Abstract
We investigated the role of Akt-1, one of the major downstream effectors of phosphoinositide 3-kinase (PI3K), in platelet function using mice in which the gene for Akt-1 had been inactivated. Using ex vivo techniques, we showed that Akt-1-deficient mice exhibited impaired platelet aggregation and spreading in response to various agonists. These differences were most apparent in platelets activated with low concentrations of thrombin. Although Akt-1 is not the predominant Akt isoform in mouse platelets, its absence diminished the amount of total phospho-Akt and inhibited increases in intracellular Ca2+ concentration in response to thrombin. Moreover, thrombin-induced platelet α-granule release as well as release of adenosine triphosphate from dense granules was also defective in Akt-1-null platelets. Although the absence of Akt-1 did not influence expression of the major platelet receptors for thrombin and collagen, fibrinogen binding in response to these agonists was significantly reduced. As a consequence of impaired αIIbβ3 activation and platelet aggregation, Akt-1 null mice showed significantly longer bleeding times than wild-type mice. (Blood. 2004;104:1703-1710)
Introduction
Under normal conditions, platelets circulate freely in the blood without interacting with each other or the vessel wall. On vascular injury, subendothelial matrix proteins, including collagens, or soluble agonists trigger platelet activation. The hallmark of platelet activation is the transformation of the major platelet glycoprotein, αIIbβ3, from its resting to active state, which serves as a fibrinogen receptor, thereby mediating platelet aggregation. One of the most potent platelet agonists, thrombin, acts via a dual system of G protein-coupled protease-activated receptors, PAR3 and PAR4. Both of these receptors are required for optimal thrombin-induced aggregation and secretion.1
The majority of platelet agonists, including thrombin and collagen, activate phosphoinositide 3-kinase (PI3K) in platelets. Inhibitors of PI3K (wortmannin and LY294002) block fibrinogen binding and platelet aggregation induced by thrombin and collagen, indicating a role for PI3K in αIIbβ3 activation.2 Platelets contain 2 major forms of PI3K, p85/p110 PI3K, composed of a p110 catalytic and p85 regulatory subunit and PI3Kγ, composed of a p110γ catalytic and p101 regulatory subunit. Both forms of PI3K are involved in the inside-out signaling that activates αIIbβ3 and induces platelet aggregation.2
Recent studies demonstrated that a deficiency in the p85α regulatory subunit in mice leads to a significant reduction of collagen-induced platelet aggregation, particularly at low doses of stimulus..3 The absence of PI3Kγ activity, indicated by the lack of Akt phosphorylation, leads to impaired platelet aggregation in response to adenosine diphosphate (ADP) and protects against thrombosis.4
PI3Ks generate phosphoinositide products that target the Tec family tyrosine kinases, serine/threonine protein kinases such asAkt, guanosine diphosphate/guanosine triphosphate exchange factors, and phospholipase γ.5,6 Among these, Akt is known to be one of the major downstream effectors of PI3K.7,8 Three isoforms of Akt, which possess more than 80% homology, have been identified, Akt-1, Akt-2, and Akt-3.7,9,10 In human platelets, the major isoform is Akt-1.9
In platelets, Akt is phosphorylated in response to a number of stimuli, including collagen, thrombin, and phorbol myristic acid (PMA).9,11,12 Several studies have shown that Akt phosphorylation occurs in thrombin- and collagen-stimulated platelets even when fibrinogen binding and platelet aggregation are inhibited, indicating that Akt might be involved in inside-out αIIbβ3 signaling.9,13 Although it is accepted that the activation of Akt in platelets depends on the phospholipid products of PI3K activity, some studies have reported an existence of a second partially PI3K-independent mechanism.9
The purpose of this study was to define the role of Akt-1 kinase, a principal downstream effector of the PI3K signaling pathway, in platelet function ex vivo and in vivo using Akt-1 null mice.
Materials and methods
Animals
Akt-1-deficient mice were generated in the laboratory of one of the authors (N.H.) and maintained on 129 R1/C57BL/6 backgrounds.14 Ten- to 14-week-old wild-type (WT) and Akt-1 null littermates were used in the study.
Preparation of washed platelets
While the mice were under anesthesia, 800 μL blood was drawn from the inferior vena cava of each mouse into a syringe containing 5 mM EDTA (ethylenediaminetetraacetic acid) and 1 μg/mL prostaglandin E1 (PGE1; final concentrations). Platelets were obtained from platelet-rich plasma of blood pooled from 3 to 5 mice by gel filtration as described previously.15
Platelet aggregation
Platelet aggregation stimulated by 15 μg/mL collagen, 10 μM ADP, 100 nM PMA, or various concentrations of thrombin (0.04-1 U/mL) was monitored using a Lumi-Aggregometer type 500 VS (Chrono-Log, Haver-town, PA). In some experiments, fibrinogen was added at a final concentration of 200 μg/mL.
Fibrinogen binding and FACS analysis
To analyze fibrinogen binding, platelets were stimulated with ADP (10 μM), PMA (100 nM), or various concentrations of thrombin (0.1-0.5 U/mL) followed by 5 μg/mL d-phenylalanyl-l-prolyl-l-argininechloromethyl ketone (PPACK). Fluorescein isothiocyanate (FITC)-labeled fibrinogen was added at a final concentration of 300 nM for 30 minutes. To analyze glycoprotein VI (GPVI), αIIbβ3, and P-selectin expression, gel-filtered platelets were incubated with FITC-labeled anti-GPVI (Emfret Analytics, Wurzburg, Germany), anti-β3 integrin (Chemicon International, Temecula, CA), or FITC-labeled anti-P-selectin (BD Biosciences PharMingen, San Diego, CA) antibodies (10 μg/mL each) for 30 minutes. Fluorescence-activated cell sorting (FACS) was performed using a FACSCalibur (Becton Dickinson, San Jose, CA) and data were analyzed using the manufacturer's CellQuest software program.
Adhesion assays
Platelet adhesion to collagen was performed as previously described.16 Briefly, coverslips were coated with 20 μg/mL Horm collagen (Hormon-Chemie, Munchen, Germany) and gel-filtered platelets (2 × 107/mL) were added in the absence of calcium and in the presence of 2 U/mL apyrase. Attached platelets stained with tetramethylrhodamine isothiocyanate (TRITC)-phalloidin were viewed using a fluorescence microscope, and the numbers of attached and spread platelets per field were counted.
Western blotting
Gel-filtered platelets (1 × 108/mL) were incubated with the integrin inhibitors echistatin (20 μg/mL) and eptifibatide (50 μg/mL) to block fibrinogen binding, then stimulated with thrombin (0.01, 0.05 or 0.1 U/mL) or collagen (20 μg/mL) for 5 and 15 minutes, respectively, followed by PPACK (5 μg/mL). Levels of total Akt and phospho-Akt were detected by Western blot analysis using antibodies against Akt or phospho-Ser473 Akt (Cell Signaling Technology, Beverly, MA), as described previously.17 To analyze thrombin receptor levels, 40 μg platelet lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with polyclonal antibody for thrombin receptor PAR-3 or PAR-4 (all from Santa Cruz Biotechnology, Santa Cruz, CA). Equal loading was confirmed by reprobing the stripped membranes with β-actin antibody (Sigma Chemical, St Louis, MO).
ATP release
The release of adenosine triphosphate (ATP) was measured in a Lumi-Aggregometer (Chrono-Log) using 250 μL gel-filtered platelets according to the manufacturer's recommendations.
Measurement of intracellular Ca2+ concentration
Gel-filtered platelets were loaded with 2 μM fura-2/am (Molecular Probes, Eugene, OR), washed, and resuspended in Tyrode buffer with 1 mM CaCl2 or 0.5 mM EGTA (ethyleneglycol biscaminoethyl ether tetraacetic acid). Fluorescence measurements were performed in a 4-clear-sided cuvette (Fisher Scientific, Pittsburgh, PA) using a dual-wavelength spectrofluorometer (Deltascan RFK6002; Photon Technology International, Lawrenceville, NJ) at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm as described.18 Fura-2 fluorescence signals were collected and analyzed using software from Photon Technology International (Felix).
Tail vein bleeding times
Tail vein bleeding times (TVBTs) were measured as described19 with modifications to provide a stronger hemostatic challenge as previously described.20 Pairs of mice were anesthetized and placed on a prewarmed pad. Because the Akt-1-null mice are smaller in size, their tails and tail veins are also about 20% to 30% smaller. To monitor bleeding times under conditions similar for WT and Akt null mice, tails were cut with a scalpel at the position where the diameter of the tail was 2.5 mm. Each tail was immersed in normal saline (37°C). The time from the incision to cessation of bleeding was recorded as the TVBT. The amount of shed blood was also measured.
Whole blood platelet counting
Blood samples from 6 mice of each genotype were analyzed using a hematology analyzer (Bayer, Tarrytown, NY). Adjusted platelet numbers are shown as means ± SE.
Statistical analysis
Values were expressed as means ± SE (or SD as indicated in figure legends). In most assays, statistical significance was evaluated using 2-sample t test. For bleeding time experiments, the difference between WT and Akt-1 null mice was analyzed using nonparametric log-rank test. Results were considered statistically significant with P less than .05.
Results
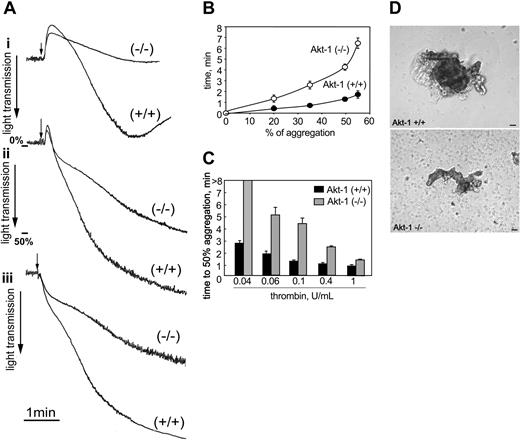
We first assessed the effects of Akt-1 deficiency on thrombin-induced platelet aggregation. Dramatic differences in aggregation were evident in response to thrombin at concentrations ranging from 0.04 U/mL to 0.4 U/mL (Figure 1). Representative aggregation curves are shown in Figure 1A (i, 0.04 U/mL; ii, 0.1 U/mL; and iii, 0.4 U/mL). Although aggregation of WT platelets was still evident with low-dose thrombin stimulation (0.04 U/mL), the same treatment did not induce a detectable response in Akt-1-null platelets (Figure 1Ai). At higher concentrations of thrombin (0.1 U/mL or 0.4 U/mL) Akt-1-null platelets also showed reduced and delayed aggregation responses compared to WT platelets (Figure 1Aii,iii). The maximal extent of aggregation of Akt-1-null platelets in response to 0.1 U/mL thrombin was reduced (55% ± 6%) compared to WT platelets (85% ± 10%) (Figure 1Aii). Furthermore, aggregation of Akt-1-null platelets was delayed compared to WT platelets, that is, the time from addition of agonist to 50% aggregation was tripled in Akt-1-null platelets (Figure 1B). Overall, the time required to achieve 50% aggregation at thrombin concentrations ranging from 0.04 U/mL to 0.4 U/mL was increased more than 2-fold in Akt-1-null platelets (Figure 1C). However, at higher doses of thrombin (1 U/mL) the extent of platelet aggregation was similar for WT and Akt-1-null platelets, although the time to complete the aggregation response was still slightly longer for Akt-1-null platelets (Figure 1C). Platelet aggregates from Akt-1-null mice were markedly smaller compared to WT, and a number of single nonaggregated platelets were present (Figure 1D).
Platelet aggregation in response to thrombin. Platelet aggregation was stimulated with thrombin and optically monitored in a Lumi-Aggregometer. (A) Representative aggregation curves in response to thrombin at 0.04 U/mL (i), 0.1 U/mL (ii), and 0.4 U/mL (iii). Arrows indicate the points of agonist addition. (B) Time from the addition of 0.1 U/mL thrombin to 20%, 35%, 50%, and 55% aggregation in WT (•) and Akt-1-null platelets (○). Means ± SD of 3 independent experiments are shown. (C) WT and Akt-1-null platelets were stimulated with thrombin at 0.04, 0.06, 0.1, 0.4, and 1 U/mL. Bars represent means ± SD of time from addition of agonist to 50% aggregation from 3 independent experiments. (D) Platelets from WT and Akt-1 null mice aggregated by 0.1 U/mL thrombin were visualized using phase contrast microscopy (Leica) and photographed (Micromax). Scale bars equal 20 μm. Images were acquired using a Leica DMIRB phase contrast microscope, objective × 20, and a Micromax RTE/CCD-1300-V-HS camera.
Platelet aggregation in response to thrombin. Platelet aggregation was stimulated with thrombin and optically monitored in a Lumi-Aggregometer. (A) Representative aggregation curves in response to thrombin at 0.04 U/mL (i), 0.1 U/mL (ii), and 0.4 U/mL (iii). Arrows indicate the points of agonist addition. (B) Time from the addition of 0.1 U/mL thrombin to 20%, 35%, 50%, and 55% aggregation in WT (•) and Akt-1-null platelets (○). Means ± SD of 3 independent experiments are shown. (C) WT and Akt-1-null platelets were stimulated with thrombin at 0.04, 0.06, 0.1, 0.4, and 1 U/mL. Bars represent means ± SD of time from addition of agonist to 50% aggregation from 3 independent experiments. (D) Platelets from WT and Akt-1 null mice aggregated by 0.1 U/mL thrombin were visualized using phase contrast microscopy (Leica) and photographed (Micromax). Scale bars equal 20 μm. Images were acquired using a Leica DMIRB phase contrast microscope, objective × 20, and a Micromax RTE/CCD-1300-V-HS camera.
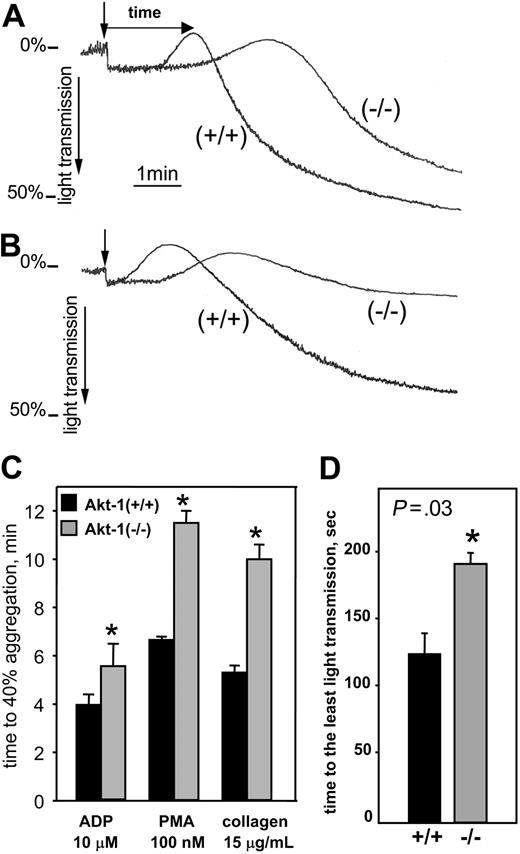
We next assessed the response of Akt-1-null platelets to other representative agonists known to cause platelet aggregation. As evident from Figure 2, Akt-1-null platelets exhibited a markedly delayed response to collagen. Representative curves of collagen-stimulated platelet aggregation are shown in the presence (Figure 2A) or absence (Figure 2B) of fibrinogen. When fibrinogen was present, platelet aggregation in response to collagen was only slightly diminished, but was markedly delayed in Akt-1-null platelets compared to WT (Figure 2A). Moreover, in the absence of fibrinogen, Akt-1-null platelets failed to form aggregates, whereas WT platelets showed a significant aggregation response (Figure 2B). In the presence of fibrinogen the time required to achieve 40% aggregation was prolonged in Akt-1-null platelets by 2-fold in response to collagen (P = .02; Figure 2C). Particularly noticeable, the response of Akt-1-null platelets was substantially slower during the earliest stages of platelet activation, which are associated with platelet shape change. The time from addition of collagen to the lowest point of light transmission (indicated by arrows in Figure 2A) was prolonged by 1.5-fold in Akt-1-deficient mice compared to WT mice (P = .03; Figure 2D). In response to PMA, the time required to achieve 40% aggregation was prolonged in Akt-1-null platelets by 1.7-fold (P = .04; Figure 2C). ADP-stimulated aggregation of Akt-1-deficient platelets was prolonged 1.3-fold compared to WT (Figure 2C; P = .045). Thus, relative to thrombin and collagen, the ADP-induced response was less affected by the absence of Akt-1.
Platelet aggregation in response to collagen, PMA, or ADP. Platelet aggregation was stimulated by collagen (15 μg/mL), PMA (100 nM), or ADP (10 μM) and optically monitored. (A-B) Representative aggregation curves in response to collagen in the presence (A) and absence (B) of 200 μg/mL fibrinogen. Arrows indicate the point of collagen addition. (C) Time from addition of agonist to 40% aggregation in response to ADP, PMA, and collagen. (D) For collagen-induced platelet aggregation, times from agonist addition to the point where light transmission was minimal (as indicated in panel A). For both panels C and D, bars show means ± SD of 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05).
Platelet aggregation in response to collagen, PMA, or ADP. Platelet aggregation was stimulated by collagen (15 μg/mL), PMA (100 nM), or ADP (10 μM) and optically monitored. (A-B) Representative aggregation curves in response to collagen in the presence (A) and absence (B) of 200 μg/mL fibrinogen. Arrows indicate the point of collagen addition. (C) Time from addition of agonist to 40% aggregation in response to ADP, PMA, and collagen. (D) For collagen-induced platelet aggregation, times from agonist addition to the point where light transmission was minimal (as indicated in panel A). For both panels C and D, bars show means ± SD of 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05).
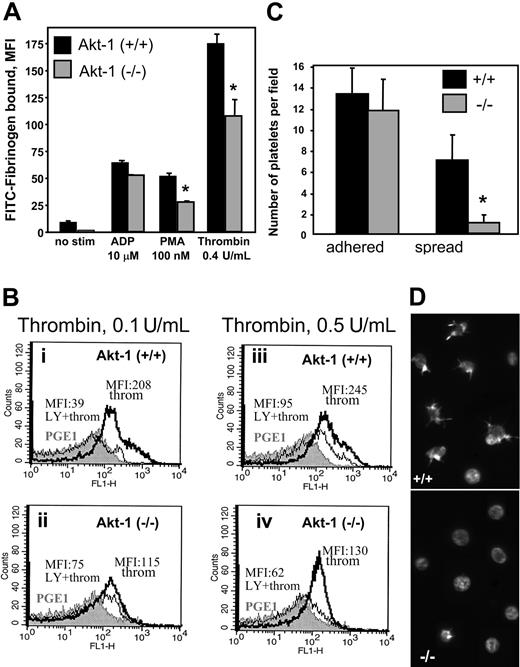
Fibrinogen binding is a critical step in platelet aggregation and serves as an indicator of αIIbβ3 activation. Disruption of the PI3K/Akt pathway by wortmannin blocks αIIbβ3 activation and, hence, fibrinogen binding.2 To evaluate the role of Akt-1 in αIIbβ3 activation, we performed an analysis of fibrinogen binding to platelets from WT and Akt-1 null mice. Stimulation of WT platelets with ADP, PMA, or thrombin as model agonists induced 7-, 5.5-, and 17-fold increases in the binding of fibrinogen, respectively, as determined by FACS analysis (Figure 3A). In Akt-1-null platelets, fibrinogen binding was reduced by 35% (P < .01) and 43% (P = .04) compared to WT in response to thrombin and PMA, respectively. Of note, the differences were most evident at the early stages of fibrinogen binding, that is, when incubation of platelets with fibrinogen was shorter than 30 minutes. Surprisingly, Akt-1-null platelets bound fibrinogen normally in response to ADP (Figure 3A), including at low concentrations of this agonist (2 μM; data not shown).
Fibrinogen binding and platelet adhesion. (A) Fibrinogen binding. Platelets were preincubated with ADP (10 μM), PMA (100 nM), and thrombin (0.4 U/mL). FITC-labeled fibrinogen was then added at a final concentration of 300 nM for 30 minutes and fibrinogen binding was analyzed by FACS. A total of 20 000 events per sample was recorded. Bars present MFI ± SD of triplicates from 2 representative experiments. (B) Fibrinogen binding in the presence of LY294002. Platelets from 3 pairs of WT (i,iii) and Akt-1 null (ii,iv) mice were stimulated with thrombin at 0.1 U/mL or 0.5 U/mL, respectively, in the presence or absence of LY294002 (80 μM). FITC-labeled fibrinogen at concentration of 300 nM was added. Fibrinogen binding was analyzed by FACS analysis and 10 000 events per sample were recorded. Gray profiles represent fibrinogen binding in the presence of PGE1; MFIs are shown in response to thrombin in the presence (thin lines) and the absence (thick lines) of LY294002, respectively. Similar results were obtained in an additional 2 experiments. (C-D) Platelet adhesion to collagen. Platelets were resuspended in calcium-free Tyrode buffer containing 2 U/mL apyrase. Platelet suspensions were added to coverslips coated with collagen at 20 μg/mL and incubated for 10 minutes at 37°C. Adherent platelets were fixed and stained with TRITC-phalloidin for actin. The adhesion was visualized and quantified by fluorescence microscopy. Bars present the mean number of platelets (means ± SD) for 6 fields of 3 independent experiments (C). The photograph illustrates adherent or spread platelets in the representative fields (D). * indicates significant difference between WT and Akt-1-null platelets (P < .05). Images were acquired using a Leica DMR fluorescence microscope with a × 100 objective lens, oil, and a × 1.6 zoom adaptor, and a Micromax RTE/CCD-1300-V/HS camera.
Fibrinogen binding and platelet adhesion. (A) Fibrinogen binding. Platelets were preincubated with ADP (10 μM), PMA (100 nM), and thrombin (0.4 U/mL). FITC-labeled fibrinogen was then added at a final concentration of 300 nM for 30 minutes and fibrinogen binding was analyzed by FACS. A total of 20 000 events per sample was recorded. Bars present MFI ± SD of triplicates from 2 representative experiments. (B) Fibrinogen binding in the presence of LY294002. Platelets from 3 pairs of WT (i,iii) and Akt-1 null (ii,iv) mice were stimulated with thrombin at 0.1 U/mL or 0.5 U/mL, respectively, in the presence or absence of LY294002 (80 μM). FITC-labeled fibrinogen at concentration of 300 nM was added. Fibrinogen binding was analyzed by FACS analysis and 10 000 events per sample were recorded. Gray profiles represent fibrinogen binding in the presence of PGE1; MFIs are shown in response to thrombin in the presence (thin lines) and the absence (thick lines) of LY294002, respectively. Similar results were obtained in an additional 2 experiments. (C-D) Platelet adhesion to collagen. Platelets were resuspended in calcium-free Tyrode buffer containing 2 U/mL apyrase. Platelet suspensions were added to coverslips coated with collagen at 20 μg/mL and incubated for 10 minutes at 37°C. Adherent platelets were fixed and stained with TRITC-phalloidin for actin. The adhesion was visualized and quantified by fluorescence microscopy. Bars present the mean number of platelets (means ± SD) for 6 fields of 3 independent experiments (C). The photograph illustrates adherent or spread platelets in the representative fields (D). * indicates significant difference between WT and Akt-1-null platelets (P < .05). Images were acquired using a Leica DMR fluorescence microscope with a × 100 objective lens, oil, and a × 1.6 zoom adaptor, and a Micromax RTE/CCD-1300-V/HS camera.
To assess the relative role of PI3K on αIIbβ3 activation by various concentrations of thrombin in WT and Akt-1-null platelets, we measured fibrinogen binding in the presence of LY294002, an inhibitor of PI3K. In WT platelets, LY294002 almost completely inhibited the rise in fibrinogen binding induced by 0.1 U/mL thrombin as indicated by the decrease in the mean fluorescence intensity (MFI) from 208 to 39 (Figure 3Bi), demonstrating the key role of PI3K in this process. As anticipated, the inhibition of PI3K by LY294002 in WT platelets had a much greater effect on fibrinogen binding than the lack of Akt-1 due to the fact that PI3K targets a number of intracellular mediators involved in platelet activation.6,9 In Akt-1-deficient platelets, treatment with LY294002 decreased MFI from 115 to 75 (Figure 3Bii). Interestingly, residual fibrinogen binding in the presence of LY294002 was greater in Akt-1-deficient platelets (MFI = 75) compared to the WT platelets (MFI = 39), which probably reflects some compensatory changes. Stimulation of WT platelets with higher concentrations of thrombin (0.5 U/mL) resulted in an expected increase of fibrinogen binding (MFI = 245), and this binding was significantly, but not completely, abrogated by LY294002 (MFI = 95; Figure 3Biii). At the same time, Akt-1-null platelets also responded to higher concentrations (0.5 U/mL) of thrombin (MFI = 130) and LY294002 inhibited the thrombin-induced response to near baseline levels (MFI = 62; Figure 3Biv), which was similar to that observed with the lower concentration (0.1 U/mL) of thrombin (Figure 3Bii). The fact that LY294002 was still effective in Akt-1-null platelets reflects the role of downstream effectors of PI3K other than Akt as well as the alternative isoforms of Akt.
When collagen was used as an adhesive substrate, the extent of attachment was similar for WT and Akt-1-null platelets (Figure 3C). However, a marked delay in spreading of Akt-1-deficient platelets was observed. The number of Akt-1-null platelets with formed filopodia and lamellipodia was about 6-fold lower after 10 minutes of incubation on collagen (Figure 3D), although no significant differences were observed after prolonged (30 minutes) incubation (data not shown).
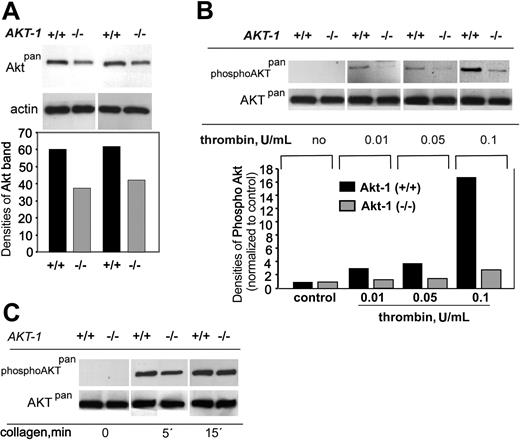
To explore the expression of Akt isoforms in WT and Akt-1-deficient platelets, we performed Western blotting analysis using an anti-Aktpan antibody, which is known to recognize all isoforms of Akt. In contrast to the published observations for human platelets,9 we found that Akt-1 is not the predominant Akt isoform in murine platelets. A reduction (from 60 to 35 densitometric units) in total Akt was observed in platelets from Akt-1-null mice compared to those from WT mice (Figure 4A). Because platelet activation with low concentrations of thrombin revealed the most significant differences between WT and Akt-1-null platelets, we performed a comparison of Akt phosphorylation in platelets stimulated with various concentrations of thrombin in the absence of fibrinogen binding (Figure 4B). Under these experimental conditions, no Akt phosphorylation was observed in unstimulated platelets. Thrombin concentrations as low as 0.01 U/mL induced weak phosphorylation of Akt (normalized band density increased from 1 to 3) in WT platelets as detected by antiphospho-Aktpan antibody. Of note, this concentration was sufficient to induce shape change, but not complete platelet aggregation of both phenotypes. Akt phosphorylation levels increased (4 and 17) when higher concentrations of thrombin (0.05 and 0.1 U/mL, respectively) were used. In contrast, virtually no response was observed in Akt-1-null platelets stimulated with 0.01 and 0.05 U/mL thrombin. Only at 0.1 U/mL thrombin were we able to detect phosphorylated Akt in Akt-1-null platelets. When collagen was used as a stimulus (Figure 4C), a 1.6-fold difference in Akt phosphorylation (although not as apparent as in the case of thrombin) between WT and Akt-1-null platelets was observed at the early time point (5 minutes after addition of agonist). At the 15-minute time point, the levels of Akt phosphorylation in WT and Akt-1-null platelets were similar. These data are in agreement with a delayed aggregation in response to collagen in Akt-1-null platelets (Figure 2). Thus, Akt-1 appears to be preferentially activated in platelets stimulated with subthreshold concentrations of thrombin or at the early time points of stimulation by collagen.
Total and phosphorylated Akt. Gel-filtered platelets (1 × 108/mL) were resuspended in the presence of integrin inhibitors to completely block fibrinogen binding. Platelet lysates were subjected to SDS-PAGE and Western blotting using antibodies against all Akt isoforms (Aktpan), phosphorylated Akt (phospho-Aktpan), or actin to ensure equal protein loading. (A) Total Akt levels (top) and their densitometric analysis (bottom) in 2 separate samples of unstimulated platelets. (B) Akt phosphorylation in response to different concentrations of thrombin (top) and densitometric analysis of phosphorylated Akt (bottom). (C) Akt phosphorylation in response to collagen (20 μg/mL) at different time points.
Total and phosphorylated Akt. Gel-filtered platelets (1 × 108/mL) were resuspended in the presence of integrin inhibitors to completely block fibrinogen binding. Platelet lysates were subjected to SDS-PAGE and Western blotting using antibodies against all Akt isoforms (Aktpan), phosphorylated Akt (phospho-Aktpan), or actin to ensure equal protein loading. (A) Total Akt levels (top) and their densitometric analysis (bottom) in 2 separate samples of unstimulated platelets. (B) Akt phosphorylation in response to different concentrations of thrombin (top) and densitometric analysis of phosphorylated Akt (bottom). (C) Akt phosphorylation in response to collagen (20 μg/mL) at different time points.
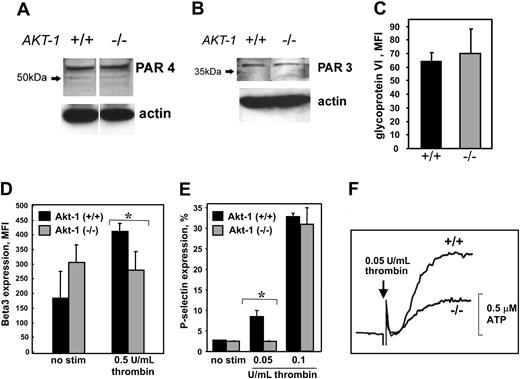
To determine whether the lack of Akt-1 affects the process of platelet development and, as a consequence, its responses to agonists, we assessed the expression of thrombin receptor protease-activated receptors, PAR4 and PAR3, on WT and Akt-1-null platelets. From Western blots, it is evident that both types of platelets express similar levels of thrombin receptors (Figure 5A-B). Likewise, no differences were observed in the expression of the activating receptor for collagen, GPVI, as determined by FACS (Figure 5C). FACS analysis using antibody against the β subunit of αIIbβ3 revealed that basal expression of this integrin is 37% higher on Akt-1-deficient platelets as compared to WT platelets (Figure 5D). However, in WT but not in Akt-1-null platelets, β3 expression was increased on stimulation even with low concentrations of thrombin. As a result, WT platelets activated with 0.5 U/mL thrombin expressed about 40% more β3 integrin than Akt-1-deficient platelets (Figure 5D).
Expression of thrombin and collagen receptors, β3 integrin, and P-selectin, and ATP release. (A-B) Expression of thrombin receptors. Thrombin receptors PAR4 (A) and PAR3 (B) were detected by Western blot. (C) Expression of collagen receptor GPVI. Platelet suspensions were incubated with FITC-labeled anti-GPVI antibody for 15 minutes at room temperature. (D) Expression of β3 integrin. Gel-filtered platelets were incubated with or without thrombin at 0.5 U/mL for 15 minutes and then fixed. After washing, platelets were incubated with anti-β3 integrin antibody for 20 minutes followed by FITC-labeled secondary antibody. (E) P-selectin expression. Platelets were stimulated with or without thrombin at 0.05 U/mL or 0.1 U/mL for 5 minutes and then fixed. After washing, platelets were incubated with FITC-labeled P-selectin antibody for 20 minutes. For panels C-E, the samples were analyzed by flow cytometry; 20 000 events were recorded. Bars present mean ± SD from 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05). (F) ATP release. The release of ATP was measured in gel-filtered platelets by a Lumi-Aggregometer type 500 VS using luciferin-luciferase. ATP served as the standard in all experiments. The addition of thrombin at a final concentration of 0.05 U/mL is indicated by the arrow (P < .05).
Expression of thrombin and collagen receptors, β3 integrin, and P-selectin, and ATP release. (A-B) Expression of thrombin receptors. Thrombin receptors PAR4 (A) and PAR3 (B) were detected by Western blot. (C) Expression of collagen receptor GPVI. Platelet suspensions were incubated with FITC-labeled anti-GPVI antibody for 15 minutes at room temperature. (D) Expression of β3 integrin. Gel-filtered platelets were incubated with or without thrombin at 0.5 U/mL for 15 minutes and then fixed. After washing, platelets were incubated with anti-β3 integrin antibody for 20 minutes followed by FITC-labeled secondary antibody. (E) P-selectin expression. Platelets were stimulated with or without thrombin at 0.05 U/mL or 0.1 U/mL for 5 minutes and then fixed. After washing, platelets were incubated with FITC-labeled P-selectin antibody for 20 minutes. For panels C-E, the samples were analyzed by flow cytometry; 20 000 events were recorded. Bars present mean ± SD from 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05). (F) ATP release. The release of ATP was measured in gel-filtered platelets by a Lumi-Aggregometer type 500 VS using luciferin-luciferase. ATP served as the standard in all experiments. The addition of thrombin at a final concentration of 0.05 U/mL is indicated by the arrow (P < .05).
The potential abnormality in platelet secretion was further addressed by analyzing the expression of another α-granule component, P-selectin, in response to low thrombin concentrations (Figure 5E). When WT platelets were stimulated with 0.05 U/mL thrombin, about 10% expressed P-selectin on their surface, whereas Akt-1-null platelets remained at levels similar to nonstimulated platelets. Higher concentrations of thrombin (0.1 U/mL) increased the number of P-selectin-positive platelets to 34% and 32% for WT and Akt-1-null platelets, respectively. As anticipated, the values for WT and Akt-1-null platelets stimulated with thrombin at 0.5 U/mL were also similar at 86% and 84%, respectively. Taken together, our results show that α-granule release is reduced in Akt-1-deficient platelets stimulated with low but not high concentrations of thrombin compared to WT. Furthermore, the dense granule secretion was also reduced in Akt-1-null platelets stimulated with low concentrations of thrombin. As shown in Figure 5F, thrombin at 0.05 U/mL induced 0.4 ± 0.07 μM ATP release in Akt-1-null platelets compared to 0.8 ± 0.12 μM in WT platelets. When high concentrations of thrombin (0.5 U/mL) were used, these values were 2.8 ± 0.7 μM for Akt-1-deficient platelets and 3.4 ± 0.9 μM for WT platelets.
Several studies indicated that an increase in the PI3K product, phosphatidylinositol 3,4,5-triphosphate, resulted in an increased basal calcium level in platelets as well as in potentiation of calcium entry.21 An increase in intracellular calcium is a prerequisite for platelet shape change and activation.22 Knowing that platelet responses to low concentrations of thrombin, including shape change and activation, are delayed in Akt-1-null platelets, we sought to determine whether Akt-1 is involved in the regulation of intracellular Ca2+ concentration ([Ca2+]i). Accordingly, we performed measurements of [Ca2+]i levels using fura-2-loaded platelets. In WT platelets, thrombin caused a dose-dependent increase in [Ca2+]i at concentrations as low as 0.01 and 0.05 U/mL. Akt-1-deficient platelets also responded to thrombin stimulation although this response was less pronounced (Figure 6A). Likewise, in the presence of the Ca2+-chelating agent EGTA, Akt-1-deficient platelets achieved lower levels of [Ca2+]i in response to thrombin stimulation compared to WT platelets, but only when low doses of thrombin (≤ 0.05 U/mL) were used (Figure 6B). At the same time, platelet stimulation with higher concentrations of thrombin (1 U/mL and more) or with ionomycin (not shown) revealed no significant differences between WT and Akt-1-null platelets in the presence or absence of extracellular Ca2+ (Figure 6B). Thus, it appears that knockout of Akt-1 results in defective mobilization of stored Ca2+ and impaired increase of [Ca2+]i in response to low concentrations of thrombin, which, in turn, may account for reduced and delayed platelet activation.
[Ca2+]i measurements. Gel-filtered platelets at 5 × 107/mL were loaded with 2 μM fura-2/am and resuspended in Tyrode buffer with 1 mM CaCl2 and 1 mM MgCl2. Platelets were stimulated with 0.01, 0.05, or 1 U/mL thrombin and fluorescence signals at excitation wavelengths of 340 and 380 were collected and the 340/380 ratio was analyzed using Felix software from Photon Technology. (A) Representative traces showing changes in F340/F380 ratio in platelets stimulated with 0.01, 0.05, and 1 U/mL thrombin. The solid and dotted lines represent WT and Akt-1-null platelets, respectively. Arrow indicates the point of thrombin addition. Curves were smoothed using the Loess technique with the sampling proportion 0.1 and with the polynomial degree 3 in SigmaPlot 8.0. (B) The average changes in fura-2 ratio (Δratio) in platelets following the addition of thrombin at indicated concentrations in the presence of 0.5 mM EGTA or 1 mM Ca2+. The Δratio was calculated as the difference between the peak ratio after agonist was added, and its level immediately before agonist addition. Data (means ± SD) were summarized from 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05).
[Ca2+]i measurements. Gel-filtered platelets at 5 × 107/mL were loaded with 2 μM fura-2/am and resuspended in Tyrode buffer with 1 mM CaCl2 and 1 mM MgCl2. Platelets were stimulated with 0.01, 0.05, or 1 U/mL thrombin and fluorescence signals at excitation wavelengths of 340 and 380 were collected and the 340/380 ratio was analyzed using Felix software from Photon Technology. (A) Representative traces showing changes in F340/F380 ratio in platelets stimulated with 0.01, 0.05, and 1 U/mL thrombin. The solid and dotted lines represent WT and Akt-1-null platelets, respectively. Arrow indicates the point of thrombin addition. Curves were smoothed using the Loess technique with the sampling proportion 0.1 and with the polynomial degree 3 in SigmaPlot 8.0. (B) The average changes in fura-2 ratio (Δratio) in platelets following the addition of thrombin at indicated concentrations in the presence of 0.5 mM EGTA or 1 mM Ca2+. The Δratio was calculated as the difference between the peak ratio after agonist was added, and its level immediately before agonist addition. Data (means ± SD) were summarized from 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05).
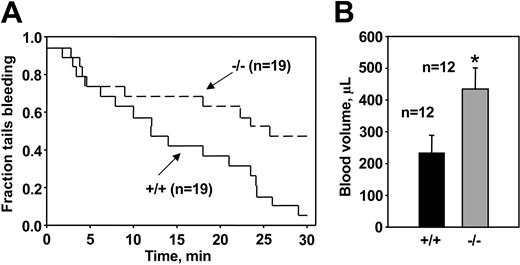
To determine whether altered platelet responses to thrombin and collagen affected hemostasis in Akt-1 null mice, bleeding time measurements were performed using a tail-cut model, which induces a relatively severe hemostatic challenge. Consistent with data from platelet aggregation studies, Akt-1 null mice had about 50% longer bleeding times than WT mice. The median bleeding times with 95% confidence interval were 19.3 minutes (7.9, 24.1 minutes) for WT mice and 29.5 minutes (9.5, 43.7 minutes) for Akt-1-deficient mice (P = .035; Figure 7A). Only 16% of the WT mice continued to bleed at 25 minutes. In contrast, 55% of Akt-1 null tails were still bleeding at this time point. Furthermore, blood loss during this test was significantly greater in Akt-1 null mice (mean ± SE, 363.6 ± 69 μL) as compared to WT mice (192.6 ± 58 μL; P = .0286; Figure 7B). The difference in tail bleeding times was not due to altered platelet production because platelet counts in peripheral blood in mice lacking Akt-1 (8.45 ± 1.92 × 108/mL) were similar to those in WT littermates (8.38 ± 2.40 × 108/mL).
TVBT and volume of shed blood. (A) TVBTs. WT and Akt-1 null mice were anesthetized and tails were amputated at a position where the diameter of the tail was 2.5 mm and immersed in saline. The time from the incision to cessation of bleeding was recorded. Shown is the fraction of tails that are bleeding as a function of time after tail transection. Genotypes and the number of mice of each genotype are indicated. The effect of Akt-1 deficiency on bleeding time was significant by log-rank test (P = .035). (B) Volume of shed blood. The amounts of shed blood during bleeding time test were measured. Bars represent the means ± SE for 12 WT mice and 12 Akt-1 null mice. *Significantly greater amount of shed blood in Akt-1 null mice compared with WT mice (P < .05).
TVBT and volume of shed blood. (A) TVBTs. WT and Akt-1 null mice were anesthetized and tails were amputated at a position where the diameter of the tail was 2.5 mm and immersed in saline. The time from the incision to cessation of bleeding was recorded. Shown is the fraction of tails that are bleeding as a function of time after tail transection. Genotypes and the number of mice of each genotype are indicated. The effect of Akt-1 deficiency on bleeding time was significant by log-rank test (P = .035). (B) Volume of shed blood. The amounts of shed blood during bleeding time test were measured. Bars represent the means ± SE for 12 WT mice and 12 Akt-1 null mice. *Significantly greater amount of shed blood in Akt-1 null mice compared with WT mice (P < .05).
Discussion
The goal of our study was to define the role of Akt-1, one of the major downstream effectors of the PI3K pathway, in platelet function ex vivo and in vivo. As demonstrated by decreased and prolonged platelet aggregation and by decreased fibrinogen binding stimulated by low concentrations of thrombin, disruption of Akt-1 activity resulted in impaired platelet responses. Although we found that Akt-1 is not the major Akt isoform in mouse platelets, its absence significantly reduced the amount of total phosphorylated Akt when suboptimal concentrations of thrombin were used. Absence of Akt-1 did not seem to influence the process of platelet development because platelet counts as well as expression of the major platelet receptors for thrombin and collagen were not altered. In contrast, platelet degranulation associated with an increase of the surface expression of αIIbβ3 and P-selectin, as well as dense granule release, was defective in Akt-1-null platelets stimulated with low concentrations of thrombin. Moreover, the increase in [Ca2+]i in response to low-dose thrombin was impaired in Akt-1-deficient platelets. Most important, defective platelet function resulted in abnormal hemostasis and prolonged bleeding times in Akt-1 null mice. Our data suggest that Akt-1 plays a significant role in the signaling pathways induced by low concentrations of thrombin leading to αIIbβ3 activation, fibrinogen binding, and platelet aggregation.
Although a deficiency in Akt-1 affected platelet responses to a number of agonists, the most significant differences were found with thrombin and collagen. Thrombin and collagen serve as major activators of the PI3K/Akt pathway, and Akt is known to undergo phosphorylation in response to these agonists.9,12 We were able to show that platelet stimulation with low concentrations of thrombin resulted in accumulation of phosphorylated total Akt in WT but not in Akt-1-deficient platelets. This reveals a differential role for Akt-1 in platelets. Despite its relatively low expression compared to other isoforms, Akt-1 is important for platelet responses and the functional overlap between Akt isoforms is not absolute. A similar tendency was observed (but not discussed) in a recently published study by Woulfe et al,23 in which a minimal decrease in total Akt was observed in Akt-1-null platelets compared to WT; however, the amount of phosphorylated Akt in response to thrombin (1 U/mL) was decreased by at least 2-fold. In our study, we demonstrate that Akt-1 plays an important and unique role in platelet responses to thrombin at concentrations lower than 0.1 U/mL.
Interestingly, thrombin-induced platelet aggregation depends on PI3K as indicated by the blocking effect of wortmannin.2 However, aggregation was not affected in the absence of the major forms of PI3K in platelets, PI3K P85α,3 or PI3Kγ,4 suggesting an involvement of alternative PI3K isoforms. Based on our results, the thrombin-stimulated pathway requires Akt-1 to achieve normal aggregation and fibrinogen binding. Remarkably, the difference between Akt-1 null and WT platelets was most evident in response to low concentrations of thrombin (which are most likely to approximate physiologic levels). Such effects also have been described in knockout mice lacking certain isoforms of PI3Ks.3,4 The platelet defect in Akt-1-deficient mice was not noted in the recent study by Woulfe et al, which might be due to the differences in the genetic background.23 Alternatively, it may be due to their use of thrombin-receptor activating peptide or high doses of thrombin (1 U/mL).23 We also observed that, under these conditions, the differences between responses of WT and Akt-1-null platelets are subtle. Our data support previously published studies demonstrating that signaling pathways leading to Akt phosphorylation are different in platelets stimulated with high versus low thrombin concentrations.9
Previous studies demonstrated that collagen activates Akt via GPVI on platelets. Akt phosphorylation in response to collagen occurs as a result of agonist-induced signaling and does not depend on platelet aggregation.12 This process is solely dependent on PI3K activity because it can be completely inhibited by PI3K inhibitor.12 Moreover, deficiency of p85α PI3K in mice causes defects in platelet aggregation in response to collagen and collagen-related peptide.3 We demonstrate that the lack of Akt-1 in platelets results in the delay of total Akt phosphorylation, platelet aggregation, and spreading induced by collagen. Although collagen and thrombin activate distinct intracellular signaling pathways and potentially act through different PI3K isoforms, it appears that they share one common participant, Akt-1.
Because PI3K targets important players other than Akt in agonist-induced signaling,6 it is not surprising that the platelet defect in Akt-1-null platelets is mild compared to the effects of PI3K inhibitors. In addition, mouse platelets express high levels of Akt-2 and Akt-3, which might compensate for the lack of Akt-1. However, despite its modest expression, Akt-1 seems to mediate an increase in [Ca2+]i in platelets stimulated with low concentrations of thrombin. Because an elevation in [Ca2+]i is an immediate consequence of thrombin stimulation and represents a crucial and necessary event in the process of platelet activation,24 the latter observation might account for the defect in platelet aggregation in Akt-1-null platelets. This conclusion is supported by the previously reported observation that phosphoinositide products of PI3K stimulate an increase in [Ca2+]i and platelet aggregation.25 Moreover, the PI3K inhibitor wortmannin interferes with thrombin-induced redistribution of cytosolic calcium as well as with calcium entry, which, in turn, results in incomplete platelet activation.26 Although the mechanism by which Akt-1 regulates [Ca2+]i remains unclear, this observation represents an initial step in delineation of the molecular pathways leading to complete platelet aggregation.
The deficiency of PI3Kγ that impaired ADP-induced platelet aggregation or the lack of p85α PI3K that decreased platelet responses to collagen did not affect the process of hemostasis.3,4 In our study, we observed that Akt-1-null platelets exhibit relatively a modest reduction in aggregation responses to thrombin compared with WT platelets. It is surprising that even a partial attenuation of platelet responsiveness had a significant effect on hemostasis as indicated by increased bleeding times in Akt-1 null mice. This may be due to the fact that Akt-1 is the convergence point of various activating pathways in platelets.3,4,12 Alternatively, this implies a prominent role for thrombin signaling in the hemostatic response. Of note, the blockade of the thrombin receptor PAR4 on platelets results in extended bleeding times and protection against systemic platelet activation.27 Moreover, it was previously reported that even a partial decrease in thrombin-induced platelet aggregation (reduced response to low concentrations of thrombin) observed in PAR3-deficient mice results in impaired hemostasis and protection against thrombosis.20 In this regard, the choice of the most appropriate experimental model to test the consequences of partially defective platelet aggregation is of particular importance. As reported by Weiss and colleagues,20 and our data are in agreement, models associated with a strong hemostatic challenge reveal relatively mild platelet defects, whereas less stringent methods fail to do so.1,20
In summary, the findings that Akt-1 activity is required for (1) optimal platelet αIIbβ3 activation, (2) thrombin and collagen-induced platelet aggregation, and (3) normal tail bleeding times, are, to our knowledge, the first demonstration that Akt-1 plays an important role in hemostasis. Further studies are required to identify the downstream effectors of Akt-1 (and other Akt isoforms) that are involved in the regulation of platelet responses.
Prepublished online as Blood First Edition Paper, April 22, 2004; DOI 10.1182/blood-2003-10-3428.
Supported by National Institutes of Health grant HL071625 (T.V.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Vicky Byers-Ward for outstanding technical assistance, Drs E. F. Plow and E. Kandel for critical reading of the manuscript, and Drs X. Peng and P. Elson for their expertise in statistical analysis.

![Figure 6. [Ca2+]i measurements. Gel-filtered platelets at 5 × 107/mL were loaded with 2 μM fura-2/am and resuspended in Tyrode buffer with 1 mM CaCl2 and 1 mM MgCl2. Platelets were stimulated with 0.01, 0.05, or 1 U/mL thrombin and fluorescence signals at excitation wavelengths of 340 and 380 were collected and the 340/380 ratio was analyzed using Felix software from Photon Technology. (A) Representative traces showing changes in F340/F380 ratio in platelets stimulated with 0.01, 0.05, and 1 U/mL thrombin. The solid and dotted lines represent WT and Akt-1-null platelets, respectively. Arrow indicates the point of thrombin addition. Curves were smoothed using the Loess technique with the sampling proportion 0.1 and with the polynomial degree 3 in SigmaPlot 8.0. (B) The average changes in fura-2 ratio (Δratio) in platelets following the addition of thrombin at indicated concentrations in the presence of 0.5 mM EGTA or 1 mM Ca2+. The Δratio was calculated as the difference between the peak ratio after agonist was added, and its level immediately before agonist addition. Data (means ± SD) were summarized from 3 independent experiments. *Significant difference between WT and Akt-1-null platelets (P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/6/10.1182_blood-2003-10-3428/6/m_zh80180466620006.jpeg?Expires=1769144155&Signature=A4i23OuxCoAekZTUFzxfHaxe3vhY~fDp~NxrugawaZQaye5YgnrEt2-qYghSYFVoxwe9U9bejx1R30ACH70KZe3v3JWI6d8SgyVm5Lof~X-HyWJ6l7e~RtD0~2HlKamQK~YqQcnFs4bfhGmjXRBqnUsvx0QvPHLQnXzrVIBXpCPDcm7pXgq-ubYMZ4rVPD6RMKjLACbrbyjGv-GLUp2pABbtu1qyBdrC0OttLxGPU0P3lDRhDHEzU7blgpipPG~6rIzdZ4Z0M6JIZ2XY4k0PzuuX3vxdsyK~Xopexidqct3xKxDrM9WCTTBSH-03rb1-9q-CtDJ1D0x0O8LR-HQHbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal