Abstract
The mutant tg/tg mice, which do not express mi transcription factor (MITF), lack mast cells in most tissues. Since MITF is expressed in both mast cells and tissues where mast cells develop, there is a possibility that the tg/tg mice may show abnormalities in both mast cell precursors and tissue environments. We examined this possibility by bone marrow and skin transplantation. When bone marrow cells of tg/tg mice were transplanted to W/Wv mice that possess normal tissue environment, mast cells did not develop in all tissues examined. The number of developing mast cells in the skin of W/Wv mice was much lower when grafted to tg/tg recipients than when grafted to normal (+/+) recipients. These results indicated that mast cell precursors of tg/tg mice were defective. When bone marrow cells of +/+ mice were transplanted, the number of developing mast cells was significantly lower in examined tissues of tg/tg recipients than in those of W/Wv recipients, suggesting that the tissue environment for mast cell development was defective in tg/tg mice. MITF appeared essential for the function of both mast cell precursors and tissue environments for their development. (Blood. 2004;104:1656-1661)
Introduction
The products encoded by 3 mouse loci influence the development of mast cells. Kit ligand (KitL) is the most important growth factor for mast cells and is encoded by Sl locus.1-4 KIT is the receptor for KitL and is encoded by W locus.1,5,6 The mutant Sl/Sld and W/Wv mice, which are deficient in KitL and KIT, respectively, lack mast cells in all examined tissues. The third product is mi transcription factor (MITF), which is encoded by mi locus. MITF is a basic helix-loop-helix leucine zipper transcription factor.7-9 The mutant tg/tg mice, which do not express MITF due to a transgene insertion to the promoter region of MITF gene, lack mast cells in the peritoneal cavity, mesentery, stomach, and spleen.10 In spite of the lack of mast cells in most tissues, the dermis of tg/tg mice has a decreased but recognizable number of mast cells (one third that of normal [+/+] littermates).11,12
KitL is expressed in tissues where mast cells develop, and KIT is expressed in mast cells and their precursors. Therefore, Sl/Sld mice have a defect in tissue environment for mast cell development, whereas W/Wv mice have a defect in mast cell precursors.1,2,5 MITF is expressed in both mast cells and tissues where mast cells develop.10 This suggested that MITF regulated not only the transcription of genes expressed in mast cells or mast cell precursors but also the transcription of genes expressed in the tissue environment. The tg/tg mice appear to show abnormalities in both mast cell precursor and tissue environment. As for the mast cell abnormality, we reported that the production of various molecules, including KIT and spermatogenic immunoglobulin superfamily (SgIGSF), a new mast cell adhesion molecule, was deficient in mast cells of tg/tg mice.11-22 The mast cell precursors of tg/tg mice have not been well characterized yet, especially in in vivo conditions. As for the tissue environment, we recently found the phenomenon suggesting defects in tg/tg mice. When bone marrow-derived cultured mast cells (BMMCs) of +/+ mice were intraperitoneally injected, mast cells survived in the mesentery of W/Wv mice but not in that of tg/tg mice. In the present study, we investigated defects in mast cell precursors and tissue environments of tg/tg mice by bone marrow and skin transplantation.
Materials and methods
Mice and cells
The original stock of VGA-9-tg/tg mice, in which the mouse vasopressin-Escherichia coli β-galactosidase transgene was integrated at the promoter region of the MITF gene, was given by Dr H. Arnheiter (National Institutes of Health, Bethesda, MD).7 The VGA-9-tg/tg mice were maintained by consecutive backcrosses to our own C57BL/6 (B6) and WB inbred colonies more than 15 generations. B6-tg/+ mice were crossed together, and WB-tg/+ mice were crossed to B6-tg/+ mice. The resulting B6-tg/tg and (WB × B6) F1 (WBB6F1)-tg/tg mice were selected by their white coat color. B6-+/+, WBB6F1-W/Wv, and WBB6F1-+/+ mice were purchased from the Japan SLC (Hamamatsu, Japan). BMMCs were obtained from bone marrow cells of WBB6F1-+/+ and -tg/tg mice as described previously.23
Bone marrow transplantation
Radioflex 350 X-ray machine (Rigaku, Tokyo, Japan) operated at 180 kV and 15 mA with 1-mm aluminum filter (0.885 Gy/min) was used. Mice were kept in a polypropylene box during irradiation. Bone marrow cells (1.0 × 107) were suspended in α-minimal essential medium (α-MEM; ICN Biomedicals, Costa Mesa, CA) and were injected intravenously to 2 Gy-irradiated WBB6F1-W/Wv mice or to 8 Gy-irradiated WBB6F1-tg/tg mice. The irradiation dose of WBB6F1-W/Wv mice was reduced because they were remarkably radiosensitive.24
Estimation of the origin of blood cells other than mast cells
At 10 weeks after bone marrow transplantation, mice were killed by decapitation after ether anesthesia. The hemoglobin pattern in cellulose acetate electrophoresis was easily distinguished between WBB6F1 mice (diffuse, Hbbd/Hbbs) and B6 mice (sharp, Hbbs/Hbbs).25 Then, we used this to identify the origin of erythrocytes. Heparinized blood sample was washed with saline 5 times, and twice the volume of distilled water and half the volume of toluene were added. After centrifugation, the lower layer of this solution was mixed with the same volume of cystamine solution containing 333.5 mM cystamine and 6.65 mM dithioerythritol, and electrophoresed on cellulose acetate membrane (Toyo Roshi, Tokyo, Japan) in the TBE buffer containing 180 mM Tris (tris(hydroxymethyl)aminomethane), 100 mM boric acid, and 2 mM EDTA (ethylenediaminetetraacetic acid, pH 8.9). Hemoglobin of B6 mice showed only fast-migrating single hemoglobin, whereas hemoglobin of WBB6F1 mice showed 3 bands: single hemoglobin was fastest, d-major hemoglobin was intermediate, and d-minor hemoglobin was slowest.
B lymphocytes (immunoglobulin M+ [IgM+]) and macrophages (CD11b+) were obtained from spleen using magnetic cell sorting system (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. T lymphocytes (Thy1.2+) and granulocytes (Gr-1+) were also obtained by MACS from lymph nodes and bone marrow, respectively. Genomic DNA was extracted from the separated cells using Wizard SV genomic DNA purification kit (Promega, Madison, WI). Polymerase chain reaction (PCR) was carried out with the genomic DNA as a template. The primers were as follows: 5′-CTTTCGCCAGCTGGCGTAATAGCGAAGAGG and 5′-CATTAAATGTGAGCGAGTAACAACCCGTCG for β-galactosidase transgene, and 5′-TAAAGACCTCTATGCCAACAC and 5′-CTCCTGCTTGCTGATCCACAT for β-actin gene.
Staining and counting of mast cells
Mast cell numbers in the peritoneal cavity, skin, glandular stomach, and mesentery were estimated as described previously.10 In brief, Tyrode buffer containing 0.1% gelatin (Sigma, St Louis, MO) was injected into the peritoneal cavity, and the fluid containing the peritoneal cells was aspirated with a Pasteur pipette. After centrifugation, the pellet was resuspended with the Tyrode buffer, and the peritoneal cells were attached to microscope slide with a Cytospin 2 centrifuge (Shandon, Pittsburgh, PA). Pieces of dorsal skin and glandular stomach were removed and smoothed onto a piece of the filter paper to keep them flat. Mesentery was stretched onto a microscope slide. All specimens were fixed in Carnoy solution. The cytospin preparation of peritoneal cells, the sections (3-μm thick) of skin and glandular stomach, and the stretch preparations of mesentery were stained with Alcian blue and nuclear fast red.
Limiting dilution analysis of mast cell precursors
The principle of the method has been described in detail.26 Bone marrow cells of WBB6F1-+/+ and -tg/tg mice were suspended in α-MEM, to which India ink was added to mark the injection sites. Various numbers of cells in 0.05 mL medium were directly injected into the dorsal skin of WBB6F1-W/Wv mice. At 5 weeks after injection, mice were killed by decapitation, and dorsal skin was peeled off. Each injection site was identified as a blotted black spot. The skins of injection sites were attached to filter paper to keep them flat, fixed in Carnoy solution, and embedded in paraffin. Serial sections (25-μm thick, cut parallel to the skin surface) were cut from the subcutaneous tissue to the epithelium and stained with Alcian blue and nuclear fast red. All serial sections were examined, and the injection cells were assumed to contain mast cell precursor(s) when a group of more than 100 mast cells was found in at least one section. Concentration of mast cell precursors was calculated from the proportion of injection sites at which such a group of mast cells did not appear (defined as “proportion of nonappearance”) for various cell doses by limiting dilution analysis according to Porter and Berry27 and Breivik.28
Skin transplantation
A piece of skin about 25 × 25 mm in its full thickness, including paniculus carnosus, was removed. A small piece (about 25 × 3 mm) was cut from the skin to determine the number of mast cells before grafting. The remaining part of the skin was grafted onto the back of the recipient in the rotation of 180 degrees, and was kept in place by wound clips.29 At various times after transplantation, mice were killed by decapitation after ether anesthesia. The skin was removed, smoothed onto a piece of the filter paper to keep it flat, and fixed in Carnoy solution. The skin sections (3-μm thick) were stained with Alcian blue and nuclear fast red, and the number of mast cells was counted. The grafted skin was identified by the direction of hair.
In vitro colony formation
Methylcellulose culture was carried out according to the method described by Nakahata et al.30 α-MEM (1 mL) containing 4 × 104 bone marrow cells, 2 × 105 spleen or peripheral blood mononuclear cells or 5 × 102 BMMCs, 1% methylcellulose (Sigma), 30% fetal calf serum (FCS; Nippon Bio-supp Center, Tokyo, Japan), 1% deionized bovine serum albumin, and 10-4 M 2-mercaptoethanol (Sigma) was plated in 35-mm dishes. The medium contained both 10 ng/mL interleukin-3 (IL-3, recombinant mouse; R&D, Minneapolis, MN) and 50 ng/mL KitL (recombinant mouse; R&D), or 10 ng/mL IL-3 alone. Mast cell colonies were counted on day 16. The colony-forming assay using dispersed skin cells was described previously.31 In brief, skin pieces were diced into fragments and were incubated in Hanks balanced salt solution buffered with Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10 mM, pH 7.4) containing collagenase (Type I, 1 mg/mL; Sigma), hyaluronidase (Type I, 1 mg/mL; Sigma), and FCS (20%) for 2.5 hours. After the incubation, cell clusters were broken up by repeated aspiration through needles of decreasing size. Then, the suspensions were passed through a nylon mesh, washed with α-MEM, and plated in 35-mm dishes, and methylcellulose culture was carried out (1.0 × 105 cells per dish with the medium containing 10 ng/mL IL-3 and 50 ng/mL KitL).
Flow cytometry
Mast cell colonies obtained from bone marrow cells or BMMCs were harvested and washed once with cold phosphate-buffered saline (PBS) containing 2% FCS and 0.1% sodium azide. The cells were incubated with fluorescein isothiocyanate (FITC)-conjugated rat anti-KIT antibody or its isotype control (BD Biosciences, San Jose, CA) and then analyzed on a FACScan (BD Biosciences).
Proliferation assay
The response to KitL of BMMCs was determined by uptake of 3H-thymidine (TdT; Amersham Bioscience, Piscataway, NJ). BMMCs were cultured overnight in 96-well plate with various concentrations of KitL and 10 ng/mL IL-3. At 4 hours before harvesting, cells were pulsed with 1 μCi/well (0.037 MBq/well) [3H]-TdT, harvested onto glass fiber filters, and counted in a scintillation counter (Hewlett-Packard, Palo Alto, CA).
Results
Bone marrow transplantation from and to tg/tg mice
Bone marrow cells of B6-+/+ and B6-tg/tg mice were transplanted to irradiated WBB6F1-W/Wv mice. At 10 weeks after transplantation, we confirmed donor origin of erythrocytes in the recipient WBB6F1-W/Wv mice. Hemoglobin of B6 mice showed a single band in electrophoresis, whereas that of WBB6F1 mice showed 3 bands (Figure 1A). When bone marrow cells of B6-+/+ mice were transplanted, the hemoglobin of WBB6F1-W/Wv recipients showed a single band (Figure 1A). The hemoglobin of WBB6F1-W/Wv mice also showed a single band after the transplantation of B6-tg/tg bone marrow cells (Figure 1A).
Origin of blood cells in the 2 Gy-irradiated WBB6F1-W/Wv mice 10 weeks after the bone marrow transplantation from B6-+/+ and B6-tg/tg mice. (A) Hemoglobin electrophoresis. Hemoglobin of WBB6F1-W/Wv recipients after the bone marrow transplantation from B6-+/+ or B6-tg/tg mice was electrophoresed in cellulose acetate membrane. In each group, 5 mice were examined, and the results of 3 in 5 were shown. Hemoglobin of B6-tg/tg mice and that of WBB6F1-W/Wv mice were also electrophoresed as controls. (B) Detection of transgene by genomic PCR. Genomic DNAs were purified from B and T lymphocytes, macrophages, and granulocytes, and the presence of the transgene, which was contained only in cells of B6-tg/tg mouse origin, was examined. In each group, 5 mice were examined with similar results (the result of a mouse is shown here). The presence of actin gene was examined as a control.
Origin of blood cells in the 2 Gy-irradiated WBB6F1-W/Wv mice 10 weeks after the bone marrow transplantation from B6-+/+ and B6-tg/tg mice. (A) Hemoglobin electrophoresis. Hemoglobin of WBB6F1-W/Wv recipients after the bone marrow transplantation from B6-+/+ or B6-tg/tg mice was electrophoresed in cellulose acetate membrane. In each group, 5 mice were examined, and the results of 3 in 5 were shown. Hemoglobin of B6-tg/tg mice and that of WBB6F1-W/Wv mice were also electrophoresed as controls. (B) Detection of transgene by genomic PCR. Genomic DNAs were purified from B and T lymphocytes, macrophages, and granulocytes, and the presence of the transgene, which was contained only in cells of B6-tg/tg mouse origin, was examined. In each group, 5 mice were examined with similar results (the result of a mouse is shown here). The presence of actin gene was examined as a control.
B lymphocytes (IgM+), T lymphocytes(Thy1.2+), macrophages (CD11b+), and granulocytes (Gr-1+) were purified from recipient WBB6F1-W/Wv mice by the magnetic cell sorting system, and the presence of β-galactosidase transgene was examined. The separated B and T lymphocytes, macrophages, and granulocytes in the recipient WBB6F1-W/Wv mice contained the transgene after the bone marrow transplantation from B6-tg/tg mice (Figure 1B).
Then, we examined the number of mast cells in various tissues of the recipient WBB6F1-W/Wv mice. Although appreciable numbers of mast cells developed after the bone marrow transplantation from B6-+/+ mice, no mast cells were detected in the peritoneal cavity, mesentery, stomach, spleen, and skin after the transplantation from B6-tg/tg mice (Table 1).
Number of mast cells in various tissues at 10 weeks after bone marrow transplantation
Tissue examined . | B6-+/+ to WBB6F1-W/Wv . |
|---|---|
| Peritoneal cavity | 49 ± 5 |
| Mesentery | 7.1 ± 0.4 |
| Stomach | 412 ± 31 |
| Spleen | 2.3 ± 0.9 |
| Skin | 119 ± 10 |
Tissue examined . | B6-+/+ to WBB6F1-W/Wv . |
|---|---|
| Peritoneal cavity | 49 ± 5 |
| Mesentery | 7.1 ± 0.4 |
| Stomach | 412 ± 31 |
| Spleen | 2.3 ± 0.9 |
| Skin | 119 ± 10 |
Values represent the number of mast cells in each combination of donors and recipients. Results are mean ± SE of 5 mice and are given as the number per 103 peritoneal cells, number per mm2 mesentery and spleen, and number per cm stomach and skin. The number of mast cells in each combination of donors and recipients was less than 0.1 for B6-tg/tg to WBB6F1-W/Wv mice. P < .01 by t test when compared with the values observed in WBB6F1-W/Wv mice transplanted from B6-+/+ donors. WBB6F1-W/Wv mice were irradiated with 2 Gy before transplantation.
There was a possibility that the nonappearance of mast cells in WBB6F1-W/Wv mice after the transplantation of B6-tg/tg bone marrow cells was due to the additive effect of strain difference (B6 versus WBB6F1 mice) and the lack of MITF (+/+ versus tg/tg). We transplanted bone marrow cells from WBB6F1-tg/tg mice to irradiated WBB6F1-W/Wv mice. As in the case of the transplantation from B6-tg/tg mice, no mast cells were detected in tissues of the recipient WBB6F1-W/Wv mice after the transplantation from WBB6F1-tg/tg mice (Table 2).
Number of mast cells in various tissues at 10 weeks after bone marrow transplantation
Tissue examined . | WBB6F1-+/+ to WBB6F1-W/Wv . | WBB6F1-+/+ to WBB6F1-tg/tg . |
|---|---|---|
| Peritoneal cavity | 59 ± 5 | 3.3 ± 0.2* |
| Mesentery | 9.1 ± 0.2 | < 0.1* |
| Stomach | 466 ± 41 | 253 ± 27* |
| Spleen | 2.9 ± 0.6 | < 0.1* |
| Skin | 150 ± 12 | 93 ± 10* |
Tissue examined . | WBB6F1-+/+ to WBB6F1-W/Wv . | WBB6F1-+/+ to WBB6F1-tg/tg . |
|---|---|---|
| Peritoneal cavity | 59 ± 5 | 3.3 ± 0.2* |
| Mesentery | 9.1 ± 0.2 | < 0.1* |
| Stomach | 466 ± 41 | 253 ± 27* |
| Spleen | 2.9 ± 0.6 | < 0.1* |
| Skin | 150 ± 12 | 93 ± 10* |
Values represent the number of mast cells in each combination of donors and recipients. Results are mean ± SE of 5 mice and are given as the number per 103 peritoneal cells, number per mm2 mesentery and spleen, and number per cm stomach and skin. The number of mast cells in each combination of donors and recipients was less than 0.1 for WBB6F1-tg/tg to WBB6F1-W/Wv. Before the transplantation, WBB6F1-W/Wv mice were irradiated with 2 Gy, and WBB6F1-tg/tg mice with 8 Gy. P < .01 by t test when compared to the values observed in WBB6F1-W/Wv mice transplanted from WBB6F1-+/+ donors.
P < .01 by t test when compared to the values observed in WBB6F1-W/Wv mice transplanted from WBB6F1-+/+ donors.
We also transplanted bone marrow cells from WBB6F1-+/+ mice to irradiated WBB6F1-tg/tg mice. When compared with the case of transplantation from WBB6F1-+/+ mice to irradiated WBB6F1-W/Wv mice, the number of mast cells was significantly lower in the peritoneal cavity, stomach, and skin when bone marrow cells were transplanted from WBB6F1-+/+ mice to irradiated WBB6F1-tg/tg mice (Table 2). Moreover, no mast cells were detected in the mesentery and spleen of the recipient WBB6F1-tg/tg mice.
Direct transplantation of bone marrow cells to the skin of WBB6F1-W/Wv mice
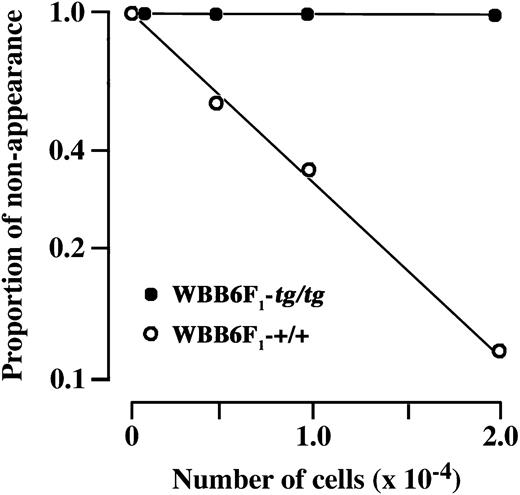
Various numbers of WBB6F1-+/+ or WBB6F1-tg/tg bone marrow cells were directly injected into the skin of WBB6F1-W/Wv mice. At 5 weeks after injection, the proportion of injection sites where mast cell clusters appeared was determined. When 5.0 × 103 and 1.0 × 104 bone marrow cells of WBB6F1-+/+ mice were injected, clusters of mast cells developed at approximately half and two thirds of injection sites, respectively (Figure 2). Mast cell clusters developed at most injection sites when 2.0 × 104 WBB6F1-+/+ bone marrow cells were injected (Figure 2). In contrast, mast cell clusters did not develop when even 2 × 104 WBB6F1-tg/tg bone marrow cells were injected (Figure 2).
Limiting dilution analysis of mast cell precursors present in bone marrow cells. Various numbers of bone marrow cells derived from WBB6F1-+/+ and -tg/tg mice were directly injected into the dorsal skin of WBB6F1-W/Wv mice. At 5 weeks after injection, the proportion of injection sites at which mast cell clusters did not appear (proportion of nonappearance) was determined and plotted to the number of injected cells. The calculated concentration of mast cell precursors in the WBB6F1-+/+ bone marrow was 10 (8-13 in 95% confidence intervals) among 105 cells. No apparent clusters develop even when 2.0 × 104 bone marrow cells of WBB6F1-tg/tg mice were injected into the skin of WBB6F1-W/Wv mice.
Limiting dilution analysis of mast cell precursors present in bone marrow cells. Various numbers of bone marrow cells derived from WBB6F1-+/+ and -tg/tg mice were directly injected into the dorsal skin of WBB6F1-W/Wv mice. At 5 weeks after injection, the proportion of injection sites at which mast cell clusters did not appear (proportion of nonappearance) was determined and plotted to the number of injected cells. The calculated concentration of mast cell precursors in the WBB6F1-+/+ bone marrow was 10 (8-13 in 95% confidence intervals) among 105 cells. No apparent clusters develop even when 2.0 × 104 bone marrow cells of WBB6F1-tg/tg mice were injected into the skin of WBB6F1-W/Wv mice.
Skin transplantation to and from tg/tg mice
When skin pieces of WBB6F1-W/Wv mice were grafted onto the back of WBB6F1-+/+ mice, mast cell number in the grafts gradually increased and reached levels comparable with the number observed in the surrounding skin of recipient WBB6F1-+/+ mice, within 5 weeks after transplantation (Figure 3). In contrast, when skin pieces of WBB6F1-W/Wv mice were grafted onto the back of WBB6F1-tg/tg mice, the number of mast cells in the grafts remained in significantly lower levels than those of the surrounding skin of WBB6F1-tg/tg recipients even at 10 weeks after the transplantation (Figure 3). Figure 3 also shows that the number of developing mast cells in the WBB6F1-W/Wv grafted skin was much lower when transplanted to WBB6F1-tg/tg recipients than when transplanted to WBB6F1-+/+ recipients.
Effect of host genotypes on the number of mast cells that developed in the skin tissues grafted from WBB6F1-W/Wv mice. The skin of WBB6F1-W/Wv mice was grafted to WBB6F1-+/+ mice or WBB6F1-tg/tg mice. The number of mast cells in the skin graft and the surrounding host skin was counted various days after transplantation. The bars represent the mean ± standard error (SE) of 5 mice. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the values of host skin.
Effect of host genotypes on the number of mast cells that developed in the skin tissues grafted from WBB6F1-W/Wv mice. The skin of WBB6F1-W/Wv mice was grafted to WBB6F1-+/+ mice or WBB6F1-tg/tg mice. The number of mast cells in the skin graft and the surrounding host skin was counted various days after transplantation. The bars represent the mean ± standard error (SE) of 5 mice. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the values of host skin.
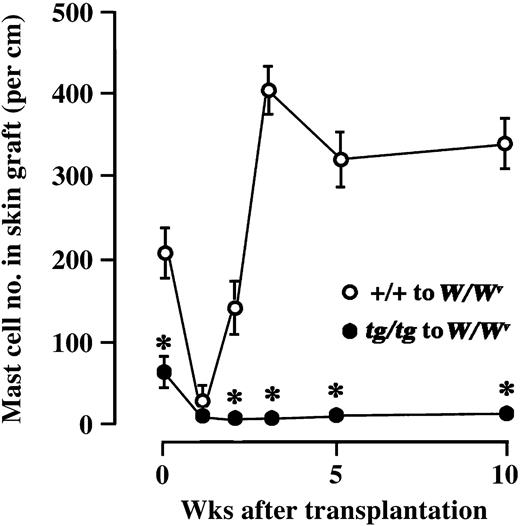
Next, we grafted skin tissues from WBB6F1-+/+ or WBB6F1-tg/tg mice onto the back of WBB6F1-W/Wv mice. The number of mast cells in WBB6F1-+/+ grafts dropped to one sixth a week after the transplantation, and then increased over the values observed in the skin of intact WBB6F1-+/+ mice (Figure 4). The number of mast cells decreased and remained in the low levels in the skin tissues grafted from WBB6F1-tg/tg mice (Figure 4).
Number of mast cells in skin tissues of WBB6F1-+/+ or WBB6F1-tg/tgmice grafted to WBB6F1-W/Wv mice. The bars represent the mean ± SE of 5 mice. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the values of skin tissues grafted from WBB6F1-+/+ mice.
Number of mast cells in skin tissues of WBB6F1-+/+ or WBB6F1-tg/tgmice grafted to WBB6F1-W/Wv mice. The bars represent the mean ± SE of 5 mice. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the values of skin tissues grafted from WBB6F1-+/+ mice.
We also compared the change of mast cell number in skin grafts transplanted from WBB6F1-W/Wv mice or from WBB6F1-tg/tg mice to the back of WBB6F1-+/+ mice. Skin grafts from WBB6F1-W/Wv mice did not contain mast cells before the transplantation, but the mast cell number in grafts remarkably increased after transplantation to WBB6F1-+/+ mice (Figure 5). Skin grafts from WBB6F1-tg/tg mice contained mast cells before the transplantation to WBB6F1-+/+ mice. Although the mast cell number gradually increased in skin tissues grafted from WBB6F1-tg/tg mice, the magnitude of increase was significantly lower than that observed in skin tissues grafted from WBB6F1-W/Wv mice (Figure 5).
Number of mast cells in skin tissues of WBB6F1-W/Wv or WBB6F1-tg/tgmice grafted to WBB6F1-+/+ recipients. The bars represent the mean ± SE of 5 mice. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the value of skin graft from WBB6F1-W/Wv mice.
Number of mast cells in skin tissues of WBB6F1-W/Wv or WBB6F1-tg/tgmice grafted to WBB6F1-+/+ recipients. The bars represent the mean ± SE of 5 mice. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the value of skin graft from WBB6F1-W/Wv mice.
In vitro colony formation
Because KitL is rich in skin tissues, the number of mast cell colonies obtained in the presence of KitL was supposed to reflect the number of mast cell precursors that could differentiate in the skin. Then, in various hematopoietic tissues, we examined the number of mast cell precursors that could differentiate in the skin. Bone marrow, spleen, and peripheral blood mononuclear cells were obtained from WBB6F1-+/+ and WBB6F1-tg/tg mice, and were cultured in methylcellulose containing IL-3 alone or both IL-3 and KitL. When cultured with IL-3 alone, numbers of mast cell colonies per plated cells were comparable between WBB6F1-+/+ and WBB6F1-tg/tg mice (Table 3). In contrast, when cultured with both IL-3 and KitL, the number of mast cell colonies developed from WBB6F1-+/+ cells was much higher than the number of mast cell colonies from WBB6F1-tg/tg cells (Table 3).
Number of mast cell colonies that developed after plating bone marrow, spleen, and peripheral blood mononuclear cells of WBB6F1-+/+ or WBB6F1-tg/tg mice in the presence of IL-3 alone or of both IL-3 and KitL
. | No. of colonies in each culture condition . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | IL-3* . | . | IL-3 + KitL† . | . | |||
| Cells . | WBB6F1-+/+ . | WBB6F1-tg/tg . | WBB6F1-+/+ . | WBB6F1-tg/tg . | |||
| Bone marrow | 2.8 ± 0.5 | 2.7 ± 0.5 | 8.0 ± 0.8 | 3.0 ± 0.5‡ | |||
| Spleen | 17.8 ± 5.4 | 11.5 ± 3.1 | 29.8 ± 6.1 | 10.0 ± 4.8‡ | |||
| Blood | 1.3 ± 0.5 | 0.8 ± 0.3 | 5.8 ± 1.4 | 1.0 ± 0.7‡ | |||
| BMMC | 4.3 ± 1.4 | 3.2 ± 0.9 | 16.2 ± 3.4 | 5.5 ± 0.8 | |||
. | No. of colonies in each culture condition . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | IL-3* . | . | IL-3 + KitL† . | . | |||
| Cells . | WBB6F1-+/+ . | WBB6F1-tg/tg . | WBB6F1-+/+ . | WBB6F1-tg/tg . | |||
| Bone marrow | 2.8 ± 0.5 | 2.7 ± 0.5 | 8.0 ± 0.8 | 3.0 ± 0.5‡ | |||
| Spleen | 17.8 ± 5.4 | 11.5 ± 3.1 | 29.8 ± 6.1 | 10.0 ± 4.8‡ | |||
| Blood | 1.3 ± 0.5 | 0.8 ± 0.3 | 5.8 ± 1.4 | 1.0 ± 0.7‡ | |||
| BMMC | 4.3 ± 1.4 | 3.2 ± 0.9 | 16.2 ± 3.4 | 5.5 ± 0.8 | |||
Values represent the mean ± SE of 6 mice (bone marrow, spleen, and blood) or of 3 cultures (BMMC) and are given as the number per 4 × 104 bone marrow cells, 2 × 105 spleen and peripheral blood mononuclear cells, and 5 × 102 CMCs.
Culture medium containing 10 ng/mL IL-3.
Culture medium containing 10 ng/mL IL-3 and 50 ng/mL KitL.
P < .01 by t test when compared with the value of WBB6F1-+/+ mice.
Since the number of mast cell colonies derived from WBB6F1-+/+ cells was higher when cultured with both IL-3 and KitL than when cultured with IL-3 alone, there was a possibility that the 2 conditions, IL-3 alone and IL-3 + KitL, detected different progenitors. To rule out this, we plated BMMCs that were considered to be the progenitor of mast cells1 and compared the colony number obtained with IL-3 alone with thatobtained with IL-3 + KitL. As observed in the culture derived from bone marrow, spleen, and peripheral blood mononuclear cells, the number of mast cell colonies developed from WBB6F1-+/+ and WBB6F1-tg/tg BMMCs was comparable when cultured with IL-3, but that developed from WBB6F1-+/+ BMMCs was greater than that developed from WBB6F1-tg/tg BMMCs when cultured with both IL-3 and KitL (Table 3). The expression level of KIT protein was lower in mast cells of colonies developed from WBB6F1-tg/tg bone marrow cells than in mast cells of colonies developed from WBB6F1-+/+ bone marrow cells (Figure 6A). The expression level of KIT was also lower in WBB6F1-tg/tg BMMCs than in WBB6F1-+/+ BMMCs when cultured with either IL-3 alone or IL-3 + KitL (Figure 6A). Consistent with the expression level of KIT, the response to KitL was defective in WBB6F1-tg/tg BMMCs (Figure 6B). The lower number of mast cell colonies developed from WBB6F1-tg/tg cells in the presence of KitL appeared to be due to the lower expression of KIT in WBB6F1-tg/tg mast cells.
Defective KIT expression and response to KitL in mast cells of WBB6F1-tg/tg mice. (A) Flow cytometry of the surface expression of KIT in mast cells obtained from colonies and BMMCs. Mast cell colonies developed from bone marrow cells (i-ii), and BMMCs cultured in the presence of IL-3 alone (iii-iv) or IL-3 + KitL (v-vi) were harvested and incubated with FITC-conjugated anti-KIT antibody or isotype control. The staining profiles with anti-KIT antibody (thick line) and with isotype control (thin line) were shown. The data were representative of 3 independent experiments. (B) Response to KitL of BMMCs derived from WBB6F1-+/+ or WBB6F1-tg/tg mice. BMMCs were incubated in the medium containing 10 ng/mL IL-3 and various concentration of KitL, and the response to KitL was detected with uptake of 3H-TdT. The bars represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars.
Defective KIT expression and response to KitL in mast cells of WBB6F1-tg/tg mice. (A) Flow cytometry of the surface expression of KIT in mast cells obtained from colonies and BMMCs. Mast cell colonies developed from bone marrow cells (i-ii), and BMMCs cultured in the presence of IL-3 alone (iii-iv) or IL-3 + KitL (v-vi) were harvested and incubated with FITC-conjugated anti-KIT antibody or isotype control. The staining profiles with anti-KIT antibody (thick line) and with isotype control (thin line) were shown. The data were representative of 3 independent experiments. (B) Response to KitL of BMMCs derived from WBB6F1-+/+ or WBB6F1-tg/tg mice. BMMCs were incubated in the medium containing 10 ng/mL IL-3 and various concentration of KitL, and the response to KitL was detected with uptake of 3H-TdT. The bars represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars.
Skin cell suspensions obtained from WBB6F1-+/+ and WBB6F1-tg/tg mice were cultured in methylcellulose containing IL-3 and KitL. The number of mast cell colonies developed from suspended skin cells of WBB6F1-+/+mice was much higher than that of mast cell colonies from suspended skin cells of WBB6F1-tg/tg mice (Table 4).
Number of mast cell colonies that developed after plating dispersed skin cells
Mice . | No. of colonies . |
|---|---|
| WBB6F1-+/+ | 152 ± 11 |
| WBB6F1-tg/tg | 5.3 ± 1.9* |
Mice . | No. of colonies . |
|---|---|
| WBB6F1-+/+ | 152 ± 11 |
| WBB6F1-tg/tg | 5.3 ± 1.9* |
Values represent mean ± SE of 7 mice and are given as the number per 1 × 105 mononuclear cells, cultured in methylcellulose containing 10 ng/mL IL-3 and 50 ng/mL KitL.
P < .01 by t-test when compared with the value of WBB6F1-+/+ mice.
Discussion
When bone marrow cells of B6-tg/tg mice were transplanted to the irradiated WBB6F1-W/Wv mice, the hemoglobin pattern was completely changed from recipient type to donor type. This indicated that erythrocytes normally developed from the hematopoietic stem cells of B6-tg/tg mice. B and T lymphocytes, macrophages, and granulocytes in the recipient WBB6F1-W/Wv mice contained transgene after the bone marrow transplantation from B6-tg/tg mice, indicating that hematopoietic stem cells of B6-tg/tg mice also differentiated to these cells in irradiated WBB6F1-W/Wv mice. In contrast, mast cells did not develop in all examined tissues after the transplantation of bone marrow cells of B6-tg/tg or WBB6F1-tg/tg mice. The hematopoietic stem cells in the bone marrow appeared to require MITF for differentiation to mast cells but not for differentiation to other blood cells.
Roundy et al32 reported that the number of mature B cells decreased in B6-mi/mi mice, in which abnormal MITF was present instead of normal MITF. B6-mi/mi mice showed more severe phenotype than B6-tg/tg mice due to an inhibitory effect of abnormal MITF.33 However, the development of B cells may not be deficient in B6-tg/tg mice. In fact, our preliminary experiment showed that the proportion of IgM+ and IgD+ B cells in the spleen was comparable between B6-tg/tg and B6-+/+ mice (E.M., K.I., T.H., and Y.K., unpublished data, November 2003).
The number of developing mast cells in the skin tissues grafted from WBB6F1-W/Wv donors was much lower when transplanted to WBB6F1-tg/tg recipients than when transplanted to WBB6F1-+/+ recipients. This was consistent with the result of bone marrow transplantation that no mast cells were detected in the recipient WBB6F1-W/Wv mice after the transplantation from WBB6F1-tg/tg mice. Since WBB6F1-W/Wv mice possess normal tissue environment for mast cell development,1,5 mast cell precursors in the bloodstream of WBB6F1-tg/tg mice appeared to have defects. There was a possibility that the precursor cells had a defect in migration from blood to tissues. This possibility was not plausible, since the direct injection of WBB6F1-tg/tg bone marrow cells into the skin did not result in development of mast cell clusters. Although mast cell precursors of WBB6F1-tg/tg mice possessed the potential to form mast cell colonies in methylcellulose containing IL-3 (Table 3), they lacked the potential for differentiating into mast cells within tissues.
Morphologically identifiable mast cells can proliferate in the skin of WBB6F1-W/Wv mice.34 Matsuda et al29 also reported that mast cells settled in the skin proliferate when the skin is transplanted to the back of another mouse. We transplanted the skin tissues of WBB6F1-+/+ and WBB6F1-tg/tg mice to the back of WBB6F1-W/Wv mice. Since in vitro mast cell colony-forming cells of WBB6F1-W/Wv mice do not differentiate to mast cells in their own skin tissue,1,35 mast cells developing in the skin tissues grafted to WBB6F1-W/Wv mice appeared to be of graft origin. As reported by Matsuda et al,29 mast cell number decreased just after the transplantation, and increased thereafter in the grafts from WBB6F1-+/+ mice. In contrast, mast cell number did not increase in the grafts from WBB6F1-tg/tg mice. Although morphologically identifiable mast cells were present in the skin tissue of WBB6F1-tg/tg mice, their proliferative potential appeared to be deficient.
When the skin tissues were grafted to WBB6F1-+/+ mice, the magnitude of the increase of mast cell number was lower in the grafts from WBB6F1-tg/tg mice than in the grafts from WBB6F1-W/Wv mice. Since mast cells in the skin grafts from WBB6F1-tg/tg mice remained in very low levels in WBB6F1-W/Wv recipients, mast cells developing in the skin grafts appeared to be of the recipient WBB6F1-+/+ mouse origin. Precursor cells migrating in blood of WBB6F1-+/+ recipients appeared to develop more easily in the grafts from WBB6F1-W/Wv mice than in the grafts from WBB6F1-tg/tg mice. These results suggested that the microenvironment for mast cell development was defective in the skin tissue of WBB6F1-tg/tg mice. This was consistent with the present result that the number of mast cells in various tissues was significantly lower in WBB6F1-tg/tg mice than in WBB6F1-W/Wv mice after transplantation of WBB6F1-+/+ bone marrow cells (Table 2). This was also consistent with our previous result that the intraperitoneally injected BMMCs derived from WBB6F1-+/+ mice survived in the mesentery and spleen of WBB6F1-W/Wv mice but not in the mesentery and spleen of WBB6F1-tg/tg mice.10 Roundy et al32 suggested that the tissue environments for development of B cells and mast cells were defective in another MITF mutant, B6-mi/mi mice.
Since KitL is present in skin tissue,1,23 the number of mast cell colonies developing in methylcellulose containing KitL appeared to reflect partly the number of mast cell precursors that could differentiate in the skin tissue. When cultured in the presence of KitL, the number of mast cell colonies that developed from WBB6F1-tg/tg bone marrow, spleen, and peripheral blood cells was approximately one third to one sixth that of mast cell colonies that developed from WBB6F1-+/+ cells. In contrast, the number of mast cell colonies that developed from suspended skin of WBB6F1-tg/tg mice was one thirtieth that of WBB6F1-+/+ mice. This also suggests the presence of defects in both precursor cells and skin tissue environment of WBB6F1-tg/tg mice.
In addition to deficient transcription of KIT and SgIGSF, which may explain the defect of mast cell precursors, transcription of molecule(s) regulating development of mast cells may be deficient in tissues of WBB6F1-tg/tg mice. Our previous reverse transcriptase-PCR results using whole tissues of mesentery and spleen suggested that the expression level of KitL appeared to be comparable between the mesentery and spleen of WBB6F1-tg/tg mice and those of WBB6F1-W/Wv mice.10 Therefore, KitL is not considered to be such a regulating molecule. WBB6F1-tg/tg mice may be useful for identifying new microenvironmental factors other than KitL that regulate development of mast cells.
Prepublished online as Blood First Edition Paper, June 1, 2004; DOI 10.1182/blood-2004-01-0247.
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, and from the Osaka Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Ms C. Murakami, Ms K. Hashimoto, Mr M. Kohara, and Ms T. Sawamura for technical assistance.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal