Abstract
Reduced-intensity conditioned (RIC) hematopoietic stem cell transplantation (HSCT) has improved the accessibility of transplantation in patients previously ineligible. We report the results of allografting following conditioning with fludarabine, busulphan, and alemtuzumab in 62 patients with myelodysplastic syndromes (MDSs) (matched sibling donors [24] or volunteer unrelated donors [VUDs, 38]). The median age for sibling recipients was 56 years (range, 41-70 years) and for VUD recipients, 52 years (range, 22-65 years), with a median follow-up (survivors) of 524 days (range, 93-1392 days) and 420 days (range, 53-1495 days), respectively. The nonrelapse mortality (NRM) at days 100, 200, and 360 was 0%, 5%, and 5%, respectively, for siblings and 11%, 17%, and 21%, respectively, for VUD. The overall survival at one year was 73% for siblings and 71% for VUDs, with a disease-free survival (DFS) of 61% and 59%, respectively. The prognostic significance of the International Prognostic Scoring System (IPSS) was preserved. Of recipients, 86% achieved full-donor chimerism. The cumulative incidence at day 100 of grades III to IV graft-versus-host disease (GVHD) for VUD recipients was 9% and for sibling recipients, 0%. There were 26 patients (16 sibling and 10 VUD) who received donor lymphocyte infusion (DLI) at a median of 273 days (range, 126-1323 days). RIC allogeneic HSCT using this protocol appears to be safe and permits durable donor engraftment. Longer follow-up is required to confirm any potential survival advantage. (Blood. 2004;104:1616-1623)
Introduction
The myelodysplastic syndrome (MDS) and its related conditions such as acute myeloid leukemia with trilineage dysplasia (TLDAML), myelodysplastic/myeloproliferative diseases, and treatment-related myelodysplasia/acute myeloid leukemia (t-MDS/AML) are diagnoses usually made late in life. Adverse prognostic features are usually present at diagnosis, and durable responses to combination chemotherapy are poor, with patients within the International Prognostic Scoring System (IPSS) high-risk group experiencing a median survival of only 0.4 years.1 The presence of multilineage dysplasia in “de novo” AML, suggesting possible transformation from undiagnosed MDS, a common feature in elderly AML, is well recognized to have an adverse impact on survival.2,3 The only curative option for these groups of patients at present is allogeneic hematopoietic stem cell transplantation (HSCT), with its attendant graft-versus-leukemia (GVL) effect. However, the advanced age at presentation and often coexisting morbidity preclude standard myeloablative conditioning in the majority of patients because of the high transplant-related toxicity.4-7 As a result, a supportive care approach to management has been the norm.
In the last 5 years, reduced-intensity conditioned (RIC) HSCT regimens have been demonstrated to be safe and efficacious in a wide range of hematologic malignancies.8-12 The low regimen-related toxicity and morbidity have dramatically extended the availability of allogeneic HSCTs to a large and important group of patients previously ineligible. All RIC protocols depend upon intensive immunosuppression in order to permit donor engraftment and to establish donor-recipient chimerism, which rapidly develops into full-donor chimerism with withdrawal of immunosuppression or donor lymphocyte infusions (DLIs). Control of disease is afforded principally by the GVL effect and to a lesser extent by the chemotherapy administered during conditioning. As most RIC regimens use relatively low doses of cytotoxics, the transplant-related toxicity is significantly reduced.
We report our experience of RIC HSCT using conditioning with fludarabine, busulphan, and alemtuzumab (CAMPATH-1H) in 62 patients with myelodysplastic syndromes who received hematopoietic stem cells (HSCs) from either matched sibling donors or volunteer unrelated donors (VUDs). This we believe is the first and largest systematic series to date of RIC HSCT in myelodysplasia using a uniform conditioning regimen incorporating alemtuzumab.
Patients, materials, and methods
Patients with a confirmed diagnosis of myelodysplastic syndrome (MDS), acute myeloid leukemia with multilineage dysplasia (TLD-AML), myelodysplastic/myeloproliferative disorders (MDS-MPDs) classified according to the World Health Organization (WHO) classification13 were eligible. The protocol was approved by our local institutional research ethics committee. Informed consent was obtained with a full explanation of the risks and alternatives, including that of no further treatment, and the experimental nature of the study protocol. HLA typing of donor and recipients was carried out by high-resolution molecular techniques. Patients with a suitable HLA-matched sibling donor received a sibling allogeneic HSCT, otherwise a suitable VUD was identified using standard criteria.14 In the presence of more than one HLA-matched sibling or VUD donor, a cytomegalovirus (CMV)-matched donor was selected.
The RIC protocol consisted of “FBC”: 30 mg/m2 fludarabine intravenously from day -9 to day -5; 4 mg/kg busulphan per day orally from day -3 to day -2; and 20 mg alemtuzumab intravenously from day -8 to day -4. Unselected peripheral blood stem cells (PBSCs) or bone marrow (BM) was infused on day 0. Immunosuppression was achieved with 1.5 mg/kg cyclosporine intravenously every 12 hours from day -1, titrated to plasma trough levels of 150 to 200 ng/L. Cyclosporin (Neorall; Novartis, Frimley, Camberley, United Kingdom) was substituted when a good oral intake was tolerated and tapered from day +56 in the absence of graft-versus-host disease (GVHD). Filgrastim (300 μg) was administered subcutaneously or intravenously from day +7 to neutrophil engraftment (neutrophils ≥ 0.5 × 109/L). Toxicity was assessed using the Common Toxicity Criteria of the National Cancer Institute (NCIC).15
All patients received standard nursing and supportive care protocols for neutropenic patients. Infection prophylaxis consisted of 200 mg itraconazole suspension orally daily and 500 mg/m2 high-dose aciclovir intravenously every 8 hours from day -5 in high-risk patients (patient and/or donor CMV positive); for all other patients, prophylaxis consisted of 200 mg aciclovir orally every 8 hours and 300 mg aerosolized pentamidine on day -10, and then monthly until cotrimoxazole could be instituted. Patients with previous or ongoing pulmonary aspergillosis received prophylactic (2 mg/kg intravenously 3 times a week) or treatment (3 mg/kg intravenously daily) dose of liposomal amphotericin B (AmBisome; Gilead, Great Abington, Cambridgeshire, United Kingdom), respectively. Blood products were universally leucodepleted and irradiated to 25 Gy. CMV-seronegative recipients received CMV-seronegative blood products.
All recipients were screened for CMV infection/reactivation at least weekly for the first 6 months then every 2 weeks until immunosuppression was completely withdrawn and no graft-versus-host disease was present. Prior to August 2000, CMV monitoring was performed using a fluorescent antigen detection methodology, cytomegalovirus early nuclear fluorescence antigen (CENFA). Subsequently a quantitative polymerase chain reaction (PCR)-based DNA assay several fold more sensitive than the CENFA was used. Indications for preemptive therapy with intravenous ganciclovir or foscarnet were 2 consecutive positive PCR assays or a single positive CENFA.
Chimerism was assessed using X, Y-FISH (fluorescent in-situ hybridization) where a donor-recipient sex mismatch existed, and/or by PCR and fluorescent analysis of short tandem repeat (STR) sequences on bone marrow, whole blood, and peripheral CD3+ and CD15+ cell fractions using the Promega PowerPlex 16 System (Promega, Madison, WI). Semiquantification of donor and recipient chimerism was calculated as a percentage derived from the areas under the curves using the mean of the informative alleles. Chimerism assessments were scheduled for days 28, 56, and 100; 6 months; and then yearly. Response assessment was made morphologically, or where a karyotypic abnormality was present at diagnosis or before transplantation, by conventional cytogenetics. Relapse in patients with refractory anemia required evidence of trilineage dysplasia in marrow and evidence of recipient chimerism or the re-emergence of a previous cytogenetic abnormality.
Graded incremental donor lymphocyte infusions (DLIs) were administered if after day 100 there remained evidence of recipient chimerism despite full withdrawal of immunosuppression. DLI was also administered in the presence of persisting or progressive disease following withdrawal of immunosuppression. Graft-versus-host disease (GVHD) was assessed clinically using the guidelines of the Consensus Conference on acute GVHD grading16 and confirmed histologically where possible. De novo GVHD is defined as the onset of GVHD prior to the delivery of DLI.
Overall survival was measured from day 0 to death from any cause; disease-free survival (DFS), from day 0 to first indicator of relapse (morphologic or cytogenetic); and treatment-related mortality or nonrelapse mortality (NRM), from day 0 to death without evidence of relapse. Survival curves were generated by the Kaplan-Meier method.
Results
There are 62 patients with myelodysplastic syndromes who have undergone RIC transplantation according to the protocol defined. Table 1 summarizes the diagnoses, IPSS scores, cytogenetic risk groups, comorbidities, and previous induction therapy. All patients were of advanced age and/or possessed relative or absolute contraindications to conventional myeloablative HSCT. In particular, 11 patients had a recent history of invasive pulmonary aspergillosis (IPA). On the basis of age alone, 24 patients were entered into the protocol.
Patient and disease characteristics
Characteristics . | Sibling . | VUD . | All . |
|---|---|---|---|
| Refractory anemia/refractory cytopenia (RA/RC) | 4 | 12 | 16 |
| Refractory anemia with excess blasts-I (RAEB-I) | 7 | 2 | 9 |
| Refractory anemia with excess blasts-II (RAEB-II) | 5 | 5 | 10 |
| Chronic myelomonocytic leukemia (CMML) | 1 | 3 | 4 |
| Acute myeloid leukemia with multilineage dysplasia (TLD-AML) | 7 | 16 | 23 |
| Total | 24 | 38 | 62 |
| IPSS | |||
| IPSS-Int1 | 6 | 9 | 15 |
| IPSS-Int2 | 5 | 10 | 15 |
| IPSS-high | 6 | 3 | 9 |
| AML | 7 | 16 | 23 |
| Cytogenetic risk1 | |||
| Good risk (normal karyotype, del(5q),-Y) | 11 | 16 | 27 |
| Intermediate risk (all other abnormalities) | 5 | 12 | 17 |
| Poor risk (complex [≥ 3 abnormalities], Chr 7 abnormalities) | 6 | 9 | 15 |
| Unknown | 2 | 1 | 3 |
| Comorbidities | |||
| Invasive pulmonary aspergillosis within 3 months of conditioning | 3 | 8 | 11 |
| Pulmonary transfer factor (diffusion capacity, TLco) less than 75% of predicted | 3 | 7 | 10 |
| Creatinine clearance, less than 75 mL/min | 2 | 1 | 3 |
| HLA antibody-mediated platelet refractoriness | 2 | 1 | 3 |
| Ulcerative colitis | 1 | 1 | 2 |
| Cardiomyopathy | 3 | 1 | 4 |
| Porphyria cutanea tarda | 0 | 1 | 1 |
| Fe overload | 0 | 3 | 3 |
| Epilepsy | 1 | 0 | 1 |
| Age alone | 8 | 16 | 24 |
Characteristics . | Sibling . | VUD . | All . |
|---|---|---|---|
| Refractory anemia/refractory cytopenia (RA/RC) | 4 | 12 | 16 |
| Refractory anemia with excess blasts-I (RAEB-I) | 7 | 2 | 9 |
| Refractory anemia with excess blasts-II (RAEB-II) | 5 | 5 | 10 |
| Chronic myelomonocytic leukemia (CMML) | 1 | 3 | 4 |
| Acute myeloid leukemia with multilineage dysplasia (TLD-AML) | 7 | 16 | 23 |
| Total | 24 | 38 | 62 |
| IPSS | |||
| IPSS-Int1 | 6 | 9 | 15 |
| IPSS-Int2 | 5 | 10 | 15 |
| IPSS-high | 6 | 3 | 9 |
| AML | 7 | 16 | 23 |
| Cytogenetic risk1 | |||
| Good risk (normal karyotype, del(5q),-Y) | 11 | 16 | 27 |
| Intermediate risk (all other abnormalities) | 5 | 12 | 17 |
| Poor risk (complex [≥ 3 abnormalities], Chr 7 abnormalities) | 6 | 9 | 15 |
| Unknown | 2 | 1 | 3 |
| Comorbidities | |||
| Invasive pulmonary aspergillosis within 3 months of conditioning | 3 | 8 | 11 |
| Pulmonary transfer factor (diffusion capacity, TLco) less than 75% of predicted | 3 | 7 | 10 |
| Creatinine clearance, less than 75 mL/min | 2 | 1 | 3 |
| HLA antibody-mediated platelet refractoriness | 2 | 1 | 3 |
| Ulcerative colitis | 1 | 1 | 2 |
| Cardiomyopathy | 3 | 1 | 4 |
| Porphyria cutanea tarda | 0 | 1 | 1 |
| Fe overload | 0 | 3 | 3 |
| Epilepsy | 1 | 0 | 1 |
| Age alone | 8 | 16 | 24 |
From day -5 to day -1, 24 patients in an initial cohort received alemtuzumab. Immediate gastrointestinal toxicity was limited to NCIC grades I to II mucositis; nausea/vomiting; and diarrhea. No patients required total parenteral nutrition.
Of the patients, 24 (13 men, 11 women) received HSCs from a sibling donor and 38 (21 men, 17 women) from a VUD. All patients had received at least one prior course of chemotherapy, except for 16 patients with refractory anemia/cytopenia who received no prior cytotoxic therapy; 4 of these had received anti-lymphocyte globulin (ALG). Those patients with TLD-AML received a median of 3 cycles of chemotherapy (range, 1 to 6 cycles; Table 2).
There were 33 patients who received at least one cycle of a fludarabine, cytarabine, granulocyte colony-stimulating factor (G-CSF), and idarubicin (FLAG)17 -containing regimen (FLAG-idarubicin; FLAG-Daunoxome [liposomal daunorubicin]; or FLAG-Mylotarg [gemtuzumab ozogamicin]) in order to achieve a remission. Of the patients, 13 received standard AML induction/consolidation therapy only, which was generally delivered according to the United Kingdom Medical Research Council (UK MRC) AML12 protocol. There were 2 patients in the sibling group who had previously received a “minimally myeloablative” HSCT at day -184 and day -108. The conditioning for this consisted of 200 cGy total body irradiation and alemtuzumab with immunosuppression using mycophenolate mofetil and cyclosporine.
Table 3 summarizes the patient and disease characteristics at transplantation. All siblings received fully HLA-matched grafts, while 13 VUD grafts were disparate; 8 of these patients received a graft with a major HLA mismatch. Of the 24 sibling HSCTs, 22 used PBSCs compared with 19 of the 38 VUD HSCTs, with a median CD34 cell count of 4.6 × 106 (range, 1.9 to 17.5 × 106/kg) and 4.2 × 106 (range, 0.4 to 17.3 × 106/kg), respectively. There was, however, no difference in engraftment latency.
Patient and disease characteristics at transplantation
. | Sibling . | VUD . | All . |
|---|---|---|---|
| Patients | 24 | 38 | 62 |
| Median age, y (range) | 56 (41-70) | 52 (22-65) | 53 (22-70) |
| Median time from diagnosis, mo (range) | 12 (5-60) | 16 (6-48) | 15 (5-60) |
| Median donor age, y (range) | 56 (40-68) | 36 (22-47) | 42 (22-68) |
| Disease status at transplantation | |||
| CKR, no. in remission/no. with cytogenetic abnormality at diagnosis | 8/11 | 6/22 | 14/33 |
| CMpR | 14 | 21 | 35 |
| PR | 3 | 2 | 5 |
| PD/relapse | 2 | 2 | 4 |
| Stable | 5 | 13 | 18 |
| CMV status, recipient/donor | |||
| Positive/positive | 14 | 4 | 18 |
| Positive/negative | 3 | 15 | 18 |
| Negative/positive | 1 | 2 | 3 |
| Negative/negative | 6 | 17 | 23 |
| Stem cell source, PBSC/BM | 22/2 | 19/19 | 41/21 |
| HLA disparity | 0 | 13 | 13 |
| Class I major, 1 Ag | 7 | 7 | |
| Class I minor, 1 Ag | 4 | 4 | |
| Class I major and minor, 2 Ag | 1 | 1 | |
| Class II minor, 1 Ag | 2 | 2 | |
| CD34 dose, × 106/kg (range) | 4.6 (1.9-17.5) | 4.2 (0.4-17.3) | 4.4 (0.44-17.5) |
| Days until neutrophil engraftment greater than 0.5 × 109/L (range) | 14 (9-57) | 15 (11-27) | 14 (9-57) |
| Days until platelet engraftment greater than 20 × 109/L (range) | 15 (7-105) | 17 (9-42) | 16 (7-105) |
. | Sibling . | VUD . | All . |
|---|---|---|---|
| Patients | 24 | 38 | 62 |
| Median age, y (range) | 56 (41-70) | 52 (22-65) | 53 (22-70) |
| Median time from diagnosis, mo (range) | 12 (5-60) | 16 (6-48) | 15 (5-60) |
| Median donor age, y (range) | 56 (40-68) | 36 (22-47) | 42 (22-68) |
| Disease status at transplantation | |||
| CKR, no. in remission/no. with cytogenetic abnormality at diagnosis | 8/11 | 6/22 | 14/33 |
| CMpR | 14 | 21 | 35 |
| PR | 3 | 2 | 5 |
| PD/relapse | 2 | 2 | 4 |
| Stable | 5 | 13 | 18 |
| CMV status, recipient/donor | |||
| Positive/positive | 14 | 4 | 18 |
| Positive/negative | 3 | 15 | 18 |
| Negative/positive | 1 | 2 | 3 |
| Negative/negative | 6 | 17 | 23 |
| Stem cell source, PBSC/BM | 22/2 | 19/19 | 41/21 |
| HLA disparity | 0 | 13 | 13 |
| Class I major, 1 Ag | 7 | 7 | |
| Class I minor, 1 Ag | 4 | 4 | |
| Class I major and minor, 2 Ag | 1 | 1 | |
| Class II minor, 1 Ag | 2 | 2 | |
| CD34 dose, × 106/kg (range) | 4.6 (1.9-17.5) | 4.2 (0.4-17.3) | 4.4 (0.44-17.5) |
| Days until neutrophil engraftment greater than 0.5 × 109/L (range) | 14 (9-57) | 15 (11-27) | 14 (9-57) |
| Days until platelet engraftment greater than 20 × 109/L (range) | 15 (7-105) | 17 (9-42) | 16 (7-105) |
CKR indicates complete karyotypic remission, cytogenetic abnormality present at diagnosis no longer detectable and marrow blasts less than 5%; CMpR, complete morphologic remission, less than 5% bone marrow blasts irregardless of karyotype; PR, partial response, greater than 50% decrease in bone marrow blasts; PD, progressive disease, greater than 50% increase in bone marrow blasts; and Ag, antigen.
Primary graft failure occurred in 2 patients (1 sibling; 1 VUD). There was no evidence of autologous recovery at the point of stem cell reinfusion. Both received stem cell reinfusions following a course of anti-lymphocyte globulin (ALG) at day +48 (CD34 = 2.47 × 106/kg) and day +45 (CD34 = 0.84 × 106/kg). The former showed neutrophil engraftment at day +57. The latter succumbed to invasive pulmonary aspergillosis (IPA) and neutropenic sepsis without evidence of engraftment.
Late graft failure without evidence of relapse or autologous reconstitution occurred in one VUD recipient with atypical CML/CMML. He failed to re-engraft following ALG and stem cell reinfusion on day +102 (CD34 = 4.7 × 106/kg). A second transplantation procedure with a different VUD was performed on day +294 and he remains fully donor chimeric.
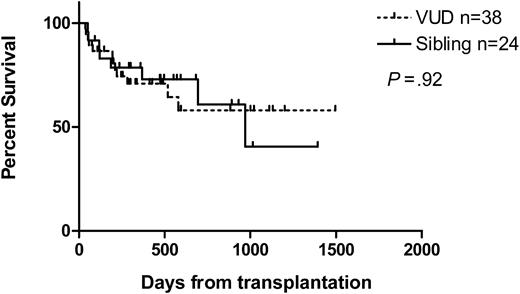
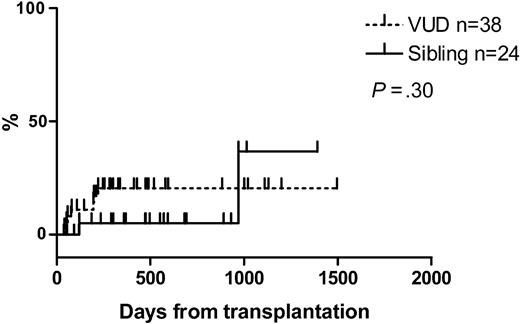
At a median follow-up of 474 days (range, 50-1392 days) for sibling recipients and 295 days (range, 37-1495 days) for VUD recipients, 67% (16 of 24) and 68% (26 of 38) were alive, respectively. The median follow-up for survivors was 524 days (range, 93-1392 days) and 420 days (range, 53-1495 days), respectively. The Kaplan-Meier estimates of overall survival (OS) at one year for sibling recipients were 73% and for VUD recipients, 71%. The estimated median overall survival was 969 days for sibling recipients. That for VUD recipients has not been reached. Similarly, at one year the nonrelapse mortality (NRM) was 5% and 21%, respectively, and disease-free survival 61% and 59%, respectively. Kaplan-Meier curves for overall survival, nonrelapse mortality, relapse, and disease-free survival are shown in Figures 1, 2, 3, 4.
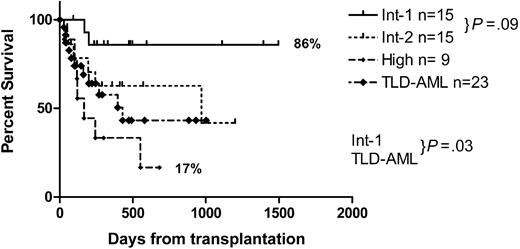
Disease-free survival correlates strongly with the IPSS with a DFS at one year of 86% for IPSS-intermediate-1 (Int1), 63% for IPSS-Int2, 33% for IPSS-high (H), and 58% for TLD-AML. The median DFS for the TLD-AML group was 1.2 years; IPSS-H, 5.5 months; and IPSS-Int2, 2.7 years. The median DFS for the IPSS-Int1 group has not been reached. Table 4 summarizes the survival follow-up.
Summary of follow-up
. | Sibling . | VUD . | All . |
|---|---|---|---|
| Median follow-up, all, d (range) | 474 (50-1392) | 295 (37-1495) | 348 (37-1495) |
| Median follow-up, survivors, d (range) | 524 (93-1392) | 420 (53-1495) | 473 (53-1495) |
| Predicted NRM, % | |||
| Day + 100 | 0 | 11 | 7 |
| Day + 200 | 5 | 17 | 13 |
| 1 year | 5 | 21 | 15 |
| Predicted DFS, % | |||
| Day + 100 | 83 | 84 | 84 |
| Day + 200 | 70 | 66 | 67 |
| 1 year | 61 | 59 | 62 |
| Predicted OS, % | |||
| Day + 100 | 92 | 87 | 89 |
| Day + 200 | 79 | 81 | 80 |
| 1 year | 73 | 71 | 74 |
. | Sibling . | VUD . | All . |
|---|---|---|---|
| Median follow-up, all, d (range) | 474 (50-1392) | 295 (37-1495) | 348 (37-1495) |
| Median follow-up, survivors, d (range) | 524 (93-1392) | 420 (53-1495) | 473 (53-1495) |
| Predicted NRM, % | |||
| Day + 100 | 0 | 11 | 7 |
| Day + 200 | 5 | 17 | 13 |
| 1 year | 5 | 21 | 15 |
| Predicted DFS, % | |||
| Day + 100 | 83 | 84 | 84 |
| Day + 200 | 70 | 66 | 67 |
| 1 year | 61 | 59 | 62 |
| Predicted OS, % | |||
| Day + 100 | 92 | 87 | 89 |
| Day + 200 | 79 | 81 | 80 |
| 1 year | 73 | 71 | 74 |
Disease-free survival also corresponds strongly with the disease status at transplantation. The outcome in patients who underwent transplantation with relapsed/progressive disease or in partial remissions was poor, with median survivals from transplantation of 68 days and 162 days, respectively (Figure 5).
Disease-free survival by status at transplantation. rel indicates relapse; progr, progressive.
Disease-free survival by status at transplantation. rel indicates relapse; progr, progressive.
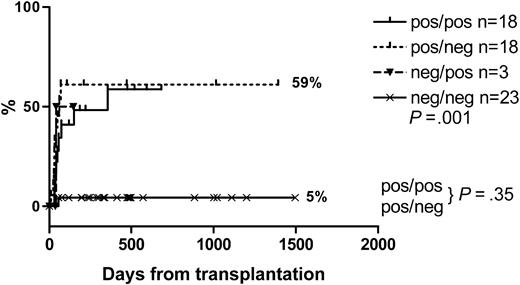
There was high incidence of CMV DNAemia/antigenemia. The median time to first positivity was 43 days (range, 30-356 days) in sibling recipients and 38 days (range, 10-70 days) in VUD recipients. In the highest risk recipient/donor pairs (recipient CMV antibody positive, donor CMV antibody positive or negative), the cumulative incidence of CMV DNAemia/antigenemia was approximately 59% at one year. With the exception of patients developing GVHD requiring treatment with steroids following DLI, the incidence of CMV positivity plateaus by day 200. Only one low-risk VUD patient (CMV-seronegative donor and recipient) developed CMV viremia and CMV disease. The cumulative incidence of CMV reactivation is shown in Figure 6.
Within the VUD group, there were 4 nonrelapse deaths of which infections were of major etiology. Of the deaths, 2 were due to viral infections (1 adenovirus, 1 respiratory syncytial virus); 1 was due to invasive pulmonary aspergillosis, and 1 was due to Epstein-Barr virus (EBV)-positive posttransplantation lymphoproliferative disease.18 In the sibling group, parainfluenza III infection and adenovirus contributed to 2 relapsed deaths, and chronic hepatitis B, to one late NRM at day 969. There was one nonrelapse death from thrombotic-thrombocytopenic purpura at day +121.
Chimerism results on unfractionated bone marrow were available on 22 (92%) of 24 sibling recipients and 35 (92%) of 38 VUD recipients. Of 22 (73%) sibling recipients, 16 achieved full-donor chimerism at first chimerism assessment at a median of 30 days (range, 25-190 days), and 28 (80%) of 35 VUD recipients, at a median of 33 days (range, 20-620 days). Overall, 18 (82%) of 22 sibling recipients achieved full-donor chimerism at a median of 30 days (range, 28-190 days), and 31 (89%) of 35 VUD recipients achieved full-donor chimerism at a median of 36 days (range, 20-620 days).
Quantitative lineage-specific (CD3, CD15) chimerism from peripheral blood cells was available on 4 sibling and 17 VUD recipients. All 21 patients achieved full-donor CD15 chimerism at a median of 35 days (range, 26-188 days) after transplantation. Of 21 patients, 18 (86%) achieved full-donor CD3 chimerism at a median of 57 days (range, 26-201 days) after transplantation. Of interest, 2 of the 3 patients who had not achieved full-donor CD3 chimerism at the time of reporting received peripheral blood stem cells from a sibling donor.
While we have insufficient data at present to determine if there is a quantitative difference in T-cell and myeloid engraftment between sibling and VUD recipients, we have identified 4 early patterns of engraftment for peripheral blood myeloid (CD15+) and T-cell chimerism (CD3+): (1) initial full-donor chimerism in both lineages at day +28, and continued stable full-donor chimerism (37%); (2) initial full-donor engraftment but loss of T-cell engraftment followed by loss of myeloid engraftment over 2 to 3 months in the absence of cytogenetic or morphologic features of relapse (23%); (3) initial incomplete engraftment followed by full T-cell and myeloid engraftment with withdrawal of immunosuppression (27%); and (4) initial full myeloid engraftment, but incomplete T-cell engraftment followed by loss of T cells and myeloid engraftment over 2 to 3 months, in the absence of cytogenetic or morphologic features of relapse (14%).
After transplantation, 16 (67%) sibling recipients received DLI at a median of 264 days (range, 127-1323 days), and 10 (26%) VUD recipients received DLI at a median of 307 days (range, 126-1290 days). The indications for DLI and the outcome are shown in Table 5. The most common indication for DLI was evidence of declining donor chimerism. In all VUD recipients and in 80% of sibling recipients this resulted in re-establishment of full-donor chimerism. There were 2 sibling recipients who appeared refractory to escalating DLI, with no evidence of GVHD or donor reconstitution. Extensive chronic GvHD (cGVHD) and “acute onset” grade IV GVHD developed in 2 VUD recipients (20%), and grade III GVHD, in 2 sibling recipients (12%). While DLI was effective against cytogenetic relapses, it was generally ineffective against established morphologic relapse.
Indications for DLI and outcome
| . | Sibling, 16 of 24 . | VUD, 10 of 38 . |
|---|---|---|
| Indication for DLI | ||
| Cytogenetic relapse | ||
| Number | 2 | 2 |
| Response | 2 CKR | 2 CKR |
| Morphologic relapse | ||
| Number | 4 | 2 |
| Response | 4 NR | 2 NR |
| Decreasing donor chimerism | ||
| Number | 10 | 6 |
| Response | 8 full donor | 6 full donor |
| Prior GVHD | 2 (Gr 1) | 1 (Gr 1) |
| Median total CD3/kg (range) | 1.1 × 107 | 8.0 × 106 |
| (6.0 × 105 to 7.2 × 107) | (1.5 × 106 to 6.2 × 107) | |
| Median days after transplantation | 264 (127-1323) | 307 (126-1290) |
| GVHD after DLI | 2 lcGVHD | 1 ecGVHD |
| 2 Gr III | 2 Gr I | |
| 1 Gr II | 1 Gr IV |
| . | Sibling, 16 of 24 . | VUD, 10 of 38 . |
|---|---|---|
| Indication for DLI | ||
| Cytogenetic relapse | ||
| Number | 2 | 2 |
| Response | 2 CKR | 2 CKR |
| Morphologic relapse | ||
| Number | 4 | 2 |
| Response | 4 NR | 2 NR |
| Decreasing donor chimerism | ||
| Number | 10 | 6 |
| Response | 8 full donor | 6 full donor |
| Prior GVHD | 2 (Gr 1) | 1 (Gr 1) |
| Median total CD3/kg (range) | 1.1 × 107 | 8.0 × 106 |
| (6.0 × 105 to 7.2 × 107) | (1.5 × 106 to 6.2 × 107) | |
| Median days after transplantation | 264 (127-1323) | 307 (126-1290) |
| GVHD after DLI | 2 lcGVHD | 1 ecGVHD |
| 2 Gr III | 2 Gr I | |
| 1 Gr II | 1 Gr IV |
CKR indicates complete karyotypic remission; NR, no response; full donor, achieved full-donor chimerism; 1cGVHD, limited chronic graft-versus-host disease; and ecGVHD, extensive chronic graft-versus-host disease.
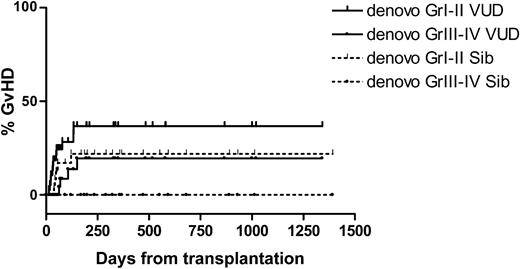
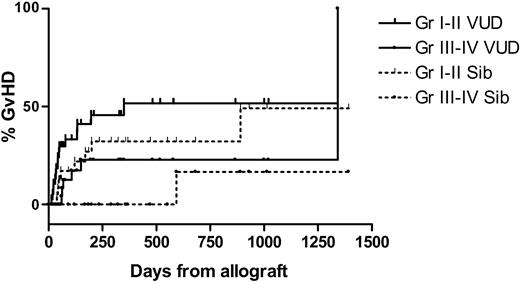
Acute de novo GVHD was limited to grade III in both groups. The cumulative incidence at day 100 of grades III to IV GVHD for VUD recipients was 9%. No sibling recipients developed acute de novo grades III-IV GVHD. Following DLI, the cumulative incidence of GVHD at 2 years for VUD was 52% (Gr I-II), 23% (Gr III-IV) for sibling recipients 32% (Gr I-II), 17% (Gr III-IV). The cumulative incidence of GVHD is shown in Figures 7, 8. One patient from each group developed extensive chronic cutaneous (sclerodermatous) GVHD. Clinically and histopathologically the onset of GVHD after day 100 resembled either acute or chronic GVHD, with skin rashes, diarrhea, abnormal liver function tests, or lichenification and sicca syndromes, and patients were therefore graded as for acute GVHD (aGVHD) if acute features were present.
Cumulative incidence of de-novo versus host disease. Sib indicates sibling.
Cumulative incidence of de-novo versus host disease. Sib indicates sibling.
Cumulative incidence of graft-versus-host disease. Pos indicates positive; neg, negative.
Cumulative incidence of graft-versus-host disease. Pos indicates positive; neg, negative.
A history of invasive pulmonary aspergillosis at transplantation did not contribute to mortality with this transplantation and supportive care protocol, and no patients developed complications related to their comorbidity.
Discussion
The myelodysplastic syndromes are potentially curable by allogeneic transplantation. Conventional myeloablative allogeneic HSCT is associated with a relapse rate of 28% to 48% and a substantial nonrelapse mortality of 34% to 54%, with transplant-related complications increasing in frequency and severity with age.19-22 These events negate the potential benefits of allogeneic HSCT. Improvements in transplantation protocols, such as the use of targeted dose oral busulphan, have improved the transplant-related mortality and disease-free survival in MDS, with an impressive 100-day TRM of 12% to 13%, a 3-year estimated TRM of 28% to 30%, and disease-free survival (DFS) of 56% to 59%.23,24
A complementary approach with reduced myeloablative chemotherapy but with increased immunosuppressive therapy, the reduced-intensity conditioned (RIC) allograft has relatively low transplant-related toxicity and good efficacy in disease control afforded mainly by a graft-versus-malignancy effect. In some series, severe (grades III-IV) aGVHD was significant at 38% to 60%,8,11,12,25-27 but not in others.28 Alemtuzumab is a monoclonal anti-CD52 antibody, and when used as an in vivo T-cell depletion agent it dramatically reduces the incidence of GVHD.9,29,30 The long half-life of alemtuzumab also results in the depletion of donor CD52+ cells including circulating antigen-presenting dendritic cells.31,32 In our series there was no mortality directly attributable to de novo GVHD with a relatively low incidence of de novo aGVHD and cGVHD compared with non-alemtuzumab-containing regimens, even in recipients of mismatched VUD grafts. However, the disadvantage of in vivo purging with alemtuzumab may be a clinically significant reduction in the GVL effect and a higher risk of opportunistic infections.
Studies using fludarabine and busulphan alone in HLA-matched sibling allografts suggest that while the incidence of de novo GVHD was increased, patients who received alemtuzumab required more DLI for mixed chimerism or relapse, with resulting GVHD. There was therefore no difference in the overall incidence of GVHD at 2 years (overall grades II-IV acute GVHD or chronic extensive GVHD), with an incidence of 57% in the alemtuzumab-free group (95% CI, 45%-71%) and 45% (95% CI, 27%-74%) in those who received alemtuzumab (P = .5) (R. Martino, Hospital Santa Creu i Sant Pau, Barcelona, written communication, July 2003). The role of alemtuzumab in HLA-matched sibling allografts therefore deserves further studies.
The incidence of CMV reactivation and other viral infections is high following conditioning with alemtuzumab because of delayed T-cell reconstitution.33-35 Our experience is consistent with this, with 57% of VUD and 50% of sibling recipients in the intermediate- and high-risk populations developing evidence of CMV viremia requiring preemptive therapy. Delays in immune reconstitution highlight the importance of the selection of the most appropriate donors with respect to CMV serology. In our series, there appears to be no statistically significant difference in CMV DNAemia (P = .35) between recipient/donor pairs who are CMV positive/positive and CMV positive/negative. In vivo donor T-cell depletion with alemtuzumab may account for this by depleting CMV-reactive T cells from CMV-positive donors.
The role of remission induction prior to transplantation conditioning has not been well defined, with some studies showing no statistical difference between the outcomes of patients who received induction chemotherapy and those who underwent allografting directly,36,37 but with others demonstrating that the absence of complete remission at transplantation was associated with a poor outcome.37,38 In our series, remission status at transplantation was highly predictive of transplantation failure and early relapse. This may be consistent with the delay in developing a significant graft-versus-leukemia effect. The principle of attainment of at least a complete morphologic response prior to allografting would therefore be desirable. We would recommend a course of intensive induction chemotherapy (eg, with FLAG-idarubicin)17 to be followed upon recovery by an RIC allogeneic HSCT.
Our data show that the NRM at day 100 compares extremely favorably with that of standard myeloablative HSCT, with an NRM in our sibling group of 0% and in the VUD group of 11%. However there were a significant number of relapse and nonrelapse events in the subsequent 100 days.39 The predicted NRM at one year for the sibling group was 5% and the VUD group, 21%. The majority of these events in this setting appears to be infective in origin, reflecting the intense and prolonged immunosuppression.
With standard allograft conditioning, the NRM has been demonstrated conclusively to be associated with age and HLA matching19-21,40-48 ; the impact of these in RIC allografts is less clear, with some suggestion that HLA mismatches are much better tolerated.9 Although the numbers in our series are still small, there appears to be no statistically significant difference between recipients of a sibling allograft compared with a VUD allograft. HLA disparity between the VUD and recipient did not appear to adversely affect the outcome.
Age and disease stage have been shown to be independent prognostic variables for NRM, DFS, and OS, with patients who underwent transplantation early in the disease course having a significantly lower relapse risk and with older patients faring worse.4 The risk of relapse was higher in patients who underwent transplantation with higher blast counts, with higher IPSS scores, and in T-cell-depleted transplantations.49 Regardless of the intensity of conditioning, the DFSs following allografting still correlate strongly with the MDS subtype, disease duration, IPSS scores, and cytogenetic abnormalities.19,42,43,46,47,50 These observations appear to be upheld in our series, with preservation of the prognostic significance of the IPSS and with the median disease-free survivals from transplantation in our TLD-AML group of 1.2 years; IPSS-H, 5.5 months; and IPSS-Int2, 2.7 years. Patients with progressive disease who underwent transplantation had a poor outcome.
Although GVL effect is demonstrable, DLI for relapse expectedly appears most effective at low tumor burdens (cytogenetic relapse), with limited effect demonstrable in our series once morphologic relapse can be identified.
The kinetics of engraftment within hematopoietic cell lineages is dependent upon the conditioning regimen, the source of hematopoietic stem cells, and posttransplantation immunosuppression.51-55 High graft natural killer (NK) cell numbers are associated with high donor T-cell chimerism,56 and the persistence of a large proportion of recipient T cells is predictive of poor engraftment,57 with loss of donor T cells being associated with subsequent loss of the graft.58 Full T-cell engraftment appears to precede myeloid engraftment, the development of GVHD, and the GVL effect.54 Dendritic cell (DC) chimerism after HSCT affects the development of GVHD, with DCs in patients developing cGVHD being exclusively of donor origin.59,60 The kinetics of engraftment following alemtuzumab-containing regimens appears to differ from other T-cell-depleted allografts possibly because of its anti-DC effect.31,32 Our limited data on lineage-specific engraftment appear to show that engraftment after RIC differs from our standard myeloablative conditioning where T-cell and myeloid engraftment is completely donor by day 28 and remains stable in the absence of relapse.
Late graft failure failed to respond to stem cell reinfusion in contrast to early DLI delivered for declining donor chimerism in this limited number of patients, probably because of recipient T-cell reconstitution. Early detection of declining donor chimerism with PCR of short tandem repeat sequences, single nucleotide polymorphisms,61 or X, Y-FISH will be invaluable for the timely guided delivery of DLI.
Our results demonstrate that RIC allogeneic HSCT with fludarabine, busulphan, and alemtuzumab is safe with minimal toxicity, even in high-risk patients of advanced age in whom conventional myeloablative HSCT would have been relatively or absolutely contraindicated, good engraftment, little severe GVHD, and good disease control except in those with aggressive disease. Coexisting conditions that would have been contraindications to conventional conditioning HSCT did not pose a barrier to RIC HSCT with this conditioning and supportive care protocol. The long-term outcome of RIC conditioning is still unknown, and further studies and follow-up are essential in order to optimize the conduct of this extremely important therapeutic modality, which still remains the only curative option for high-risk patients with myelodysplasia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, April 1, 2004; DOI 10.1182/blood-2003-12-4207.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal