Comment on Arbab et al, page 1217
To advance and exploit recent advances in experimental cell-based therapies, a method to monitor the migratory patterns of therapeutic cells that compels the development of clinically relevant cellular labeling and imaging techniques is necessary.
Recent advances in experimental cell-based therapies envisage the evolution of a new class of pharmaceuticals for the treatment of human disease.1 The need for a method by which to monitor and assess in vivo the biodistribution and migratory patterns of therapeutic cells has impelled the parallel development of cellular labeling and imaging techniques. The capabilities of magnetic resonance imaging (MRI) for high spatial resolution and accurate quantitation distinguish this imaging modality as particularly suited for such applications. The full exploitation of these benefits requires strategies that allow highly efficient cell labeling with limited cytotoxicity or impairment of cellular function. Several different superparamagnetic iron oxide particle (SPIO)–based preparations have demonstrated suitable characteristics in the magnetic labeling of cells. These particles effect susceptibility-based contrast enhancement within the tissue of interest, which is proportional to the concentration of iron in the cells. SPIO-based preparations with particles ranging in diameter from 17 to 900 nm have been used successfully to label and create images of a number of human cell types used for cell-based therapies including CD34+ hematopoietic progenitor cells,2,3 CD4+ T lymphocytes,2,4 CD8+ T lymphocytes,4,5 and mesenchymal stem cells.4 While cell-based therapies are currently undergoing clinical trials and the FDA has adjusted its policies to foster their development, a magnetic labeling strategy using FDA-approved agents has yet to be described. The question remains: will the magnetic labeling of cells remain a preclinical research tool, or will it evolve into a clinically relevant technique that is ready for prime time?
Writing in this issue of Blood, Arbab and colleagues strongly suggest an affirmative answer to the latter question in their use of a combination of FDA-approved agents to label several human and murine cell types. The authors use the commonly used transfection agent protamine sulfate to modify a commercially available suspension of dextran-coated SPIOs known as ferumoxides. Protamine sulfate is FDA approved for the treatment of heparin overdoses, while ferumoxides have been FDA approved for use as an MR contrast agent in the diagnosis of liver cancer for more than 7 years in the form of Feridex IV (Berlex Laboratories, Wayne, NJ). The article establishes that the combination of these agents results in an effective preparation of magnetically labeled cells. In the article, the authors initially define ferumoxide–protamine sulfate ratios that provide optimal relaxation properties for contrast enhancement, and proceed to demonstrate that this combination permits extremely high labeling efficiency as well as minimal short- and long-term effects on cellular toxicity, proliferation, and differentiation in the examined cell types. In so doing, this research identifies a promising magnetic cell-labeling strategy that is appropriate for potential clinical development and delineates a paradigm for the evaluation of such strategies prior to clinical implementation. This group has pioneered the use of transfection agents in combination with SPIOs for cell labeling and MR-based cell tracking.4 The authors' progress in this area affords an example of the systematic development of bench research for bedside application. The work of Arbab et al represents an important step in this effort, suggesting the prospective design of future clinical trials.FIG1
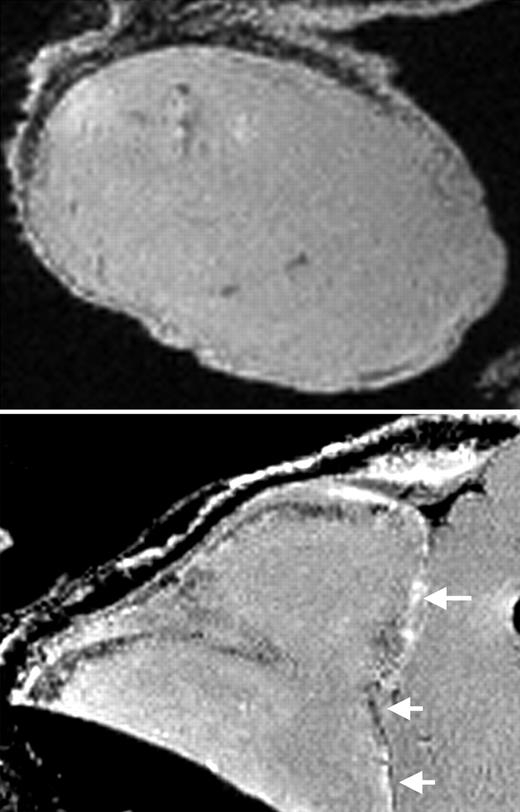
MRI of labeled CD34+ cells in tumors. See the complete figure in the article beginning on page 1217.
MRI of labeled CD34+ cells in tumors. See the complete figure in the article beginning on page 1217.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal