Abstract
To study interleukin-7 (IL-7) in early thymocyte development, we generated mice transgenic (Tg) for the IL-7 gene under control of the lck proximal promoter. Founder line TgA, with the lowest level of IL-7 overexpression, showed enhanced αβ T-cell development. In contrast, in the highest overexpressing founder line, TgB, αβ T-cell development was disturbed with a block at the earliest intrathymic precursor stage. This was due to decreased progenitor proliferation as assessed by Ki-67 staining and in vivo bromodeoxyuridine (BrdU) incorporation. Bcl-2 was up-regulated in T-cell–committed progenitors in all Tg lines, and accounted for greater numbers of double positive (DP), CD4 single positive (SP), and CD8SP thymocytes in TgA mice where, in contrast to TgB mice, thymocyte progenitor proliferation was normal. Mixed marrow chimeras using TgB+ and congenic mice as donors, and experiments using anti–IL-7 monoclonal antibody (MAb) in vivo, confirmed the role of IL-7 protein in the observed TgB phenotype. In conclusion, at low Tg overexpression, IL-7 enhanced αβ T-cell development by increasing thymocyte progenitor survival, while at high overexpression IL-7 reduces their proliferation, inducing a dramatic block in DP production. These results show for the first time in vivo a dose effect of IL-7 on αβ T-cell development and have implications for IL-7 in the clinical setting.
Introduction
Interleukin-7 (IL-7) is a nonredundant cytokine in thymic development. It has been implicated in both proliferation and survival of early T cells.1-3 After the transition from the multipotent to the T-cell–committed stage, thymocyte progenitors become dependent on IL-7 for normal cell cycle progression and cell survival through inhibition of apoptosis via up-regulation of the Bcl-2 expression.1 Consequently, in several mouse models of IL-7 signal disruption such as IL-7–/–,2 IL-7 receptor α–/– (IL-7Rα–/–),3,4 γc–/–,5-7 Jak3–/–,8-10 and Jak1–/–,11 progression beyond double negative–2 (DN2) stage is severely diminished. However, despite its role on proliferation, studies evaluating the need for IL-7 during lymphocyte development led to the conclusion that the primary role of this cytokine was rather in maintaining cell survival.12 Additionally, IL-7 controls T-cell receptor γ (TCRγ) rearrangement by regulating locus accessibility,13 such that γδ T-cell production is abrogated in the absence of IL-7 signaling,4,5,9,13-15 demonstrating a complete reliance on IL-7 by this lineage.
Many in vitro studies have shown an effect of IL-7 on thymocyte progenitors.16-19 However, the effect of IL-7 on αβ T-cell development yielded somewhat conflicting results. Varas et al observed, with rat fetal thymic organ culture (FTOC) grown in the presence of 2000 U/mL IL-7, an enhancement of αβ thymocyte maturation.19 In contrast, Plum et al's study, which used mouse FTOC treated with different doses of human recombinant IL-7 (rIL-7, 100-5000 U/mL), showed significantly lower numbers of αβ T cells with increasing IL-7 doses.17 Interestingly, even though γδ T-cell numbers were found to be consistently increased in in vitro culture systems in the presence of IL-7, it is unclear if this enhancement is due to increased proliferation, survival, or a developmental bias from αβ to γδ T-cell lineage.16-19
To date, 4 IL-7 transgenic (Tg) mouse models have been described in which expression of an IL-7 transgene is driven by different promoters including the immunoglobulin κ (Igκ) chain,20 the Ig heavy chain promoter and enhancer,21 the Eα promoter of murine major histocompatibility complex (MHC) class II,22 and the human K14 promoter.23 In 3 models and despite different spatial patterns of IL-7 expression, mice developed lymphoproliferative diseases of B21,22 or T21,23 origin. However, the effect of IL-7 transgene on early thymocyte development has not been extensively characterized.
In order to target IL-7 overexpression to the T lineage, we generated Tg mice with an IL-7 transgene under the control of the lck proximal promoter. This promoter is up-regulated early in thymocyte development.24,25 We established 3 founder lines, each with a different level of thymic IL-7 Tg expression. We found, for the first time, a dose-dependent, thymocyte subpopulation–specific effect of IL-7 on αβ T-cell development in vivo.
Materials and methods
Construction of the lck–IL-7 transgene
A 533-bp IL-7 gene cDNA containing the entire coding murine sequence was cloned by polymerase chain reaction (PCR) using mRNA from cell line J558 (gift from Dr Terry Fry, National Cancer Institute, Bethesda, MD). The IL-7 fragment was then inserted into the BamHI site of the lck proximal promoter construct.26 The 5.5-kb fragment containing the IL-7 transgene construct was then removed from the plasmid using NotI and purified over a sucrose gradient for oocyte injection.
Production of transgenic mice
Microinjection was carried out on B6 oocytes. Transgenic C57BL/6 strain mice were generated and housed at the Science Application International Corporation transgenic core facility in Frederick, MD, according to National Institutes of Health (NIH) guidelines. Transgenic mice were maintained at the heterozygous state on a C57BL/6 background. There were 3 different founder lines of IL-7 Tg mice showing 3 different integration sites and IL-7 expression levels that were determined. We designated them as IL-7 TgA, H, and B lines.
Quantitative PCR for IL-7 expression
IL-7 quantitation was performed in day-1 newborn and in 6- to 8-week-old mice. In newborn mice, RNA was extracted from total thymocyte tissue. In adult mice, thymocyte suspensions were prepared from the 3 IL-7 Tg lines and their negative littermates. To eliminate non–T cells in adult thymi, CD4+, CD8+, and CD4+CD8+ thymocytes were positively selected using magnetic beads coated with antibodies (Abs) specific for CD4 and CD8, and Miltenyi columns (Miltenyi, Auburn, CA). RNA was extracted (Trizol; Invitrogen, Carlsbad, CA) and DNase digested (DNase I; Invitrogen).
Real-time quantitative PCR was performed using a Lightcycler Instrument (Roche Molecular Biochemicals, Indianapolis, IN). The real-time PCR conditions were as follows: 95° C for 5 minutes, then 35 cycles with 95° C for 5 seconds, 58° C for 20 seconds, and 72° C for 25 seconds. HPRT was used as a positive control and to quantitate total amplified RNA. Results were expressed as the ratio of IL-7 mRNA (fg) to HPRT mRNA (fg). The primer (Invitrogen) and the hybridization probe (Idaho Technology, Salt Lake City, UT) sequences were as follows: mIL7P1 sense (S), 5′-ggaattcctccactgatccttg-3′ and mIL7P2 antisense (AS), 5′-ctcagtagtctctttagg-3′; HPRTP1 S, 5′-cagtcaacgggggacataaa-3′; HPRTP2 AS, 5′-ttgttgttggatatgcccttg-3′; mIL7 probe 1, 5′ (LC red)–tggtgaactgcacaagtaagg-3′; mIL7 probe 2, 5′-gtatcacaaggcacacaaaca (F)–3′; HPRT2 probe 1, 5′ (LC red)–gccccaaaatggttaaggttgc; and HPRT2 probe 2, 5′-gctggtgaaaaggacctctcg (F)–3′.
In situ hybridization for IL-7 expression
Thymi from IL-7 transgenic mice were Bouin fixed, paraffin embedded, and sectioned in RNAse-free conditions. In situ hybridization (ISH) on the sections was performed as described by Faust et al.27 Analysis was performed with an Olympus Provis AX70 microscope (Olympus, Melville, NY), and images were taken with a SPOTRT color camera and SPOT imaging software (Diagnostic Instruments, Sterling Heights, MI). Original magnification was × 100, and 10 × /0.30 Olympus UPlan FL objectives were used.
Antibodies and flow cytometry
The 4- to 5-multicolor flow cytometry experiments were performed either on a multilaser FACStar Plus or FACSVantage SE (Becton Dickinson, San Jose, CA). Dead cells were excluded from analysis by propidium iodide staining and forward light scatter gating. Data on 5 to 50 × 104 live cells were collected. Anti-TCRδ (GL-3), anti-TCRβ (H57), anti-CD69, anti-B220, and anti–natural killer (NK; DX5) Abs were fluorescein isothiocyanate (FITC) conjugated. Anti-CD4 and -CD8 Abs were, respectively, phycoerythrin (PE) and cyanin 5 (Cy-5) labeled. For immature thymocyte progenitor staining, Abs specific for all lineage markers (lin: CD3, CD4, CD8, B220, IgM, Mac-1, NK1.1, GR-1) were FITC conjugated or biotinylated and labeled with streptavidin-Alexa 594, while CD44 and CD25 were PE and Cy-5 labeled, respectively. For lin– cell analysis, 1 to 5 × 106 cells were collected. All antibodies were supplied by BD Biosciences) except for anti-CD8 Cy-5, which was purchased from Caltag Laboratories (Burlingame, CA). Cells were initially incubated with 2.4G2 to block Fc receptors, then stained with Abs. Fluorescence data were displayed as dot plots or histograms using CELLQuest software (BD Biosciences). Anti–IL-7Rα Ab was kindly provided by Shin-Ichi Nishikawa (Kyoto University, Japan) and Dr David Allman28 (University of Pennsylvania School of Medicine, Philadelphia) and was FITC conjugated. For intracellular Bcl-2 and Ki-67 staining, thymocytes were first stained for membrane surface antigens. Cells were then fixed, permeabilized (Cytoperm/Cytofix; BD Biosciences), and stained with FITC-conjugated hamster antimouse Bcl-2 Ab (clone 3F11) or antihuman Ki-67 (clone B56) or their monoclonal isotype control Ab (BD Biosciences).
Western blot for Bcl-2
Double-positive (DP) cells were sorted from each transgenic line and normal B6 mice. Cells were washed in phosphate-buffered saline, lysed at 20 × 106 cells/mL in standard radioimmunoprecipitation assay buffer, solubilized in sodium dodecyl sulfate sample buffer, boiled for 5 minutes and resolved (12% gel) using polyacrylamide gel electrophoresis (PAGE), and transferred to polyvinylidene fluoride membrane (Amersham, Piscataway, NJ) using a Bio-rad (Hercules, CA) semidry transfer apparatus. Membranes were blocked with 5% milk and immunoblotted at 0.5 μg/mL with horseradish peroxidase (HRP)–labeled Abs against Bcl-2 (C-2, 26 kDa) or actin (C-11, 37 kDa) as a control (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were developed using enhanced chemiluminescence (Pierce Chemical, Rockford, IL).
BrdU in vivo incorporation experiment
To study thymocyte proliferation, 6- to 8-week-old Tg and 3 negative control mice were intraperitoneally injected with 1 mg bromodeoxyuridine (BrdU; BD Biosciences). Then 4 hours later, the mice were killed and thymocyte progenitors were stained for surface antigens lineage markers CD44 and CD25, as described in “Materials and methods.” After fixation and permeabilization, cells were stained with anti-BrdU FITC Ab or an isotype control (BD Biosciences).
Mixed bone marrow chimeras
Bone marrow cells were harvested from 8-week-old Tg+ (CD45.2) or Tg– (CD45.2) and congenic (CD45.1) mice. Bone marrow cells were T-cell depleted prior to intravenous injection, using rabbit antimouse brain (RAMB) and guinea pig complement (GPC; Invitrogen) as previously described.29 Briefly, freshly harvested bone marrow cells were incubated with RAMB diluted to 1:50 for 30 minutes, then with diluted GPC (1:4) for 30 minutes. B6 mice (3 months old) were lethally irradiated (10 Gy) up to 6 hours prior to intravenous injection with a mixture of 5 × 106 cells from IL-7 Tg+ (CD45.2) or Tg– (CD45.2) bone marrow cells mixed to 5 × 106 cells from congenic Ly5.2 (CD45.1) cells. Thymocytes from individual lobe recipients were analyzed as indicated 7 to 12 weeks after the procedure.
Intraperitoneal injection of anti–IL-7 or isotype control Abs
Anti–IL-7 monoclonal antibody (MAb, M25; 1 mg)30 or its isotype control (CC57; American Type Culture Collection [ATCC], Manassas, VA) catalog number: HB-268) was injected 3 times weekly up to a total of 10 mg. In each group, 3 IL-7 TgB mice were included for analysis. The mice were 4 weeks old at the beginning of the treatment and were killed 2 to 3 days after the last injection.
Results
Establishment of 3 founder lines with varying transgene expression
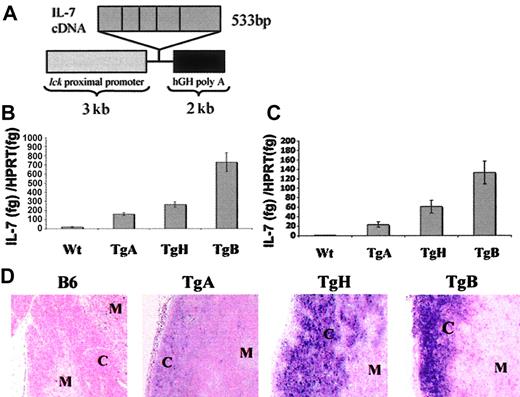
Transgenic mice were generated using a cDNA encoding the IL-7 gene in a lck proximal promoter expression vector26 (Figure 1A). There were 3 founder lines of transgenic mice established, designated TgA, TgB, and TgH.
Construction of the lck–IL-7 transgene and IL-7 expression in Tg+ and B6 mice. (A) The construct included 3 kb of the lck proximal promoter, 533 bp of IL-7 murine cDNA containing the whole coding murine sequence, and 2 kb of the intron post stop codon of the 3′ human growth hormone (hGH).26 (B-C) Comparison of thymic IL-7 expression among the 3 founder lines of IL-7 Tg and negative controls in newborn and 6- to 8-week-old mice, respectively. Real-time PCR was performed for IL-7 expression and normalized to HPRT expression. Results were presented as the ratio of IL-7 (fg)/HPRT (fg) expression. (B) Total thymocyte tissue was used to determine IL-7 expression in day-1 newborn mice. The numbers of mice were as follow: TgA = 5, TgH = 3, TgB = 4, and negative littermates n = 8. (C) DP, CD4SP, and CD8SP cells from adult thymi were positively selected as described in “Materials and methods” and compared with thymic tissue from normal B6 mice. Error bars indicate SEM. (D) In situ hybridization with IL-7 RNA probe. Representative sections from the thymus of TgA, TgH, TgB, and normal B6 mice showing the localization of IL-7 expression to the cortex “C” and the medulla “M.”
Construction of the lck–IL-7 transgene and IL-7 expression in Tg+ and B6 mice. (A) The construct included 3 kb of the lck proximal promoter, 533 bp of IL-7 murine cDNA containing the whole coding murine sequence, and 2 kb of the intron post stop codon of the 3′ human growth hormone (hGH).26 (B-C) Comparison of thymic IL-7 expression among the 3 founder lines of IL-7 Tg and negative controls in newborn and 6- to 8-week-old mice, respectively. Real-time PCR was performed for IL-7 expression and normalized to HPRT expression. Results were presented as the ratio of IL-7 (fg)/HPRT (fg) expression. (B) Total thymocyte tissue was used to determine IL-7 expression in day-1 newborn mice. The numbers of mice were as follow: TgA = 5, TgH = 3, TgB = 4, and negative littermates n = 8. (C) DP, CD4SP, and CD8SP cells from adult thymi were positively selected as described in “Materials and methods” and compared with thymic tissue from normal B6 mice. Error bars indicate SEM. (D) In situ hybridization with IL-7 RNA probe. Representative sections from the thymus of TgA, TgH, TgB, and normal B6 mice showing the localization of IL-7 expression to the cortex “C” and the medulla “M.”
Relative IL-7 mRNA expression was determined in the thymi of newborn mice (day 1) by real-time PCR and expressed relative to that of the gene HPRT. TgA, TgH, and TgB mice had 9-fold, 14-fold, and 39-fold, respectively, increased IL-7 message levels compared with normal thymic tissue expression (Figure 1B). To determine if IL-7 overexpression is maintained in adult mice, quantitative reverse-transcriptase (RT)–PCR was also performed on thymocyte subsets from 6- to 8-week-old mice. We excluded non–T-lineage cells by positive selection of DP and SP thymocytes in order to better compare intrathymic IL-7 mRNA in the absence of non–IL-7 transgene–expressing cells (Figure 1C). Thymocytes from TgA mice consistently had the smallest increase (38-fold) in IL-7 expression, the TgH mice maintained an intermediate increase of 115-fold, and the IL-7 TgB line showed the greatest increase, 230-fold, over IL-7 levels in thymic tissue from normal control animals. Positively selected DP + SP cells from control B6 mice showed 100-fold less IL-7 expression than total thymic tissue (data not shown), consistent with the observation that IL-7 is normally produced mainly by thymic epithelial cells.31,32
IL-7 overexpression in Tg+ mice was also confirmed by in situ hybridization (Figure 1D). An antisense IL-7 RNA probe hybridized predominantly to the thymic cortex of the Tg+ mice, which corresponds to the spatial distribution of lck proximal promoter expression. Consistent with the RT-PCR results, the strongest hybridization signal was seen in TgB, the weakest in TgA, and an intermediate signal in TgH mice. In contrast, in the normal B6 thymus, the IL-7 probe hybridized predominantly in the medulla where the staining was faint and dispersed. Interestingly, TgB mice showed a decreased medullary IL-7 expression, suggestive of a down-regulation of the endogenous IL-7 gene, and marked cortical atrophy confirmed by light microscopy.
Phenotypic changes also varied with transgene expression levels. TgB mice showed the most pronounced phenotype, with thymic cortical atrophy without other significant abnormalities (data not shown and Figure 1D). Spleen and lymph nodes were normal in size and histologic appearance. By 4 to 5 months of age, several of these mice developed a lymphoblastic lymphoma involving the hematopoietic tissues (bone marrow, thymus, spleen, lymph nodes), the kidney, the liver, and the lungs. In contrast, TgA and TgH mice did not develop lymphoma.
The effect of IL-7 overexpression on thymocyte development is expression-level dependent
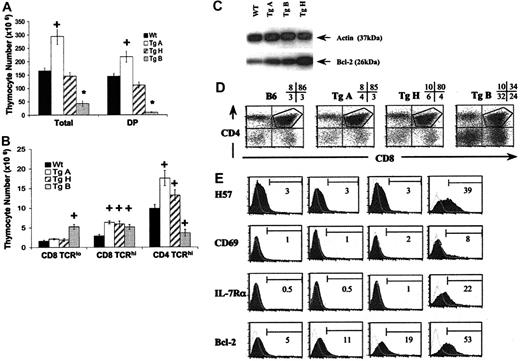
To analyze the effect of IL-7 overexpression on thymocyte development, we studied by flow cytometry CD4/CD8-defined thymic subpopulations from 6- to 12-week-old IL-7 Tg+ mice and their wild-type littermates (Figure 2). Cellularity was markedly different in the 3 lines (Figure 2A). TgA mice showed a significant increase in total thymocytes, DP cells, CD4SP TCRhi, and CD8SP TCRhi (Figure 2A). In contrast, thymocyte numbers in TgB were 3.9-fold lower than wild-type littermates. This was primarily due to a dramatic decrease in the DP population (16-fold). Within the SP population, the CD4+TCRhi subset was significantly decreased, while the CD8+TCRhi subset was augmented compared with normal controls (Figure 2A-B). This resulted in a reversed CD4/CD8 ratio in the thymus, spleen, lymph node, and blood (data not shown). In addition, the CD8+TCRlo cells that developmentally precede the DP stage were increased in number in the TgB mice (P < .0001, Figure 2B). The TgH thymi demonstrated an increase only in CD8SP TCRhi thymocytes (Figure 2B). In all 3 founder lines, there was an increase in the percentage of DN, CD4SP, and CD8SP populations along with a relative decrease of DP thymocytes (Figure 2D). Thus, consideration of the total cell numbers revealed a striking difference among the Tg+ lines. The TgA line, with the lowest IL-7 overexpression, in fact demonstrated an increase in the absolute numbers of both DP and SP cells. In contrast, the TgB line, with the highest levels of IL-7 overexpression, had a marked decrease in the absolute numbers of DP and CD4SP cells.
Effect of IL-7 overexpression on αβ T-cell development. Panels A-B represent absolute thymocyte counts from 6- to 12-week-old TgA (n = 11), TgH (n = 7), TgB (n = 12), and negative littermates (n = 15). *P < .0001; +P < .05. The 2 bar graphs depict absolute numbers (mean ± SEM) of total thymocytes and DP cells (A) and CD8+TCRlo, CD8+TCRhi, and CD4+TCRhi thymocytes (B) from Tg+ and Tg– mice. Error bars indicate SEM. (C) Western blot for Bcl-2 on sorted DP thymocytes from all 3 Tg lines and normal B6 mice. Actin was used as a control. (D) Dot plot analysis of CD4 and CD8 expression on thymocytes from representative 8-week-old Tg+ (A, H, and B lines) versus Tg– control mice. The percentages of DN, DP, CD4SP, and CD8SP in each sample are indicated. The gate is used to analyze the DP cells. (E) Maturation markers, IL-7 Rα, and Bcl-2 expression in DP thymocytes from representative Tg+ and Tg– mice. The shaded areas represent the marker of interest, and the open areas represent the negative isotype controls. The percentage of positive cells for each marker is shown.
Effect of IL-7 overexpression on αβ T-cell development. Panels A-B represent absolute thymocyte counts from 6- to 12-week-old TgA (n = 11), TgH (n = 7), TgB (n = 12), and negative littermates (n = 15). *P < .0001; +P < .05. The 2 bar graphs depict absolute numbers (mean ± SEM) of total thymocytes and DP cells (A) and CD8+TCRlo, CD8+TCRhi, and CD4+TCRhi thymocytes (B) from Tg+ and Tg– mice. Error bars indicate SEM. (C) Western blot for Bcl-2 on sorted DP thymocytes from all 3 Tg lines and normal B6 mice. Actin was used as a control. (D) Dot plot analysis of CD4 and CD8 expression on thymocytes from representative 8-week-old Tg+ (A, H, and B lines) versus Tg– control mice. The percentages of DN, DP, CD4SP, and CD8SP in each sample are indicated. The gate is used to analyze the DP cells. (E) Maturation markers, IL-7 Rα, and Bcl-2 expression in DP thymocytes from representative Tg+ and Tg– mice. The shaded areas represent the marker of interest, and the open areas represent the negative isotype controls. The percentage of positive cells for each marker is shown.
The first step of thymic positive selection includes an up-regulation of the TCRs to medium-high levels.33,34 This process begins at the DP stage,35,36 where it is accompanied by up-regulation of CD69,37,38 IL-7R,39 and the Bcl-2 protein.40 To study the effect of IL-7 on thymocyte maturation, DP thymocytes were analyzed for expression of maturation markers (H57 and CD69), as well as for the Bcl-2 and IL-7Rα expression (Figure 2E). While DP thymocytes showed a normal maturation profile in TgA and TgH lines, maturation was altered in TgB mice, in which DP cells had higher levels of H57 and CD69 expression. Also, Bcl-2 protein and IL-7Rα expression were up-regulated in this population. However, while the percentage of this mature DP is increased in TgB mice, its total number was not significantly higher than normal controls. Interestingly, Bcl-2 was also slightly increased in TgA and TgH DP cells compared with normal controls without IL-7R overexpression or CD69 up-regulation (Figure 2E). This was confirmed by Western blot (Figure 2C). These data indicate that at high IL-7 expression (TgB), the enhanced maturation of DP thymocytes reflects more a decrease of DP turnover than an increase in maturation, the mature cells corresponding to cells that have undergone a positive selection.
Because the lck proximal promoter activity is stronger in early and mature thymocytes than in mature T cells, transgene effects in the periphery might be expected to be less dramatic than in the thymus. While characterization of peripheral T splenocytes showed a tendency to higher CD4+ and CD8+ cells in transgenic mice with the lowest IL-7 overexpression, a significant decrease in both CD4+ naive and memory T cells was observed in mice with highest IL-7 overexpression. In this latter case, CD8 memory T cells were significantly higher than in normal controls, resulting in inverted CD4/CD8 ratio in spleen and lymph nodes (data not shown).
High IL-7 affects early thymocyte progenitor progression
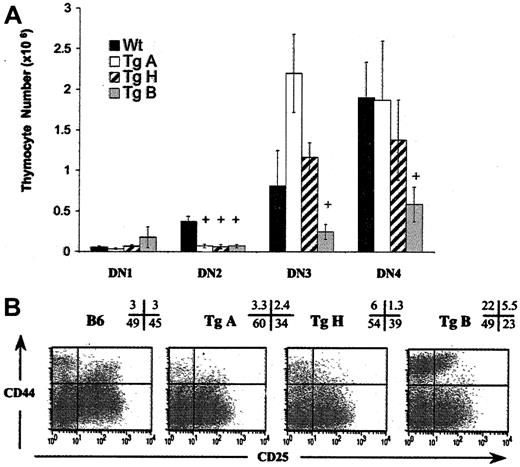
To specifically evaluate early thymocyte progenitors, we studied CD44 and CD25 expression in lineage-negative (lin–) cells from adult mice (6-12 weeks old) using 4-color flow cytometric analysis. Lin– cells were defined as negative for lineage marker surface expression. Previous studies have demonstrated that these cells differentiate through the following stages: CD44+CD25–(DN1) → CD44+CD25+ (DN2) → CD44–CD25+ (DN3) → CD44–CD25– (DN4).41,42 DN1 cells contain precursors capable of differentiation into T, B, NK, and dendritic cells.18,42-44 With CD25 molecule acquisition, progenitor cells (DN2) lose the potential to differentiate into B cells.18,45,46 By the end of the DN3 stage, the cells have finished their TCRβ rearrangement and signal through their pre-Tα/TCRβ/CD3+ complex.42,45,47
We compared the 4 DN subpopulations from each Tg line with those from wild-type littermates (Figure 3). In TgA mice, DN2 cell number was decreased (5-fold, P = .003) with normal DN3 and DN4 counts, while in TgB mice, the total lin– cell number was reduced (P = .04) (data not shown), due to a significant decrease in all T-cell–committed progenitors (DN2, DN3, and DN4 cells) (Figure 3A). Only DN1 cells were unaffected in absolute cell number, resulting in an increase in percentage (mean average, 14.9% vs 1.1%) (Figure 3B). Interestingly, in these TgB mice, the majority of DN2 cells did not slightly down-regulate CD44 as in normal B6 mice, and they only dully expressed CD25. This CD44hiCD25inter DN2 population, absent in normal mice (Figure 3B), did express Thy1.2, denoting its T-lineage origin (data not shown). In TgH mice, DN2 and DN3 populations were decreased in number. In summary, at low IL-7 overexpression, the DN progenitor progression through DN3 and DN4 is normal. However, at high IL-7 overexpression, DN1 to DN2 stage transition is impaired, resulting in severe depletion of all T-cell–committed progeny.
Early T-cell development is affected in all Tg+ founder lines. Total immature progenitor thymocytes (DN) were gated on lineage-negative cells as described in “Materials and methods,” and classified from DN1 through DN4 according to the expression of CD44 and CD25 surface molecules. (A) Absolute DN counts from 6- to 12-week-old TgA (n = 8), TgH (n = 5), TgB (n = 9), and negative littermates (n = 15). +P < .05. Error bars indicate SEM. (B) Appearance of a prominent population of CD44hi cells within DN1 and DN2 subpopulations in TgB line. Dot plots depict expression of CD44 versus CD25 on DN thymocytes from all 3 Tg+ lines versus Tg– individual mice. The percentage of events corresponding to each quadrant is shown.
Early T-cell development is affected in all Tg+ founder lines. Total immature progenitor thymocytes (DN) were gated on lineage-negative cells as described in “Materials and methods,” and classified from DN1 through DN4 according to the expression of CD44 and CD25 surface molecules. (A) Absolute DN counts from 6- to 12-week-old TgA (n = 8), TgH (n = 5), TgB (n = 9), and negative littermates (n = 15). +P < .05. Error bars indicate SEM. (B) Appearance of a prominent population of CD44hi cells within DN1 and DN2 subpopulations in TgB line. Dot plots depict expression of CD44 versus CD25 on DN thymocytes from all 3 Tg+ lines versus Tg– individual mice. The percentage of events corresponding to each quadrant is shown.
IL-7 up-regulates Bcl-2 expression in T-cell–committed progenitors of all 3 Tg lines but decreases proliferation of thymocyte progenitors only in TgB
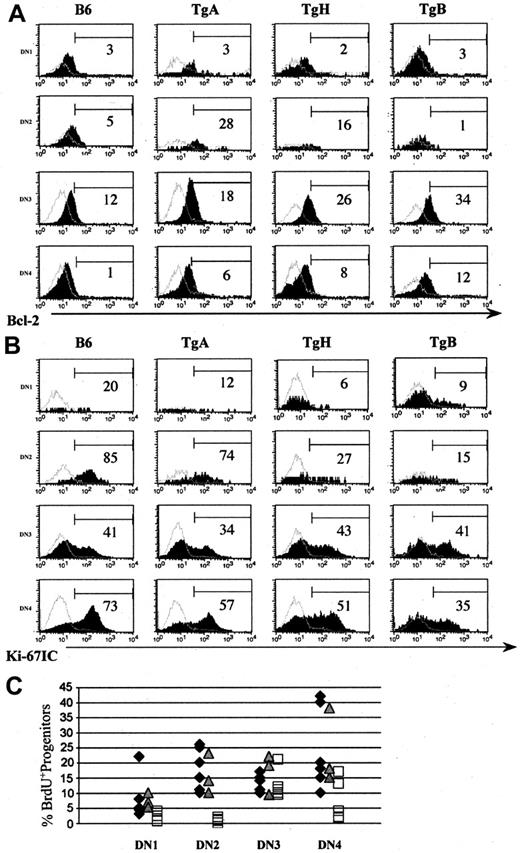
In normal mice, Bcl-2 expression is IL-7 dependent in DN2, DN3, and DN4 subsets,39 and its overexpression rescues T lymphopoiesis in IL-7 receptor–deficient mice.39,48 As expected, a Bcl-2 up-regulation was observed in DN2 (except for TgB), DN3, and DN4 in all 3 Tg lines (Figure 4A).
IL-7 up-regulates Bcl-2 in T-cell–committed progenitors in all 3 Tg lines and decreases their proliferation only at high IL-7 overexpression. Mice (6 weeks old) derived from all 3 Tg+ lines and normal control mice were studied for Bcl-2 (A) and Ki-67 (B) expression in DN1 to DN4 progenitors. Panels A-B show 1 of 4 representative experiments. Shaded histograms indicate the marker of interest; and open histograms, the negative isotype controls. Thymocyte progenitor proliferation was also determined by in vivo BrdU incorporation in 6- to 8-week-old TgA, TgB, and normal control mice (C). Panel C shows the percentage of BrdU+ cells in DN1 to DN4 in all 5 experiments. ♦ represents normal B6, represents TgA, and □ represents TgB mice.
IL-7 up-regulates Bcl-2 in T-cell–committed progenitors in all 3 Tg lines and decreases their proliferation only at high IL-7 overexpression. Mice (6 weeks old) derived from all 3 Tg+ lines and normal control mice were studied for Bcl-2 (A) and Ki-67 (B) expression in DN1 to DN4 progenitors. Panels A-B show 1 of 4 representative experiments. Shaded histograms indicate the marker of interest; and open histograms, the negative isotype controls. Thymocyte progenitor proliferation was also determined by in vivo BrdU incorporation in 6- to 8-week-old TgA, TgB, and normal control mice (C). Panel C shows the percentage of BrdU+ cells in DN1 to DN4 in all 5 experiments. ♦ represents normal B6, represents TgA, and □ represents TgB mice.
Since Bcl-2 not only inhibits cell death but also reduces Ki-67 proliferative expansion and slows the turnover of CD3–CD4–CD8– thymocyte progenitors,49 we determined their proliferation rate in all 3 IL-7 Tg and negative littermate mice using Ki-67 intracellular (IC) Ab staining. Ki-67 is a nuclear and nucleolar protein tightly associated with somatic cell proliferation.50 Its rate, distribution, and phosphorylation status are correlated with mitosis. We observed a constant decrease in positive cells in DN1, DN2, and DN4 subsets in TgH and TgB mice compared with normal controls (Figure 4B), while DN3 progenitors showed often the same division rate as normal controls. In TgA mice, division rate in DN progenitors was similar to normal B6 (Figure 4B). These results showed that the decrease of thymocyte progenitors in TgB is due to their decreased proliferation.
To confirm these results, we studied thymocyte progenitor turnover by labeling proliferating cells with the thymidine analog BrdU. TgA and TgB mice (6 weeks old) and their negative littermates were killed 4 hours after intraperitoneal injection of 1 mg BrdU (Figure 4C). As with Ki-67 staining, DN1, DN2, and DN4 but not DN3 in TgB mice showed a decreased proliferation rate compared with normal controls, especially in DN2 CD44hiCD25inter abnormal subpopulation. The percentage of BrdU+ thymocyte progenitors in TgA mice was not different from normal mice.
In conclusion, Ki67 and BrdU data confirmed that the decrease of TCRαβ cells observed in TgB mice is due to decreased thymocyte progenitor proliferation. However, in TgA, the normal proliferation rate favors increased thymocyte progenitor survival by Bcl-2 up-regulation as responsible for the increase of DP and SP thymocytes.
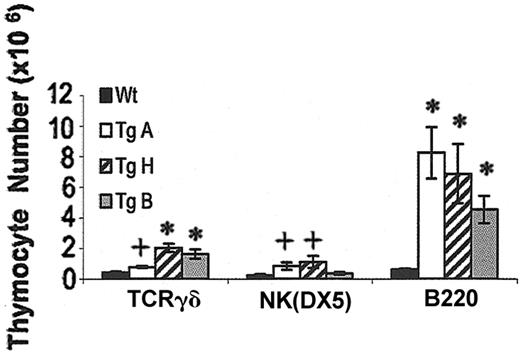
Increased B220, TCRγδ, and NK(DX5) cells in IL-7 transgenic thymi
All 3 IL-7 Tg founder lines demonstrated an abnormally large population of thymic B cells (7.3- to 13.2-fold increase over control) (Figure 5). All stages of B-cell development were overrepresented in adult Tg+ mice, from the CD43+ pro-B cells to the immature B220+IgM+ cells (Hardy classification)51 (data not shown). TCRγδ cells were also increased by 1.9-, 4.9-, and 3.9-fold in TgA, TgH, and TgB lines, respectively. NK cell number was significantly augmented in TgA and TgH thymi at 6 to 12 weeks, while only the percentage (1.25% vs 0.13%) was increased in the TgB mice (Figure 5 and data not shown).
Effect of IL-7 overexpression on TCRγδ, NK(DX5), and B220 cells. The bar graph shows the TCRγδ, NK(DX5), and B220 cell counts in Tg+ compared with Tg– mice. *P < .0001; +P < .05. Error bars indicate SEM.
Effect of IL-7 overexpression on TCRγδ, NK(DX5), and B220 cells. The bar graph shows the TCRγδ, NK(DX5), and B220 cell counts in Tg+ compared with Tg– mice. *P < .0001; +P < .05. Error bars indicate SEM.
Changes in T- and B-cell development induced by IL-7 overexpression in the thymus are not cell autonomous
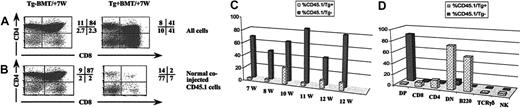
To determine whether IL-7 overexpression acts cell autonomously or as bystander effects, we performed 2 different sets of experiments. The first involved mixed bone marrow chimeras in which lethally irradiated mice were reconstituted with a 1:1 mixture of T-cell–depleted bone marrow cells from either IL-7 Tg+ mice or Tg– littermates (expressing CD45.2) and wild-type congenic mice (expressing CD45.1). Mice were killed 7 to 12 weeks later and analyzed by flow cytometry (Figure 6). In recipients of Tg+ mixed bone marrow, the majority (range, 74%-97%) of the total reconstituted thymocytes was derived from the IL-7 Tg+ (CD45.2) progenitors, whereas only 3% to 26% of thymocytes developed from the coinjected normal (CD45.1) bone marrow (Figure 6C). In contrast to what was observed in the thymocyte population, in the periphery, CD45.2 and CD45.1 marrow contributed equally to the splenic B-cell population (data not shown), confirming that comparable numbers of donor cells (CD45.1 and CD45.2) were injected. In the control group, significantly fewer thymocytes (15%-57%) were derived from Tg– (CD45.2) bone marrow donor cells. Importantly, in Tg+ recipients, the thymocyte population derived from the normal coinjected CD45.1 bone marrow cells demonstrated the same abnormalities observed in the Tg+ (CD45.2) population, with a decrease in the percentage of the DP subset and an increase in the percentages of the DN, CD8SP, and CD4SP subsets compared with the distribution seen in the control group (Figure 6D). Also, CD45.1-derived thymocytes evidenced significantly higher numbers of B220+ cells in Tg+ recipients (average, 36%; range, 12%-60%) than did Tg– recipients (average, 0.46%; range, 0.18%-0.81%). A similar increase was observed in thymic TCRγδ cells (constituting 2.0% vs 0.2% in Tg+ vs Tg– recipients) and NK cells (constituting 4.5% vs 1.1% in Tg+ vs Tg– recipients) (Figure 6D). These findings suggest that the transgene exerted its observed effect through IL-7 protein secretion. However, the advantage of proliferation of Tg+ cells over the normal coinjected bone marrow cells suggests an autocrine effect as well.
Changes in T-cell development in TgB are induced by IL-7 overexpression in the thymus. In bone marrow chimera experiments, adult B6 lethally irradiated mice were injected intravenously with a 1:1 ratio of Tg+ (CD45.2) or Tg– (CD45.2) bone marrow cells to congenic Ly5.2 (CD45.1) cells. Thymocytes from individual lobes were analyzed as indicated 7 to 12 weeks after the procedure. Dot plots of CD4/CD8 profiles on (A) total thymocytes or (B) on cells derived from normal coinjected CD45.1 donors. The percentage of events corresponding to each quadrant is shown. (C) Bar graph showing the percentage of the normal coinjected CD45.1 cells in the thymus of mixed bone marrow chimera recipients. (D) Bar graph showing the induction of the transgenic phenotype on the normal coinjected CD45.1 cells in Tg+ recipients in a representative experiment. BMT indicates bone marrow transplantation.
Changes in T-cell development in TgB are induced by IL-7 overexpression in the thymus. In bone marrow chimera experiments, adult B6 lethally irradiated mice were injected intravenously with a 1:1 ratio of Tg+ (CD45.2) or Tg– (CD45.2) bone marrow cells to congenic Ly5.2 (CD45.1) cells. Thymocytes from individual lobes were analyzed as indicated 7 to 12 weeks after the procedure. Dot plots of CD4/CD8 profiles on (A) total thymocytes or (B) on cells derived from normal coinjected CD45.1 donors. The percentage of events corresponding to each quadrant is shown. (C) Bar graph showing the percentage of the normal coinjected CD45.1 cells in the thymus of mixed bone marrow chimera recipients. (D) Bar graph showing the induction of the transgenic phenotype on the normal coinjected CD45.1 cells in Tg+ recipients in a representative experiment. BMT indicates bone marrow transplantation.
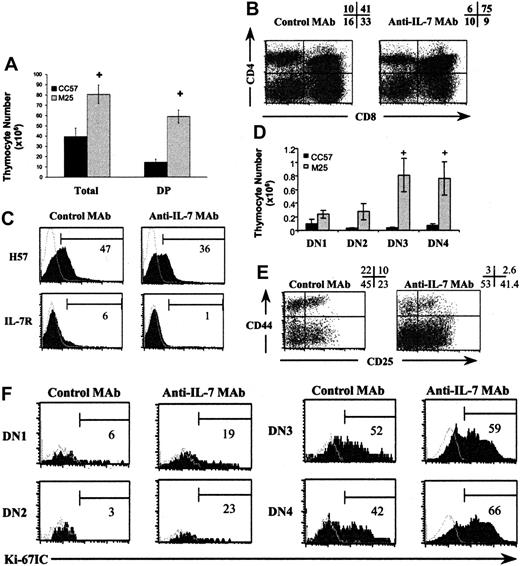
The second set of experiments used a neutralizing anti–IL-7 MAb (M25)30 to block IL-7 activity in vivo. TgB mice (1 month old) were given 10 injections of 1 mg MAb on a schedule of 3 injections per week. At 2 to 3 days after the last injection, 3 mice from each group were killed. Most of the phenotypic abnormalities previously described in TgB mice were partially reversed (Figure 7), compared with TgB mice treated with the isotype control MAb. An increase in T-cell development with significantly higher total thymocyte, DP (P = .003) (Figure 7A-B), DN3 (P = .03), and DN4 (P = .02) (Figure 7D-E) counts was observed. Using Ki-67 staining, we noticed an increase in DN1, DN2, and DN4 proliferation rates (Figure 7F). Interestingly, after anti–IL-7 MAb injection, the H57 staining on DP cells showed the appearance of cells with a normal H57–/lo profile. These cells were also IL-7R– compared with the control group (Figure 7C).
Injection of anti–IL-7 MAb (M25) induces a partial reversal of the phenotypic abnormalities observed on early and late thymic development. After 10 injections of 1 mg anti–IL-7 MAb (M25) or its isotype control (CC57), 3 mice from each group were killed 2 to 3 days after the last injection. (A) Total thymocytes and DP counts in the groups injected with M25 and CC57, respectively. (B) Modification of CD4/CD8 profile percentages after anti–IL-7 MAb injection. (C) Appearance of DP cells with normal maturation markers. The shaded areas represent the marker of interest, and the open areas represent the negative control. The percentage of positive cells for each marker is shown. Increase of absolute numbers (D) and percentages (E) of the T-cell committed progenitors DN3 and DN4 after anti–IL-7 MAb injection compared with control group mice. Error bars indicate SEM. (F) Enhancement of DN1, DN2, and DN4 cell proliferation by Ki-67 IC staining after injection of anti–IL-7 MAb in one representative experiment. +P < .05. The shaded histograms show the marker of interest; and the open histograms, the negative isotype controls. The number beneath each bracket represents the percentage of positive events.
Injection of anti–IL-7 MAb (M25) induces a partial reversal of the phenotypic abnormalities observed on early and late thymic development. After 10 injections of 1 mg anti–IL-7 MAb (M25) or its isotype control (CC57), 3 mice from each group were killed 2 to 3 days after the last injection. (A) Total thymocytes and DP counts in the groups injected with M25 and CC57, respectively. (B) Modification of CD4/CD8 profile percentages after anti–IL-7 MAb injection. (C) Appearance of DP cells with normal maturation markers. The shaded areas represent the marker of interest, and the open areas represent the negative control. The percentage of positive cells for each marker is shown. Increase of absolute numbers (D) and percentages (E) of the T-cell committed progenitors DN3 and DN4 after anti–IL-7 MAb injection compared with control group mice. Error bars indicate SEM. (F) Enhancement of DN1, DN2, and DN4 cell proliferation by Ki-67 IC staining after injection of anti–IL-7 MAb in one representative experiment. +P < .05. The shaded histograms show the marker of interest; and the open histograms, the negative isotype controls. The number beneath each bracket represents the percentage of positive events.
The results of both sets of experiments support the conclusion that IL-7 is not only present at the RNA transcript level within TgB thymocytes, but that the protein is responsible for the aberrations observed in the thymus of TgB animals.
Discussion
Different models of IL-7 Tg mice have shown the importance of this cytokine on T-cell numbers,20-23 but overexpression was dominated by its effect on B-cell development in 3 models,20-22 and the consequences on thymic development have not been extensively studied in IL-7 Tg mice with the K14 promoter.23 Under the lck proximal promoter in the current study, IL-7 overexpression was targeted specifically to thymocytes. With 3 founder lines having different IL-7 expression levels in the thymus, we were able to characterize, for the first time, a dose effect of IL-7 in vivo on αβ T-cell development. This is in accord with in vitro studies in which significantly lower numbers of αβ T cells with increasing IL-7 doses were observed.17
The principal IL-7 effect on thymocyte development is enhancing survival through the up-regulation of Bcl-2. Indeed, early progenitors express IL-7Rα and Bcl-2.1,39,48 This survival effect has made it difficult to dissect additional IL-7 roles using knock-out and transgenic models due to the paucity of thymocytes and the early appearance of T- and B-cell lymphomas. Additionally, in Bcl-2 Tg mice, Bcl-2 overexpression has been shown to slow the turnover of thymocyte progenitors.49 In our study, both mechanisms may explain the IL-7 dose effect that was particularly marked on DN progression. At low transgene overexpression (TgA), IL-7 more likely increased DP and SP thymocyte production by enhancing DN2 through DN4 subset survival through Bcl-2 up-regulation (Figure 4A). The Ki-67 and BrdU data do not favor increased DN cell proliferation in these mice (Figure 4B-C). In contrast, at high IL-7 expression (TgB), proliferation of DN1, DN2, and DN4 was decreased, and transition from the DN1 to DN2 stage was severely marked. Consistent with this, the numbers of DN2 through DN4 populations were decreased, with augmented DN1 cell percentage (Figure 3A-B). Moreover, DN2 cell differentiation was disturbed, with the appearance of an abnormal population of CD44hi CD25int (Figure 3B) Thy1.2+ cells (data not shown). The DN3 subset showed a normal proliferation rate, consistent with its usual dependency on TCR signaling for progression into the DN4 stage. A few cells escaped into mature SP thymocytes through DN3 and DN4 stages and this may be due to Bcl-2 up-regulation (Figure 4A).40 This decreased proliferation may not be explained by Bcl-2 up-regulation only. First, Bcl-2 expression in DN1 cells is IL-7 independent.1 Consistent with this, Bcl-2 was not up-regulated in the DN1 cells from the Tg mice in our study (Figure 4A). Moreover, using a model of Bcl-2 Tg mice, Bcl-2 overexpression has been shown to decrease DN4 but not DN1 through DN3 cell proliferation.49 As a consequence of the severe block in DN progression, thymic development was severely impaired in a cell subset–specific manner in TgB mice, with a dramatic drop in DP count. Interestingly, the DP subset in these mice showed an increase of their maturation markers (H57, CD69), and an up-regulation of IL-7R and Bcl-2, consistent with their late stage and the absence of production of new DP cells. The CD8 TCRlo cells in these mice showed also a decreased proliferation and Bcl-2 up-regulation (data not shown), which explains their increased numbers in these mice.
An alternative explanation for the “block” in TgB mice could be that IL-7 is skewing DN1 and/or DN2 cell differentiation toward other lineages such as B and/or TCRγδ. This may explain the increase of B and TCRγδ cells observed in all 3 Tg lines, as well as the reduced number of DN2 in all Tg. Consistent with a skewing toward the B lineage, Miller et al have shown that IL-7 promotes in vitro differentiation of the common lymphoid progenitor (CLP) into B220+CD19+ cells over T cells.52 This mechanism might be associated with other IL-7 effects as well.
The ratio of mature CD4SP/CD8SP was also reversed in the thymus of TgB mice, with a significant increase of CD8+TCRhi cells. High mature CD8+ thymocyte counts may be explained by coreceptor reversal favored by the direct presence of IL-7 as described.53 The increased number of CD8TCRhi thymocytes was also observed in FTOC, after 5-day culture of day-16 thymi from TgB mice and in day-18 thymi (data not shown).
The mixed bone marrow chimera and the injection of anti–IL-7 MAb experiments indicate that the severe phenotype observed in the TgB line is due to IL-7 overexpression and that IL-7 protein itself is involved in the phenotype. It should be noted that in the normal thymus, thymocyte exposure to IL-7 may be regulated not only by the amount produced but also by the geographic localization of production, which is altered in these Tg+ animals by virtue of the gene construct used. Progenitors are exposed to IL-7 in the cortex in the Tg+ mice, while IL-7 seems to be expressed primarily in the medulla of normal animals (Figure 1D). If IL-7 overexpression induces a down-regulation of IL-7R as soon as the DN2 development stage, this may cause a lack of survival signals due to the absence of the IL-7R/IL-7 ligand complex. However, because αβ T-cell development is not disturbed in TgA mice compared with TgB mice, it appears that the dose effect of IL-7 is more important than its geographic localization.
In conclusion, we describe, for the first time, a dose effect of IL-7 on αβ T-cell development in vivo. At low overexpression, IL-7 likely increases the survival of DN2, DN3, and DN4 cells by Bcl-2 up-regulation, which augments the DP and mature SP numbers. In contrast, at high IL-7 expression, there is a disruption of αβ T-cell development due to an early defect at DN1 to DN2 transition. This disturbance might be associated with a bias toward γδ T-cell and B-cell populations. This work is relevant to the recent increased consideration of IL-7 use in clinical settings.54,55
Prepublished online as Blood First Edition Paper, May 20, 2004; DOI 10.1182/blood-2004-01-0201.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Alfred Singer, Remy Bosselut, Nancy Hardy, and Yu-Waye Chu for expert review of the manuscript. We also acknowledge the expertise and the contributions of Filomena Medeiros for the quantitative PCR, Genevieve Sanchez and Brooke Davis in the animal experiments, Lionel Feigenbaum in the generation of the transgenic mice, Karen Byrne in the generation of the vector construct, and Michael Eckhaus in the preparation and interpretation of tissue sections.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal