Abstract
To develop a model for Epstein-Barr virus (EBV) pathogenesis in immunosuppressed hosts, we studied experimental infections of immunocompetent versus SHIV 89.6P–infected, immunosuppressed rhesus macaques with the EBV-related rhesus lymphocryptovirus (LCV). Primary LCV infection after oral inoculation of 4 immunocompetent animals was characterized by an acute viremia and seroconversion followed by asymptomatic LCV persistence. Four immunosuppressed macaques infected orally with LCV failed to develop an LCV-specific humoral response and viremia was more pronounced, but there was no evidence of LCV-induced lymphoproliferative disease. A more aggressive primary challenge was administered by intravenous inoculation of 108 autologous, LCV-immortalized B cells in 4 additional immunosuppressed animals. Two animals with modest immunosuppression remained asymptomatic, and 1 of 2 severely immunosuppressed animals developed an aggressive, monoclonal LCV-positive lymphoma. These studies demonstrate the potential for lymphomagenesis in an experimental model system for EBV infection and underscore the strength and depth of immune control in limiting LCV-induced lymphoproliferative disease.
Introduction
Primary Epstein-Barr virus (EBV) infection is the most common cause of infectious mononucleosis, and, by adulthood, nearly all humans are persistently and asymptomatically infected with EBV.1 In a small percentage of individuals, EBV infection is associated with the development of Burkitt lymphoma, AIDS, transplantation-associated B-cell lymphomas, Hodgkin lymphoma, nasopharyngeal carcinoma, leiomyosarcoma, and gastric carcinoma.1
The viral and host factors important for development and control of EBV-associated malignancies are poorly understood. EBV infects most humans by adulthood, yet the overwhelming majority of EBV-infected individuals do not suffer from EBV-associated malignancies. The degree of immunosuppression is one risk factor for EBV-infected B-cell lymphomas as demonstrated by the increased frequency of EBV-associated lymphomas in patients aggressively treated with immunosuppressive drugs after organ transplantation2 and in patients with poorly controlled or more advanced stages of AIDS.3-5 However, immunosuppression alone is not sufficient for EBV-induced lymphomagenesis, because EBV-induced B-cell lymphomas still develop in only a minority of patients who receive transplants and patients with AIDS. Furthermore, EBV-associated lymphomas occur in patients without overt immunosuppression (eg, Burkitt lymphoma and Hodgkin lymphoma). Chromosomal abnormalities, such as c-myc translocations in Burkitt lymphoma and aberrant somatic hypermutation of cellular oncogenes in postgerminal center B-cell lymphomas have been hypothesized as second steps that act in concert with EBV infection to increase the risk of B-cell lymphomas.6,7
Existing models for studying EBV infection are limited in their ability to address the multiple factors leading to the development and control of EBV-associated malignancies in vivo. Tissue culture systems are valuable for dissecting molecular pathways usurped by specific viral genes to immortalize B-cell growth,8 but tissue culture systems are unable to address why some EBV-infected individuals develop malignancies whereas others do not. Inoculation of severe combined immunodeficient (SCID) mice with EBV-infected B cells can produce tumors in a small animal model. However, in this model system, host cells are never infected with EBV, and tumorigenesis in the immunodeficient animal is inevitable if sufficient numbers of EBV-infected B cells are inoculated.9 EBV infection of cotton-top tamarins, a New World nonhuman primate, can induce lymphomagenesis in some animals in which the tamarin B cells are infected and immortalized by EBV.10,11 This is an important animal model for establishing the oncogenic potential of EBV infection, but the tamarin immune response to a cross-species virus infection may not accurately model the human immune response to EBV infection.
There is considerable biologic, genetic, and molecular evidence that the EBV-related herpesvirus, or lymphocryptovirus, that naturally infects rhesus macaques (rhesus LCV) is a relevant and useful model for EBV infection.12 Like EBV, rhesus LCV infection is highly prevalent and persistent in adult rhesus macaques.13 Rhesus LCV infection is associated with B-cell lymphomas that develop in simian immunodeficiency virus (SIV)–infected, immunosuppressed monkeys.14 In vitro, rhesus LCV infection efficiently immortalizes rhesus B cells into permanently growing cell lines similar to EBV-immortalized lymphoblastoid cell lines.15 The complete rhesus LCV genome sequence has been determined, revealing an identical repertoire of viral genes as EBV.16 Individual studies of the transformation-associated rhesus LCV latent infection genes show that they use the same molecular pathways for B-cell growth transformation as the EBV homologues.17-22
We have shown that experimental oral infection of naive, immunocompetent rhesus macaques results in an acute viral syndrome and persistent infection similar to EBV infection in humans.23 The ability to manipulate rhesus LCV infection in an experimental model system (eg, controlled inoculation of rhesus LCV infection, manipulation of the host immune system prior to infection, and infection with mutant rhesus LCV), will be valuable for dissecting different aspects of EBV pathogenesis. The current studies were undertaken to evaluate the effect of host immunosuppression on primary LCV infection and the potential for lymphomagenesis in the setting of lentivirus-induced immunosuppression as a potential experimental model for EBV infection and AIDS-related lymphomas.
Materials and methods
Virus and animal infections
Cell-free supernatants containing the rhesus LCV (cercopithicine herpesvirus 15) were prepared from LCL8664 cells15 as previously described.23 Cell lines were derived by infection of peripheral blood mononuclear cells (PBMCs) in vitro and culture in RPMI with 10% fetal calf serum (FCS) and 0.5 μg/mL cyclosporin A. Autologous, LCV-immortalized B cells used for intravenous infusions were derived, expanded, and infused within 6 months of in vitro culture. LCV seronegative rhesus macaques were identified by serologic screening of the pathogen-free colony at the New England Primate Research Center (NEPRC).13 One milliliter of a 1:1000 dilution of the SHIV 89.6P distribution stock (kindly provided by Dr Keith Reimann,24 , Beth Israel Deaconess Medical Center, Boston, MA) was infused intravenously to infect animals in groups II and III. Two to 4 weeks after SHIV infection, animals were inoculated with approximately 106 transforming units of rhesus LCV containing supernatants applied nontraumatically into the oral cavity or with 108 autologous LCV-transformed B cells resuspended in 10 mL phosphate-buffered saline (PBS) and slowly infused intravenously. All animal experiments were performed with approval from the Committee on Animals for Harvard Medical School, and animals were maintained in compliance with federal and institutional guidelines for animal care.
Serologic assays
Serum antibodies against the rhesus LCV small viral capsid antigen were detected with use of peptide immunoassays as previously described.13
CD4+/– T-cell phenotyping
Peripheral blood lymphocytes were immunophenotyped with use of CD4-specific (clone SK3; BD Biosciences, San Jose, CA) and CD3-specific (SP34; BD Biosciences) antibodies by flow cytometry with use of a standard whole blood lysis technique. Absolute lymphocyte counts were derived from an automated hematology analyzer.
Viral load assays
DNA was extracted from 2 × 106 peripheral blood mononuclear cells (QIAamp; Qiagen, Valencia, CA) and quantitated by spectrophotometry. Rhesus LCV DNA was amplified from 1 μg DNA by real-time polymerase chain reaction (PCR) with use of primers RHEBER32F (5′-GGAGGAGATGAGTGTGACTTAAATCA-3′) and RHEBER148R (5′-TGAACCGAAGAGAGCAGAAACC-3′) with a fluorogenic Taqman probe (5′-CCCGTCTTCACCACCCGGGA-3′). As an internal control, cell glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA was also amplified from samples with use of primers C2G971F (5′-AAGGCTGAGAACGGGAAGCT-3′) and C2G971R (5′-CAGCATCACCCCATTTGATCT-3′) with a fluorogenic Taqman probe (5′-TCCCATCACCATCTTCCAGGAGCG-3′). PCR mixtures (50 μL) consisted of 10 μL DNA extract, 15 nM of each primer, 200 nM TaqMan probe, and 1 × Taqman Universal Master Mix (PE Applied Biosystems, Foster City, CA) and were amplified for 40 cycles (15 seconds at 95° C, 30 seconds at 60° C, and 30 seconds at 72° C). All reactions were performed in duplicate. Viral DNA loads were normalized and expressed as the number of viral DNA copies per 106 PBMCs ([average viral DNA copy number/average GAPDH copy number] × 2 GAPDH copies/cell × 106 cells).
Measurements of SHIV RNA in plasma were performed by Chiron Diagnostics (Emeryville, CA) with use of a branched DNA amplification assay similar to the Quantiplex HIV-RNA branched DNA assay.25
Immunohistochemistry, in situ hybridization, and Southern blot analyses
Immunohistochemistry on fixed tissue sections were performed as described with use of the following antibodies: PE2 (EBNA-2), rabbit antihuman CD3 (A0452; Dako, Carpinteria, CA), clone DK25 (CD8; M0707; Dako), clone 124 (bcl2; M0887; Dako), and clone MIB-1 (Ki-67; M7240; Dako).26 Rhesus LCV DNA and rhesus LCV EBER RNA in situ hybridizations were performed as described.26 Images were acquired using an Olympus BX41 microscope with 4 × 0.10, 10 × 0.25, and 20 × 0.40 Olympus Plan and 40 × 0.75 Olympus UplanFL objectives, and an Olympus QColor3 DP11 camera (all from Olympus, Melville, NY). The following acquisition software was used: QCapture 2.60 (Quantitative Imaging, Burnaby, BC, Canada), Camedia Master 4.0 (Olympus), and Adobe Photoshop (Adobe). Rhesus LCV terminal repeat analysis was performed using a P32-labeled rhesus LCV BamHI, terminal repeat (TR)–containing DNA fragment hybridized to BamHI-digested genomic DNA that had been transferred by Southern blotting as previously described.27
RT-PCR for rhesus LCV latent infection mRNA expression
RNA extractions and reverse transcription–polymerase chain reaction (RT-PCR) were performed as previously described28 with use of the following primer pairs (PCR product size in bp): LMP1, 5′-TGAGGTAGAACCAGACAGCG-3′ and 5′-ACAGTCCTCAGCTCCTTTGC-3′ (201 nucleotides [nt's]); LMP2A, 5′-GGATCCACCTCCTTACG-3′ and 5′-AGCACTAACGGAGGCTGAAA-3′ (285 nt's); EBNA-LP, 5′-GCACGCTCTAGGCAAAGAAC-3′, in combination with EBNA-1, 5′-TCTGCCTCGTCCTCGTCCACG-3′ (488 nt's); EBNA-2, 5′-GTTCTGGTGGAGGAGGTTGA-3′ (551 nt's); EBNA-3A, 5′-AAGGTGCCTGCTGAGTAGGA-3′ (976 nt's); EBNA-3B, 5′-TGTAAGTCACTCTGTGGCGC-3′ (1050 nt's); and EBNA-3C, 5′-CGTGGTCTTGAGATCGTCTG-3′ (782 nt's).
Results
Oral rhesus LCV infection of immunocompetent (group I) and SHIV-immunosuppressed (group II) macaques
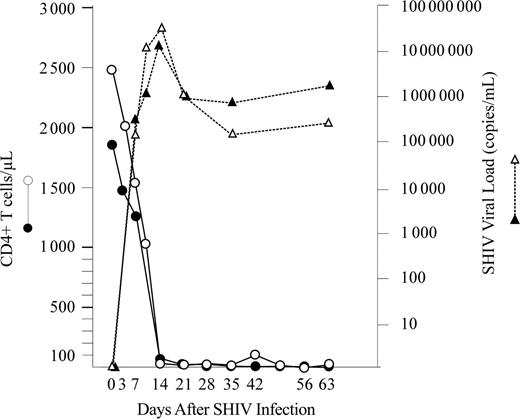
Four immunocompetent (group I) and 4 immunosuppressed (group II) rhesus macaques were orally inoculated with rhesus LCV to compare the outcome of primary rhesus LCV infection in immunocompetent with immunosuppressed hosts. To induce immunosuppression, macaques in group II were inoculated intravenously with SHIV 89.6P 2 to 4 weeks prior to LCV challenge. The pathogenic SHIV 89.6P strain was used because profound CD4+ T-cell depletion typically occurs 14 to 21 days after acute SHIV infection versus a more variable time course of CD4+ T-cell depletion after SIV infection.24 SHIV replication and CD4+ T-cell depletion in 2 representative animals in group II are shown in Figure 1. The nadir of CD4+ T-cell depletion in the SHIV-infected animals in group II was between 3 and 35 CD4+ T cells/μL (Table 1).
CD4+T-cell counts and SHIV viral loads in 2 representative animals in group II. CD4+ T-cell counts (circles) and SHIV viral loads (triangles) from Mm255-96 (filled symbols) and Mm343-96 (open symbols) are shown.
CD4+T-cell counts and SHIV viral loads in 2 representative animals in group II. CD4+ T-cell counts (circles) and SHIV viral loads (triangles) from Mm255-96 (filled symbols) and Mm343-96 (open symbols) are shown.
Summary of rhesus LCV infections
Animal no. . | SHIV infection . | CD4+ T-cell nadir, cells/μL . | Rhesus LCV infection . | LCV seroconversion . | Deceased . | Days after LCV infection . | Comments . |
|---|---|---|---|---|---|---|---|
| Group I | |||||||
| 211-98 | None | ND | Oral | Yes | Yes | 415 | Asymptomatic, killed for +PPD |
| 309-98 | None | ND | Oral | Yes | No | 827 | Alive and well |
| 141-97 | None | ND | Oral | Yes | No | 1177 | Alive and well |
| 144-97 | None | ND | Oral | Yes | No | 1177 | Alive and well |
| Group II | |||||||
| 255-96 | 89.6P | 10 | Oral | No | Yes | 122 | PCP, calcinosis |
| 166-97 | 89.6P | 3 | Oral | No | yes | 138 | Lymphocytic pneumonia |
| 343-96 | 89.6P | 35 | Oral | NA | Yes | 415 | PCP, failure to thrive |
| 165-97 | 89.6P | 11 | Oral | No | Yes | 511 | Failure to thrive |
| Group IIIa | |||||||
| 192-98 | 89.6P | 67 | B cells | Yes | No | 700 | Alive, asymptomatic |
| 260-98 | 89.6P | 121 | B cells | Yes | Yes | 799 | Failure to thrive |
| Group IIIb | |||||||
| 321-98 | 89.6P | 10 | B cells | No | Yes | 57 | Tissue invasion, Staphylococcus sepsis |
| 256-96 | 89.6P | 4 | B cells | No | Yes | 155 | LCV+ tumor |
Animal no. . | SHIV infection . | CD4+ T-cell nadir, cells/μL . | Rhesus LCV infection . | LCV seroconversion . | Deceased . | Days after LCV infection . | Comments . |
|---|---|---|---|---|---|---|---|
| Group I | |||||||
| 211-98 | None | ND | Oral | Yes | Yes | 415 | Asymptomatic, killed for +PPD |
| 309-98 | None | ND | Oral | Yes | No | 827 | Alive and well |
| 141-97 | None | ND | Oral | Yes | No | 1177 | Alive and well |
| 144-97 | None | ND | Oral | Yes | No | 1177 | Alive and well |
| Group II | |||||||
| 255-96 | 89.6P | 10 | Oral | No | Yes | 122 | PCP, calcinosis |
| 166-97 | 89.6P | 3 | Oral | No | yes | 138 | Lymphocytic pneumonia |
| 343-96 | 89.6P | 35 | Oral | NA | Yes | 415 | PCP, failure to thrive |
| 165-97 | 89.6P | 11 | Oral | No | Yes | 511 | Failure to thrive |
| Group IIIa | |||||||
| 192-98 | 89.6P | 67 | B cells | Yes | No | 700 | Alive, asymptomatic |
| 260-98 | 89.6P | 121 | B cells | Yes | Yes | 799 | Failure to thrive |
| Group IIIb | |||||||
| 321-98 | 89.6P | 10 | B cells | No | Yes | 57 | Tissue invasion, Staphylococcus sepsis |
| 256-96 | 89.6P | 4 | B cells | No | Yes | 155 | LCV+ tumor |
ND indicates not done; PPD, purified protein derivative (of tuberculin); PCP, Pneumocystis carinii pneumonia; and NA, not applicable.
Serologic responses to rhesus LCV infection were uniformly detected in the immunocompetent animals in group I with antibodies to the small viral capsid antigen (sVCA) appearing 45 to 60 days after inoculation and peaking at 90 days after infection (Figure 2). As in humans, antibodies to the sVCA remained persistently positive in the experimentally infected animals (maximum follow-up > 3 years). In the immunosuppressed animals in group II, 3 of 4 macaques were rhesus LCV naive prior to experimental infection. All 3 macaques remained rhesus LCV seronegative after experimental inoculation (Mm255-96, Mm165-97, Mm166-97; Figure 2). The fourth animal (Mm343-96) was inadvertently found to be rhesus LCV seropositive prior to infection, and serologic titers fell after immunosuppression and experimental rhesus LCV inoculation. Thus, the decreased antibody titer in this animal and the lack of antibody responses after experimental infection in 3 naive animals demonstrate the severe functional, in addition to the numerical, CD4+ T-cell deficit.
Serum antibody responses to the rhesus LCV sVCA. (A) Animals in group I: Mm141-97 (♦), Mm144-97 (•), Mm211-98 (▪), Mm309-98(▴). (B) Animals in group II: Mm343-96 (♦), Mm165-97 (•), Mm255-96 (▪), Mm166-97 (▴). (C) Animals in group III: Mm260-98 (♦), Mm256-96 (•), Mm192-98 (▪), Mm321-98 (▴).
Serum antibody responses to the rhesus LCV sVCA. (A) Animals in group I: Mm141-97 (♦), Mm144-97 (•), Mm211-98 (▪), Mm309-98(▴). (B) Animals in group II: Mm343-96 (♦), Mm165-97 (•), Mm255-96 (▪), Mm166-97 (▴). (C) Animals in group III: Mm260-98 (♦), Mm256-96 (•), Mm192-98 (▪), Mm321-98 (▴).
There was no obvious effect on survival in immunocompetent animals in group I after experimental rhesus LCV infection (maximum follow-up > 3 years). However, no immunosuppressed animals in group II survived after experimental rhesus LCV infection (mean survival, 297 days). No control group of SHIV-infected animals without LCV inoculation was included in these studies, but there did not appear to be significant differences from previously published studies in which SHIV-infected macaques were killed at 7 months because of wasting illnesses, opportunistic infections, and viral pneumonia.24 In the current studies, 2 animals were killed at approximately 4 months. The first animal (Mm255-96) acquired a Pneumocystis pneumonia and an unusual disseminated cutaneous calcinosis. The second animal (Mm166-97) experienced a lymphocytic pneumonia. LCV infection was not found to be associated with either the calcinosis or lymphocytic pneumonia by immunohistochemistry or in situ hybridization studies. Two animals survived more than 1 year with minimally higher CD4+ T-cell counts (Mm343-96, Mm165-97). Both animals were eventually killed because of a general failure to thrive, and one animal was found to have acquired a Pneumocystis pneumonia. There was no evidence of LCV-induced lymphoproliferative disease by gross evaluation at necropsy or by routine histopathologic evaluation of various tissues in either animal.
Rhesus LCV viral loads were assayed to compare viral kinetics in immunosuppressed with immunocompetent animals. PBMC-associated viral DNA was quantitated by real-time PCR from 2 immunocompetent animals in group I (Mm141-97, Mm144-97; Figure 3A). Low amounts of viral DNA were detected in the immunocompetent animals, on the order of 100 copies or less per 106 cells with peak values of 268 and 298 copies. DNA was first detected at 21 days after inoculation and remained positive for several weeks before becoming undetectable at days 70 and 126 after inoculation. Evidence for persistent virus infection below the level of DNA PCR detection was obtained at all time points after day 21 with use of a more sensitive RT-PCR assay13 for the rhesus LCV homologue of the small EBV-encoded RNAs (EBERs; data not shown).
Rhesus LCV DNA viral loads in peripheral blood mononuclear cells after experimental infection. (A) Animals in group I: Mm144-97 (▪), Mm141-97 (▴) (B) Animals in group II: Mm165-97 (•), Mm255-96 (▪), Mm166-97 (▴).
Rhesus LCV DNA viral loads in peripheral blood mononuclear cells after experimental infection. (A) Animals in group I: Mm144-97 (▪), Mm141-97 (▴) (B) Animals in group II: Mm165-97 (•), Mm255-96 (▪), Mm166-97 (▴).
Rhesus LCV DNA load measurements in the 3 SHIV-immunosuppressed animals in group II with primary rhesus LCV infection showed that viral DNA could be detected as early as day 3, much earlier than the immunocompetent animals in group I (Mm255-96, Mm165-97, Mm166-97; Figure 3B). Viral loads were approximately one log higher in Mm255-96 and Mm166-97 and remained elevated for a prolonged period until the animals succumbed to SIV-related illnesses at days 122 and 138. Mm165-97 had viral load levels comparable to immunocompetent animals (Figure 3B), eventual clearance of viral DNA loads to undetectable levels with persistent infection documented by positive rhesus LCV EBERs RT-PCR (data not shown), and a much longer survival (511 days; Table 1).
Thus, despite severe CD4+ T-cell depletion (< 50 cells/μL) and an inability to mount an antibody response to rhesus LCV infection, primary rhesus LCV infection in immunosuppressed animals resulted in viral DNA loads that were only modestly elevated and even controlled to undetectable levels in one case. These results suggest that innate immune responses or other immune pathways independent of CD4+ T cells and humoral immunity may be important for preventing LCV-induced lymphoproliferative disease.
Intravenous challenge of SHIV-immunosuppressed macaques with autologous rhesus LCV-immortalized B cells (group III)
To test whether residual immune responses could be overwhelmed by a more aggressive viral infection, 4 additional LCV-naive animals were SHIV infected and then challenged intravenously with autologous B cells immortalized in vitro with rhesus LCV (group III). DNA viral loads were assayed from 2 animals 3 to 7 days after intravenous inoculation, and PBMCs showed viral DNA load copy numbers between 104 and 106, ie, 1 to 3 logs higher than peak viral loads after oral inoculation of animals in group II (data not shown), likely because of the input cells. Immunofluorescent staining for EBNA-2 expression in PBMCs from one animal at day 3 revealed detectable virus-infected cells in the peripheral blood consistent with the high viral loads (data not shown). Thus, as expected, intravenous administration of autologous in vitro LCV-immortalized B cells resulted in a high viral inoculum early after infection.
Two animals had moderate immunosuppression after SHIV infection with CD4+ T-cell nadirs of 67 and 121 cells/μL, and these animals responded serologically to the intravenous rhesus LCV-infected B-cell inoculation (Table 1; group IIIa). Serum antibodies to the sVCA appeared earlier after intravenous inoculation as compared with oral inoculation, ie, first positive titers on day 7 and 28 versus days 45 to 60 in animals of group I infected orally. The rapid development of high-titer antibodies to a lytic antigen after intravenous inoculation of latently infected B cells suggests that there was a significant degree of lytic viral replication associated with the inoculation of LCV-infected B cells. Despite this aggressive challenge with large numbers of immortalized B cells and evidence for viral replication at an early time point, both animals in group IIIa survived the acute infection. One animal continues to survive with no obvious ill effects (> 2 years), and the other animal was killed 799 days after inoculation for a failure to thrive. Necropsy revealed no abnormal tumor growth and no evidence of lymphoproliferative disease by microscopic examination of multiple tissue and lymph node sections. Thus, autologous, LCV-immortalized B cells derived in vitro may not be inherently tumorigenic in vivo after intravenous inoculation, even in immunosuppressed hosts.
Tissue invasion and lymphomagenesis after intravenous challenge with autologous rhesus LCV-infected B cells (group IIIb)
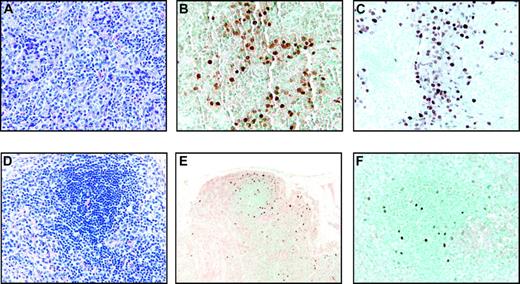
Two other animals responded to SHIV infection with severe CD4+ T-cell depletion (< 50 cells/μL, group IIIb). These 2 animals failed to develop an sVCA antibody response after intravenous inoculation of immortalized B cells consistent with the profound CD4+ T-cell depletion (Table 1). One animal (Mm321-98) was killed at day 57 because of bacterial sepsis. Histopathologic examination of a lymph node biopsy performed on day 36 revealed collections of rhesus LCV-infected cells detected by EBNA-2 immunohistochemistry and EBERs in situ hybridization (Figure 4A-C). This was in contrast to a lymph node biopsy performed at day 36 after LCV inoculation in an animal in group IIIa (Mm260-98) which revealed no evidence of tissue invasion by EBNA-2 immunohistochemistry and EBERs in situ hybridization (data not shown). At necropsy of the animal (Mm321-98) in group IIIb, scattered LCV-infected cells could be detected in the spleen, epicardium, kidney, and lymph node (Figure 4D-F; and data not shown), but there were no significant lymphoproliferations that effaced normal tissue structures. The detection of rhesus LCV–infected cells on random tissue sections and in multiple organs is consistent with tissue invasion by virus-infected cells after intravenous inoculation. However, even with severe CD4+ T-cell depletion, there was no evidence for malignant proliferation of virus-infected B cells in vivo and no obvious progression between day 36 and 57 in this animal.
Tissue invasion of rhesus LCV-positive cells in Mm321-98. (A) Hematoxylin and eosin–stained section of a lymph node biopsy performed on day 36 after intravenous inoculation of autologous rhesus LCV-immortalized B cells. Original magnification × 100. (B) EBERs in situ hybridization (original magnification × 200) and (C) EBNA-2 immunohistochemistry on sections of the same lymph node biopsy (original magnification × 200). (D) Hematoxylin and eosin–stained section from the spleen obtained at necropsy on day 57 (original magnification × 100). (E) EBERs in situ hybridization (original magnification × 20) and (F) EBNA-2 immunohistochemistry on sections from the spleen (original magnification × 100).
Tissue invasion of rhesus LCV-positive cells in Mm321-98. (A) Hematoxylin and eosin–stained section of a lymph node biopsy performed on day 36 after intravenous inoculation of autologous rhesus LCV-immortalized B cells. Original magnification × 100. (B) EBERs in situ hybridization (original magnification × 200) and (C) EBNA-2 immunohistochemistry on sections of the same lymph node biopsy (original magnification × 200). (D) Hematoxylin and eosin–stained section from the spleen obtained at necropsy on day 57 (original magnification × 100). (E) EBERs in situ hybridization (original magnification × 20) and (F) EBNA-2 immunohistochemistry on sections from the spleen (original magnification × 100).
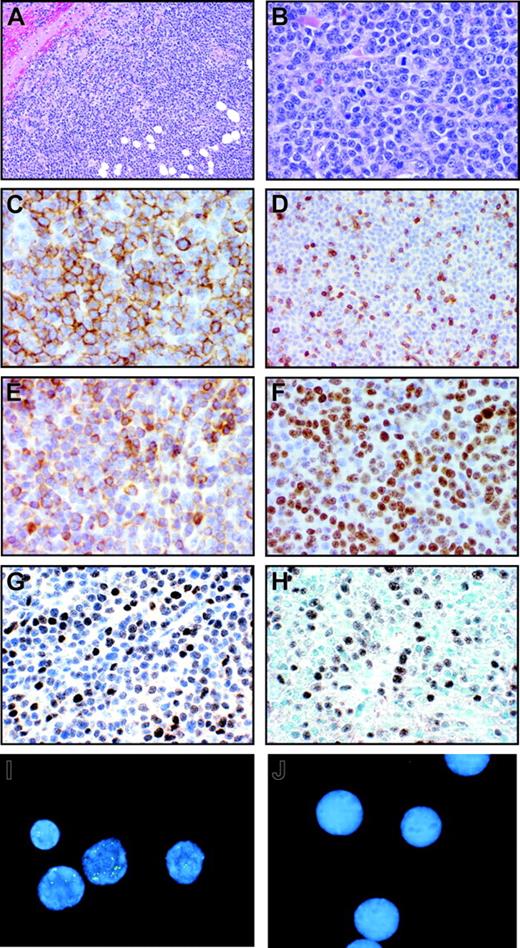
The second animal (Mm256-96) developed a grossly visible submandibular mass at approximately 18 weeks after LCV inoculation. This focal tumor mass expanded rapidly, and the animal was killed at 22 weeks. At necropsy, a well-circumscribed, round tumor mass measuring 8 cm in diameter and weighing 94 g was found under the mandible surrounding, but not invading, the esophagus and trachea. Histopathologic examination of the tumor revealed an infiltrate of large immunoblastic cells with prominent nucleoli (Figure 5A-B). Mitotic cells could be frequently observed along with occasional areas of necrosis consistent with a rapidly growing tumor. The monomorphic cells had replaced any normal tissue structure, making it difficult to determine whether the tumor mass had originated in a lymph node. Muscle and connective tissue were compressed at the periphery, and there were areas of fat, suggesting that the tumor mass may have extended beyond a potential lymph node origin. Immunohistochemical analysis indicated that most of the cells were positive for CD20, consistent with a B-cell lymphoma (Figure 5C). Most of the cells stained strongly with antibodies against bcl-2 and Ki-67 (Figure 5E-F), consistent with rapidly proliferating tumor cells. In addition, staining with CD3 (Figure 5D) and CD8 markers (data not shown) indicated a significant amount of CD8+ T-cell infiltration in the tumor mass.
Rhesus LCV-positive B-cell lymphoma from Mm256-96. (A-B) Hematoxylin and eosin–stained section of submandibular tumor mass from necropsy on day 155 after intravenous inoculation of autologous rhesus LCV-immortalized B cells. Original magnification × 40 (A) and × 200 (B). (C-G) Immunohistochemical staining of tumor section for CD20 (C), CD3 (D), bcl-2 (E), Ki-67 (F), and EBNA-2 (G). Original magnification × 200. (H) In situ hybridization of tumor section for EBER expression. Original magnification × 200. (I-J) Fluorescent in situ hybridization with a rhesus LCV DNA probe on touch preps prepared from the (I) tumor section or (J) EBV-negative BJAB B lymphoma cells. Original magnification × 400.
Rhesus LCV-positive B-cell lymphoma from Mm256-96. (A-B) Hematoxylin and eosin–stained section of submandibular tumor mass from necropsy on day 155 after intravenous inoculation of autologous rhesus LCV-immortalized B cells. Original magnification × 40 (A) and × 200 (B). (C-G) Immunohistochemical staining of tumor section for CD20 (C), CD3 (D), bcl-2 (E), Ki-67 (F), and EBNA-2 (G). Original magnification × 200. (H) In situ hybridization of tumor section for EBER expression. Original magnification × 200. (I-J) Fluorescent in situ hybridization with a rhesus LCV DNA probe on touch preps prepared from the (I) tumor section or (J) EBV-negative BJAB B lymphoma cells. Original magnification × 400.
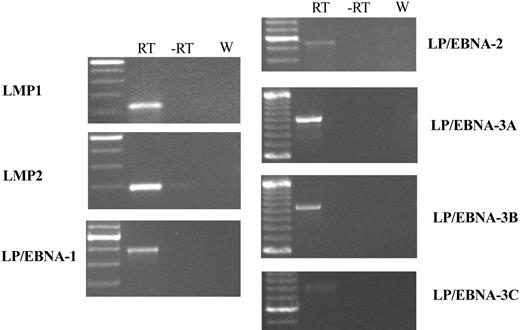
EBERs in situ hybridization demonstrated that most of the cells in the tumor mass were rhesus LCV positive (Figure 5H). Strong nuclear signals consistent with expression of the small viral RNAs were detected in a high percentage of cells comparable to the number of CD20+ B cells. Control in situ hybridizations with a sense EBERs probe were negative on adjacent sections (data not shown). A high percentage of tumor cells also expressed the EBNA-2 latent infection nuclear protein that is essential for LCV-induced B-cell immortalization (Figure 5G). Fluorescent in situ DNA hybridization (FISH) with a rhesus LCV DNA probe revealed that most of the cells from a touch prep of the tumor section were positive for intranuclear foci typical for episomal viral DNA in latently infected cells (Figure 5I). Evidence for a clonal proliferation of virus-infected cells was obtained by Southern blot analysis for the number of terminal repeats (TRs).29 A single TR band consistent with a monoclonal proliferation of virus-infected cells was detected in genomic DNA prepared directly from the tumor (Figure 6 lane 2) and from a cell line propagated in tissue culture from the tumor material (Figure 6 lane 3). The original input cell line from which cells were taken for the intravenous infusion consisted of an oligoclonal population of virus-infected cells as demonstrated by the presence of 2 or 3 major bands detected with a TR probe (Figure 6 lane 4). RT-PCR analysis for latent infection rhesus LCV gene expression revealed that mRNA transcripts for all 6 EBNAs and 2 latent membrane proteins typical for the type III latency program were expressed in the tumor tissue (Figure 7). These DNA, RNA, and protein assays demonstrate that the B-cell lymphoma arising in this animal was tightly associated with rhesus LCV infection.
Southern blot analysis for viral clonality. Genomic DNA from the rhesus LCV-producing B-cell line LCL8664, Mm256-96 tumor tissue, a cell line derived from the Mm256-96 tumor tissue, and the autologous, rhesus LCV–infected cell line used for intravenous inoculation were separated on a 0.6% agarose gel, transferred to a nylon membrane, and hybridized with a rhesus LCV DNA probe for the terminal repeats. Molecular weight markers in kilobases are shown to the right.
Southern blot analysis for viral clonality. Genomic DNA from the rhesus LCV-producing B-cell line LCL8664, Mm256-96 tumor tissue, a cell line derived from the Mm256-96 tumor tissue, and the autologous, rhesus LCV–infected cell line used for intravenous inoculation were separated on a 0.6% agarose gel, transferred to a nylon membrane, and hybridized with a rhesus LCV DNA probe for the terminal repeats. Molecular weight markers in kilobases are shown to the right.
RT-PCR analysis for rhesus LCV latent infection gene expression in Mm256-96 tumor tissue. RT-PCR was performed on RNA extracted from the Mm256-96 tumor tissue with the use of primers specific for the mRNAs of rhesus LCV latent membrane protein 1 (LMP1), latent membrane protein 2A (LMP2A), and mRNAs spliced from the terminal exon of EBNA-LP to the coding region for EBNA-1,-2, -3A, -3B, and -3C. Control reactions consisting of all reagents except reverse transcriptase (-RT) or with water in place of RNA (W) are included. Molecular weight markers are shown in the leftmost column, with the brightest band representing 500 bp and minor bands at increments of 100 bp.
RT-PCR analysis for rhesus LCV latent infection gene expression in Mm256-96 tumor tissue. RT-PCR was performed on RNA extracted from the Mm256-96 tumor tissue with the use of primers specific for the mRNAs of rhesus LCV latent membrane protein 1 (LMP1), latent membrane protein 2A (LMP2A), and mRNAs spliced from the terminal exon of EBNA-LP to the coding region for EBNA-1,-2, -3A, -3B, and -3C. Control reactions consisting of all reagents except reverse transcriptase (-RT) or with water in place of RNA (W) are included. Molecular weight markers are shown in the leftmost column, with the brightest band representing 500 bp and minor bands at increments of 100 bp.
Discussion
These studies compare the outcome of experimental LCV infection in the presence or absence of lentivirus-induced immunosuppression. We previously reported that naive, immunocompetent rhesus macaques could be experimentally infected with rhesus LCV by oral inoculation, the natural route of infection.23 Those experiments demonstrated that experimental infection of rhesus macaques could be used to model acute and persistent EBV infection in humans. The additional oral infections of immunocompetent animals in group I in the current report extend the findings of this experimental system by detecting DNA viremia in the PBMCs of immunocompetent hosts between 3 and 12 weeks after oral inoculation and by demonstrating the durability of persistent infection after experimental inoculation with up to 4 years of follow-up. Thus, experimental rhesus LCV infection in immunocompetent animals can provide an important animal model for studying the early time points in acute infection and how changes in specific viral genes may affect persistent virus infection and immune evasion after experimental infections.
The current studies also provide insights into the pathogenesis of LCV infection in the setting of immunosuppression. Primary LCV infection in SHIV-infected macaques resulted in LCV DNA viremia that appeared earlier, was slightly higher, and lasted slightly longer than infection of immunocompetent animals, suggesting that CD4+ T cells contribute to the control of viremia in primary infection. The potential importance of the CD4+ T-cell responses was further highlighted by the animals challenged intravenously with LCV-infected B cells. Two animals with reduced CD4+ T-cell counts (67 and 121 cells/μL; animals in group IIIa) were asymptomatic after LCV challenge and survived in excess of 2 years, whereas the animals with severe CD4+ T-cell depletion (< 50 cells/μL) had evidence of tissue invasion with LCV-positive B cells or the development of a lymphoma. However, the severe CD4+ T-cell depletion induced by SHIV infection while blocking normal development of humoral immunity was not sufficient for the efficient induction of LCV-induced lymphoproliferative disease after primary LCV infection, as demonstrated by the animals in group II. In fact, one SHIV-immunosuppressed animal was able to resolve primary rhesus LCV infection to undetectable DNA PCR levels and maintained a persistent LCV infection for more than 1.5 years despite a severe CD4+ T-cell deficit that prevented an antibody response to the rhesus LCV infection. These results suggest that immune responses independent of CD4+ T cells may also be important for preventing the efficient induction of LCV-induced lymphomas after primary LCV infection. In addition to CD8+ T cells, innate immune responses, such as natural killer (NK) cell responses, may contribute to the immediate control of primary EBV infection. Identifying immune responses capable of controlling LCV infection in the absence of CD4+ T-cell help may provide therapeutic alternatives for patients with EBV-associated lymphoproliferative disease.
The results from the current study also establish that experimental infection with this rhesus LCV isolate can result in a virus-positive lymphoma. The most common outcome of experimental infection, even with severe CD4+ T-cell depletion, is resolution of acute infection followed by an asymptomatic persistent infection without evidence for LCV-induced lymphoproliferative disease. However, in this study, lymphomagenesis did occur after intravenous inoculation of rhesus LCV–immortalized autologous B cells into a naive animal with severe CD4+ T-cell depletion because of SHIV infection, thereby establishing the potential for a malignant outcome after experimental infection. Further studies will be required to more precisely determine the frequency of lymphomagenesis, the degree of immunosuppression required, the differences between immunosuppressive drugs and SHIV infection, and the importance of the mode and degree of LCV challenge. The focal presentation and viral clonality of the Mm256-96 lymphoma suggests that most of the originally infused LCV-immortalized B cells were not directly tumorigenic. Instead, it appears that the malignancy evolved as a clonal proliferation from a single virus-infected cell that acquired a malignant phenotype. This is similar to humans whereby EBV infection often appears as a cofactor for malignant conversion and may not be sufficient for oncogenesis in vivo.
Genomic instability (eg, chromosomal translocations and aberrant somatic hypermutation), is a hallmark of tumorigenesis and has been hypothesized as a second step in EBV-associated oncogenesis.6,7 In B cells, the normal processes of V(D)J recombination, somatic hypermutation, and class-switching that target the immunoglobulin (Ig) loci generate genomic instability as an essential part of creating and selecting diverse, high-affinity antibody molecules.30 In Burkitt lymphoma, a c-myc translocation in an EBV-infected B cell can result in malignant transformation. Aberrant somatic hypermutation of immunoglobulin and non-Ig loci has been frequently observed in many postgerminal center B-cell lymphomas, including EBV-related lymphoproliferative disease.6,7,31 The ability of EBV-infected B cells to persist for life may increase the risk of accumulating nucleotide changes in genes controlling cell growth, differentiation, and cell death, leading to uncontrolled cell proliferation and lymphomagenesis. EBV infection itself may also increase genomic instability by enhancing recombinase activity.32
Thus, immunosuppression may increase the risk of EBV-associated lymphomagenesis by way of 2 related mechanisms. First, elimination of critical immune components may directly result in uncontrolled growth of EBV-infected B cells and lymphoproliferative disease. The current studies suggest that there are redundancies built into the immune response and that multiple immune pathways contribute to the control of EBV-infected B cells. Increased numbers of EBV-infected B cells resulting from immunosuppression may indirectly lead to a second mechanism for increasing the risk of EBV-associated lymphomagenesis. The higher number of EBV-infected B cells may stochastically increase the risk of accumulating genetic changes to critical, growth-related genes. The association of lymphomagenesis with the infusion of large numbers of LCV-infected B cells in these animal studies may be modeling the random accumulation of genetic hits over time in EBV-infected B cells. Infusing high numbers of susceptible, at-risk B cells in experimental infections may increase the likelihood of an infrequent event, resulting in overt lymphomagenesis.
The rhesus LCV animal model for EBV pathogenesis is particularly powerful because of the unusually high degree of biologic and genetic similarity between rhesus LCV and EBV, the likeness of the immune responses in humans and these nonhuman primates, and the uniform pathogenesis of viral infection in macaques and humans. The current studies demonstrate the strength and depth of immune control over LCV-infected B cells and the potential for lymphomagenesis in an experimental system. The development of an experimental animal model system to study LCV infection in immunocompetent and immunosuppressed hosts is an important step to better understand EBV pathogenesis, to dissect the risk factors for EBV-associated lymphomagenesis, and to develop potential therapeutics to treat or reduce the risk of EBV-induced lymphomas.
Prepublished online as Blood First Edition Paper, May 18, 2004; DOI 10.1182/blood-2004-01-0342.
Supported by grants from the US Public Health Service (CA68051, CA65319, and DE14388). Services from the New England Primate Research Center were supported by a base grant to the institution (USPHS P51RR00168).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal