Abstract
Graft-versus-host disease (GVHD) is a major complication of allogeneic bone marrow transplantation (BMT). When GVHD is controlled by T-cell–depleted grafts or immunosuppressants, BM transplant recipients often suffer from an increased rate of leukemic relapse and impaired reconstitution of immunity. Using a mouse BMT model, we investigated the effects of hepatocyte growth factor (HGF) gene transfection on the severity of GVHD, the graft-versus-leukemia effect, and the reconstitution of T cells after BMT. After HGF gene transfer, acute GVHD was reduced, while mature donor T-cell responses to host antigens were preserved, resulting in a significant improvement of leukemia-free survival. HGF gene transfer promoted regeneration of bone marrow–derived T cells and the responsiveness of these cells to alloantigens. Furthermore, HGF preserved the thymocyte phenotype and thymic stromal architecture in mice with GVHD. This suggested that HGF exerts a potent protective effect on the thymus, which in turn promotes reconstitution of bone marrow–derived T cells after allogeneic BMT. These results indicate that HGF gene transfection can reduce acute GVHD preserving the graftversus-leukemia effect, while promoting thymic-dependent T-cell reconstitution after allogeneic BMT.
Introduction
Donor T cells contaminating hematopoietic stem cells (HSCs) contribute both positively and negatively to the outcome of allogeneic bone marrow transplantation (BMT). Donor T cells play a positive role by facilitating engraftment of the allograft and contributing to antitumor immunity, but are also primarily responsible for graft-versus-host disease (GVHD).1-8 To date, approaches to control acute GVHD have employed methods that attempt to remove or suppress the function of all T cells regardless of their immunologic specificity. Although the administration of immunosuppressants achieves an acceptable rate of engraftment with reasonable control of GVHD, these drugs induce a generalized decrease of immunocompetence with its attendant morbidity and mortality. Immunosuppressants also often have significant acute and chronic adverse effects on organ function.9,10 Ex vivo T-cell depletion of donor HSCs appears to be more effective at ameliorating or even eliminating GVHD, but this approach is complicated by an unacceptable incidence of graft failure, profound and protracted immunodeficiency, and loss of antitumor immunity.5-8 New methods of reducing toxicity while retaining the antitumor potential of BMT, including donor lymphocyte infusion and nonmyeloablative conditioning, have led to a significant decrease in the occurrence of acute GVHD. Unfortunately, along with the decline of acute GVHD, chronic GVHD has begun to emerge as a major complication of BMT.11
An important cause of impaired reconstitution of immunity after allogeneic BMT is the thymic damage initiated by hostreactive donor T cells, because reconstitution of T-cell immunity depends on the differentiation of donor HSCs in the thymus.12,13 It is likely that thymic epithelial cells (TECs) affected by GVHD fail to provide certain signals that are critical for T-cell differentiation in the thymus.14 Thus, protecting TECs from host-reactive donor T cells may ameliorate GVHD-associated impairment of immune reconstitution.
Hepatocyte growth factor (HGF) was originally identified and cloned as a potent mitogen for hepatocytes.15,16 It has mitogenic, motogenic, and morphogenic effects on various epithelial tissues, including the liver, kidneys, lungs, and intestines.17-19 HGF also shows antiapoptotic activity20 and plays a role in enhancing hematopoiesis.21 We recently demonstrated that repeated transfection of the human HGF gene into skeletal muscle induced the continuous production of HGF, strongly inhibited acute GVHD, and promoted donor HSC engraftment in a well-characterized mouse model of GVHD.22 The present study was performed to examine the influence of HGF on the graft-versus-leukemia (GVL) effect and on T-cell reconstitution after BMT. We found that HGF gene transfection inhibited acute GVHD after BMT, while retaining the GVL effect of mature donor T cells, and also promoted immune reconstitution by born marrow (BM)–derived donor T cells. HGF gene transfection preserved the thymocyte phenotype and thymic stromal architecture in mice with GVHD, suggesting that HGF has a potent protective effect on TECs, which in turn enhances T-cell reconstitution after allogeneic BMT.
Materials and methods
Animals
C57BL/6 (B6, Ly5.2+, H-2b) mice or B6.SJL-PtprcaPep3bBoyJ (B6, Ly5.1+, H-2b) mice were used as BM transplant donors and (B6 × DBA/2)F1 (BDF1, Ly5.2+, H-2bxd) mice were used as recipients. C3H/HeJ (C3H, H-2k) mice were used as stimulators. All of the mice were 8 to 12 weeks old. B6 (Ly5.2+), C3H, and BDF1 mice were purchased from the Shizuoka Laboratory Animal Center (Shizuoka, Japan), while B6 (Ly5.1+) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were maintained in a pathogen-free facility at the Hyogo College of Medicine (Nishinomiya, Hyogo, Japan). Animal experiments were done in accordance with the guidelines of the National Institutes of Health (Bethesda, MD), as specified by the animal care policy of Hyogo College of Medicine.
Bone marrow transplantation
BDF1 mice were exposed to total body irradiation (TBI) from an x-ray source (900 cGy at a dose rate of 50 cGy/min), after which T-cell–depleted (TCD) BM cells (5 × 106) (BMT control group) or BM cells (5 × 106) plus spleen cells (2.5 × 107) (GVHD control group) from B6 (Ly5.2+) or B6 (Ly5.1+) donors were injected via the tail vein. T-cell depletion was performed by treatment with anti-Thy1.2 monoclonal antibody (mAb) (clone 30-H-12) plus rabbit complement. For the induction of leukemia, P815 cells (1 × 104) derived from a mastocytoma in a DBA/2 mouse (H-2d) were injected together with the donor BM cells on the day of BMT. To assess the protective effect of HGF against thymic damage, nonirradiated BDF1 (Ly5.2+, H-2bxd) mice injected with B6 (Ly5.1+) spleen cells were used.
Expression vector and preparation of liposomes containing hemagglutinating virus of Japan (HVJ liposomes)
Human HGF cDNA (2.2 kilobase [kb]) was inserted into the EcoRI and NotI sites in the plasmid pUC-SRα under control of the SRα promoter.23 HVJ liposomes containing plasmid DNA and high-mobility group 1 nonhistone chromosomal protein purified from calf thymus were constructed as described previously.22 Briefly, phosphatidylserine, phosphatidylcholine, and cholesterol were mixed at a weight ratio of 1:4.8:2. This lipid mixture (1 mg) plus plasmid DNA (20 to 40 μg), which had previously been complexed with 6- to 12-μg high-mobility group 1, was sonicated to form liposomes and then mixed with ultraviolet-irradiated HVJ. Excess free virus was subsequently removed from the HVJ liposomes by sucrose density gradient centrifugation.
Gene transfer
BDF1 mice were injected into the gluteal muscle with either HVJ liposomes containing 8 μg human HGF expression vector (HGF-HVJ liposomes) or mock vector. Gene transfer was repeated once a week after BMT (Figures 1A and 4A).
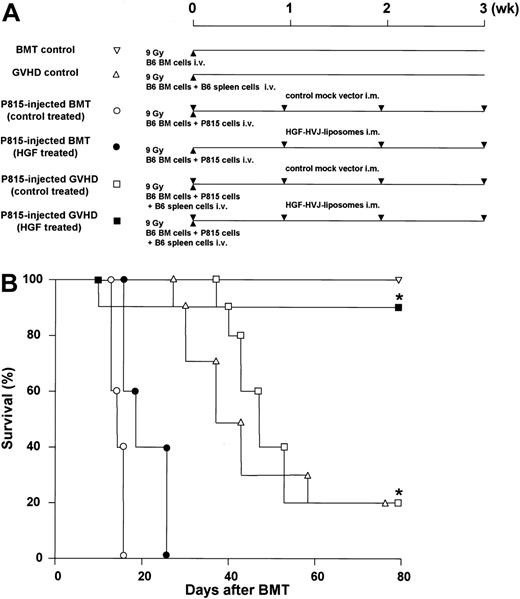
Reduction of GVHD and preservation of GVL activity. (A) Experimental protocol. BDF1 recipients received TBI (9 Gy) followed by intravenous injection of TCD B6 bone marrow cells (5 × 106) and P815 cells (104) on day 0. In addition, some recipients received B6 spleen cells (2.5 × 107) on day 0. BDF1 recipients that received TCD B6 bone marrow cells without P815 cells were used as BMT controls. BDF1 recipients that received TCD B6 bone marrow cells and B6 spleen cells without P815 cells were used as GVHD controls. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0, +7, +14, and +21 of BMT. (B) The survival of HGF-treated GVHD recipients was significantly better than that of all other groups. Shown are BMT controls (▿, n = 5); GVHD controls (▵, n = 5); P815-injected BM transplant (control treated) (○, n = 5); P815-injected BM transplant (HGF treated) (▪, n = 5); P815-injected GVHD (control treated) (□, n = 10); P815-injected GVHD (HGF treated) (▪, n = 10). *P < .01 for survival of P815-injected GVHD (control treated) versus P815-injected GVHD (HGF treated). Representative data from 2 similar experiments are shown.
Reduction of GVHD and preservation of GVL activity. (A) Experimental protocol. BDF1 recipients received TBI (9 Gy) followed by intravenous injection of TCD B6 bone marrow cells (5 × 106) and P815 cells (104) on day 0. In addition, some recipients received B6 spleen cells (2.5 × 107) on day 0. BDF1 recipients that received TCD B6 bone marrow cells without P815 cells were used as BMT controls. BDF1 recipients that received TCD B6 bone marrow cells and B6 spleen cells without P815 cells were used as GVHD controls. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0, +7, +14, and +21 of BMT. (B) The survival of HGF-treated GVHD recipients was significantly better than that of all other groups. Shown are BMT controls (▿, n = 5); GVHD controls (▵, n = 5); P815-injected BM transplant (control treated) (○, n = 5); P815-injected BM transplant (HGF treated) (▪, n = 5); P815-injected GVHD (control treated) (□, n = 10); P815-injected GVHD (HGF treated) (▪, n = 10). *P < .01 for survival of P815-injected GVHD (control treated) versus P815-injected GVHD (HGF treated). Representative data from 2 similar experiments are shown.
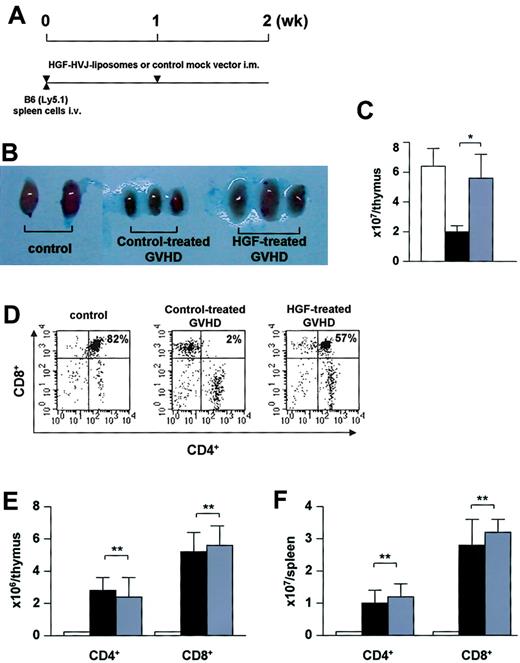
Reduction of thymic GVHD. (A) Experimental protocol. Nonirradiated BDF1 (Ly5.2) recipients received an intravenous injection of B6 (Ly5.1) spleen cells (2.5 × 107) on day 0. BDF1 recipients that received BDF1 spleen cells (2.5 × 107) were used as BMT controls. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0 and +7 of GVHD induction. (B) On day 14 after GVHD induction, the thymi harvested from HGF-treated GVHD recipients were clearly larger than those from control-treated GVHD recipients. (C) Cellularity of the thymus on day 14 after GVHD induction. Total thymus cells are shown. (D) Cell surface expression of CD4 and CD8. (E-F) Donor T-cell infiltration into the thymus (E) and spleen (F). Donor-derived T cells were distinguished from host cells by the expression of Ly5.1. □ indicates controls; ▪, control-treated GVHD recipients; and ▦, HGF-treated GVHD recipients. Data represent the mean ± SD from 5 mice. *P < .05. **P not significant. Representative results from 2 similar experiments are shown.
Reduction of thymic GVHD. (A) Experimental protocol. Nonirradiated BDF1 (Ly5.2) recipients received an intravenous injection of B6 (Ly5.1) spleen cells (2.5 × 107) on day 0. BDF1 recipients that received BDF1 spleen cells (2.5 × 107) were used as BMT controls. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0 and +7 of GVHD induction. (B) On day 14 after GVHD induction, the thymi harvested from HGF-treated GVHD recipients were clearly larger than those from control-treated GVHD recipients. (C) Cellularity of the thymus on day 14 after GVHD induction. Total thymus cells are shown. (D) Cell surface expression of CD4 and CD8. (E-F) Donor T-cell infiltration into the thymus (E) and spleen (F). Donor-derived T cells were distinguished from host cells by the expression of Ly5.1. □ indicates controls; ▪, control-treated GVHD recipients; and ▦, HGF-treated GVHD recipients. Data represent the mean ± SD from 5 mice. *P < .05. **P not significant. Representative results from 2 similar experiments are shown.
Histopathology
Tissues were fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin and were examined by light microscopy. For detection of medullary TECs by immunohistochemistry, the thymus was embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek, Torrance, CA). The tissue was cut into 5-μm sections, mounted on microscope slides, and fixed in pure acetone. The slides were hydrated with phosphate-buffered saline (PBS) for 15 minutes and then incubated with 1% bovine serum albumin (BSA) in PBS for 30 minutes. Next, the sections were incubated with anti-MTS10 mAb (PharMingen, SanDiego, CA) in 0.2% BSA/0.05% sodium azide in PBS for 45 minutes at room temperature in a humidified chamber. After washing 3 times (10 minutes each) with 0.05% Tween 20 in PBS, the sections were incubated for 45 minutes at room temperature with fluorescein isothiocyanate (FITC)–labeled antirat immunoglobulin (Dako, Carpinteria, CA). Then the slides were washed another 3 times (10 minutes each) and mounted with 75% glycerol in PBS (pH 9.5). Images were obtained with a Nikon OPTIPHOTO-2 fluorescence microscope (Nikon, Chiyoda, Japan). For detection of the c-Met HGF receptor, a Vector MOM Immunodetection Kit (Vector Laboratories, Burlingame, CA) was used. In brief, sections were stained with an anti–c-Met mAb (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour. After washing, the sections were incubated with horseradish peroxidase–labeled antimouse immunoglobulin G (IgG), incubated further with 3,3′-diaminobenzidine (DAB) (Dako), and counterstained with hemalaun. For staining of cytokeratin, sections were stained with biotinylated anti–cytokeratin-18 mAb (Ks 18.04) (Progene, Heidelberg, Germany) and horseradish peroxidase–labeled streptavidin.
Flow cytometry
Cell suspensions were prepared in PBS containing 1% fetal calf serum (FCS) and 0.1% sodium azide. The cells were incubated with an anti–Fc receptor mAb (2.4G2) for 10 minutes at 4°C, and then incubated with a FITC-conjugated mAb and a phycoerythrin (PE)–conjugated mAb for 30 minutes. The stained cells were washed twice, resuspended, and analyzed by means of a FACScan (Becton Dickinson, Mountain View, CA). The anti–Fc receptor (2.4G2) mAb, FITC-conjugated antimouse H-2Kb (clone AF6-88.5) mAb, antimouse CD3 (clone 145-2C11) mAb, antimouse CD4 (clone GK1.5) mAb, antimouse CD8 (clone 53-6.7) mAb, PE-conjugated antimouse H-2Kd (clone SF1-1.1) mAb, and antimouse CD45.2/Ly5.1 (clone 104) mAb were all purchased from PharMingen. Multicolor flow cytometry was performed as described previously, with some modifications.22 Channel numbers for the integration of data were chosen on the basis of the staining pattern of normal spleen cells. Staining of normal F1 spleen cells with anti–major histocompatibility complex (anti-MHC) antibodies gave a unimodal positive profile when compared with the negative controls. When donor cells were detected in the GVHD mice, these cells were identified as subpopulations that were clearly negative for F1-specific MHC markers.
ELISA for HGF and interleukin-2 (IL-2)
The human HGF level was measured in plasma by enzyme-linked immunosorbent assay (ELISA) using an antihuman HGF mAb, and the plasma level of mouse HGF was measured by another ELISA using an antimouse HGF mAb (Institute of Immunology, Tokyo, Japan). The human HGF ELISA system specifically detected human HGF, but not mouse HGF. The mouse IL-2 level in culture supernatants was measured by ELISA using an antimouse IL-2 mAb (Genzyme Pharmaceuticals, Cambridge, MA). Each assay was performed according to the manufacturer's protocol.
Mixed lymphocyte reaction (MLR) and in vitro cytokine production
Spleen cells from BM transplant recipients (3 × 105/200 μL/well) were cultured with irradiated (20 Gy) BDF1 (H-2b×d) spleen cells (3 × 105/200 μL/well) or C3H (H-2k) spleen cells (3 × 105/200 μL/well) for 72 hours in 96-well flat-bottomed plates (Falcon Labware, Lincoln Park, NJ), and then pulsed with [3H]thymidine (1 μCi [0.037 MBq] per well). Depletion of Ly5.1+ cells or Ly5.2+ cells was performed by treatment of spleen cells with either anti-Ly5.1 mAb (clone A20) or anti-Ly5.2 mAb (clone 104), respectively, plus rabbit complement. Proliferation was investigated after 20 hours, and the stimulation index (SI) was calculated according to the following formula: sample with stimulation cpm/sample without stimulation cpm. The IL-2 concentration in the culture supernatant was measured by ELISA.
51Cr release assay
Effector cytotoxic T lymphocyte (CTL) activity was tested with the use of spleen cells freshly harvested from BM transplant recipients without in vitro sensitization. CTL activity was assessed on the basis of the lysis of concanavalin A (ConA) blasts from BDF1 mice or P815 cells in a 4-hour 51Cr release assay, as described elsewhere.24 Donor CD8+ T cells in each group were determined and the numbers normalized. They were added at varying effector-target cell (E/T) ratios and incubated for 4 hours with either ConA blasts from BDF1 mice or P815 cells labeled with 100 μCi (3.7 MBq) 51Cr. Effector cells were tested in triplicate, and the percentage of lysis was calculated according to the following formula: [(sample cpm - spontaneous cpm)/(maximum cpm - spontaneous cpm)] × 100%. Results are shown as the mean percentage of lysis at a given E/T ratio for each treatment group.
Statistical analysis
Mean group values were compared by the 2-tailed Student t test. Survival data were plotted by the Kaplan-Meier method and were analyzed by the log-rank test. P less than .05 was considered significant.
Results
HGF promotes leukemia-free survival after BMT by reducing GVHD while preserving the GVL effect
We previously reported that repeated transfection of the human HGF gene into skeletal muscle of BM transplant recipients reduced tissue damage and the subsequent inflammatory responses caused by acute GVHD.22 Because both GVHD and GVL effects are initiated in the presence of graft-versus-host alloreactivity associated with MHC mismatches, we examined the effect of HGF on leukemia-free survival after BMT using a GVL model. BDF1 mice underwent BMT and either HGF gene or a control transfection, as described in “Materials and methods” (Figure 1A). Repeated transfection of the human HGF gene achieved a plasma HGF level (human and mouse HGF) between 1.07 and 1.35 ng/mL during the 4-week period after BMT. Both control-treated and HGF-treated BM transplant recipients injected with P815 cells died by day 30 after BMT. In the GVHD control recipients and control-treated GVHD recipients injected with P815 cells, death occurred from day 27 and day 35, respectively; only 20% of these animals survived to day 80. In contrast, 90% of the HGF-treated GVHD recipients injected with P815 cells were alive on day 80 (P < .01) (Figure 1B). Histopathologic examination revealed prominent P815 cell infiltration in the liver in all of the control-treated and HGF-treated BM transplant recipients injected with P815 cells. Both control-treated and HGF-treated GVHD recipients injected with P815 cells showed no evidence of P815 infiltration in the liver. But, control-treated GVHD recipients injected with P815 cells developed significantly more severe GVHD than HGF-treated GVHD recipients injected with P815 cells (not shown). These results indicate that HGF gene transfection promoted leukemia-free survival after BMT by reducing GVHD while preserving GVL activity.
HGF preserves the reactivity of mature donor T cells to host antigens after BMT
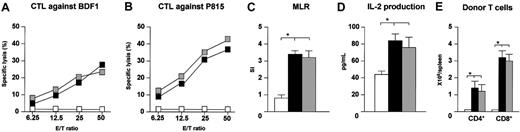
The host-specific response of donor T cells has been demonstrated to be crucial for the GVL effect.25 To investigate whether transfection of HGF gene had an effect on the response of mature donor T cells to host antigens in vivo, the CTL activity of spleen cells from HGF-treated GVHD recipients against host antigens was examined. Spleen cells were harvested from BDF1 recipients on day 14 after BMT, and the CTL activity against host antigens was tested without in vitro sensitization. Strong CTL activity against host BDF1 spleen ConA blasts and P815 cells was demonstrated by the spleen cells from control and HGF-treated GVHD recipients injected with P815 cells (Figure 2A-B). Transfection of HGF also had no effect on donor T-cell proliferation and IL-2 production in response to host antigens (Figure 2C-D). Next, we examined the number of donor CD4+ and CD8+ T cells in the spleen, because earlier studies have demonstrated that donor CD8+ T cells show a more potent GVL effect than CD4+ T cells in several models.26,27 The number of donor CD4+ and CD8+ T cells in the spleens of both control and HGF-treated GVHD recipients injected with P815 cells was significantly increased compared with the BM transplant control recipients, and the increase of donor CD8+ T cells was greater than that of donor CD4+ T cells (Figure 2E). These results indicate that HGF treatment of GVHD recipients maintains the number and reactivity of mature donor T cells against host antigens after BMT.
Preservation of mature donor T-cell expansion and responsiveness to host antigens after BMT. BDF1 recipients received TBI (9 Gy) followed by intravenous injection of TCD B6 bone marrow cells (5 × 106) and B6 spleen cells (2.5 × 107) plus P815 cells (104) on day 0. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0 and +7 of BMT. BDF1 recipients that received TCD B6 bone marrow cells alone were used as BMT controls. On day 14, CTL activity against host antigens and P815 cells (A-B), proliferation (C), and IL-2 production (D) in response to host antigens, and the number of donor CD4+ and CD8+ T cells (E) were determined with the use of spleen cells of recipients as described in “Materials and methods.” □ indicates BMT controls; ▪, P815-injected GVHD (control treated); and ▦, P815-injected GVHD (HGF treated). Mean counts per minute were as follows: unstimulated cultures for BMT controls, 966 cpm; P815-injected GVHD (control treated), 1644 cpm; and P815-injected GVHD (HGF treated), 1799 cpm. Data represent the mean ± standard deviation (SD) for 3 mice. *P < .05 for BMT control versus P815-injected GVHD (control treated and HGF treated). Data of CTL activity are shown as the mean percentage of lysis at a given E/T ratio for 3 mice. Representative results from 2 similar experiments are shown.
Preservation of mature donor T-cell expansion and responsiveness to host antigens after BMT. BDF1 recipients received TBI (9 Gy) followed by intravenous injection of TCD B6 bone marrow cells (5 × 106) and B6 spleen cells (2.5 × 107) plus P815 cells (104) on day 0. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0 and +7 of BMT. BDF1 recipients that received TCD B6 bone marrow cells alone were used as BMT controls. On day 14, CTL activity against host antigens and P815 cells (A-B), proliferation (C), and IL-2 production (D) in response to host antigens, and the number of donor CD4+ and CD8+ T cells (E) were determined with the use of spleen cells of recipients as described in “Materials and methods.” □ indicates BMT controls; ▪, P815-injected GVHD (control treated); and ▦, P815-injected GVHD (HGF treated). Mean counts per minute were as follows: unstimulated cultures for BMT controls, 966 cpm; P815-injected GVHD (control treated), 1644 cpm; and P815-injected GVHD (HGF treated), 1799 cpm. Data represent the mean ± standard deviation (SD) for 3 mice. *P < .05 for BMT control versus P815-injected GVHD (control treated and HGF treated). Data of CTL activity are shown as the mean percentage of lysis at a given E/T ratio for 3 mice. Representative results from 2 similar experiments are shown.
HGF promotes immune reconstitution via BM-derived donor T cells after BMT
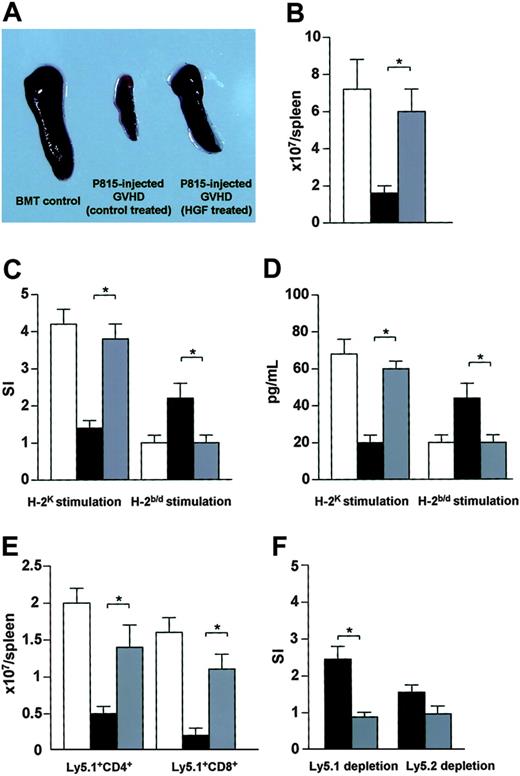
A previous study demonstrated that HGF treatment of GVHD recipients promotes donor cell engraftment after BMT.22 In the present study, we examined whether HGF treatment of GVHD recipients could promote immune reconstitution by BM-derived donor T cells after BMT. Spleens harvested from HGF-treated GVHD recipients injected with P815 cells on day 56 after BMT were clearly larger than spleens from control-treated GVHD recipients injected with P815 cells (Figure 3A). The number of spleen cells from HGF-treated GVHD recipients injected with P815 cells was also higher than in the control-treated GVHD recipients injected with P815 cells (Figure 3B). We next examined donor T-cell functions after BMT. In contrast to control-treated GVHD recipients injected with P815 cells, a marked increase of proliferation and IL-2 production was observed in HGF-treated GVHD recipients injected with P815 cells after stimulation with allogeneic C3H spleen cells (Figure 3C-D). Spleen cells from control-treated GVHD recipients injected with P815 cells proliferated and produced IL-2 in response to host BDF1 spleen cells, while spleen cells from HGF-treated GVHD recipients injected with P815 cells did not react to host BDF1 spleen cells (Figure 3C-D). To investigate BM-derived reconstitution of T-cell immunity, irradiated BDF1 (Ly5.2+) mice received TCD BM cells from B6 (Ly5.1+) donors plus spleen cells from B6 (Ly5.2+) donors. HGF treatment led to a significant increase in the number of Ly5.1+ BM-derived CD4+ and CD8+ T cells (Figure 3E). Spleen cells from control-treated GVHD recipients injected with P815 cells proliferated to a greater extent in response to host BDF1 spleen cells after depletion of Ly5.1+ cells compared with depletion of Ly5.2+ cells (Figure 3F). These results suggest that peripherally derived donor T cells from control-treated GVHD recipients injected with P815 cells still possessed reactivity to host antigens, leading these recipients to develop manifestations associated with chronic GVHD. In contrast, HGF treatment of GVHD recipients established immunologic tolerance to host antigens and promoted immune reconstitution by BM-derived donor T cells.
Promotion of T-cell reconstitution after BMT. (A) BDF1 recipients were treated as described in the Figure 1 legend. On day 56, the spleens harvested from P815-injected GVHD recipients (HGF treated) were clearly enlarged relative to those from P815-injected GVHD recipients (control treated). (B) Total number of spleen cells. (C) MLR. Proliferative response of spleen cells to host antigens and alloantigens on day 56 after BMT. The mean counts per minute were as follows: unstimulated cultures for BMT controls, 1120 cpm; P815-injected GVHD (control treated), 1055 cpm; and P815-injected GVHD (HGF treated), 1098 cpm. (D) IL-2 production in response to host antigens and to alloantigens on day 56 after BMT. (E-F) BDF1 (Ly5.2) recipients were irradiated (9 Gy) and received TCD bone marrow cells (5 × 106) from B6 (Ly5.1) donors and spleen cells (2.5 × 107) from B6 (Ly5.2) donors plus P815 cells. Recipients were treated with HGF cDNA or a mock vector as described in the Figure 1 legend. BDF1 recipients that received TCD bone marrow cells (5 × 106) from B6 (Ly5.1) donors were used as BMT controls. On day 56 after BMT, the number of Ly5.1+CD4+ T cells and Ly5.1+CD8+ T cells in the spleen were analyzed by flow cytometry (E). Spleen cells were treated with either anti-Ly5.1 mAb or anti-Ly5.2 mAb plus rabbit complement, and proliferative responses to host antigens were examined. The mean counts per minute were as follows: unstimulated cultures for P815-injected GVHD (control treated), 1551 cpm; and P815-injected GVHD (HGF treated), 1389 cpm (F). □ indicates BMT controls; ▪, P815-injected GVHD (control treated); and ▦, P815-injected GVHD (HGF treated). Data represent the mean ± SD from 3 mice. *P < .05 for P815-injected GVHD (control treated) versus P815-injected GVHD (HGF treated). Representative results from 2 similar experiments are shown.
Promotion of T-cell reconstitution after BMT. (A) BDF1 recipients were treated as described in the Figure 1 legend. On day 56, the spleens harvested from P815-injected GVHD recipients (HGF treated) were clearly enlarged relative to those from P815-injected GVHD recipients (control treated). (B) Total number of spleen cells. (C) MLR. Proliferative response of spleen cells to host antigens and alloantigens on day 56 after BMT. The mean counts per minute were as follows: unstimulated cultures for BMT controls, 1120 cpm; P815-injected GVHD (control treated), 1055 cpm; and P815-injected GVHD (HGF treated), 1098 cpm. (D) IL-2 production in response to host antigens and to alloantigens on day 56 after BMT. (E-F) BDF1 (Ly5.2) recipients were irradiated (9 Gy) and received TCD bone marrow cells (5 × 106) from B6 (Ly5.1) donors and spleen cells (2.5 × 107) from B6 (Ly5.2) donors plus P815 cells. Recipients were treated with HGF cDNA or a mock vector as described in the Figure 1 legend. BDF1 recipients that received TCD bone marrow cells (5 × 106) from B6 (Ly5.1) donors were used as BMT controls. On day 56 after BMT, the number of Ly5.1+CD4+ T cells and Ly5.1+CD8+ T cells in the spleen were analyzed by flow cytometry (E). Spleen cells were treated with either anti-Ly5.1 mAb or anti-Ly5.2 mAb plus rabbit complement, and proliferative responses to host antigens were examined. The mean counts per minute were as follows: unstimulated cultures for P815-injected GVHD (control treated), 1551 cpm; and P815-injected GVHD (HGF treated), 1389 cpm (F). □ indicates BMT controls; ▪, P815-injected GVHD (control treated); and ▦, P815-injected GVHD (HGF treated). Data represent the mean ± SD from 3 mice. *P < .05 for P815-injected GVHD (control treated) versus P815-injected GVHD (HGF treated). Representative results from 2 similar experiments are shown.
HGF decreases thymic GVHD
Reconstitution of cell-mediated immunity after BMT depends on differentiation of donor HSCs in the thymus, so thymic dysfunction as a consequence of GVHD is likely to impair T-cell reconstitution.12,13 To investigate the protective effect of HGF against thymic GVHD, a nonirradiated parent-into-F1 transplantation model was studied in which thymic injury was independent of the damage caused by TBI (Figure 4A). On day 14 after GVHD induction, a significant decrease in the size and cellularity of the thymus was observed in control-treated GVHD recipients, while HGF treatment significantly inhibited this GVHD-induced thymic injury (Figure 4B-C). CD4+CD8+ double-positive thymocytes were markedly diminished, while CD4+, CD8+ single-positive thymocytes were increased in control-treated GVHD recipients. In contrast, HGF treatment significantly inhibited GVHD-induced changes of the thymocyte populations (Figure 4D). Next, we examined the infiltrating donor T cells in the thymus. Thymocytes from Ly5.2+ recipients were analyzed by flow cytometry to detect the presence of Ly5.1+ T cells, showing that HGF treatment diminished the percentage of donor CD4+ and CD8+ T cells infiltrating the thymus in GVHD recipients. However, the absolute number of infiltrating donor CD4+ and CD8+ T cells was not affected by HGF (Figure 4E). We next assessed the extent of donor CD4+ and CD8+ T-cell infiltration in the spleen treated either with HGF gene or control mock vector. Although HGF treatment diminished the percentage of donor CD4+ and CD8+ T cells infiltrating the spleen in GVHD recipients, the absolute number of infiltrating donor CD4+ and CD8+ T cells was not affected by HGF (Figure 4F).
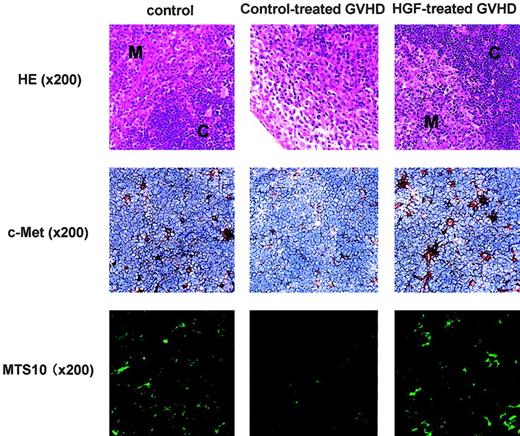
HGF diminishes TEC injury
On day 14 after GVHD induction, histology of control-treated GVHD recipients showed the loss of a clear separation between cortex and medulla, while HGF treatment significantly inhibited this GVHD-induced thymic injury (Figure 5, top). To define the cells targeted by HGF in the thymus, expression of the HGF receptor c-Met was examined immunohistochemically with the use of a mAb directed against c-Met. Adjacent sections (not shown) were stained for cytokeratin to verify the presence of TECs. It was found that c-Met was expressed exclusively by TECs in both the cortex and medulla, and was not expressed by thymocytes (Figure 5, middle). Because the effect of HGF on the thymus is likely to be mediated via binding to its receptor expressed on TECs, the cellular stromal composition and architecture of the thymic microenvironment were investigated in control-treated GVHD recipients. Immunohistochemistry using a mAb for the major type of medullary TEC (MTS10) revealed severely diminished cell numbers in the GVHD recipients. In contrast, HGF treatment preserved this population of cells at a level similar to that in the thymus of control recipients (Figure 5, bottom). These results indicate that HGF has a protective effect against TEC damage caused by GVHD.
TEC expression of c-Met and the effect of HGF treatment. The HGF receptor c-Met is expressed by TECs and HGF treatment reduces TEC injury. Top row: BDF1 (Ly5.2) recipients were treated as described in the Figure 4 legend. On day 14 after GVHD induction, thymic tissue sections from the 3 groups that received transplants were analyzed for histology with the use of hematoxylin and eosin (HE) stain. M indicates medulla; and C, cortex. Middle row: On day 14 after GVHD induction, thymic tissue sections from the 3 groups that received transplants were analyzed for the expression of HGF receptor c-Met with the use of an anti–c-Met antibody. Adjacent sections (not shown) were stained for cytokeratin to verify the presence of TECs. Bottom row: On day 14 after GVHD induction, thymic tissue sections from the 3 groups that received transplants were analyzed for medullary TECs with the use of an anti-MTS10 antibody. Original magnification × 200 (indicated in parentheses). Image acquired using a dual-model cooled CCD camera C4880 (Hamamatsu Photonics, Hamamatsu, Japan), numerical aperture 0.75, and ARGUS (Hamamatsu Photonics).
TEC expression of c-Met and the effect of HGF treatment. The HGF receptor c-Met is expressed by TECs and HGF treatment reduces TEC injury. Top row: BDF1 (Ly5.2) recipients were treated as described in the Figure 4 legend. On day 14 after GVHD induction, thymic tissue sections from the 3 groups that received transplants were analyzed for histology with the use of hematoxylin and eosin (HE) stain. M indicates medulla; and C, cortex. Middle row: On day 14 after GVHD induction, thymic tissue sections from the 3 groups that received transplants were analyzed for the expression of HGF receptor c-Met with the use of an anti–c-Met antibody. Adjacent sections (not shown) were stained for cytokeratin to verify the presence of TECs. Bottom row: On day 14 after GVHD induction, thymic tissue sections from the 3 groups that received transplants were analyzed for medullary TECs with the use of an anti-MTS10 antibody. Original magnification × 200 (indicated in parentheses). Image acquired using a dual-model cooled CCD camera C4880 (Hamamatsu Photonics, Hamamatsu, Japan), numerical aperture 0.75, and ARGUS (Hamamatsu Photonics).
Effect of HGF on thymopoiesis after BMT without GVHD
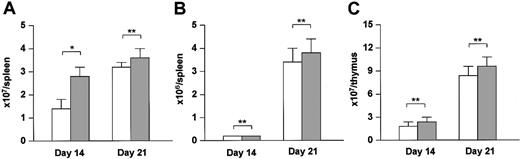
To determine whether the enhancement of immune reconstitution by BM-derived donor T cells was due to an effect of HGF on thymopoiesis or was an indirect effect secondary to the amelioration of thymic GVHD, we transferred the HGF gene into a BMT model and examined its effect on reconstitution of T-cell immunity. Irradiated BDF1 (Ly5.2+) mice received TCD BM cells from B6 (Ly5.1+) donors. HGF treatment led to significant increase in the number of Ly5.1+ BM-derived cells in the spleen on day 14 after BMT (Figure 6A), but did not promote BM-derived T-cell reconstitution on day 14 or day 21 after BMT (Figure 6B). HGF treatment also did not promote BM-derived thymocyte reconstitution on day 14 or day 21 after BMT (Figure 6C). These results indicate that HGF does not directly promote thymopoiesis, but can facilitate the reconstitution of T-cell immunity through its protective effect on thymic GVHD.
Direct effect of HGF on thymopoiesis. BDF1 (Ly5.2) recipients were irradiated (9 Gy) and received TCD bone marrow cells (5 × 106) from B6 (Ly5.1) donors. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0, +7, and +14 of BMT. On days 14 and 21 after BMT, the number of Ly5.1+ cells (A) and Ly5.1+CD3+ cells (B) in the spleen and the number of Ly5.1+ cells in the thymus (C) were analyzed by flow cytometry. □ indicates control-treated BM transplant recipients; and ▦, HGF-treated BM transplant recipients. Data represent the mean ± SD from 3 mice. *P < .05. **P not significant. Representative results from 2 similar experiments are shown.
Direct effect of HGF on thymopoiesis. BDF1 (Ly5.2) recipients were irradiated (9 Gy) and received TCD bone marrow cells (5 × 106) from B6 (Ly5.1) donors. HVJ liposomes containing 8 μg human HGF cDNA or a mock vector were injected intramuscularly on days 0, +7, and +14 of BMT. On days 14 and 21 after BMT, the number of Ly5.1+ cells (A) and Ly5.1+CD3+ cells (B) in the spleen and the number of Ly5.1+ cells in the thymus (C) were analyzed by flow cytometry. □ indicates control-treated BM transplant recipients; and ▦, HGF-treated BM transplant recipients. Data represent the mean ± SD from 3 mice. *P < .05. **P not significant. Representative results from 2 similar experiments are shown.
Discussion
This study demonstrated that HGF gene transfer could markedly reduce GVHD, while preserving the GVL effect against leukemic cells (Figure 1). Much of the therapeutic potential of allogeneic BMT is related to its GVL effect, which is mediated primarily by the cytotoxicity of donor T cells.25 Many approaches to controlling acute GVHD, such as pharmacologic immunosuppression or ex vivo T-cell depletion of the donor HSCs, reduce GVL activity because these methods inhibit donor T-cell responses to host antigens.5-8 HGF treatment maintained the number of donor CD8+ T cells in the spleen on day 14 after BMT and also maintained the responses of these cells to host antigens such as proliferation, CTL activity, and IL-2 production (Figure 2). Conditioning-induced inflammation of the epithelial target tissues of GVHD plays an important role in inducing host-reactive donor T cells to leave the lymphohematopoietic system and infiltrate to these tissues. The gastrointestinal tract has an important role in the amplification of inflammatory effectors, because normal bowel flora and their products (such as endotoxin) can serve as potent triggers of inflammatory cytokine production during GVHD. Furthermore, the Peyer patches of the small intestine provide an important site for antigen presentation to CD8+ donor T cells.28 It is generally accepted that interactions between donor T cells and host antigen-presenting cells (APCs) occur in the secondary lymphoid organs and that host APCs are both necessary and sufficient to cause GVHD.29 The Peyer patches are an integral component of gut-associated lymphoid tissue, which constitutes the largest secondary lymphoid organ in the body and is the major sites of luminal antigen and microorganism sampling, which leads to the continuous induction of immune responses.30 It is possible that the production of endotoxin or inflammatory cytokines in the Peyer patches during injury caused by GVHD may activate host APCs and thereby accelerate the presentation of antigens to donor CD8+ T cells. Thus, protection against intestinal injury and subsequent inflammatory immune responses might be able to confine host-reactive donor T cells to the lymphohematopoietic tissues and subsequently separate GVHD from the GVL effect. Various therapeutic strategies for separation of the GVL effect from the occurrence of GVHD after BMT have been examined in animal models. These approaches include (1) inhibition of inflammatory cytokines with the use of mAbs,31 (2) protection against intestinal damage due to GVHD by using shielding cytokines,26,32 (3) administration of a single dose of IL-12 on the day of BMT,27 and early administration of IL-18 after BMT.33 Recombinant human IL-11 and keratinocyte growth factor (KGF) provided effective protection against intestinal injury caused by acute GVHD and preserved the responsiveness of donor T cells to host antigens, thus promoting leukemia-free survival after BMT by reducing acute GVHD while preserving GVL activity.26,32 Our approach was to reduce the damage to the epithelial target tissues of GVHD and the subsequent inflammatory response caused by acute GVHD through the antiapoptotic action of HGF,22 while preserving the responsiveness of donor T cells to host antigens and thus promoting leukemia-free survival after BMT.
We found that HGF treatment protected against the thymic damage caused by GVHD after BMT (Figures 4 and 5) and promoted T-cell reconstitution (Figure 3). The beneficial effects of HGF on the thymus may be mediated by various mechanisms, but these remain to be defined. First, HGF may block damage to TECs initiated by host-reactive donor T cells via its antiapoptotic action. In this context, we have recently shown that HGF has an antiapoptotic effect on intestinal epithelial cells, thereby blocking intestinal injury caused by GVHD.22 HGF may also promote epithelial cell regeneration after damage has been caused by cellular and inflammatory mediators. Second, endothelial regeneration is essential for the tissue repair process. Since HGF is a potent stimulator of endothelial regeneration, its actions that promote the repair of damaged endothelial cells may not only inhibit inflammatory cell activation by the damaged cells but may also promote regeneration of the thymus. Third, endotoxin and inflammatory cytokines accelerate donor T-cell expansion in the epithelial tissues targeted by GVHD. In our previous study, we demonstrated that HGF inhibits intestinal injuriy caused by GVHD, thereby decreasing inflammatory cytokine production and donor T-cell expansion in epithelial target tissues.22 The protective effect of HGF on the thymus may therefore be in part secondary to the blocking of inflammatory cytokine production. Indeed, we observed a decrease in the thymic expression of interferon-γ (IFN-γ) mRNA (not shown) in HGF-treated GVHD recipients compared with GVHD control recipients. However, IFN-γ mRNA expression in the thymus was still significantly higher in HGF-treated GVHD recipients than in normal mice (not shown), and considerable donor CD8+ T-cell infiltration of the thymus was seen in the HGF-treated GVHD recipients (Figure 4). These results suggest that HGF does not merely have an indirect effect by blocking inflammatory cytokine production and donor T-cell infiltration, but directly protects the thymus from injury caused by GVHD.
Chronic GVHD is characterized by long-lasting immunodeficiency, autoantibody production, and increased collagen deposition, resulting in symptoms similar to those seen in patients with autoimmune diseases. The pathology of chronic GVHD includes the development of host-reactive donor T cells from transplanted donor HSCs in a thymus damaged by acute GVHD.12 T cells generated in irradiated F1 mice reconstituted with parental HSCs show strong tolerance of host antigens, and it has been suggested that contact between T cells and TECs induces the clonal deletion of host-reactive T cells.34 However, TEC damage caused by acute GVHD in the recipient might result in a failure of clonal deletion of host-reactive donor HSC–derived T cells.35 Indeed, chronic GVHD is reported to be the most important factor predicting thymic dysfunction after allogeneic BMT in humans.36 On day 56 after BMT, spleen cells from BM transplant control recipients did not proliferate in response to BDF1 spleen cells, but spleen cells from control-treated GVHD recipients injected with P815 cells showed a proliferative response (Figure 3). This suggests that on day 56 after BMT, donor cells from control-treated GVHD recipients injected with P815 cells were still reacting to host antigens, thereby causing chronic GVHD. In contrast, spleen cells from HGF-treated GVHD recipients injected with P815 cells did not react to host antigens. These results suggest that immunologic tolerance of host antigens was established in the HGF-treated mice by protection against thymic damage caused by acute GVHD, thereby preventing the progression to chronic GVHD.
HGF treatment promoted BM-derived T-cell reconstitution after allogeneic BMT. T-cell immune reconstitution is established through thymic and extrathymic pathways. The thymic pathway involves differentiation of lymphoid precursor cells and selection of functional mature T cells in the thymus, while the extrathymic pathway involves peripheral expansion of the mature donor T cells cotransfused with HSCs. Data from human and murine studies have demonstrated that the extrathymic pathway of T-cell regeneration is limited and that complete T-cell reconstitution depends on the thymus.37,38 Thymic dysfunction as a consequence of GVHD is likely to contribute to severe immunodeficiency after BMT, because TECs, BM-derived thymic stromal cells, and thymocytes are targeted by acute GVHD, and this leads to the loss of normal thymic repertoire selection.39,40 Previously, we have demonstrated that HGF directly promotes hematopoiesis after BMT.22 Since c-Met is expressed on hematopoietic progenitor cells, HGF may directly stimulate lymphoid precursor cells and promote T-cell reconstitution after BMT. To investigate this point, we examined the effect of the reconstitution of cell-mediated immunity using a BMT model. Although HGF treatment promoted donor cell engraftment in the spleen at 14 days after BMT, it did not affect BM-derived T-cell reconstitution in the spleen and the thymus at 14 and 21 days after BMT (Figure 6). Recent studies have demonstrated that KGF treatment is protective against thymic injury caused by GVHD.41 In contrast to our findings, KGF also significantly increased BM-derived T-cell reconstitution in a BMT model.42 Accordingly, HGF directly promotes hematopoiesis of engrafted donor cells, while it indirectly promotes T-cell reconstitution through protection against thymic injury caused by GVHD.
In this report, we have described a new approach to separate the GVL effect from GVHD using HGF gene transfection. HGF transfection also promoted T-cell reconstitution and inhibited chronic GVHD, which are essential objectives after allogeneic BMT. It may be possible to achieve successful transplantation from major human leukocyte antigen–mismatched donors by using HGF gene transfection in combination with other approaches, such as inhibition of T-cell activation or prevention of cytokine up-regulation.
Prepublished online as Blood First Edition Paper, April 20, 2004; DOI 10.1182/blood-2003-12-4309.
Supported by Grants for Scientific Research (nos. 11671019, 14657120, and 15591071) from the Ministry of Education, Science and Culture of Japan.
T. Iwasaki and T. Imado contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hirotsugu Kubo and Masahito Yagi for assistance in preparing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal