Advances in AL amyloidosis: improved quality of life for patients responding to stem cell transplantation and honed patient selection and prognostication using sensitive cardiac biomarkers.
Primary systemic amyloidosis (AL) is a complex multisystem disease that demands both clinical alertness for prompt recognition and a personalized therapeutic strategy. The hematologic complete response rate, improvement of target organ dysfunction, and survival after high-dose therapy followed by autologous peripheral blood stem cell transplantation (PBSCT) have been reported in several nonrandomized trials including approximately 500 treated patients. Seldin and colleagues now report that PBSCT also produces measurable and sustained improvement in health-related quality of life (QOL), particularly in patients who achieve hematologic complete response (CR). The results attained with PBSCT in selected AL patients, namely high rate of CR, improvement of organ system dysfunction and QOL, and long-term survival, set a benchmark against which new antiplasma cell and antiamyloid interventions must be measured. These brilliant results come at a price, since transplantation-related mortality, although progressively decreasing, is still 13% to 14% at experienced centers.1,2 The paper by Dispenzieri and colleagues raises the possibility that this toll might be further reduced by refining patient selection using new markers. They report that simple measurements of sensitive serum cardiac biomarkers (N-terminal pro-brain natriuretic peptide [NT-proBNP] and troponins) can identify a substantial subgroup of high-risk patients even within the selected cohort undergoing PBSCT. Their staging system has several potential applications and contributes to honing patient selection. This necessary selection limits the applicability of PBSCT to a minority of AL patients. Early diagnosis remains the key to effective therapy because it allows full treatment options and potential reversal of organ damage. Hematologists should promote clinical alertness among their colleagues in other specialties.FIG1
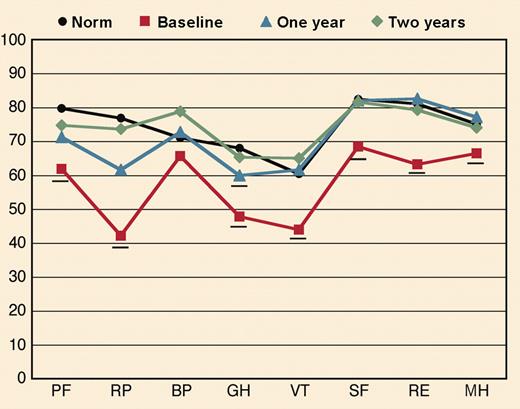
Quality of life of patients who achieve a hematologic complete response to high dose chemotherapy by one year progressively improved and equalled that of population norms at two years. See the complete figure in the article beginning on page 1888.
Quality of life of patients who achieve a hematologic complete response to high dose chemotherapy by one year progressively improved and equalled that of population norms at two years. See the complete figure in the article beginning on page 1888.
Seldin and colleagues observed better outcomes of PBSCT in patients who achieved CR than in those who did not. However, they did report improvements in non-CR patients as well, indicating that partial responses can be accompanied by stabilization or improvement of organ function. This is relevant, since it was reported that reducing the amyloidogenic serum-free light chain (FLC) concentration by more than 50% is often sufficient to lead to stabilization or regression of amyloid deposits, with potential for improved organ function and extended survival.3 Ultimately, progression or improvement of amyloid will be determined by changes in organ function. Although we can now rapidly monitor the clonal response by measuring serum FLC, changes in organ function occur over a longer period. Ideally, we should titrate treatment to reduce the concentration of the circulating amyloidogenic FLC below the threshold resulting in improvement, or at least stabilization, of organ function. Do we have the tools to do so in the clinical setting? As shown by Dispenzieri et al and ourselves,4 serum concentrations of cardiac biomarkers are the most powerful predictors of survival. We have recently observed that reduction of the concentration of serum FLC below thresholds, varying in different patients, translates into concurrent reduction of NT-proBNP and, later, into clinical cardiac improvement in most patients, although the short follow-up prevents evaluation of the impact on survival.5 Serial measurement of these 2 markers might enable clonal and organ (cardiac) response to be evaluated rapidly, minimizing exposure to chemotherapy and detecting refractory patients early, thus gaining precious time for possible alternative treatments.FIG2
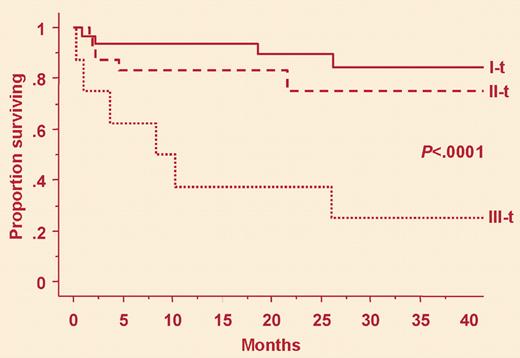
Patients selected for high dose chemotherapy can have elevated levels of two sensitive cardiac markers, and are at high risk (group III-t). See the complete figure in the article beginning on page 1881.
Patients selected for high dose chemotherapy can have elevated levels of two sensitive cardiac markers, and are at high risk (group III-t). See the complete figure in the article beginning on page 1881.
Certainly more data, desirably from randomized studies, such as the ongoing randomized multicenter French trial comparing PBSCT versus the less toxic regimen of oral melphalan and dexamethasone, are needed before settling the therapeutic strategy in AL. Navigation in the sea of AL remains perilous, but we now have several landmarks, represented by sensitive biomarkers, which orient us in the optimal choice and best use of the increasing number of effective therapies. Combined with early diagnosis, this should lead to extended and better life for all AL patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal