Abstract
Persistent levels of plasma nontransferrin bound iron (NTBI) have been associated with tissue iron overload and toxicity. We characterized NTBI's susceptibility to deferoxamine (directly chelatable iron [DCI]) and redox activity (labile plasma iron [LPI]) during the course of long-term, continuous L1 (deferiprone) treatment of patients with hemoglobin E disease and β-thalassemia (n = 17). In 97% of serum samples (n = 267), the LPI levels were more than 0.4 μM (mean ± SEM, 3.1 ± 0.2 μM) and the percent transferrin (Tf) saturation more than 85 (111 ± 6), whereas only in 4% of sera were the LPI levels more than 0.4 μM for Tf saturation less than 85%. Daily administration of L1 (50 mg/kg) for 13 to 17 months caused both LPI and DCI to decrease from respective initial 5.1 ± 0.5 and 5.4 ± 0.6 μM to steady mean levels of 2.18 ± 0.24 and 2.81 ± 0.14 μM. The steady lowest levels of LPI and DCI were attained after 6 to 8 months, with a half time (t1/2) of 2 to 3 months. Serum ferritin and red cell membrane–associated iron followed a similar course but attained steady basal levels only after 10 to 12 months of continuous treatment, with a t1/2 of 5 to 7 months. These studies indicate that LPI and DCI can serve as early indicators of iron overload and as measures for the effectiveness of iron chelation in reducing potentially toxic iron in the plasma.
Introduction
Iron chelation therapy with deferoxamine (DFO) results in a significant improvement in the life expectancy of patients with transfusional iron overload. This is largely attributed to the prevention of heart disease in well-treated thalassemia major patients and, in a few, to the reversal of existing heart disease by aggressive DFO therapy.1,2 The orally active L1 (deferiprone) is more convenient for patients because, unlike DFO, it does not require a special transfusion pump or costly disposable infusers. Since its introduction in 1987,3 the orally active L1 has undergone clinical trials for treating thalassemia major patients.4,5 Although in some patients it might be as effective as DFO given subcutaneously, in others higher doses of DFO or combined therapy with L1 may be needed to provide adequate iron chelation.6
L1 seems to be particularly useful for patients with thalassemia intermedia, who accumulate iron at a much slower rate in the absence of transfusions. Early studies on those patients indicated a reduction in serum ferritin7 and liver iron stores8 following L1 treatment of up to 12 months. A more recent study of L1 has shown significant falls in serum ferritin and red cell membrane iron followed periodically over an 80-week period.9 Long-term treatment with L1 also led to a major reduction in end-point levels of liver iron and circulating forms of labile iron, collectively known as nontransferrin bound iron (NTBI),10 that are potentially harmful to the heart and other organs.
The present prospective study was undertaken with the aim of assessing the possibility that the basal levels of plasma NTBI could serve as early indicators of iron overload in iron-overloaded β-thalassemia/hemoglobin E (HbE) patients and of efficacy of chelation treatment with the oral chelator L1. In a broad sense, NTBI encompasses forms of iron that are not tightly associated with transferrin (Tf) or other proteins.10 Different detection methods exploited diverse properties of iron and probably measured overlapping subfractions of a larger iron pool collectively termed “NTBI.” We have focused on NTBI fractions in plasma or serum of iron-overloaded patients that are both labile—namely, redox active and chelatable—and refer to it as LPI, labile plasma iron. We determined NTBI at least 10 hours after the last dose of L1 was taken, to ensure that the measurements were not compromised by possible contamination with residual Fe chelates or, conversely, with residual free chelator. For that purpose, we applied 2 complementary assays. The first assay, DCI (directly chelatable iron), detects the NTBI component that is directly chelatable by DFO, as measured with a fluoresceinated derivative of DFO.11 DCI measures all the components of NTBI that are accessible to DFO, which includes endogenous ligands such as albumin and citrate as well as exogenous ones such as L1.11 The second assay, LPI, detects the fraction of NTBI that is redox active and eliminated by chelators such as L1 or DFO.12 Thus, in the absence of residual amounts of a chelator (eg, L1) or chelates (eg, L1-Fe complexes) in the plasma, DCI and LPI should measure the same component of NTBI. The study was carried out over a 2-year period during which thalassemia intermedia patients were treated daily with 50 mg/kg L1 for up to 17 months and NTBI was followed periodically together with the more established markers of iron overload. The study indicates that periodic measures of LPI and DCI can serve as early indicators of the efficacy of chelation treatment.
Patients, materials, and methods
Reagents
The following materials were obtained and used without further purification: ascorbic acid, ferrous ammonium sulfate, bovine serum albumin fraction V (Sigma, St Louis, MO), dihydrorhodamine 123, dihydrochloride salt (DHR) (Biotium, Hayward, CA), L1 (deferiprone; Apotex, Weston, ON, Canada), nitrilotriacetic acid (NTA; Fluka, Seelze, Germany), deferrioxamine (DFO) (Novartis, Basel, Switzerland), Chelex-100 chelating resin (Bio-Rad, Hercules, CA), and FL-DFO (N-(fluorescein-5-thiocarbamoyl) desferrioxamine; Molecular Probes, Eugene, OR). Iron-free HEPES-buffered saline (HBS; HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] 20 mM, NaCl 150 mM, pH 7.4) was obtained by treatment with 1 g per 100 mL Chelex-100. Ascorbate (free acid form) was prepared as a concentrated stock of 20 mM in water. DHR was prepared as a concentrated 50 mM stock in acidified dimethyl sulfoxide (produced by adding 0.05 mL 2 M HCl to 1.0 mL dimethyl sulfoxide).
Analyses
Chemical measurement of liver iron was carried out as previously described.13 Red cell ghost membrane iron was determined with the ferrozine-based method in the presence of denaturant sodium dodecyl sulfate and sodium metabisulphite as reducing agent.14,15 Serum erythropoietin was measured by an enzyme-linked immunosorbent assay (ELISA) kit, Quantikine IVD human erythropoietin (EPO) immunoassay (R&D Systems, Minneapolis, MN). Serum ferritin was measured as described by Flowers et al.16 Serum iron and total iron-binding capacity were measured as recommended by the International Committee for Standardization in Hematology (ICSH).17
LPI component of NTBI
The method is based on the conversion of the nonfluorescent dihydrorhodamine DHR to the fluorescent form by various oxidants, such that the generation of reactive oxygen species can be followed as an increase in DHR fluorescence.12 In the LPI assay (Aferrix, Rehovot, Israel), each serum sample is tested under 2 conditions: with 40 μM ascorbate alone and with 40 μM ascorbate in the presence of 50 μM iron chelator. The difference in the rate of oxidation of DHR in the presence and absence of chelator represents the component of plasma NTBI that is redox active. The slopes (r) of DHR fluorescence intensity with time were calculated from measurements taken between 15 and 40 minutes. LPI calculation: Duplicate values of r in the presence of L1 (rL1), and in the absence of L1 (r), were averaged, and the LPI concentration (μM) was determined from calibration curves relating the difference in slopes with and without L1 against Fe concentration as follows: LPI =Δr/rst = (r-rL1)/rst, where Δr and rst denote the L1-sensitive component of r and the calibration factor relating Δr to the Fe concentration, respectively. The calibration factor rst was derived from calibration curves generated using iron concentration standards (0-20 μM) prepared in HBS containing 20 mg/mL bovine serum albumin, which were assayed for LPI similarly to serum samples.
Solutions for iron concentration standards were prepared from a stock solution of stable Fe(III)–NTA complex, formed by mixing equal volumes of 100 mM nitrilotriacetic acid (NTA), titrated to pH 7.0 with NaOH, and 10 mM ferrous ammonium sulfate to produce a molar ratio of Fe/NTA of 1:10 and allowing the Fe(II) to oxidize to Fe(III) in ambient air for 30 minutes.
Directly chelatable iron (DCI) component of NTBI
The concentration of directly chelatable iron (DCI) in the sera of patients was determined with an assay obtained from Aferrix. The method, which was published previously,11 is based on the relative quenching of fluoresceinated DFO by plasma NTBI. Calibration was carried out using the same iron concentration standards used in the LPI assay.
Patient treatment and sampling
Seventeen nontransfusion-dependent β-thalassemia/HbE patients from Thailand who were previously untreated (for at least 1 year) were engaged in the study over a 7- to 17-month period. The patients were recommended to take 50 mg/kg L1 (deferiprone; Apotex) daily in 2 doses, before or after meals. The patients were seen every week for the initial 3 months and then every month throughout the study for clinical examination and laboratory tests. Ophthalmic and auditory examinations were performed both before and at the end of the trial. A complete blood count and renal and liver function tests were performed on each monthly visit. Serum iron, total iron-binding capacity, ferritin, red cell membrane iron, LPI and DCI, and 24-hour urine were measured every 2 months. Serum samples were taken from the patients in the morning, at least 10 hours after the previous drug intake. A liver biopsy for liver iron estimation was performed at the beginning of deferiprone therapy, and the final biopsy was performed within 20 weeks of discontinuing deferiprone. Of the 17 patients, only 14 completed at least 15 weeks of treatment, some in a discontinuous fashion. The trial was approved by the Medical Ethical Committee of Mahidol University, Bangkok, and each of the patients gave full informed consent.
Statistical and graphic analyses were performed using the program Origin (version 7; OriginLab, Northampton, MA).
Results
Table 1 summarizes the parameters obtained in a 13- to 17-month follow-up study of 14 nontransfusion-dependent β-thalassemia/HbE patients from Thailand treated with 50 mg/kg daily doses of L1. Indicated are mean values of each parameter at the beginning and end of the study, the percent change of those values, as well as the length of treatment (t) required for attaining a final steady value and/or half the maximum attainable change (t1/2) in the indicated parameter.
Parameters associated with iron overload in L1-treated patients
Parameters . | Initial values . | Final values . | % change . | t1/2 decay, mo, range . | tmax decay, mo, range . |
|---|---|---|---|---|---|
| Liver iron, mg/g | 23.7 ± 4.3 | 11.4 ± 3.4 | -52* | NA | NA |
| SI, μM | 37 ± 19 | 36 ± 16 | 0 | NA | NA |
| Tf sat. % | 109 ± 23 | 107 ± 33 | 0 | NA | NA |
| SF, ng/mL | 3352 ± 343 | 899 ± 599 | -73* | 4-6 | 10-12 |
| RBCM iron, nmol/mg | 37.2 ± 2.6 | 8.0 ± 1.5 | -78* | 3-5 | 10-12 |
| LPI, μM | 5.1 ± 0.5 | 2.2 ± 0.2 | -57* | 2-3 | 5-7 |
| DCI, μM | 5.4 ± 0.6 | 2.8 ± 0.2 | -49* | 2-3 | 5-7 |
| EPO, U/L | 164 ± 43 | 264 ± 54 | 61* | NA | NA |
| Hb level, g/L | 66 ± 11 | 74 ± 11 | 12† | NA | NA |
Parameters . | Initial values . | Final values . | % change . | t1/2 decay, mo, range . | tmax decay, mo, range . |
|---|---|---|---|---|---|
| Liver iron, mg/g | 23.7 ± 4.3 | 11.4 ± 3.4 | -52* | NA | NA |
| SI, μM | 37 ± 19 | 36 ± 16 | 0 | NA | NA |
| Tf sat. % | 109 ± 23 | 107 ± 33 | 0 | NA | NA |
| SF, ng/mL | 3352 ± 343 | 899 ± 599 | -73* | 4-6 | 10-12 |
| RBCM iron, nmol/mg | 37.2 ± 2.6 | 8.0 ± 1.5 | -78* | 3-5 | 10-12 |
| LPI, μM | 5.1 ± 0.5 | 2.2 ± 0.2 | -57* | 2-3 | 5-7 |
| DCI, μM | 5.4 ± 0.6 | 2.8 ± 0.2 | -49* | 2-3 | 5-7 |
| EPO, U/L | 164 ± 43 | 264 ± 54 | 61* | NA | NA |
| Hb level, g/L | 66 ± 11 | 74 ± 11 | 12† | NA | NA |
The table depicts the initial and final values of parameters associated with iron overload in 14 patients who completed a course of 13 to 17 months of treatment with L1 (50 mg/kg daily doses). The figures were taken from the analyses presented in Figures 2, 3, 4 and are depicted as mean ± SEM. The tmax decay indicates the time required to reach the lowest steady level for a given parameter; t1/2, the decay time to reach the half-maximum change; SI, total serum iron; Tf sat. %, percent transferrin saturation; and NA, nonapplicable.
P < .01.
No statistical significance.
It is clear from the table that whereas the percent transferrin saturation values and those of serum iron hardly changed over the L1 treatment period, all other iron serum parameters such as serum ferritin (SF), red blood cell membrane (RBCM) iron, LPI, DCI, as well as liver iron were reduced significantly by 50% to 80%.
Profiles of NTBI in L1-treated patients
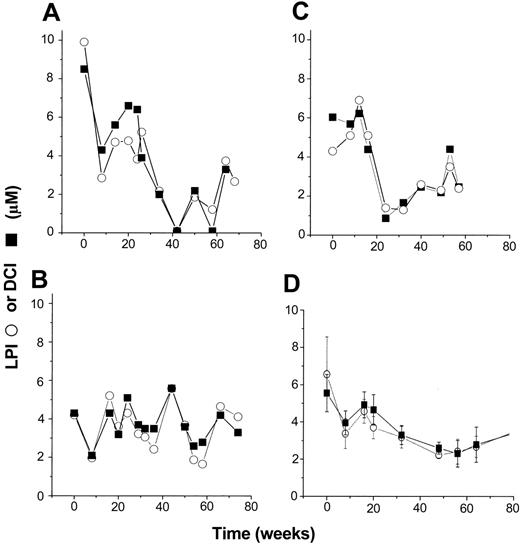
The profiles of basal levels of LPI and DCI in 3 selected L1-treated patients are shown in Figure 1. The term “basal levels” is used to indicate that the samples, which were taken more than 10 hours after L1 administration, were relatively free of chelator and chelates, which presumably cleared from circulation. We based this assumption on the fact that LPI values, which reflect only labile iron, were very close to those of DCI, which detects both labile iron and L1 iron chelates.11 The profiles shown are representative of the different patterns observed. For each individual treatment, the trends obtained are not significantly different for both parameters, indicating a substantial degree of overlap exists between LPI and DCI. However, the values of neither parameter declined with time of treatment in a monotonous fashion. In some individuals the fluctuations were relatively higher than in others, possibly due to temporary (1 to 2 months) lack of full compliance and/or to intrinsic variations in iron utilization during chelation treatment. On the other hand, the hemoglobin levels of the treated individuals remained relatively stable or changed steadily (± 20% to 30%) during the entire treatment, including the periods of highest fluctuations in LPI (P.P. and Z.I.C., unpublished observations, October 2003). This is also line with the fact that these patients did not undergo blood transfusions in the course of treatment. Much of the fluctuations were reduced by averaging the individual profiles at each time point (6- to 10-week periods), as shown in Figure 1D. Based on this paradigm, all the following profiles are given as mean values of parameters obtained from all the patients in the study in a given month or 2-month period.
Effect of oral L1 administration on basal LPI and DCI levels. Individual profiles. Serum samples from patients obtained at least 10 hours after the last L1 oral administration were assayed for LPI (○) and DCI (▪) as described in “Patients, materials, and methods.” The figure depicts results obtained monthly or bimonthly with 3 patients (A-C), each representative of the different types of individual responses observed in the whole group. (D) The mean ± SEM values for LPI and DCI (both in μM) for the entire group. The respective levels at the onset and after 57 to 80 weeks (13 to 17 months) of the treatment were 6.54 ± 2.00 and 3.48 ± 0.34 for LPI and 5.55 ± 1.00 and 2.4 ± 0.20 for DCI. The differences in the profiles of LPI and DCI for each individual were not significant at P < .01 (paired t test analysis).
Effect of oral L1 administration on basal LPI and DCI levels. Individual profiles. Serum samples from patients obtained at least 10 hours after the last L1 oral administration were assayed for LPI (○) and DCI (▪) as described in “Patients, materials, and methods.” The figure depicts results obtained monthly or bimonthly with 3 patients (A-C), each representative of the different types of individual responses observed in the whole group. (D) The mean ± SEM values for LPI and DCI (both in μM) for the entire group. The respective levels at the onset and after 57 to 80 weeks (13 to 17 months) of the treatment were 6.54 ± 2.00 and 3.48 ± 0.34 for LPI and 5.55 ± 1.00 and 2.4 ± 0.20 for DCI. The differences in the profiles of LPI and DCI for each individual were not significant at P < .01 (paired t test analysis).
The profiles of LPI and DCI (mean values ± SEM) obtained for the entire group of patients engaged in the study (n = 17) are shown as a function of duration of L1 treatment (Figure 2). After 6 to 8 months of daily treatment with 50 mg/kg, LPI reached basal levels of 2.2 ± 0.2 μM and DCI 2.8 ± 0.3 μM. The difference between LPI and DCI mean values was 0.3 to 0.6 μM over the entire profile and reflects probably the effects of various serum components on the assays—in particular, interference of radical scavengers with LPI (W.B. and Z.I.C., unpublished observations, July 2002). This is also observed in the high correlation found between these 2 parameters, as depicted in the inset of Figure 2A. Despite the larger number of patients followed, the average profiles still showed fluctutations even after LPI and DCI reached apparently steady basal levels. As in the previous case, we do not attribute those fluctuations to lack of reproducibility of the assay, which was applied on the same samples twice independently, yielding similar results. We rather suspect a variable compliance on the part of some individuals. Unlike LPI and DCI levels that responded to L1 within 1 to 2 months of treatment and significantly decreased thereafter, Tf saturation and total serum iron showed no significant changes over the same time period (Figure 2B).
Effect of oral L1 administration. Grouped profiles. Effect on basal LPI and DCI levels (A) and serum iron and transferrin saturation (B). (A) Results obtained either monthly or bimonthly with 17 patients treated for 7 to 17 months showing mean ± SEM values for LPI and DCI (both in μM). The decay lines were fitted to single exponentials y = A1*exp(–x/t1) + y0 by nonlinear regression analysis (A1 representing the initial y values, x the time, y0 the finally attained values, and t1 the decay correlation time in months [= 1/rate constant]) yielding for LPI: A1 = 5.1 ± 0.5, t1 = 1.5 ± 0.3, and y0 = 2.18 ± 0.14 (r = 0.78); and for DCI: A1 = 5.5 ± 0.6, t1 = 1.84 ± 0.3, and y0 = 2.8 ± 0.14 (r = 0.84). The LPI (○) and DCI (▪) values (μM) at the onset of the treatment were 5.1 ± 0.5 and 5.4 ± 0.6, respectively, and 17 months later they dropped to 2.18 ± 0.24 and 2.8 ± 0.14, respectively, with a half time (t1/2) of 1 to 2 months (t1/2 = t1 * ln2). The inset depicts the correlation between DCI and LPI (corresponding mean ± SEM values plotted against each other) and the best linear fit (slope, 0.8 ± 0.1; intercept, 0.7 ± 0.5; r = 0.8). (B) The mean ± SEM values of serum iron (SI, in μM; •) (range, 31-46; mean, 39 ± 1) and percent transferrin saturation (Tf sat., %; □) (range, 87-116; mean, 108 ± 2) of the serum samples used for the analysis shown in panel A.
Effect of oral L1 administration. Grouped profiles. Effect on basal LPI and DCI levels (A) and serum iron and transferrin saturation (B). (A) Results obtained either monthly or bimonthly with 17 patients treated for 7 to 17 months showing mean ± SEM values for LPI and DCI (both in μM). The decay lines were fitted to single exponentials y = A1*exp(–x/t1) + y0 by nonlinear regression analysis (A1 representing the initial y values, x the time, y0 the finally attained values, and t1 the decay correlation time in months [= 1/rate constant]) yielding for LPI: A1 = 5.1 ± 0.5, t1 = 1.5 ± 0.3, and y0 = 2.18 ± 0.14 (r = 0.78); and for DCI: A1 = 5.5 ± 0.6, t1 = 1.84 ± 0.3, and y0 = 2.8 ± 0.14 (r = 0.84). The LPI (○) and DCI (▪) values (μM) at the onset of the treatment were 5.1 ± 0.5 and 5.4 ± 0.6, respectively, and 17 months later they dropped to 2.18 ± 0.24 and 2.8 ± 0.14, respectively, with a half time (t1/2) of 1 to 2 months (t1/2 = t1 * ln2). The inset depicts the correlation between DCI and LPI (corresponding mean ± SEM values plotted against each other) and the best linear fit (slope, 0.8 ± 0.1; intercept, 0.7 ± 0.5; r = 0.8). (B) The mean ± SEM values of serum iron (SI, in μM; •) (range, 31-46; mean, 39 ± 1) and percent transferrin saturation (Tf sat., %; □) (range, 87-116; mean, 108 ± 2) of the serum samples used for the analysis shown in panel A.
Correlations between LPI and other parameters of iron overload: group analysis
The mean values of serum ferritin (SF) and RBCM-associated iron were also obtained during the course of L1 treatment, as shown in Figure 3. SF is the more commonly accepted parameter for assessing iron overload, whereas RBCM iron has been proposed to be an indicator of red cell dysfunction in thalassemia.15
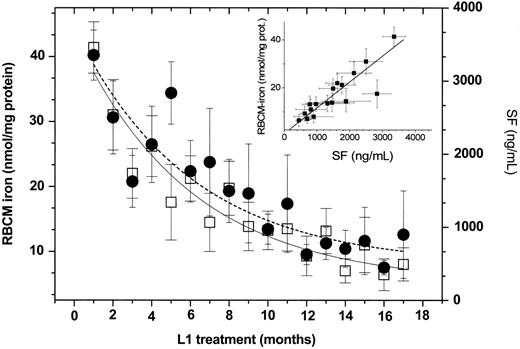
Effect of oral L1 administration on serum ferritin (SF) and red bloodcell membrane (RBCM) iron levels. Grouped profiles. The figure depicts results obtained either monthly or bimonthly with 17 patients showing mean ± SEM values for serum ferritin SF (in nanograms per milliliter, • and broken line) and RBCM (nanomoles per milligram of protein, □ and solid line). The lines represent the nonlinear least square fit based on a single exponential decay function as described in the legend of Figure 2; for RBCM iron, A1 = 37.2 ± 2.6, y0 = 8.0 ± 1.5, and t1 = 5.7 ± 0.8 (r = 0.94); and for SF, A1 = 3407 ± 868, y0 = 550 ± 250, and t1 = 8 ± 0.8 (r = 0.90). The linear correlation between SF and RBCM iron given in the inset yielded a slope of 0.011 ± 0.001, intercept 0.9 ± 1.5. and r = 0.92.
Effect of oral L1 administration on serum ferritin (SF) and red bloodcell membrane (RBCM) iron levels. Grouped profiles. The figure depicts results obtained either monthly or bimonthly with 17 patients showing mean ± SEM values for serum ferritin SF (in nanograms per milliliter, • and broken line) and RBCM (nanomoles per milligram of protein, □ and solid line). The lines represent the nonlinear least square fit based on a single exponential decay function as described in the legend of Figure 2; for RBCM iron, A1 = 37.2 ± 2.6, y0 = 8.0 ± 1.5, and t1 = 5.7 ± 0.8 (r = 0.94); and for SF, A1 = 3407 ± 868, y0 = 550 ± 250, and t1 = 8 ± 0.8 (r = 0.90). The linear correlation between SF and RBCM iron given in the inset yielded a slope of 0.011 ± 0.001, intercept 0.9 ± 1.5. and r = 0.92.
The patterns of decay of SF and RBCM iron obtained in the present study are qualitatively similar to those observed earlier.9 However, a quantitative analysis of the new data reveals that the time required for reducing the initial levels by 50% (t1/2) was 6 to 7 months for SF and 5 to 6 months for RBCM. Both parameters reached a steady basal value within 10 to 12 months. Although there is a parallel decay in SF and RBCM iron, which is evident from the linear relationship found between these 2 parameters (Figure 3 inset), the causal relationship is yet to be elucidated.
A comparison between the decay profiles of LPI and those of SF and RBCM iron following L1 treatment is depicted in Figure 4 in terms of parameter normalized values. We arbitrarily divided the profiles into 2 phases demarcated by the period of treatment required for LPI to reach a constant value. Analysis of the profiles of the individual phases fitted to single exponential decays revealed that the normalized initial decline rate was similar for both LPI and either SF and RBCM iron (RBCM-I) (Figure 4) despite the fact that the half time of decay (t1/2) was 2 to 3 months shorter for LPI than for RBCM-I or SF. Moreover, whereas fluctuations in LPI were noticeable in the plateau phase of the profile (second phase), those in SF and RBCM iron were highest at the initial phase.
Comparative decay kinetics of basal LPI, serum ferritin, and red blood cell membrane (RBCM) levels in patients treated with L1. Data taken from Figures 2 and 3 were normalized to the value at the onset of the L1 treatment, plotted against the length of L1 treatment, and analyzed in terms of single exponential decay functions as shown in the legend for Figure 2. The complete profiles were divided into 2 phases at the 8-month point, at which time LPI reached a steady basal level (A). The profiles for the different phases are depicted separately in panels B and C. Time to reach steady basal levels: for LPI (★) 7 to 8 months, and for either SF (○) or RBCM iron (□) 10 to 12 months. The half time of decline (t1/2) was 1 to 2 months for LPI, 3 to 5 months for RBCM iron, and 4 to 6 months for SF.
Comparative decay kinetics of basal LPI, serum ferritin, and red blood cell membrane (RBCM) levels in patients treated with L1. Data taken from Figures 2 and 3 were normalized to the value at the onset of the L1 treatment, plotted against the length of L1 treatment, and analyzed in terms of single exponential decay functions as shown in the legend for Figure 2. The complete profiles were divided into 2 phases at the 8-month point, at which time LPI reached a steady basal level (A). The profiles for the different phases are depicted separately in panels B and C. Time to reach steady basal levels: for LPI (★) 7 to 8 months, and for either SF (○) or RBCM iron (□) 10 to 12 months. The half time of decline (t1/2) was 1 to 2 months for LPI, 3 to 5 months for RBCM iron, and 4 to 6 months for SF.
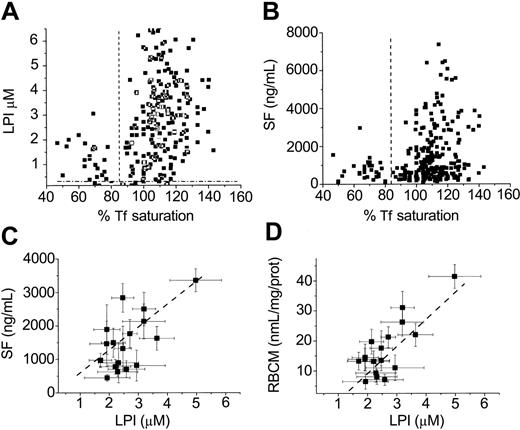
The present studies offered the possibility of using the course of chelation as means to assess when LPI appears relative to the other parameters of iron overload—namely, percent Tf saturation, serum ferritin, and the more recently identified parameter RBCM iron14,15 (Figure 5). In the group of patients treated for up to 17 months with L1, the average value of LPI (μM) in 267 samples was 3.1 ± 1.4 SEM and percent Tf saturation 111 ± 12. The correlation between percent Tf saturation values and LPI is shown in Figure 5A. LPI levels higher than 0.4 μM, the threshold level found in a variety of iron overload conditions,12 was detected in 97% of the patients with Tf saturations higher than 85%. Similar results were obtained in patients with other forms of transfusion-induced hemosiderosis (W.B. and Z.I.C., unpublished observations, February 2004). In patients with Tf saturations lower than 85%, only 4% had LPI at levels higher than 0.4 μM.
Effect of oral L1 administration: correlations between basal LPI, Tfsaturation, serum ferritin, and red blood cell membrane (RBCM) iron levels. The figure depicts results obtained either monthly or bimonthly with patients treated with L1 showing actual values of percent Tf saturation versus those of LPI (μM) (A) or those of percent Tf saturation versus those of SF (nanograms per milliliter) (B) and mean ± SEM values for LPI (μM) versus the respective mean ± SEM values of either SF (nanograms per milliliter) (C) or RBCM (nanomoles per milligram of protein) (D). The broken lines denote, for panels A and B, the value of 85 for percent Tf saturation; for panel A, 0.4 for LPI, and for panel B, 450 for SF. The correlations (corresponding mean ± SEM values plotted against each other) analyzed by the best linear fits yielded the following. (A) For LPI and RBCM iron (top): slope, 9 ± 1; intercept, –9 ± 3, r = 0.74; and (B) for LPI and SF (middle): slope, 660 ± 204; intercept, –100 ± 120, r = 0.78.
Effect of oral L1 administration: correlations between basal LPI, Tfsaturation, serum ferritin, and red blood cell membrane (RBCM) iron levels. The figure depicts results obtained either monthly or bimonthly with patients treated with L1 showing actual values of percent Tf saturation versus those of LPI (μM) (A) or those of percent Tf saturation versus those of SF (nanograms per milliliter) (B) and mean ± SEM values for LPI (μM) versus the respective mean ± SEM values of either SF (nanograms per milliliter) (C) or RBCM (nanomoles per milligram of protein) (D). The broken lines denote, for panels A and B, the value of 85 for percent Tf saturation; for panel A, 0.4 for LPI, and for panel B, 450 for SF. The correlations (corresponding mean ± SEM values plotted against each other) analyzed by the best linear fits yielded the following. (A) For LPI and RBCM iron (top): slope, 9 ± 1; intercept, –9 ± 3, r = 0.74; and (B) for LPI and SF (middle): slope, 660 ± 204; intercept, –100 ± 120, r = 0.78.
Discussion
Nontransferrin bound iron (NTBI) has been detected in diseases associated with dysfunctions of iron metabolism caused by genetic factors or therapeutic interventions.10,18,19 The presence of NTBI in plasma has been assumed to be of potential risk to the heart and to other organs due to its propensity for permeating cells by unregulated mechanisms and thereby causing iron overload and engaging in oxidative damage. Most of those studies have relied on models of iron overload obtained by imposing heavy loads of iron on animals with nonnatural sources of the metal or administering various types of iron salts into the medium of cultured cells. Although all of those maneuvers lead to global iron overload and ensuing tissue damage, they do not address the issue of NTBI's involvement in organ damage in the clinical state.
To what extent the presence of NTBI in plasma has clinical implications depends, first and foremost, on whether or not it could be correlated with other clinical parameters associated with iron disorders. However, firstly, NTBI needs to be defined in clinical chemistry terms irrespective of its chemical composition. Originally NTBI was detected in sera from thalassemic patients as a chemical fraction of iron not associated with Tf.18 Attempts to improve the detection assays included the use of mobilizing agents in conjunction with chelators/detectors10,19-24 or high concentrations of ascorbate.21 The results indicated that NTBI is heterogeneous and variable not only quantitatively but also in composition, depending on the pathological condition in question.10 For example, in hereditary hemochromatosis, NTBI (0.4- to 3-μM levels) mobilized with agents such as nitrilotriacetate or oxalate is detected in patients with Tf saturations lower than 85% and even as low as 55%, none of which is directly chelatable by agents such as DFO or L1 or by Tf itself.19,20 In thalassemia, unlike in hemochromatosis, NTBI levels span a wider range of values (0.4-10 μM), Tf saturation levels can reach 80% to 100% and even higher, and most of the NTBI is chelated by agents such as DFO.10,11,23 This fraction of NTBI, which in thalassemia is referred to as directly (or DFO-) chelatable iron (DCI),11 has recently been shown to be redox active and was therefore referred to as labile plasma iron (LPI).12 Regarding the chemical nature of NTBI per se, an early study carried out on serum from a hemochromatosis patient24 suggested citrate iron as the major NTBI component. However, that suggestion was probably not supported by data obtained by the same authors who showed that the putative component was barely accessible to DFO, whereas citrate Fe is readily chelated. Other candidates have been recently proposed25 but remain to be experimentally validated.
The present study was undertaken with the aim of assessing whether any of the labile forms of NTBI have clinical implications for iron overload conditions. Specifically, does NTBI represent a parameter that could be quantitatively correlated with established parameters of iron overload that also have diagnostic as well as therapeutic value and, if so, could it be used for assessing the efficacy of iron chelation therapy. We have chosen for this study a group of patients with overt iron overload and no previous treatment with chelator or recent (more than 2 years) blood transfusion who enrolled in a program of iron chelation therapy with L1. All patients had β-thalassemia intermedia or hemoglobin E disease. For NTBI measurements we applied both the DCI and the LPI methods, which in recent studies11,12 allowed the simultaneous detection of chelatable and labile iron in the plasma during treatment with L1. The measurements were carried out on blood samples withdrawn from patients at least 10 to 12 hours after the last intake of the drug to allow the NTBI to recover to “basal levels” following drug withdrawal.26 The study lasted for about 2 years in which 17 patients were treated daily with L1 (50 mg/kg in 2 doses), of whom 14 completed (cumulatively) 13 to 17 months of L1 treatment, with clinical and biochemical/hematologic tests taken monthly or bimonthly. A similar study showed recently that long-term treatment with L1 led to marked reductions in the final score of the biochemical/hematologic parameters, but no kinetic analysis was provided.9
It is clear from Table 1 that, unlike serum iron and Tf saturation, the basal levels of DCI and LPI were reduced by L1 treatment to a similar extent (percent change) as liver iron, the classical parameter of iron overload.27,28 The final level of DCI or LPI observed after 13 to 17 months of treatment was already attained after 5 to 7 months, with a t1/2 of 2 to 3 months for either parameter. The decrease in LPI (Figure 4) and DCI (not shown) could be fitted to a single exponential decay function. On the other hand, the reduction in SF and RBCM iron levels was of a bimodal nature (Figure 4). The reduction in the last 2 parameters was detectable within 2 to 3 months of treatment (as LPI or DCI), but its second phase was more prolonged (10 to 12 months) (Figure 4). From the calculated t1/2 values and from the profiles shown in Figure 4, it can be deduced that the decrease in LPI preceded that of RBCM iron and in turn preceded that of SF. However, with presently available information, it is not possible to attach to the above temporal sequence any mechanistic linkage; nor is it possible to link LPI and liver iron, despite the results shown in Table 1. Nonetheless, the fact remains that substantial basal levels of liver iron and of both NTBI parameters LPI and DCI persisted in the plasma (2 to 3 μM) even when both SF and RBCM iron continued to decrease. This might be associated with the relatively mild regimen of L1 applied (only 50 mg/kg daily), which might be sufficient for reducing SF and RBCM iron to relatively low levels but apparently is not sufficient for eliminating basal LPI or excess liver iron.3 Because the L1 concentration attained in plasma 2 to 3 hours after oral intake was still sufficient for complete elimination of LPI for at least 2 hours following administration of similar doses,12 it can be deduced that (1) LPI basal levels are steady-state levels of LPI that were replenished within 10 to 12 hours following drug intake and, if so, (2) the LPI basal levels represent the balance between L1's capacity for removing iron and the body's for replenishing it. It should also be possible to establish a temporal correlation between LPI and body iron stores by quantifying changes in liver iron levels using noninvasive techniques such as magnetic resonance imaging (MRI) or a superconducting quantum interference device27-29 (SQUID) and correlating them with changes in LPI.
From the profiles depicted in Figures 1 and 2, it can deduced that LPI and DCI are highly correlated; in some cases they are almost identical, and in others they differ at most by a value of 0.3 to 0.6 μM. Although that difference represents at most 25% lower values in LPI as compared with those of DCI, it is statistically different (P < .01). We tentatively attribute that difference to the possible presence of residual L1-Fe complexes that are detected by DCI as NTBI but not by the LPI assay or to presence of minor components of DCI that are not redox active.
The present studies offered the possibility of using the course of chelation as means to assess the parameters of iron overload with which changes in LPI could be compared—namely, percent Tf saturation and serum iron (Figure 2) and serum ferritin and the more recently identified parameter RBCM iron14 (Figure 5). In the group of patients treated for up to 17 months with L1, the average value of LPI (μM) in 267 samples was 3.1 ± 1.4 SEM and, of percent Tf saturation, 111 ± 12. The correlation between percent Tf saturation values and LPI is shown in Figure 5A. LPI levels higher than 0.4 μM, the threshold level found in a variety of iron overload conditions,12 were detected in 97% of the patients with Tf saturations higher than 85%. Similar results were obtained in patients with other forms of transfusion-induced hemosiderosis (W.B. and Z.I.C., unpublished observations, February 2004). In patients with Tf saturation values lower than 85%, only 4% had LPI at levels higher than 0.4 μM. This indicates that for most thalassemia intermedia patients whose average percent Tf saturation values were in the range of 87 to 116 (mean ± SEM, 108 ± 2), LPI was present only when Tf saturation exceeded 85% ± 5%. A similar picture was obtained for the correlation between SF and percent Tf saturation, except that only 75% of the samples had values higher than 85% Tf saturation and 750 ng/mL SF, and 17% had Tf saturations lower than 85% and SF levels higher than 720 ng/mL. It would appear paradoxical that SF (and RBCM iron) levels continued to decrease dramatically while Tf saturations decreased to a much lesser extent and remained high throughout the treatment period (Figure 5). One explanation for this discrepancy is that SF and Tf saturation represent iron pools with different rates of turnover and a much slower response to changes in the iron load. Thus, iron stores that induce secretion of ferritin into the circulation have a slow turnover (possibly days to weeks), while serum iron, and with it LPI and DCI, is replenished rapidly and continually within hours. Despite those differences between LPI and SF, which are also reflected in the decay profiles shown in Figure 4, these 2 parameters showed fair to good correlations (Figure 5C) as well as those between LPI and RBCM iron values (Figure 5D). The analysis indicates that for a particular treatment of daily L1 doses of 50 mg/kg, a decline of 9 nanomoles of iron per milligram of RBCM protein or 660 ng/mL SF was equivalent to a decline in 1μM LPI.
In conclusion, NTBI assayed periodically as DCI and LPI or sequentially during treatment of thalassemia intermedia patients with L1 can serve not only as an early indicator of iron overload but also as a measure of the efficacy of the iron chelation treatment. Further studies remain to be conducted to establish whether the steady levels of LPI and liver iron attained even after prolonged treatment could be further reduced with higher doses of L1 alone or in combination with DFO. Recent observations obtained with thalassemia major patients in Italy treated with standard doses of either DFO given nightly (intravenously or subcutaneously), L1 (75 mg/kg/d) given orally 3 times during the day, or a combination of both (DFO nightly and L1 daily) indicate that only patients given combination therapy showed no detectable levels of LPI when followed 24 hours at 4- to 6-hour intervals (G. Zanninelli et al, manuscript in preparation).
To our knowledge, the present study is the first one in which an attempt has been made to correlate the effect of iron chelating therapy on LPI and DCI with conventional measures of iron overload such as serum ferritin, liver iron, and RBC membrane iron prospectively, over a period of 13 to 17 months. Our findings that the decrease in DCI and LPI is significantly faster than that of serum ferritin and RBC iron support the concept that LPI and DCI represent a labile and highly chelatable iron compartment with a rapid turnover. Furthermore, the sharp increase observed in the magnitude of LPI when transferrin saturation exceeds 85% indicates a threshold or overflow phenomenon wherein the harmful effects of iron overload are activated when iron accumulation exceeds a certain limit. Our conclusions are strongly supported by the recent studies of Jensen et al,29 in which threshold values of liver iron (more than 400 mmol/g dry weight), serum ferritin (more than 2500 ng/mL), and Tf saturation (more than 75%) have been found, beyond which hepatic and myocardial toxicity is regularly encountered in the form of increased serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and abnormal myocardial iron concentrations. Conversely, the rapid decrease in DCI and LPI following the introduction of effective iron chelation therapy may imply early evidence of a beneficial protective effect to the heart, liver, and other vital organs. However, such correlations are yet to be found and require further prospective studies of well-documented clinical benefit.
Prepublished online as Blood First Edition Paper, May 20, 2004; DOI 10.1182/blood-2004-02-0630.
Supported by the Incubator Program Aferrix sponsored by the Israeli Ministry of Industry and Resources and The European Community 5th Framework QLRT-2001-00444 (W.B., Z.I.C.). P.P. was supported by the National Research Council of Thailand (Israel-Thailand joint project) and Apotex (Weston, ON, Canada). M.S. is a research student of The Hebrew University of Jerusalem and was supported by Apotex.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The kits for DCI and LPI detection were developed and registered through support from the Incubator Program Aferrix.

![Figure 2. Effect of oral L1 administration. Grouped profiles. Effect on basal LPI and DCI levels (A) and serum iron and transferrin saturation (B). (A) Results obtained either monthly or bimonthly with 17 patients treated for 7 to 17 months showing mean ± SEM values for LPI and DCI (both in μM). The decay lines were fitted to single exponentials y = A1*exp(–x/t1) + y0 by nonlinear regression analysis (A1 representing the initial y values, x the time, y0 the finally attained values, and t1 the decay correlation time in months [= 1/rate constant]) yielding for LPI: A1 = 5.1 ± 0.5, t1 = 1.5 ± 0.3, and y0 = 2.18 ± 0.14 (r = 0.78); and for DCI: A1 = 5.5 ± 0.6, t1 = 1.84 ± 0.3, and y0 = 2.8 ± 0.14 (r = 0.84). The LPI (○) and DCI (▪) values (μM) at the onset of the treatment were 5.1 ± 0.5 and 5.4 ± 0.6, respectively, and 17 months later they dropped to 2.18 ± 0.24 and 2.8 ± 0.14, respectively, with a half time (t1/2) of 1 to 2 months (t1/2 = t1 * ln2). The inset depicts the correlation between DCI and LPI (corresponding mean ± SEM values plotted against each other) and the best linear fit (slope, 0.8 ± 0.1; intercept, 0.7 ± 0.5; r = 0.8). (B) The mean ± SEM values of serum iron (SI, in μM; •) (range, 31-46; mean, 39 ± 1) and percent transferrin saturation (Tf sat., %; □) (range, 87-116; mean, 108 ± 2) of the serum samples used for the analysis shown in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-02-0630/5/m_zh80170465910002.jpeg?Expires=1769126356&Signature=eN39aOZWcDmtwl3oDjCPy~Rn4yrHfJHIbz1MV-0E1vGjevOBsZ0a4hZrTwKJZZXDNNBLlZBEZhHb9aqyncSgvmr6DVGNiI2agk~gXN8QjfETiKQQuxtxnWQWFXP7ZagTHHN74WFEWEm5unFZebRd2epfz4c7RL~vgRWiVW9vhejpQ7OEIaUC5mURkixyonZiglz-WkUtYcob9BxEhMHtex8fR6lmO2ZFKyja9kagI4dw1oL-XcwQ-v0vi-Ul3~P531Ehn3EA~NSesP8QR~iosNZtOfO0Fcm3wlknko-2D6xXSfDHCUCvcc3w2ZM6AFEBOUJs8i8dznp1CyNq8aeHew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal