. Data shown are means ± SEM of a pool of 3 experiments where each group from each experiment was performed in triplicate. *P < .02 compared with Nil; †P < .0001 compared with thrombin alone. (B) EC monolayers were stained to demonstrate changes in PKCζ localization following: (i) no treatment (Nil); treatment with thrombin (0.2 U/mL) either alone (ii; Thrombin), after pretreatment with Ang-1 (iii; 0.1 μg/mL; Ang-1+ T), or after pretreatment with PKC inhibitor bisindolylmaleimide I (iv; 6 μM; BIS + T). Counterstaining for VE-cadherin is shown in panels v-viii. (C) Dominant-negative PKCζ inhibits thrombin-stimulated PKC phosphorylation. ECs were infected with pAdEasy-1 constructs encoding dominant-negative PKCζ (DN) or constitutively active PKCζ (CA). DN indicates cells overexpressing dominant-negative PKCζ with no treatment; DN + T, cells overexpressing dominant-negative PKCζ and treated with thrombin; CA, cells overexpressing constitutively active PKCζ with no treatment; CA + T, cells overexpressing constitutively active PKCζ treated with thrombin. Proteins were separated by SDS-PAGE before transfer to PVDF membrane and probing with an antibody directed against phosphorylated Thr410 of the activation loop of PKCζ (top panel). Membranes were stripped and reprobed with an anti-PKCζ antibody (bottom panel). (D) Ang-1 blocks thrombin-stimulated PKCζ phosphorylation. ECs were infected with pAdEasy-1 constructs encoding wild-type PKCζ. Cells were lysed after no treatment (Nil), treatment with thrombin (0.2 U/mL) either alone (T) or after pretreatment with Ang-1 (Ang-1 + T; 0.1 μg/mL) or bisindolylmaleimide I (Bis + T; 6 μM). Proteins were separated by SDS-PAGE before transfer to PVDF membrane and probing with an antibody directed against phosphorylated Thr410 of the activation loop of PKCζ (top panel). Membranes were stripped and reprobed with an anti-PKCζ antibody (bottom panel).
Staining of confluent quiescent ECs with the anti-PKCζ antibody showed PKCζ to be distributed throughout the cytoplasm (Figure 4Bi), and thrombin stimulation caused PKCζ to be localized to the cell membrane (Figure 4Bii). When ECs were pretreated with Ang-1 at 0.1 μg/mL for 30 minutes (Figure 4Biii), or high-dose bisindolylmaleimide I, for 15 minutes (Figure 4Biv), followed by thrombin treatment, PKCζ relocalization was dramatically decreased, suggesting inhibition of PKCζ activation by Ang-1.
To confirm the Ang-1 effect to inhibit PKCζ activation, another indicator of activation, the phosphorylation status of PKCζ was investigated. Phosphorylation of the threonine in the activation loop of PKCs is the critical first step of PKC activation. This is rapidly followed by autophosphorylation of a threonine and a serine residue within the catalytic domain and concomitant translocation from the cytoplasm to the cell membrane.23,37 Phosphorylated PKCζ could not be detected using an antibody specific for the phosphorylated Thr 410 within the activation loop of PKCζ following thrombin treatment of normal ECs, presumably because the levels of the phosphorylated PKCζ are low. Wild-type PKCζ was then overexpressed by adenoviral infection. After 24 hours of serum starvation, ECs infected with dominant-negative PKCζ showed no PKCζ phosphorylation, either basally or in response to thrombin, whereas those expressing constitutively active PKCζ showed constitutive PKCζ phosphorylation that was not altered by thrombin stimulation (Figure 4C). In cells overexpressing wild-type PKCζ, thrombin treatment for 15 minutes increased the phosphorylation of PKCζ (Figure 4D), whereas pretreatment with Ang-1 at 0.1 μg/mL for 30 minutes or bisindolylmaleimide I at 6 μM for 15 minutes inhibited thrombin-stimulated PKCζ phosphorylation (Figure 4D). Thus, thrombin stimulates PKCζ activation (phosphorylation and translocation), and Ang-1 inhibits activation.
Ang-1 inhibits actin cytoskeleton reorganization, Rho activation, and the Ca++ flux induced by thrombin
Having determined that Ang-1 can inhibit the PKCζ activation induced by thrombin we next investigated whether Ang-1 can inhibit other signaling pathways involved in thrombin-induced permeability.
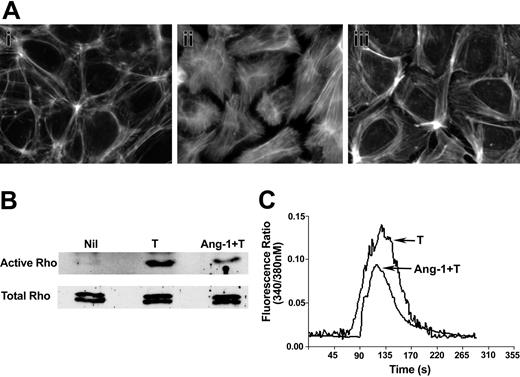
Cell contraction and shape change are dependent on actin reorganization, which can be regulated by the small GTPase Rho.10,11,28 Unstimulated cells displayed a cortical actin-type staining (Figure 5Ai). Thrombin induced the classic pattern of stress fiber formation (Figure 5Aii), which was inhibited by Ang-1 (Figure 5Aiii), showing the typical cortical actin-type staining pattern seen in untreated ECs (Figure 5Ai), a characteristic of an intact impermeable monolayer.10,13 Consistent with these actin-based changes, Ang-1 also inhibited thrombin-induced activation of Rho (Figure 5B), as measured in pull-down assays. In the 3 experiments performed, Ang-1 induced a 40% to 60% inhibition of the active Rho compared with thrombin alone.
Ang-1 regulates thrombin-induced actin stress fiber formation, Rho activity, and Ca++ flux. (A) EC monolayers were unstimulated (i), stimulated with thrombin (0.2 U/mL) alone for 10 minutes (ii), or pretreated with Ang-1 (0.1 μg/mL) for 30 minutes followed by thrombin (iii). Cells were stained using rhodamine-phalloidin for detection of F-actin. (B) Cells were either unstimulated (Nil), stimulated with thrombin alone (T), or pretreated with Ang-1 and then stimulated with thrombin (Ang-1 + T) and analyzed for active Rho (top panel). Total Rho in the cell lysates is shown in the bottom panel. (C) Cells were loaded with fura 2 either in the absence or presence of Ang-1 (0.1 μg/mL) for 60 minutes. Cells were then treated with thrombin (0.2 U/mL) and fluorescence was measured. The pooled results, expressed as fluorescence ratio, are given for 100 cells from each of 3 experiments.
Ang-1 regulates thrombin-induced actin stress fiber formation, Rho activity, and Ca++ flux. (A) EC monolayers were unstimulated (i), stimulated with thrombin (0.2 U/mL) alone for 10 minutes (ii), or pretreated with Ang-1 (0.1 μg/mL) for 30 minutes followed by thrombin (iii). Cells were stained using rhodamine-phalloidin for detection of F-actin. (B) Cells were either unstimulated (Nil), stimulated with thrombin alone (T), or pretreated with Ang-1 and then stimulated with thrombin (Ang-1 + T) and analyzed for active Rho (top panel). Total Rho in the cell lysates is shown in the bottom panel. (C) Cells were loaded with fura 2 either in the absence or presence of Ang-1 (0.1 μg/mL) for 60 minutes. Cells were then treated with thrombin (0.2 U/mL) and fluorescence was measured. The pooled results, expressed as fluorescence ratio, are given for 100 cells from each of 3 experiments.
Thrombin-induced activation of PAR-1, among other events, raises intracellular Ca++ concentration through mobilization of Ca++ from intracellular stores, and this increase in intracellular Ca++ controls the activity of the Ca++/calmodulin-dependent MLCK which also affects the actin-based cytoskeletal machinery.46 To determine whether Ang-1 affects this arm of thrombin signaling, the thrombin-induced Ca++ flux was measured in fura 2-loaded ECs in the presence or absence of Ang-1. Ang-1 at 0.1 μg/mL was used as we have previously demonstrated that this is optimal for inhibition of thrombin-induced permeability. Figure 5C shows that the Ang-1 significantly inhibited the thrombin-induced Ca++ flux.
Rho activation pathways are not involved in thrombin-induced permeability changes through PKCζ
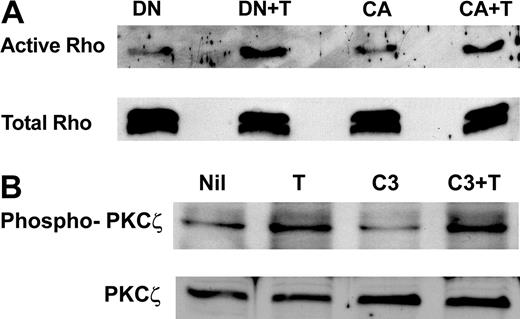
Since there are reports that Rho and PKCζ are able to regulate the activity of each other,42,47 we first sought to determine whether Rho is a downstream target for PKCζ. The results in Figure 6A show that the dominant-negative PKCζ does not block thrombin-induced Rho activation nor does constitutively active PKCζ regulate basal Rho activity. When cells expressing constitutively active or dominant-negative PKCζ were stained for actin stress fiber formation, no alteration from basal unstimulated cells was observed (data not shown).
Rho activation is not involved in thrombin-induced permeability changes mediated by PKCζ (A) Blocking PKCζ activation does not block thrombin-induced Rho activation. ECs were infected with pAdEasy-1 constructs encoding dominant-negative PKCζ or constitutively active PKCζ. DN indicates cells overexpressing dominant-negative PKCζ with no treatment; DN + T, cells overexpressing dominant-negative PKCζ and treated with thrombin; CA, cells overexpressing constitutively active PKCζ with no treatment; CA + T, cells overexpressing constitutively active PKCζ treated with thrombin. One milligram of each total cell lysate was analyzed for active Rho (top panel). Total Rho is shown in the bottom panel. (B) Inhibition of Rho does not inhibit thrombin-induced PKCζ phosphorylation. ECs were infected with a pAdEasy-1 construct encoding wild-type PKC-ζ, or pretreated with the Rho inhibitor Clostridium botulinum C3 transferase followed by thrombin. Nil indicates untreated; T, thrombin alone; C3, C3 transferase alone; C3 + T, pretreated with C3, then treated with thrombin. Proteins were separated by SDS-PAGE before transfer to PVDF membrane, and probing with an antibody directed against phosphorylated Thr410 of the activation loop of PKCζ (top panel). Membranes were stripped and reprobed with an anti-PKCζ antibody (bottom panel).
Rho activation is not involved in thrombin-induced permeability changes mediated by PKCζ (A) Blocking PKCζ activation does not block thrombin-induced Rho activation. ECs were infected with pAdEasy-1 constructs encoding dominant-negative PKCζ or constitutively active PKCζ. DN indicates cells overexpressing dominant-negative PKCζ with no treatment; DN + T, cells overexpressing dominant-negative PKCζ and treated with thrombin; CA, cells overexpressing constitutively active PKCζ with no treatment; CA + T, cells overexpressing constitutively active PKCζ treated with thrombin. One milligram of each total cell lysate was analyzed for active Rho (top panel). Total Rho is shown in the bottom panel. (B) Inhibition of Rho does not inhibit thrombin-induced PKCζ phosphorylation. ECs were infected with a pAdEasy-1 construct encoding wild-type PKC-ζ, or pretreated with the Rho inhibitor Clostridium botulinum C3 transferase followed by thrombin. Nil indicates untreated; T, thrombin alone; C3, C3 transferase alone; C3 + T, pretreated with C3, then treated with thrombin. Proteins were separated by SDS-PAGE before transfer to PVDF membrane, and probing with an antibody directed against phosphorylated Thr410 of the activation loop of PKCζ (top panel). Membranes were stripped and reprobed with an anti-PKCζ antibody (bottom panel).
To determine whether PKCζ is regulated by Rho in our system, we used the specific Rho inhibitor Clostridium botulinum C3 transferase. Although this inhibitor totally blocked thrombin-induced actin cytoskeleton reorganization (data not shown), it had no effect on thrombin-induced PKCζ phosphorylation (Figure 6B), suggesting that Rho was not involved in PKCζ activation.
Discussion
The mechanisms involved in determining the barrier function of vascular ECs have been the subject of much investigation. Thrombin leads to increased EC permeability mediated through its receptor PAR-1 activating multiple downstream signaling intermediates. At least 2 of these signaling pathways, the Rho/Rho kinase pathway and the elevation of intracellular Ca++, both of which result in MLCK activation, have been firmly linked to permeability increases mediated through changes in the actin cytoskeleton. Our data described herein identify a new pathway involving the atypical PKC isoform, PKCζ, in the regulation of thrombin-induced EC leakiness. We have previously shown that the vasculogenic factor Ang-1 inhibits thrombin-induced permeability increases.8 Our results here show that Ang-1 achieves its inhibition of EC permeability by acting on the above thrombin-stimulated pathways linked to permeability; that is, Ang-1 inhibits Ca++ flux, inhibits Rho activation, and inhibits activation of PKCζ. These results add to our previous findings,8 where we showed that Ang-1 also acts to inhibit the thrombin-induced tyrosine phosphorylation of VE-cadherin and PECAM-1,8 suggesting stabilization of the EC junctions by Ang-1.
Thrombin is a serine protease with multiple roles central to vascular biology, acting on platelets, ECs, and circulating clotting factors.48 Thrombin is a potent activator of ECs, increasing intercellular gap formation and the permeability of confluent EC monolayers.4,14 Thrombin signaling is mediated by the protease-activated receptor PAR-1.9 PAR-1 can activate a number of downstream signaling pathways, with the molecules activated depending on the G proteins recruited to the receptor, with PKC shown to be one such downstream intermediate.48 In HUVE cells, all PKC classes are represented, with the classic isoform PKCα, the novel isoform PKCϵ, and the atypical isoform PKCζ predominating.43,49 Previous studies of the effects of PKC activation on EC permeability have produced conflicting results, with some authors concluding that PKC activation does not have a significant role in permeability changes in ECs,10,11 while others conclude that PKC activation is important.49,50 Studies using phorbol-12-myristate-13-acetate as a diacylglycerol surrogate to stimulate PKC activation have shown both inhibition10,51 and stimulation10,22,52 of EC permeability in a concentration-dependent manner.10,11 We chose a range of PKC inhibitors for which the mechanism of inhibition is well characterized.53 This allowed us to narrow down the class of PKC that is likely to be involved in the thrombin stimulation under our experimental conditions as an atypical PKC, and not a classic or novel isoform. Subsequently, by relocalization studies, we showed that the distribution of PKCα and PKCϵ isoforms, reported by some groups to be involved in thrombin-induced permeability, was not altered after thrombin treatment, in agreement with our inhibitor studies, which suggested that classic and novel PKC isoforms are not involved in our system. The localization studies showed that PKCζ distribution is altered by thrombin, while PKCλ is not, and fractionation experiments demonstrated that this redistribution was from the cytosol to the membrane. Since membrane localization typically indicates activation, these results suggested the activation of PKCζ by thrombin. Finally, with the use of specific gene tools, namely dominant-negative and constitutively active PKCζ constructs, we confirmed the essential role of PKCζ in the control of EC permeability.
There are now a number of important pathways activated by thrombin that are critical in permeability increases. One involves the thrombin-induced elevation of intracellular Ca++, which regulates the calcium-dependent calmodulin MLC kinase, resulting in a transient increase in MLC phosphorylation. A second pathway results in activation of Rho/Rho kinase, which regulates MLC phosphatase activity, and further controls activation of MLC. MLC is the common intermediate between these 2 pathways, and its phosphorylation appears to be the key event that regulates EC contractile forces acting through the actin cytoskeleton, with impact on EC-EC and EC-matrix interactions. Another pathway, reported by Van Nieuw Amerongen and colleagues,10,11 involves protein tyrosine kinase-dependent activation of proteins such as VE-cadherin that control junctional integrity. Their work also suggests that the Ca++, Rho kinase, and protein tyrosine kinase pathways induced by thrombin and involved in permeability reflect separate pathways. Whether the PKCζ pathway induced by thrombin is another separate pathway or interacts with these aforementioned pathways remains to be determined.
In an attempt to determine the mechanism of PKCζ-dependent control of permeability, we reasoned that Rho was a likely downstream target of PKCζ. However, we show that PKCζ activation by thrombin resulting in permeability changes does not involve Rho, acting either upstream or downstream of PKCζ (Figure 6). This is interesting given that chemoattractant-induced leukocyte adhesion and chemotaxis are mediated through Rho-dependent activation of PKCζ.39 In addition, the Ras-mediated activation of NIH-3T3 cells, which results in profound disassembly of actin stress fibers, acts through Rac-dependent activation of PKCζ.42 How then does PKCζ regulate EC permeability? The identification of downstream effectors of PKCζ will help to answer this question. One possibility is that PKCζ directly associates with and phosphorylates junctional adhesion molecules such as PECAM, which is important in EC integrity and is known to be altered in both localization and phosphorylation status following thrombin stimulation. Indeed, serine/threonine phosphorylation of PECAM is important for its function, for its association with proteins such as γ catenin, and for its localization.54 However, our initial experiments have not shown any physical association between PKCζ and PECAM. An alternative possibility is that PKCζ may be in complexes with proteins such as PAR3. In epithelial cells atypical PKCs are known to associate in a PAR3/PAR6/aPKC complex, which localizes to tight junctions and regulates cell polarity.55-58 PAR3 also associates directly with the junctional adhesion molecule JAM.59,60 In ECs, JAM-2, which is localized at intercellular contacts, promotes leukocyte transendothelial cell migration,61 a process that, like altered cell permeability, involves junctional alteration.
In terms of the activation of the endothelium, it is interesting to note that PKCζ may be an important pivot for regulation of the inflammatory response. PKCζ is involved in tumor necrosis factor-induced intercellular adhesion molecule-1 gene transcription62 through nuclear factor (NF)-κB activation. More recently, it has been shown that PKCζ is involved in the protein-synthesis-independent activation of ICAM-1, resulting in the early onset of enhanced neutrophil adhesion to ECs. This activation occurs via PKCζ-dependent phosphorylation of ICAM-1.63 Furthermore, Ras-induced transcriptional activation of VEGF synthesis is through PKCζ.47 Thus, PKCζ is involved in permeability changes as shown here, in leukocyte adhesion and in angiogenesis, all of which are important aspects of inflammation.
Ang-1 is a powerful anti-inflammatory, antipermeability agent,8 as well as mediating vascular EC survival and angiogenic properties.28,32 Ang-1 signals via the protein tyrosine kinase receptor Tie2 through a phosphoinositide 3 kinase (PI3K)-AKT-dependent pathway to mediate its prosurvival activity. The PI3K pathway leading to the generation of endothelial nitric oxide (NO) is involved in the angiogenic response to Ang-1.64 A recent report shows that Tie2 can bind ABIN-2, the A20 binding inhibitor that is involved in inhibition of the NF-κB-dependent inflammatory response in ECs.65 However, very little is known about other signaling pathways involved in Tie2-dependent events, especially signals that may influence permeability changes. At present, we have not resolved whether Ang-1 regulates the permeability pathways of Ca++ flux, Rho/Rho kinase, junctional protein regulation, and PKCζ activation through separate signaling events or through a common upstream regulator, for example, an adaptor molecule. What is clear from our work, and that of other researchers, is that the anti-inflammatory, antiangiogenic properties of Ang-1 are broad and play a key role in vascular control. Therefore, the signals emanating from receptor-ligand interaction are likely to be many and varied, and their elucidation awaits further investigation.
Prepublished online as Blood First Edition Paper, June 1, 2004; DOI 10.1182/blood-2003-11-3744.
Supported by grants from the National Health and Medical Research Council of Australia, National Heart Foundation of Australia, and the Anti-Cancer Foundation of South Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Drs Pu Xia, Michel Aurrand-Lion, Wayne Rankin, and Charles Hii for helpful discussions; Dr Roland B Gregory for help with the Ca++ flux measurements; and Dr Alex Toker for the gift of PKCζ constructs and antiphospho PKCζ antibody. The collection of umbilical cords by the staff of the delivery wards of the Women's and Children's and Burnside War Memorial Hospitals, Adelaide, and the growth of endothelial cells by Anna Sapa are greatly appreciated.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal