Abstract
Because de novo purine synthesis (DNPS) is a target of widely used antileukemic agents (eg, methotrexate, mercaptopurine), we determined the rate of DNPS and the expression of genes involved in purine metabolism in different subtypes of acute lymphoblastic leukemia (ALL). Among 113 children with newly diagnosed ALL, lymphoblasts with the TEL-AML1 translocation had significantly lower DNPS than all other genetic subtypes of B-lineage ALL or T-lineage ALL (352 ± 57 versus 1001 ± 31 or versus 1315 ± 76 fmol/nmol/h, P < .0001). By assessing the expression of 82 genes involved in purine metabolism (KEGG pathway database) in ALL blasts from 38 patients with B-lineage ALL (14 with TEL-AML1, 24 without), we identified 16 genes that were differentially expressed in TEL-AML1–positive and TEL-AML1–negative ALL (P < .001, false discovery rate [FDR] = 5%). The pattern of expression of these 16 genes discriminated TEL-AML1–positive ALL with a true accuracy of 84% in an independent test set (n = 17, confidence interval 70% to 94%, P < .001). Western blots of selected genes documented corresponding levels of the proteins encoded. Differentially expressed genes included HPRT, IMPDH, PAICS, and GART, all of which were expressed at a significantly lower level in TEL-AML1 ALL. These findings have established that TEL-AML1 ALL has significantly lower de novo purine synthesis and differential expression of genes involved in purine metabolism.
Introduction
Pediatric acute lymphoblastic leukemia (ALL) is a heterogeneous disease comprising different immunophenotypes and various genetic subtypes caused by chromosomal translocations with aberrant gene fusions and inappropriate expression of oncogenes. These genetic abnormalities have important prognostic and therapeutic implications, but the underlying mechanisms are not fully understood.1 De novo purine synthesis (DNPS) is higher in ALL cells compared with normal bone marrow cells and peripheral blood lymphocytes, and T-lineage ALL has higher DNPS than B-lineage ALL.2,3 It is not known, however, whether DNPS differs among the genetic subtypes of B-lineage ALL.
Nucleotides play a key role in many biochemical processes related to their importance as DNA and RNA precursors, energy carriers, coenzyme components, and metabolic regulators.4 Cells obtain purine nucleotides through 2 separate metabolic pathways, de novo purine synthesis and salvage of extracellular purine bases and nucleotides. DNPS and several enzymes involved in the purine metabolism pathway are important targets for antimetabolites (eg, mercaptopurine and methotrexate) that are the cornerstone of continuation therapy for childhood ALL.1
DNPS is a complex pathway in which nucleotides are synthesized from amino acids and tetrahydrofolate in a multienzymatic process, starting with phosphoribosyl pyrophosphate (PRPP), primarily formed from ribose-5-phosphate (from the pentose phosphate pathway) and adenosine triphosphate (ATP).4 Previous studies have shown that the pattern of gene expression in ALL blasts differs among the major genetic and lineage subtypes of childhood ALL5 and is able to discriminate treatment-induced changes in gene expression.6 The present investigation was undertaken to assess differences in the rate of DNPS in bone marrow leukemic blasts from patients with different genetic and lineage subtypes of ALL and to determine whether there is differential expression of purine pathway genes in ALL with low versus high DNPS.
Patients, materials, and methods
Patients and genetic characterization of leukemia cells
A total of 113 children aged 18 years or younger with newly diagnosed ALL, enrolled on the St Jude Total Therapy XIIIB and XV protocols, were included in this study. The investigation was approved by the Institutional Review Board at St Jude Children's Research Hospital, and signed informed consent was obtained from parents or legal guardians before enrollment. The diagnosis of ALL was based on morphology, cytochemical staining, immunophenotyping, cytogenetic analysis, and genetic characterization using methods previously described.1,5 These include B-lineage leukemias that contain t(1;19)[E2A-PBX1], t(12;21)[TEL-AML1] translocations, or a hyperdiploid karyotype (ie, more than 50 chromosomes), and T-lineage ALL (T-ALL). For the DNPS analysis we divided the patients into 5 subgroups according to different genetic subtypes (n = 30, TEL-AML1; n = 26, hyperdiploid B-lineage; n = 7, E2A-PBX; n = 33, B-lineage ALL with no defined genetic subtypes; and n = 17, T-lineage). For the microarray analysis, we studied 55 B-lineage ALL patients divided into a “training set” of 38 patients (14 with and 24 without the TEL-AML1 fusion) (Supplemental Document; see the Supplemental Document link at the top of the online article on the Blood website) and a “test set” of 17 patients (7 with TEL-AML1 and 10 without the TEL-AML1 fusion) (Supplemental Document).
Isolation of ALL blasts from bone marrow aspirate
For all patients, ALL blasts were obtained from bone marrow aspirates at diagnosis. Samples consisted of 5 to 10 mL bone marrow collected in syringes containing 800 units of heparin and kept on ice until processed. Leukemic cells were obtained by density separation over a Ficoll-Hypaque gradient and washed 3 times with a solution of HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), Hanks buffered solution, and heparin, as previously described in detail.7 The final cell yield was determined by hemocytometer and percent viability by trypan blue exclusion.
Measurement of purine bases and rate of DNPS in bone marrow ALL cells
The concentration of purine bases from hydrolyzed nucleotides and the rate of DNPS in ALL blasts were simultaneously determined by quantifying unlabeled and radiolabeled purine bases (adenine and guanine) after acid hydrolysis of a 2-hour ex vivo incubation of 5 × 106 lymphoblasts with 14C-formate, as previously described.8,9 The method for quantitating DNPS was modified to yield higher overall median values when compared with the method previously used to quantitate DNPS in ALL blasts.7,9 However, the previously observed lineage differences in DNPS (T-ALL greater than B-lineage ALL) remained evident with the current DNPS assay. The concentrations of unlabeled adenine and guanine were determined from peak areas of UV chromatograms against a linear calibration curve of standards (1 to 75 nmol on column) prepared in phosphate-buffered saline. Newly synthesized purines were determined by the concentration of 14C-adenine and 14C-guanine, which were calculated on the basis of the total disintegrations per minute (dpm) corresponding to the relevant peaks of the chromatograms, with a specific activity of 50 dpm/pmol. The detection limit for the radiolabeled purines was 0.02 pmol on column. The rate of de novo adenine plus guanine synthesis (DNPS) was calculated as the femtomoles of newly synthesized adenine plus guanine per nanomole of total intracellular adenine plus guanine per hour of incubation (fmol/nmol/h). Under conditions used, the rate of DNPS (14C incorporation from formate) remained linear for incubation times of 30 to 120 minutes in human CEM-CCRF leukemia cells (American Type Culture Collection [ATCC], Manassas, VA) (R2 = 0.986 ± 0.009, mean of 3 experiments).
Cell cycle distribution
We determined the percentage of cells in S phase of bone marrow leukemic blasts obtained at diagnosis in all patients for whom an adequate number of cells was available (n = 83). Propidium iodide–stained DNA content was measured by flow cytometry with a Coulter EPICS-V flow cytometer (Coulter Electronics, Hialeah, FL). The computer program ModFit (Verity Software, Topsham, ME) was used to calculate the percentages of cells in G0/G1, S, and G2+M phase.
RNA extraction and gene expression profiling
All 55 patients (38 for the training set and 17 for the test set) in whom ALL gene expression was determined had sufficient blasts in their bone marrow aspirates to permit RNA isolation from 5 million to 10 million leukemia cells (median, 97% blasts). We extracted high-quality total RNA with TriReagent (MRC, Cincinnati, OH) from cryopreserved mononuclear cell suspensions from bone marrows at diagnosis. Using methods previously described,5,6,10 total RNA was processed and hybridized to the HG-U133A oligonucleotide microarray, which contains 22 283 gene probe sets, representing approximately 12 357 human genes, plus approximately 3820 expressed sequence tag clones (EST) with unknown function (Affymetrix, Santa Clara, CA; see manufacturer's manual for detailed protocol).11 We used the default settings of Affymetrix Microarray Suite software version 5 (MAS 5.0; Affymetrix) to calculate scaled gene expression values. To further establish the validity of gene expression determined by microarray analysis, we used quantitative real-time polymerase chain reaction (RT-PCR) to quantitate mRNA in 4 patient samples for 4 genes (phosphoribosylaminoimidazole carboxylase, PAICS; inosine-5′-monophosphate dehydrogenase, IMPDH2; fragile histidin triad protein, FHIT; and nonmetastatic protein NM23H1, NME1), yielding correlation coefficients of 0.97, 0.94, 0.87, and 0.89, respectively, between RT-PCR and the oligonucleotide microarray results (Supplemental Document).
Statistical analysis and bioinformatics
We selected 129 probes sets (82 genes) involved in purine metabolism pathways according to the Kyoto Encyclopedia of Genes and Genomes (KEGG)12 (Supplemental Document).
Pairwise comparisons using the Wilcoxon rank sum test were used to assess differences in DNPS rates among the 5 ALL subtypes. The Holm method was used to adjust for multiple testing. Gene expression for the 129 purine metabolism probes was log transformed, and the t test, distinction calculation,13 and Wilcoxon rank sum test were used to select probe sets discriminating TEL-AML1 versus non–TEL-AML1 ALL. We estimated the false discovery rate (FDR) and significance by permutation (n = 1000). We used support vector machine as the classifier in leave-one-out cross-validation analysis and in the estimation of true accuracy by an independent test set (n = 17). Because the independent test set samples were analyzed on the Affymetrix U95Av2 array,5 we first selected the U95Av2 probe sets (n = 88) that correspond to the discriminating U133A probe sets (n = 129) based on the best match information provided by Affymetrix. A bootstrap method was used to obtain the 95% confidence interval of the true accuracy. Multiple linear regression was used to evaluate the significance of selected genes after adjusting for TEL-AML1. R 1.7.0 statistical software was used to perform the above analyses. Principal components analysis (PCA) and hierarchical clustering were performed using GeneMaths 1.5 software (AppliedMaths, Sint-Martens-Latem, Belgium).
Western blot analysis
Bone marrow blast cells from 2 patients with TEL-AML1–positive ALL and 3 patients with non–TEL-AML1 ALL (2 hyperdiploid B-lineage and 1 B-lineage ALL with no defined genetic subtypes) were lysed on ice in RIPA lysis buffer (150 mM NaCl, 1.0% Nonidet P-40 [NP-40], 50 mM Tris [tris(hydroxymethyl)aminomethane] [pH 8.0]) containing freshly added proteinase inhibitors (phenylmethylsulfonyl fluoride [PMSF], sodium orthovanadate, aprotinin [Sigma, St Louis, MO; A6279]). Lysates were centrifuged at 8000g (12 000 rpm) for 10 minutes at 4°C, and protein concentration in the supernatant was determined using the Biorad Bradford method. Equivalent amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen, Carlsbad, CA; NP0321), transferred to a polyvinylidene difluoride membrane (Invitrogen; LC2005), and blocked overnight in T-TBS (10 mM Tris-HCl [pH 8], 150 mM NaCl, 0.05% Tween 20) containing 5% nonfat dry milk. Subsequently, blots were probed with primary antibodies (anti-NME1 1:4000 [Santa Cruz Biotechnology, Santa Cruz, CA; sc-343]; anti-FHIT 1:200 [Upstate Biotechnology, Lake Placid, NY; no. 07-172], anti–β-actin 1:10.000 [Sigma]) at room temperature for 1 hour, washed, and then incubated with the appropriate secondary antibodies conjugated to horseradish peroxidase. Immunoreactive bands were visualized by chemiluminescence with the ECL-plus Western blotting System (Amersham Biosciences, Freiburg, Germany; RPN 2132). The intensity of bands corresponding to FHIT and NME1 protein was normalized to the β-actin signal. Bands were visualized and quantified by PhosphorImager with the ImageQuaNT Software system (Amersham Biosciences) using blue fluorescence/chemifluorescence at 450-nm excitation.
Results
Differences in DNPS rate among ALL lineage and genetic subtypes
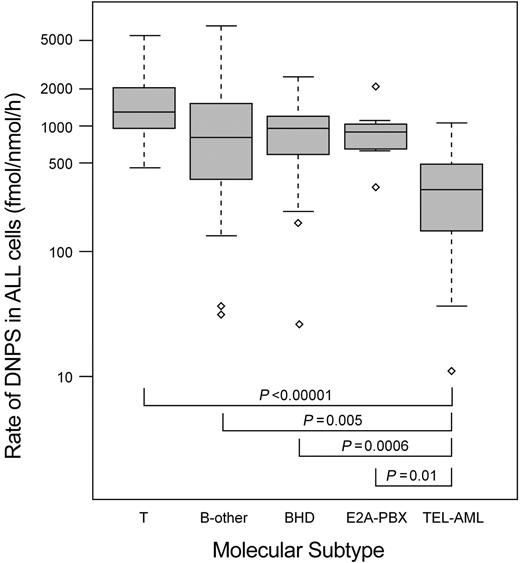
DNPS rates in pretreatment ALL blasts from 113 patients are summarized in Table 1, subgrouped by ALL lineage and genetic subtype (B-lineage ALL with TEL-AML1, n = 30; hyperdiploid B-lineage, n = 26; E2A-PBX, n = 7; no defined abnormality, n = 33; and T-lineage, n = 17).
Rate of de novo purine synthesis (DNPS) in leukemia cells of different lineage, ploidy, and genetic subtypes of ALL
Subtype . | No. of patients . | Median DNPS rate, fmol/nmol/h (range) . |
|---|---|---|
| T-ALL | 17 | 1315 (515-5018) |
| B-hyperdiploid | 26 | 1023 (34-2474) |
| E2A-PBX | 7 | 941 (367-2048) |
| TEL-AML1 | 30 | 326 (11-1104) |
| B-other | 33 | 873 (40-5933) |
Subtype . | No. of patients . | Median DNPS rate, fmol/nmol/h (range) . |
|---|---|---|
| T-ALL | 17 | 1315 (515-5018) |
| B-hyperdiploid | 26 | 1023 (34-2474) |
| E2A-PBX | 7 | 941 (367-2048) |
| TEL-AML1 | 30 | 326 (11-1104) |
| B-other | 33 | 873 (40-5933) |
DNPS was significantly lower in patients whose ALL blasts had the TEL-AML1 fusion (TEL-AML1) compared with each of the other ALL subtypes (Figure 1), whereas there was no statistical difference among patients with E2A-PBX translocation (E2A-PBX), hyperdiploid B-lineage ALL (BHD), and B-lineage ALL with no defined genetic abnormalities. The DNPS rate was higher in patients with T-lineage ALL compared with each of the other ALL subtypes, and the difference was significant when T-lineage ALL was compared with all cases of B-lineage ALL (P = .01) or when compared with only B-lineage ALL without TEL-AML1 (P = .02).
Rate of de novo purine synthesis (DNPS) in ALL cells of different genetic and lineage subtypes. The rate of de novo purine synthesis (DNPS) (femtomoles per nanomole per hour) is shown on a log scale for hyperdiploid B-lineage ALL (BHD, n = 26), ALL with the E2A-PBX translocation (E2A-PBX, n = 7), ALL with TEL-AML1 translocation (TEL-AML, n = 30), no defined genetic or chromosomal abnormalities (B-other, n = 33), and T-lineage (T, n = 17). The horizontal lines indicate the median for each subgroup; the top and bottom of each box depict the 25th and 75th percentile value. The dashed lines depict 1.5 times the interquartile range. The outlier values are shown with diamond symbols. The P values were determined by the Wilcoxon rank sum test adjusted for multiple testing.
Rate of de novo purine synthesis (DNPS) in ALL cells of different genetic and lineage subtypes. The rate of de novo purine synthesis (DNPS) (femtomoles per nanomole per hour) is shown on a log scale for hyperdiploid B-lineage ALL (BHD, n = 26), ALL with the E2A-PBX translocation (E2A-PBX, n = 7), ALL with TEL-AML1 translocation (TEL-AML, n = 30), no defined genetic or chromosomal abnormalities (B-other, n = 33), and T-lineage (T, n = 17). The horizontal lines indicate the median for each subgroup; the top and bottom of each box depict the 25th and 75th percentile value. The dashed lines depict 1.5 times the interquartile range. The outlier values are shown with diamond symbols. The P values were determined by the Wilcoxon rank sum test adjusted for multiple testing.
There were no significant differences in white blood cell (WBC) count (P = .19) or percent of bone marrow leukemia cells in S phase (P = .47) between TEL-AML1 and non–TEL-AML1 B-lineage ALL. Multiple linear regression analysis showed that a higher percent of bone marrow leukemia cells in S phase was significantly associated with a higher rate of DNPS (R2 = 0.20, P = .0032) in addition to TEL-AML1 status (Supplemental Document).
Differences in gene expression between TEL-AML1 versus non–TEL-AML1 B-lineage ALL
According to 3 independent statistical algorithms (t test, distinction calculation, and Wilcoxon rank sum test) and the estimated false discovery rate (FDR), we identified 18 purine pathway gene probe sets (corresponding to 16 genes listed in Figure 2A) that discriminated TEL-AML1 from non–TEL-AML1 B-lineage ALL (P < .001, FDR = 5%). These probe sets discriminated the 2 groups of patients with a prediction accuracy of 85% by leave-one-out cross-validation using support vector machine (SVM). Hierarchical clustering using the 18 selected probes clearly separated patients into 2 distinct groups, with TEL-AML1 ALL clustered together (Figure 2A). The principal components analysis (PCA) illustrates the degree of discrimination between the 2 groups of patients (Figure 2B). These 16 genes were able to identify TEL-AML1–positive ALL with a true accuracy of 84% in an independent test set (n = 17, P < .001), with discrimination comparable to the training set (Figure 2C).
“Supervised” hierarchical clustering and principal components analysis (PCA) using purine pathway genes discriminating TEL-AML1 and non–TEL-AML1 B-lineage ALL. (A) Each patient's ALL cells are depicted as vertical columns, with green symbols indicating ALL with TEL-AML1 fusion (n = 14) and red indicating non–TEL-AML1 (n = 24) ALL. Eighteen gene probe sets (rows, with gene names shown) were used for the hierarchical clustering. The relative level of gene expression is depicted from lowest (green) to highest (red) according to the scale shown at the bottom. (B) PCA plot using the 18 selected purine pathway gene probe sets (U133A array) discriminating TEL-AML1 versus non–TEL-AML1 in the training set. (C) PCA plot using purine pathway gene probe sets on the U95Av2 array that correspond to the probe sets discriminating TEL-AML1 versus non–TEL-AML1 identified in the training set.
“Supervised” hierarchical clustering and principal components analysis (PCA) using purine pathway genes discriminating TEL-AML1 and non–TEL-AML1 B-lineage ALL. (A) Each patient's ALL cells are depicted as vertical columns, with green symbols indicating ALL with TEL-AML1 fusion (n = 14) and red indicating non–TEL-AML1 (n = 24) ALL. Eighteen gene probe sets (rows, with gene names shown) were used for the hierarchical clustering. The relative level of gene expression is depicted from lowest (green) to highest (red) according to the scale shown at the bottom. (B) PCA plot using the 18 selected purine pathway gene probe sets (U133A array) discriminating TEL-AML1 versus non–TEL-AML1 in the training set. (C) PCA plot using purine pathway gene probe sets on the U95Av2 array that correspond to the probe sets discriminating TEL-AML1 versus non–TEL-AML1 identified in the training set.
Discriminating genes included PRPS2, GART, IMPDH2, PAICS, and HPRT1, each of which was expressed at a lower level in TEL-AML1–positive cases, whereas only 4 purine pathway genes, AK1, FHIT, GUCY1B3, and POLR2C, were expressed at a higher level in TEL-AML1 ALL.
Correlation between gene expression and DNPS rate after adjusting for TEL-AML1 translocation
For each of the 16 genes that discriminated TEL-AML1 from non–TEL-AML1 B-lineage ALL, we applied multiple regression analysis that included TEL-AML1 status and gene expression level as covariates. It revealed that the expression levels of 2 genes were significantly linearly correlated with DNPS, even after adjusting for TEL-AML1 status, which are IMPDH2 (R2 = 0.09, P = .009) and PAICS (R2 = 0.10, P = .003). The correlation between expression levels of these 2 genes and DNPS was more significant without adjusting for TEL-AML1 status (IMPH2, R2 = 0.42, P < .0001; and PAICS, R2 = 0.52, P < .0001) (Figure 3). A multiple linear regression model including these 2 genes in addition to TEL-AML1 status as covariates accounted for 67% of total variation in DNPS among B-lineage ALL (P < .0001).
Gene expression versus rate of DNPS after adjusting for TEL-AML1 in 2 discriminating genes. The relation between gene expression and rate of DNPS in 38 patients is depicted for 2 discriminating genes (PAICS and IMPDH2). Green symbols are for ALL cells with the TEL-AML1 translocation; red represents ALL without the TEL-AML1 translocation.
Gene expression versus rate of DNPS after adjusting for TEL-AML1 in 2 discriminating genes. The relation between gene expression and rate of DNPS in 38 patients is depicted for 2 discriminating genes (PAICS and IMPDH2). Green symbols are for ALL cells with the TEL-AML1 translocation; red represents ALL without the TEL-AML1 translocation.
Concordance of protein levels and gene expression in TEL-AML1–positive versus TEL-AML1–negative ALL cells
As depicted in Figure 4, there was good concordance between the level of gene expression (mRNA) and cellular FHIT and NME1 protein levels in TEL-AML1–positive versus TEL-AML1–negative ALL cells. There was a significant linear correlation between protein levels measured by Western blot analysis and mRNA expression by oligonucleotide microarray in these 5 patients (R2 = 0.98 for NME1 and R2 = 0.34 for FHIT) (Supplemental Document). NME1 mRNA and protein levels were significantly lower in TEL-AML1 leukemia cells compared with TEL-AML1–negative blasts; mRNA and protein levels of the putative tumor suppressor FHIT were significantly higher in TEL-AML1–positive ALL. These 2 genes were selected as representative of genes underexpressed or overexpressed in TEL-AML1 ALL, because there was not a sufficient number of cells to quantitate the level of all proteins encoded by the 16 discriminating genes.
Concordance of protein levels and gene expression for 2 purine genes discriminating TEL-AML1–positive and TEL-AML1–negative ALL cells. FHIT and NME1 protein levels in whole cell lysates of 2 TEL-AML1–positive ALL and 3 TEL-AML1–negative ALL samples (from left to right, 2 hyperdiploid B-lineage and 1 B-lineage ALL with no defined genetic subtype) assessed by Western blotting. FHIT protein level was significantly higher and NME1 was significantly lower in TEL-AML1–positive ALL, which is concordant with the mRNA expression in these 2 groups.
Concordance of protein levels and gene expression for 2 purine genes discriminating TEL-AML1–positive and TEL-AML1–negative ALL cells. FHIT and NME1 protein levels in whole cell lysates of 2 TEL-AML1–positive ALL and 3 TEL-AML1–negative ALL samples (from left to right, 2 hyperdiploid B-lineage and 1 B-lineage ALL with no defined genetic subtype) assessed by Western blotting. FHIT protein level was significantly higher and NME1 was significantly lower in TEL-AML1–positive ALL, which is concordant with the mRNA expression in these 2 groups.
Discussion
Acute lymphoblastic leukemia (ALL) is a heterogeneous disease in terms of clinical presentation, genetic subtypes, and response to therapy.1 Specific genetic abnormalities (eg, chromosomal gains or losses, resulting in hyperdiploidy or hypodiploidy, respectively; chromosomal translocations leading to aberrant gene fusions and dysregulation of gene expression; and deletion or functional inactivation of tumor suppressor genes) are found in leukemic cells of 75% to 80% of patients with ALL. Discovery of these abnormalities has been important in elucidating the pathogenesis, defining the prognosis, and guiding the treatment of patients with ALL.1,14
Acute lymphoblastic leukemia cells, in contrast to normal bone marrow cells and peripheral blood lymphocytes, have a relatively high rate of de novo purine synthesis (DNPS).15,16 Purine nucleotides participate in a variety of cellular functions, including synthesis of RNA and DNA, regulation of enzymatic activity, protein synthesis and function, and mediation of energy transfer in cells.4 Several in vitro and in vivo studies suggest that there is a lineage difference in the rate of DNPS in leukemic cells, with T-lineage ALL having higher DNPS than B-lineage ALL.7,17,18 Recent studies19,20 show that the gene encoding methylthioadenosine phosphorylase, an enzyme involved in the purine salvage pathway, is more frequently deleted in T-lineage ALL than in B-lineage ALL because of its close genomic proximity to the tumor suppressors p16INK4A/p15INK4B. In addition, hypoxanthine exerts feedback inhibition on DNPS, and the lower intracellular hypoxanthine plus inosine concentrations in T-lineage ALL compared with B-lineage ALL suggest that lower purine recycling and salvage activity may be compensated by increased DNPS in T-lineage ALL.7
To date, there have been no studies to determine whether there are differences in the rate of DNPS among genetic subtypes of ALL. The current work is the first to reveal that leukemia cells with the TEL-AML1 fusion have a significantly lower rate of DNPS when compared with other genetic subtypes of childhood ALL. No statistical difference was evident among ALL with E2A-PBX translocation, hyperdiploid B-lineage, and B-lineage ALL without defined genetic abnormalities. As previously observed,7 DNPS was higher in patients with T-lineage ALL compared with the other ALL subtypes (B-lineage versus T-lineage ALL, P = .01; B-lineage without TEL-AML1 versus T-lineage ALL, P = .02).
The cryptic t(12; 21) chromosomal translocation with TEL-AML1 fusion is detectable in approximately 25% of pediatric B-lineage ALL,21,22 most frequently occurring in patients between 1 and 5 years of age.23,24 This translocation results in an “in-frame” fusion of 2 critical regulators of hematopoiesis, TEL (ETV6) at chromosome 12p13 and AML1 (CBFA2) at chromosome 21q22.13,25-27 This gene fusion has been detected in neonatal blood spots of patients who years later developed ALL, suggesting its origin in fetal development.13,28,29 It is not clear, however, whether the occurrence and pathogenicity of this translocation is limited to a particular stage of early B-cell development. Although the TEL-AML1 fusion is considered to have a relatively good prognosis,24,30,31 the biologic nature of leukemias carrying the t(12; 21) is heterogeneous.22,24,32,33
It is not known whether the TEL-AML1 fusion is the cause or effect of low DNPS or whether the lower rate of DNPS contributes to the favorable prognosis of TEL-AML1 ALL. As a potential cause of leukemogenesis, it is possible that a low rate of DNPS results in lower purine pools in such cells and that an inadequate source of purines could compromise DNA repair and predispose to chromosomal translocations.34 It is possible that the rate of cell proliferation or DNA synthesis could influence the rate of cellular DNPS, which would be consistent with our observation of a significant correlation between percentages and rate of DNPS. However, this did not explain the difference between TEL-AML1 and non–TEL-AML1, because there was not a significant difference in percentage of cells in the S phase of the cell cycle in TEL-AML1 and non–TEL-AML1 ALL. It is also plausible that the lower rate of DNPS renders TEL-AML1 ALL more susceptible to antileukemic agents that inhibit DNPS (eg, methotrexate, mercaptopurine) and to more avid incorporation of fraudulent nucleotides (eg, thioguanine, cytosine arabinoside) after mercaptopurine or cytarabine therapy, thereby contributing to their favorable prognosis.22,35 Future studies will be needed to elucidate the importance of low DNPS in the pathogenesis and treatment outcome of TEL-AML1 ALL.
Because TEL-AML1 ALL was found to have significantly lower DNPS, we used oligonucleotide microarrays to identify purine metabolism pathway genes that are differentially expressed in B-lineage ALL with or without the TEL-AML1 fusion. This study identified 16 purine pathway genes (18 probe sets) that discriminated B-lineage ALL with or without TEL-AML1 fusion, revealing a new biologic difference among the genetic subtypes of ALL. It is noteworthy that only 2 of the differentially expressed purine pathway genes (PRPS2, NME2) were identified among those genes previously found to discriminate TEL-AML1 ALL from other genetic subtypes in a genome-wide approach.5 Thus, it was necessary to focus our assessment of gene expression on candidate purine pathway genes to elucidate the differences in gene expression governing purine metabolism.
Interestingly, some of the discriminating genes have been previously implicated in the pathogenesis of ALL. For example, aberrant FHIT transcription has been reported in acute myeloid leukemia and ALL.36,37 The FHIT protein has been implicated as a tumor suppressor gene, but the exact biologic function is not known.38,39 FHIT was 1 of only 4 discriminating purine pathway genes expressed at a higher level in ALL with the TEL-AML1 fusion. In contrast, NME1 (nm23-H1) and NME2 (nm23-H2) were expressed at a lower level in TEL-AML1 ALL. NME1 and NME2 show 88% amino acid sequence homology40,41 and are located in tandem on the same region of chromosome 17q21. Their expression was inversely correlated with tumor metastatic potential in experimental rodent cells and in some human tumors.42 Reduced NME expression was associated with increased metastatic potential and more aggressive disease in human breast,43 hepatocellular,44 ovarian,45 and gastric carcinoma44 and in melanoma.46 It has been reported that high-grade non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma exhibit significantly higher levels of NME expression compared with low-grade NHL.47 High expression of both NME1 and NME2 has been associated with a poor prognosis in AML and with disease progression of CML and especially in AML-M5, where NME1 overexpression was the most important poor prognostic factor.48 These results suggest that there is a connection between NME function and more aggressive phenotypes in leukemia, such as malignant growth, differentiation resistance, and chemotherapy resistance. Whether their lower expression contributes to the favorable prognosis in TEL-AML1 ALL remains to be determined.
IMPDH2 and HPRT1 were both expressed at a lower level in ALL with the TEL-AML1 fusion. The protein encoded by IMPDH2 catalyzes the first step in formation of guanine ribonucleotides from inosine monophosphate, which is important for cell proliferation. IMPDH activity correlates positively with increased proliferation of both normal and malignant cells49 as well as malignant transformation.49,50 Previous reports have shown that depletion of guanine nucleotide pools, after inhibition of IMPDH by mycophenolic acid or mizoribine, potently inhibits cell cycle progression by arresting activated T lymphocytes in the G1 phase of the cell cycle.51 HPRT1 is essential for the purine nucleotide salvage pathway and, like IMPDH2, plays an essential role in mercaptopurine activation. Hypoxanthine guanine phosphoribosyltransferase activity in ALL cells has been shown to be inversely related to leukocyte (WBC) count and tumor load in untreated ALL patients.52
Among the 18 discriminating probe sets, 2 probe sets (IMPDH2 and PAICS) showed the best linear correlation between gene expression and DNPS rate. PAICS (AIR carboxylase/SAICAR synthetase) is a bifunctional enzyme, the activities of which are required for steps 6 and 7, respectively, of purine biosynthesis.4 To our knowledge, there have been no studies establishing a role of this gene in leukemogenesis or prognosis in ALL.
One additional case of ALL was not included in our analysis of TEL-AML1 because of an equivocal genetic diagnosis. ALL cells from this patient did not have a TEL-AML1 fusion by RT-PCR or other known chromosomal translocation. However, these ALL cells had a genome-wide pattern of gene expression indicative of TEL-AML1 ALL,5 and fluorescence in situ hybridization (FISH) analysis subsequently revealed that these ALL cells had a split TEL gene due to a cryptic 3-way translocation. This case had a low rate of DNPS (516 fmol/nmol/h) and clustered among the TEL-AML1 cases by hierarchical clustering using the 18 discriminating purine pathway genes and by principal components analysis (Supplemental Document). This finding indicates that disruption of the TEL gene may be responsible for differential expression of purine pathway genes and lower DNPS in ALL cells.
Taken together, these findings reveal previously unrecognized differences in the expression of genes involved in purine metabolism with or without the TEL-AML1 fusion and concordant differences in de novo purine synthesis in ALL with or without the TEL-AML1 fusion. This provides new insights into biologic differences in TEL-AML1 ALL and to the favorable prognosis of this common genetic subtype of B-lineage ALL.
Prepublished online as Blood First Edition Paper, May 13, 2004; DOI 10.1182/blood-2003-12-4306.
Supported by National Institutes of Health (NIH) grants R37 CA36401 (W.E.E., M.V.R., C.-H.P.), R01 CA78224 (W.E.E., M.V.R., C.-H.P.), RO1 CA51001 (M.V.R., C.-H.P.), R01 CA71907 (J.D.), U01 GM61393 (M.V.R., W.E.E.), U01 GM61394, and Cancer Center Support Grant CA21765; an F.M. Kirby Clinical Research Professorship from the American Cancer Society (C.-H.P.); Erwin Schroedinger grants FWFJ2110 and J2304-B13 (L.K.); and the American Lebanese Syrian Associated Charities (ALSAC).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge the outstanding support of Sanne Lugthart, Nancy Kornegay, Mark Wilkinson, John Morris, and Dr Clayton Naeve and his staff in the Hartwell Center for Bioinformatics and Biotechnology at St Jude Children's Research Hospital. We also thank Yaqin Chu, May Chung, Emily Melton, Margaret Needham, Allison Gratzer, John Bienvenu, Paxton Baker, Tania Brooks, Cong Ding, and Nancy Duran for excellent technical assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal