Abstract
We recently reported that chronic lymphocytic leukemia (CLL) cells synthesize and release vascular endothelial growth factor (VEGF) under normoxic and hypoxic conditions. CLL B cells also express VEGF membrane receptors (VEGF-R1 and VEGF-R2), suggesting that they use VEGF as a survival factor. To assess the mechanism of apoptosis resistance related to VEGF, we determined the impact of VEGF on CLL B cells, and we studied the impact of epigallocatechin-3-gallate (EGCG), a known receptor tyrosine kinase (RTK) inhibitor, on VEGF receptor status and viability of CLL B cells. VEGF165 significantly increased apoptotic resistance of CLL B cells, and immunoblotting revealed that VEGF-R1 and VEGF-R2 are spontaneously phosphorylated on CLL B cells. EGCG significantly increased apoptosis/cell death in 8 of 10 CLL samples measured by annexin V/propidium iodide (PI) staining. The increase in annexin V/PI staining was accompanied by caspase-3 activation and poly–adenosine diphosphate ribose polymerase (PARP) cleavage at low concentrations of EGCG (3 μg/mL). Moreover, EGCG suppressed the proteins B-cell leukemia/lymphoma-2 protein (Bcl-2), X-linked inhibitor of apoptosis protein (XIAP), and myeloid cell leukemia-1 (Mcl-1) in CLL B cells. Finally, EGCG (3-25 μg/mL) suppressed VEGF-R1 and VEGF-R2 phosphorylation, albeit incompletely. Thus, these results suggest that VEGF signaling regulates survival signals in CLL cells and that interruption of this autocrine pathway results in caspase activation and subsequent leukemic cell death.
Introduction
Angiogenesis is recognized as playing a role in the pathophysiology of many human malignancies.1 While solid tumors have been studied extensively for abnormal angiogenesis, primary hematologic disorders including myeloma, myelodysplasia, and acute and chronic leukemias have been the focus of recent work.2 These studies have consistently detected increased or abnormal angiogenesis in the marrow of patients with hematologic disorders. This finding has suggested that vascularity can contribute to disease pathogenesis, since it is likely that the bone marrow microenvironment sustains the survival and growth of the leukemic cell. Thus, in pathologic disorders such as diabetic retinopathies, rheumatoid arthritis, and malignancy, there is an uncontrolled proliferation of vessels that subsequently develop in a chaotic fashion.3-5
The etiology of the disordered angiogenesis is at least the unbalanced production of angiogenic factors from the leukemic cells. Angiogenesis is influenced by a number of positive and negative regulatory factors, including cytokines, extracellular matrix, and other cellular constituents including pericytes.6 However, a key mediator of the abnormal angiogenesis in many hematologic malignancies has been the cytokine designated as vascular endothelial growth factor (VEGF). Of interest, it has been shown that VEGF is secreted by diverse types of malignant cells.6-8 VEGF is a relatively small molecule (∼ 45 kDa) with diverse biologic activities that include the regulation of embryonic vascular development, extracellular matrix remodeling, generation of inflammatory cytokines, and enhancement of vascular permeability.9,10 VEGF is also a survival factor for endothelial cells. This latter feature is of interest since apoptosis resistance is a seminal feature of the chronic lymphocytic leukemia (CLL) B cell.11
Recently, we and others have shown that CLL B cells spontaneously secrete VEGF.12,13 In addition, several reports show that increased marrow and lymph node angiogenesis exists in B-cell CLL (B-CLL) patients. We have found that this abnormal angiogenesis increases with increasing disease stage.14 This latter finding is consistent with the possibility that tumor vascularity assists or is permissive for disease progression in B-CLL. An alternative role for VEGF in B-CLL is that of an autocrine growth/survival factor. Thus, the VEGF molecule secreted by CLL B cells appears to be the 121 and 165 isomers known to be high-affinity ligands for certain VEGF receptors.13 Among the 3 cell-surface receptors that bind VEGF, which include VEGF-R1 (fms-like tyrosine kinase-1 [Flt-1]), VEGF-R2 (fetal liver kinase-1 [Flk-1] or kinase domain receptor [KDR]), and VEGF-R3, CLL B cells express both VEGF-R1 and VEGF-R2 on their cell surfaces.12 This constitutive expression of VEGF and its receptors by CLL B cells strongly suggested that there is a VEGF-based autocrine pathway.
In the present work, we report further experiments designed to determine whether a VEGF autocrine pathway exists in CLL B cells and plays a role in maintenance of their apoptosis resistance. To do this, we examined the ability of exogenous VEGF to increase the in vitro apoptosis resistance in CLL B cells, the status of proapoptotic and antiapoptotic molecules in CLL B cells exposed to VEGF, and the activation status of VEGF receptors 1 (VEGF-R1) and 2 (VEGF-R2) in CLL B cells. In addition, we determined the ability of epigallocatechin-3-gallate (EGCG), a known receptor tyrosine kinase (RTK) inhibitor among its other cellular functions, to modulate VEGF receptor activation status and CLL B-cell apoptosis.
Materials and methods
Patient selection and purification of lymphocytes
The laboratory study was approved by the Mayo Clinic Institutional Review Board according to the Declaration of Helsinki. Blood was obtained from healthy donors or untreated B-CLL patients who had provided written informed consent. The B-CLL patients all had a confirmed diagnosis using the National Cancer Institute (NCI) working group definition.15 The total cohort studied in this investigation included 20 patients who had not been treated for at least 6 weeks prior to blood processing for the study and represented all Rai stages. The patients were predominantly patients who had not required therapy in the course of their disease. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized venous blood by density gradient centrifugation. To remove adherent cells, PBMCs were suspended in RPMI 1640 supplemented with 10% fetal calf serum and incubated in plastic dishes at 37° C for 1 hour prior to collection of nonadherent cells. To obtain at least 95% purity of CLL B cells, nonadherent cells were depleted of T cells by incubation with sheep erythrocytes. For some experiments, we purified CLL B cells from magnetic bead columns. In brief, highly purified CD19+ B cells (> 95%) were obtained from PBMCs by standard negative selection using a cocktail of subset-specific antibodies conjugated with magnetic beads (Miltenyi Biotech, Auburn, CA). These purified CLL B cells were then used immediately for the laboratory studies or cryopreserved in RPMI 1640, 20% fetal calf serum, and 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen until use.
Reagents
Immunologic reagents that recognize the following antigens were purchased from the indicated suppliers: procaspase-3 (CPP32), B-cell leukemia/lymphoma-X protein (Bcl-X), and myeloid cell leukemia-1 (MCl-1) from BD Pharmingen (San Diego, CA); phosphorylated extracellular signal-related kinase (ERK) from Cell Signaling (Beverly, MA); Bcl-2 from Dako (Carpinteria, CA); X-linked inhibitor of apoptosis protein (XIAP) from R&D Systems (Minneapolis, MN); poly–adenosine diphosphate ribose polymerase (PARP) from Biomol (Plymouth Meeting, PA); actin from Novus Biologicals (Littleton, CO); and phosphotyrosine (clone 4G10) from Upstate Biotechnology (Lake Placid, NY). Antibodies to fms-like tyrosine kinase-1 (FLT) (6.12R1) and KDR (3.83R2) were kindly provided by Imclone (New York, NY). EGCG as well as all other chemicals and reagents were obtained from Sigma Chemical (St Louis, MO).
Cell culture and ELISA methods
Primary CLL B cells or human splenic B cells were cultured in serum-free RPMI 1640. Cells were maintained at 37° C in an atmosphere containing 95% air–5% CO2 (vol/vol). A commercially available enzyme-linked immunosorbent assay (ELISA) was used according to instructions of the supplier (R&D Systems) to assay for VEGF secreted into the culture medium of CLL B cells during a 24-hour exposure to diluent or various EGCG concentrations in vitro.
Immunoblot analysis
Primary CLL B cells were cultured in 6-well tissue culture dishes and treated with either vehicle or drug for 24 hours. These treated cells were then washed 3 times with PBS (calcium, magnesium-free Dulbecco phosphate-buffered saline) and solubilized in alkylation buffer (6 M guanidine hydrochloride, 250 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.4, at 21° C, and 10 mM EDTA [ethylenediaminetetraacetic acid] supplemented immediately before use with 150 mM 2-mecaptoethanol and 1 mM phenylmethylsulfonyl fluoride). Samples were then dialyzed into 4 M urea and then 0.1% sodium dodecyl sulfate (SDS). Protein extracts were then separated on 5% to 15% SDS–polyacrylamide gel electrophoresis (PAGE) acrylamide gels and transferred to nitrocellulose and then blocked with 10% Tris-saline milk (TSM). Primary antibodies were diluted in the 10% TSM milk and incubated overnight on the shaker at room temperature. After extensive washing with 1 × PBS containing 0.05% Tween 20 for 3 × 15 minutes, the blot was then incubated with horseradish peroxidase (HRP)–conjugated secondary antibody (KPL, Gaithersburg, MD) diluted 1:8000 in 10% TSM for 1 hour at room temperature. After extensive washing with 1 × PBS containing 0.05% Tween 20 for another 30 minutes, bound antibodies were detected by enhanced chemiluminescence (ECL; Amersham Pharmacia, Piscataway, NJ).
Immunoprecipitation
Freshly isolated primary leukemic cells were treated with either vehicle or drug for 24 hours prior to VEGF165 (50 ng/mL) activation for 30 minutes. After this brief stimulation, total protein extracts from primary leukemic cells were obtained by lysing in the cold lysis buffer (30 mM Tris-HCL, pH 7.4, 150 mM NaCl, 1% glycerol, 1% Triton X-100, and 2 mM EDTA) in the presence of phosphatase inhibitors (100 mM NaF, 10 mM Na4P2O4, 1 mM Na2VO4), and protease inhibitors (10 μg/mL Leupeptin, 10 μg/mL Pepstatin A, and 1 mM phenylmethylsulfonyl fluoride [PMSF]). After centrifugation to remove all cell debris, protein extracts were immunoprecipitated overnight at 4° C on the rotator with either anti-FLT (6.12R1) or anti-KDR (3.83R2). The beads were washed with lysis buffer 3 × and resuspended into loading buffer and subjected to SDS-PAGE acrylamide gel electrophoresis (5%-15%) under reducing conditions. Proteins were subsequently transferred to nitrocellulose membrane. Blots were then blocked with 10% TSM milk and followed by incubation with primary, phosphotyrosine 4G10, and secondary antibodies. Secondary antimouse immunoglobulin G (Ig-G)–HRP for both VEGF-R1 (FLT) and VEGF-R2 (KDR) was used at 1:8000. The ECL chemiluminescence detection system and ECL film were used to visualize the presence of proteins on the cellulose blots.
Apoptosis/cell death assay
Primary CLL B cells (1 × 106 cells) were treated with either vehicle or drug for 24 hours. After the treatment, cells were rinsed with 1 × PBS and then subjected to apoptosis by means of a fluorescein isothiocyanate (FITC)–labeled annexin V/propidium iodide assay. These cells were directly analyzed in a FACScan (Becton Dickinson, Sunnyvale, CA) with a sample size of 10 000 cells gated on the basis of forward and side scatter. Storing and processing of data were done with FACScan software as described previously.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was isolated from primary CLL B cells using Trizol Reagent (Life Technologies, Rockville, MD) according to established procedures.16 The following were heated to 65° C for 10 minutes: 5 μg RNA, 1 μL random hexamer primer, 1 μL dithiothreitol (DTT), and 8 μL diethyl pyrocarbonate (DEPC) water. Bulk 1st strand mix (5 μL) from Pharmacia 1st strand synthesis (Amersham Pharmacia, Piscataway, NJ) was added into the mixture, which was then incubated at 37° C for 1 hour. The mixture (2 μL) was then supplemented with 25 μL Qiagen Hotstart Master mix reagent containing 200 μM deoxynucleoside triphosphate (dNTP), 1 × PCR buffer at 1.5 mM MgCl2, and 0.25 U Hotstart Taq. Additionally, 1.25 μL of each primer (forward and reverse) and 20.5 μL water were added. PCR was performed for 34 cycles with Hotstart at 94° C for 15-minute extension at 72° C (1 minute), denaturation at 94° C (30 seconds), and annealing at 58° C (1 minute) using Perkin Elmer 9600 thermocycler (Warrington, United Kingdom). After amplification, samples were subjected to electrophoresis on 1.5% agarose gel containing ethidium bromide, visualized under ultraviolet (UV) light, and phosphographed using an Alpha-Imager 3400 (Alpha Innotech, Essex, United Kingdom). The controls for the PCR methods included a blank control for all runs and are included in our figures.
The following primers were used: β-actin 5′ GGA TCC GAC TTC GAG CAA GAG ATG GCC AC-3′ (forward), 5′-CAA TGC CAG GGT ACA TGG TGG TG-3′ (reverse), 299-bp product; MCL-1 5′-CGC CAT CAT GTC GCC CGA AGA (forward), 5′-GGA AGG CCG TCT CGT GGT TGC-3′ (reverse), 335-bp product; XIAP 5′-CAG CAT CAA CAC TGG CAC GAG-3′ (forward), 5′-GTC TCA GAT GGC CTG TCT AAG-3′ (reverse), 277-bp product; BCL2 5′-TAG TGT GGT CTC CGA ATG TCT-3′ (forward), 5′-GCC ATT GCC TAT CCT CAC GTT-3′ (reverse), 249-bp product.
Statistical analysis
The probability of a statistically significant difference between the mean value of 2 data sets was determined by a 2-tailed Student t test using Statview Software (Statview Software, Cary, NC).
Results
VEGF rescue of CLL B cells from spontaneous and drug-induced apoptosis in chlorambucil by induction of Mcl-1 and XIAP
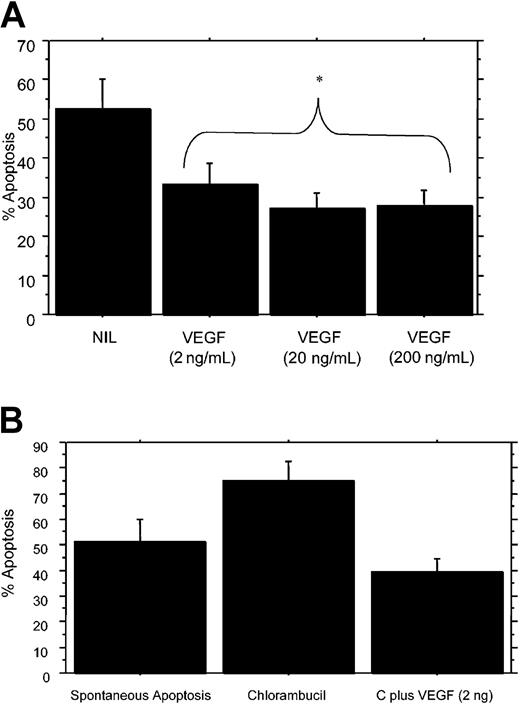
Based on previous results showing that CLL B cells spontaneously secrete VEGF and that these cells also express VEGF-R1 and VEGF-R2 on their cell membranes,12 initial experiments were designed to determine whether VEGF could influence resistance of CLL B cells to apoptosis. CLL B cells were cultured with VEGF165 from 2 to 200 ng/mL for 24 hours and then examined for spontaneous apoptosis levels in the subsequent 24 hours. Figure 1A illustrates the results of 7 separate experiments on samples from 8 patients, where on average 52.4% (standard error, 7.6%) of CLL B cells spontaneously underwent apoptosis without VEGF. However, for CLL B cells exposed to VEGF (2, 20, and 200 ng/mL) there was a substantial decrease in the level of spontaneous apoptosis, with levels of 33.3%, 26.9%, and 27.6%, respectively.
VEGF165 protects CLL B cells from spontaneous apoptosis/cell death and overcomes chlorambucil-induced apoptosis/cell death. (A) CLL B cells from 7 separate individuals were cultured without (NIL) and with the indicated concentrations of VEGF (ng/mL indicated in parentheses) and the mean level of spontaneous apoptosis/cell death was determined after 24 hours in culture. The mean decrease in apoptosis with VEGF-exposed CLL B cells was substantially lower when compared with control cells. (B) CLL B cells were first cultured with VEGF165 (2 ng/mL) or diluent for 24 hours and then incubated with chlorambucil (10 μM) for an additional 24 hours before assessing levels of apoptosis/cell death. Data (mean ± 1 SEM) shown are representative of 4 different patients. C indicates chlorambucil; NIL, B cells cultured without VEGF; and *, statistical analysis.
VEGF165 protects CLL B cells from spontaneous apoptosis/cell death and overcomes chlorambucil-induced apoptosis/cell death. (A) CLL B cells from 7 separate individuals were cultured without (NIL) and with the indicated concentrations of VEGF (ng/mL indicated in parentheses) and the mean level of spontaneous apoptosis/cell death was determined after 24 hours in culture. The mean decrease in apoptosis with VEGF-exposed CLL B cells was substantially lower when compared with control cells. (B) CLL B cells were first cultured with VEGF165 (2 ng/mL) or diluent for 24 hours and then incubated with chlorambucil (10 μM) for an additional 24 hours before assessing levels of apoptosis/cell death. Data (mean ± 1 SEM) shown are representative of 4 different patients. C indicates chlorambucil; NIL, B cells cultured without VEGF; and *, statistical analysis.
Further experiments on 4 patients evaluated whether VEGF could protect CLL B cells from drug-induced apoptosis. CLL B cells were first cultured with VEGF165 (2 ng/mL) or diluent for 24 hours and then incubated with chlorambucil (10 μM) for an additional 24 hours. While chlorambucil alone increased the level of apoptosis from 51.2 ± 8.7% to 74.9 ± 7.4%, the VEGF-exposed cultures were substantially protected from apoptosis with a mean apoptotic level of only 39.3 ± 5.4% (Figure 1B). In preliminary experiments, we have also tested the ability of VEGF165 to rescue CLL B cells (n = 2) from in vitro exposure to F-ara-A (the active moiety of fludarabine). We found that preincubation of 20 ng VEGF165 for 24 hours with CLL B cells was able to restore 55% and 39.5% of the apoptosis resistance to CLL B cells subsequently exposed to 10 μM F-ara-A when compared with F-ara-A–exposed CLL B cells but not pre-exposed to VEGF165.
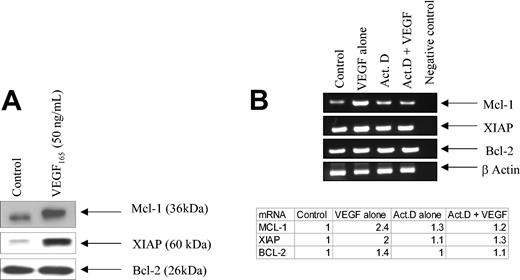
To determine if these antiapoptotic effects might reflect the ability of VEGF to alter expression levels of various apoptotic regulators in CLL B cells, CLL B cells were incubated with VEGF for 24 hours and examined by immunoblotting. Figure 2 shows that VEGF was very effective at increasing both Mcl-1 and XIAP in CLL B cells. In contrast, other apoptotic regulators, including Bcl-2, cellular inhibitor of apoptosis protein 1 (cIAP1), cIAP2, Bax, Bak, and TRAF-1, were not altered by VEGF (Figure 2 and data not shown). As illustrated in Figure 2B, the elevated Mcl-1 and XIAP levels reflected VEGF-induced increases in the corresponding mRNAs. The actinomycin-D–exposed CLL B cells were not able to increase mRNA for Mcl-1 and XIAP when exposed to VEGF (Figure 2B). This latter experiment is, therefore, indicating that VEGF was inducing mRNA for these 2 antiapoptotic genes.
VEGF increases both protein synthesis and mRNA for Mcl-1 and XIAP in CLL B cells. (A) The blots illustrate the increase in both Mcl-1 and XIAP in CLL B cells exposed to VEGF. CLL B cells were cultured in serum-free RPMI for 24 hours and then stimulated with VEGF165 (50 ng/mL) for 30 minutes prior to being lysed. Protein extracts (50 μg) were subjected to SDS-PAGE and then transferred to nitrocellulose. The blot was then probed with the antibody of interest. Data shown are representative of 1 of 5 different experiments. The results were consistent and reproducible. (B) VEGF stimulates increased expression of Mcl-1 and XIAP mRNA. Freshly isolated CLL B cells were pretreated with actinomycin D (1000 nM) for 24 hours prior to VEGF165 (50 ng/mL) stimulation for an additional 30 minutes. RNA was then isolated and RT-PCR was used to determine Mcl-1, XIAP, and Bcl-2 mRNA levels. The far right lane in panel B indicates our blank control for this set of experiments. The ratio (mRNA/actin) is used to determine the incremental mRNA of interest based on densitometry. The value from the table indicates the fold mRNA of interest based on densitometry. Each value is normalized to the control. This experiment has been repeated twice on 2 different patients with CLL. Act D indicates actinomycin D.
VEGF increases both protein synthesis and mRNA for Mcl-1 and XIAP in CLL B cells. (A) The blots illustrate the increase in both Mcl-1 and XIAP in CLL B cells exposed to VEGF. CLL B cells were cultured in serum-free RPMI for 24 hours and then stimulated with VEGF165 (50 ng/mL) for 30 minutes prior to being lysed. Protein extracts (50 μg) were subjected to SDS-PAGE and then transferred to nitrocellulose. The blot was then probed with the antibody of interest. Data shown are representative of 1 of 5 different experiments. The results were consistent and reproducible. (B) VEGF stimulates increased expression of Mcl-1 and XIAP mRNA. Freshly isolated CLL B cells were pretreated with actinomycin D (1000 nM) for 24 hours prior to VEGF165 (50 ng/mL) stimulation for an additional 30 minutes. RNA was then isolated and RT-PCR was used to determine Mcl-1, XIAP, and Bcl-2 mRNA levels. The far right lane in panel B indicates our blank control for this set of experiments. The ratio (mRNA/actin) is used to determine the incremental mRNA of interest based on densitometry. The value from the table indicates the fold mRNA of interest based on densitometry. Each value is normalized to the control. This experiment has been repeated twice on 2 different patients with CLL. Act D indicates actinomycin D.
Tyrosine phosphorylation status of VEGF-R1 and VEGF-R2 in CLL B cells
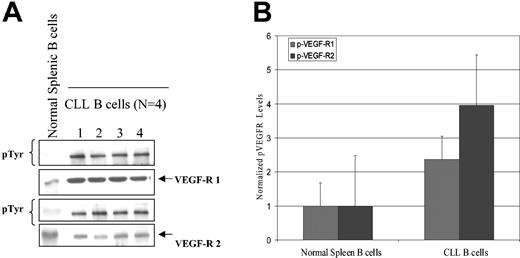
Since VEGF is known to activate VEGF-R1 and VEGF-R2 and induce receptor autophosphorylation with subsequent initiation of a signaling cascade,9,17 we next wished to evaluate the phosphorylation status of these receptors on CLL B cells. As shown in Figure 3, VEGF-R1 and VEGF-R2 could be readily immunoprecipitated from purified CLL B cells. Of interest, both receptors were tyrosine phosphorylated, with the levels of phosphorylation appearing to be equivalent for both VEGF-R1 and VEGF-R2 in all cases tested. Further analysis on samples from 4 patients demonstrated that this level of receptor autophosphorylation in CLL B cells was substantially higher than that observed in normal splenic B cells (Figure 3).
Normal splenic B and CLL B cells express differences in VEGF receptor autophosphorylation status. Protein and phosphorylation level of VEGF-R1 and VEGF-R2 in CLL B cells and splenic B cells are shown. (A) Isolated CLL B cells or spleen cells from a healthy donor were cultured in serum-free RPMI for 24 hours. Lysates (500 μg) were prepared and immunoprecipitated with either anti–VEGF-R1 or anti–VEGF-R2 antibody and then transferred to nitrocellulose. Blots were probed with phosphotyrosine antibody. Note the protein levels of VEGF-R1 and VEGF-R2 were approximately equivalent in both cell types, but the levels of spontaneous phosphorylation for VEGF-R1 and VEGF-R2 in spleen cells are much lower than in CLL B cells. (B) Densitometry was used to normalize the phosphorylated VEGF-R (p-VEGF-R) levels in the immunoblot and shows that the CLL B cells still retain a higher overall phosphorylation value for both VEGF-R1 and VEGF-R2 when compared with spleen cells.
Normal splenic B and CLL B cells express differences in VEGF receptor autophosphorylation status. Protein and phosphorylation level of VEGF-R1 and VEGF-R2 in CLL B cells and splenic B cells are shown. (A) Isolated CLL B cells or spleen cells from a healthy donor were cultured in serum-free RPMI for 24 hours. Lysates (500 μg) were prepared and immunoprecipitated with either anti–VEGF-R1 or anti–VEGF-R2 antibody and then transferred to nitrocellulose. Blots were probed with phosphotyrosine antibody. Note the protein levels of VEGF-R1 and VEGF-R2 were approximately equivalent in both cell types, but the levels of spontaneous phosphorylation for VEGF-R1 and VEGF-R2 in spleen cells are much lower than in CLL B cells. (B) Densitometry was used to normalize the phosphorylated VEGF-R (p-VEGF-R) levels in the immunoblot and shows that the CLL B cells still retain a higher overall phosphorylation value for both VEGF-R1 and VEGF-R2 when compared with spleen cells.
EGCG can down-regulate the phosphorylation of VEGF-R1 and VEGF-R2 on CLL B cells
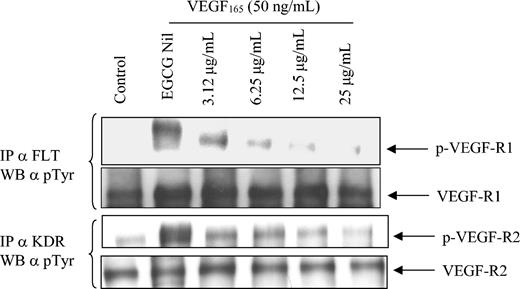
EGCG has been shown to reduce angiogenesis, decrease VEGF binding to its receptor,18 and inhibit the kinase activity of VEGF-R.19 Therefore, we were interested in determining if EGCG could alter the phosphorylation status of either VEGF-R1 or VEGF-R2. To evaluate this, we preincubated CLL B cells with increasing concentrations of EGCG (3.12-25 μg/mL) for 24 hours prior to stimulating cells with VEGF165 for one half hour. We then immunoprecipitated VEGF-R1 and VEGF-R2 using antibodies directed to FLT and KDR, respectively, and assessed receptor activation status using an anti–phosphorylated Tyrosine (p-Tyr) antibody. Figure 4 illustrates that exogenous VEGF is able to significantly increase the level of VEGF-R1 and VEGF-R2 activation (compare lanes 1 and 2, panels 1 and 3). However, preincubation with EGCG was able to significantly block receptor activation in a dose-dependent manner (Figure 4). To determine if VEGF could block the ability of EGCG to down-regulate the VEGF-R phosphorylation status, we added VEGF165 (50 ng) to the CLL B cells previously incubated with EGCG for 30 minutes prior to the p-Tyr blot. Figure 4 indicates that at the concentrations used in this experiment, VEGF165 was unable to prevent the down-regulation of receptor tyrosine phosphorylation by EGCG.
VEGF can induce increased phosphorylation of VEGF-R1 and VEGF-R2 receptors on CLL B cells, and phosphorylation of these receptors can be inhibited by EGCG. Primary CLL B cells were cultured in the presence or absence of EGCG for 24 hours and then stimulated with VEGF165 (50 ng/mL) for an additional 30 minutes prior to preparation of the protein extract. Protein extract from the immunoprecipitation (500 μg) was subjected to SDS-PAGE and transferred to nitrocellulose. The blots were initially probed with the p-Tyr antibody and then stripped with erase buffer. The blots were then reprobed with the various antibodies of interest. The data are representative of 1 of 3 different experiments. IP indicates immunoprecipitation.
VEGF can induce increased phosphorylation of VEGF-R1 and VEGF-R2 receptors on CLL B cells, and phosphorylation of these receptors can be inhibited by EGCG. Primary CLL B cells were cultured in the presence or absence of EGCG for 24 hours and then stimulated with VEGF165 (50 ng/mL) for an additional 30 minutes prior to preparation of the protein extract. Protein extract from the immunoprecipitation (500 μg) was subjected to SDS-PAGE and transferred to nitrocellulose. The blots were initially probed with the p-Tyr antibody and then stripped with erase buffer. The blots were then reprobed with the various antibodies of interest. The data are representative of 1 of 3 different experiments. IP indicates immunoprecipitation.
EGCG can suppress VEGF production and induce CLL B-cell apoptosis
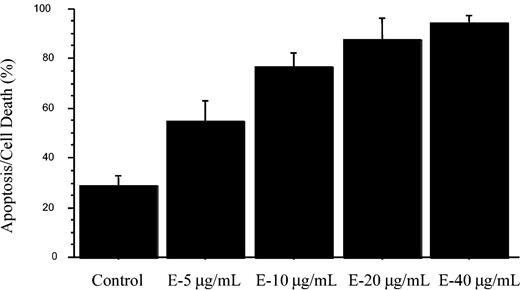
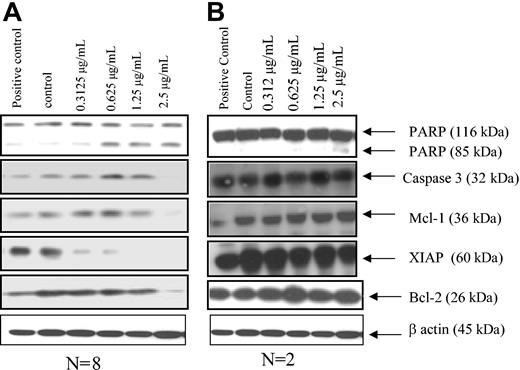
Since both green tea and the catechin EGCG have been shown to inhibit tumor growth,20,21 and the results shown here demonstrated the ability of EGCG to inhibit activation of VEGF-R1 and VEGF-R2, we were next interested in determining whether EGCG could induce apoptosis in CLL B cells. To accomplish this, we cultured purified CLL B cells with EGCG and determined the extent of apoptosis and cell death using annexin V/propidium iodide flow analysis. Figure 5 demonstrates the results for these experiments on 7 B-CLL patients. This figure shows the dose-dependent induction of cell death in the CLL B-cell clones. The level of cell death found in these experiments exceeded 80% with EGCG concentrations as low as 20 μg/mL. However, significant increases over the diluent-tested cultures were found at doses as low as 5 μg/mL, and P < .0001 was found for EGCG (40 μg/mL vs the control). Interestingly, these concentrations were within the range of EGCG that we also found to inhibit VEGF-R phosphorylation (Figure 3). Because of this association and because we found that VEGF increased both Mcl-1 and XIAP, we then tested if EGCG could modulate levels of these antiapoptotic proteins. Figure 6 illustrates that there are both EGCG-“sensitive” and EGCG-“insensitive” B-cell clones. Both Mcl-1 and XIAP were down-regulated when EGCG was added to sensitive CLL B-cell cultures using samples from 8 patients. This down-regulation of the 2 proteins was dose dependent while not usually affecting other apoptotic proteins, including Bcl-2. By contrast, expression levels of other antiapoptotic proteins, including survivin, were not substantially altered with the addition of EGCG to CLL B cells (data not shown). The impact of EGCG on XIAP consistently occurred at lower doses than that for Mcl-1. Figure 6 also shows that PARP cleavage occurs along with decreases in procaspase-3, consistent with apoptosis induction in the EGCG-sensitive CLL B-cell group. There was a small percentage of CLL B-cell clones not responsive to EGCG (designated as insensitive), with 2 of 10 CLL B-cell clones having no PARP cleavage, no caspase-3 level changes, and no alteration of apoptotic proteins (Figure 6B). The PARP cleavage data in Figure 6 are consistent with the flow cytometry analysis in that 10 of 15 patients were found to have substantial increases in apoptosis death with EGCG (data not shown).
EGCG can induce apoptosis in CLL B cells. Freshly isolated CLL B cells (n = 7) were cultured in serum-free RPMI with the presence of EGCG for 24 hours. After the 24-hour exposure to EGCG, the cells were subjected to flow analysis with annexin V/propidium iodide to measure apoptosis and cell death. The data are shown as mean ± standard error of the mean. The difference between level of apoptosis for the control versus the highest dose of EGCG (40 μg/mL) was significant (P < .0001). E indicates EGCG.
EGCG can induce apoptosis in CLL B cells. Freshly isolated CLL B cells (n = 7) were cultured in serum-free RPMI with the presence of EGCG for 24 hours. After the 24-hour exposure to EGCG, the cells were subjected to flow analysis with annexin V/propidium iodide to measure apoptosis and cell death. The data are shown as mean ± standard error of the mean. The difference between level of apoptosis for the control versus the highest dose of EGCG (40 μg/mL) was significant (P < .0001). E indicates EGCG.
Effects of EGCG on Mcl-1 and XIAP proteins in CLL B cells. (A) Representative results for sensitive CLL B cells when EGCG is added to the majority of CLL B-cell cultures (n = 8 of 10). Thus, PARP cleavage decreases in Mcl-1, XIAP, and caspase-3, and little or no change in Bcl-2 is seen. Freshly isolated CLL B cells (n = 8 patients) were treated with either vehicle or EGCG for 24 hours. Protein extracts (50 μg) were prepared and subjected to SDS-PAGE, following transfer to nitrocellulose. The blot was probed with the antibodies of interest. (B) Representative blot for the EGCG-insensitive CLL B-cell populations. Note there was no change in the apoptotic proteins or PARP cleavage in the insensitive CLL patients.
Effects of EGCG on Mcl-1 and XIAP proteins in CLL B cells. (A) Representative results for sensitive CLL B cells when EGCG is added to the majority of CLL B-cell cultures (n = 8 of 10). Thus, PARP cleavage decreases in Mcl-1, XIAP, and caspase-3, and little or no change in Bcl-2 is seen. Freshly isolated CLL B cells (n = 8 patients) were treated with either vehicle or EGCG for 24 hours. Protein extracts (50 μg) were prepared and subjected to SDS-PAGE, following transfer to nitrocellulose. The blot was probed with the antibodies of interest. (B) Representative blot for the EGCG-insensitive CLL B-cell populations. Note there was no change in the apoptotic proteins or PARP cleavage in the insensitive CLL patients.
Since the results thus far suggested that an autocrine VEGF pathway may exist in B-CLL cells and functions to promote cell viability, we next wished to determine if EGCG would be able to interrupt this putative autocrine loop in CLL B cells by decreasing VEGF levels. CLL B cells from samples on 4 patients were incubated with varying concentrations of EGCG (0.6-2.5 μg) for 24 hours. After CLL B cells were incubated with EGCG for 24 hours, the culture medium was assayed for VEGF levels by ELISA. EGCG induced a dose-dependent decrease in VEGF secretion (eg, 63.1 ± 12 pg/mL for Nil vs 45.8 ± 8.9 pg/mL for 2.5 μg/mL EGCG). These lower doses of EGCG were chosen as we were concerned about the VEGF levels falling due to increasing apoptosis of the CLL B cells with EGCG at levels of 5 μg/mL or greater as shown in Figures 4 and 5. Thus, the levels of EGCG-exposed CLL B-cell apoptosis were not altered significantly from control levels in this set of experiments or in others in which we have used similar levels of EGCG (data not shown). Nevertheless, we did see incremental decreases in VEGF synthesis with higher doses (data not shown).
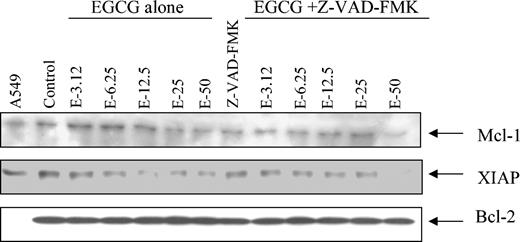
Because Mcl-1 and XIAP are substrates for caspases, we next assessed whether caspase inhibitors could prevent EGCG-induced down-regulation of these apoptosis-regulating proteins. The results shown in Figure 7 demonstrate that the presence of the caspase inhibitor [Z-VAD(oME)-FMK] could inhibit the ability of EGCG to down-regulate Mcl-1 and XIAP.
Caspase inhibitor, Z-VAD(oMe)-FMK, prevents EGCG from down-regulating Mcl-1 and XIAP. Primary CLL B cells were pretreated with Z-VAD(oMe)-FMK (25 μM) for 30 minutes prior to the EGCG treatment for 24 hours. Protein extract (50 μg) was prepared and then subjected to SDS-PAGE (7.5%). The blots were blotted with the antibody of interest. Data shown are representative of 2 different experiments.
Caspase inhibitor, Z-VAD(oMe)-FMK, prevents EGCG from down-regulating Mcl-1 and XIAP. Primary CLL B cells were pretreated with Z-VAD(oMe)-FMK (25 μM) for 30 minutes prior to the EGCG treatment for 24 hours. Protein extract (50 μg) was prepared and then subjected to SDS-PAGE (7.5%). The blots were blotted with the antibody of interest. Data shown are representative of 2 different experiments.
Discussion
Various autocrine pathways in CLL B cells provide important survival advantages for these cells. Our current studies strongly suggest that a VEGF autocrine pathway exists in CLL B cells. The evidence for this includes our finding that recombinant VEGF165 can rescue CLL B cells from both spontaneous and drug-induced apoptosis or cell death. The addition of VEGF to CLL B cells also resulted in significant increases in the antiapoptotic proteins Mcl-1 and XIAP. In addition we found that the 2 VEGF receptors (VEGF-R1 and VEGF-R2) on CLL B cells are spontaneously phosphorylated. The combination of VEGF-induced increases in antiapoptotic proteins combined with the finding of phosphorylated VEGF receptors strongly suggested that a VEGF-based pathway is linked to CLL B-cell survival. Additional evidence for this is that the addition of the catechin EGCG did result in significant increases in apoptosis/cell death levels for CLL B cells; EGCG was able to down-regulate the phosphorylation status of both VEGF-R1 and VEGF-R2 and decrease secreted VEGF levels of CLL B cells. Thus, the VEGF autocrine pathway likely plays a role in the maintenance of CLL B-cell apoptosis resistance.
The addition of VEGF165 to CLL B cells was used in multiple patients, and an increase was noted consistently of VEGF-R phosphorylation; however, the level of VEGF-R phosphorylation enhancement did vary (data not shown). Importantly, in all our experiments we consistently found that the antiapoptotic protein Mcl-1 was increased when CLL B cells were exposed to exogenous VEGF165. Potential explanations for this include that VEGF is known to bind to other membrane receptors such as neuropilin-1.22,23 In fact, we have found that CLL B cells express easily detectable levels of neuropilin-1 (NRP-1) using flow cytometry (data not shown). NRPs are known to be expressed in endothelial cells and bind VEGF165. NRP-1 is a coreceptor for VEGF receptor-2 (VEGF-R2) that enhances the binding of VEGF165 to VEGF-R2 and VEGF165-mediated chemotaxis.24 While controversial, there is also recent evidence that VEGF165 can recruit NRP-1 to form coreceptors that complex with VEGF-R1.25 Thus, the primary activation of exogenous VEGF on CLL B cells may be via its binding and activation of NRP-1. We have also examined whether B-CLL cells express VEGF-R3, an additional receptor that can also bind VEGF. However, we have been unable to detect consistent expression of this receptor on CLL B cells (data not shown).
VEGF elaboration by CLL B cells is not only present spontaneously but is up-regulated when CLL cells are exposed to hypoxia.12 We do not yet understand the mechanism for the basal levels of synthesis and secretion of VEGF by CLL B cells; however, signal transducer and activator of transcription 3 (Stat3) has been found to regulate VEGF synthesis,26,27 perhaps through binding to the VEGF promoter region. Interestingly, both Stat1 and Stat3 proteins have been found to be constitutively phosphorylated in CLL B cells.28 Thus, it is possible that an activated STAT protein could be generating an increase in mRNA for VEGF resulting in the spontaneous VEGF secretion seen so often in CLL B cells.12 We do know that the VEGF molecules secreted from CLL B cells include the isomers 121 and 165.13 These 2 molecules are known to be the most active isomers for binding to and activation of the VEGF-R1 and VEGF-R2 receptors.9 VEGF is a potent mitogen and survival factor for endothelial cells and promotes endothelial cell proliferation and survival.9 In addition, VEGF has been shown to regulate myeloid leukemic cell growth. Recently, VEGF165 has been shown to induce phosphorylation of VEGF-R2 and induce increased proliferation of myeloid leukemic cells.17
In addition to coexpression of VEGF and its receptors, the prerequisite for autocrine loops is frequently found in lymphoma, myeloma, as well as myeloid leukemia,7,8,17 suggesting that autocrine loops play a role in several other hematologic malignancies. VEGF production has been correlated with disease progression in patients with a variety of hematologic malignancies.29-31 Therefore, we were particularly interested in the ability of VEGF to alter CLL B-cell apoptosis and cell death. Since we have previously shown that CLL patients have abnormal angiogenesis in their bone marrow,14 we did not do a similar analysis of the bone marrow vascularization in this investigation. While of interest, we also could not do correlative studies of the VEGF pathways described here and the extent of marrow neovascularization and/or angiogenesis in our patient cohort.
We found that addition of recombinant VEGF165 to CLL B cells can promote survival of either spontaneous or chemotherapy-induced apoptosis and cell death. While the exact mechanism of VEGF protection from apoptosis/cell death is unclear, it appears to reside, at least, in its ability to induce Mcl-1. Most of VEGF survival activity on endothelial cells appears to be mediated via VEGF-R2 and includes the expression of the antiapoptotic proteins Bcl-2 and A1.9 There is one report that demonstrates a negative correlation between VEGF and Bcl-2 intracellular levels in CLL B cells.32 While that may seem counterintuitive, we in fact do not find any significant change in CLL Bcl-2 levels when we add VEGF to these cells in vitro. Thus, we saw little or no change in the Bcl-2 levels or other related apoptosis family members compared with the increase in Mcl-1 and XIAP when VEGF was added to CLL B cells. In addition, we did not find phosphorylated ERK1/2 or AKT proteins even when various doses of VEGF were added to CLL B cells (data not shown). Interestingly, VEGF has been found to prevent the apoptotic death of hematopoietic cells induced by exposure to chemotherapeutic drugs by increasing the levels of Mcl-1.33 That study also showed that overexpression of Mcl-1 in a leukemic cell line prevented or reduced the ability of chemotherapy-induced cell killing. Higher Mcl-1 levels have also been found to correlate with inability to obtain a complete remission with chemotherapy B-CLL patients.34 More recently, it was found that exposure of CLL B cells to cell lines, including a dendritic cell line, can subsequently induce enhanced apoptosis resistance by increasing Mcl-1 levels.35 Finally, the monoclonal antibody Rituximab has been reported to reduce Mcl-1 and XIAP levels but not Bcl-2 protein levels in B-CLL patients.36 Thus, the evidence is growing that while Bcl-2 is elevated in most CLL B cells, the presence of Mcl-1 appears to be critical in the survival of these cells. Any agent that can be shown to be capable of down-regulating Mcl-1 should be of interest in the treatment of B-CLL. Therefore, it would be important to evaluate by antisense oligonucleotides or siRNA the impact of down-regulation of the VEGF gene to see if specific decreases in Mcl-1 and/or XIAP occur and are linked to significant increases in the apoptosis of CLL B cells.
To that end, we explored the ability of a catechin, EGCG, to induce apoptosis in CLL B cells. This molecule was chosen for study because of its reported ability as a receptor tyrosine kinase inhibitor,19 although it has been reported to have several other anticarcinogenic functions.37 Since we found that CLL B cells commonly exhibit spontaneous phosphorylation of both VEGF-R1 and VEGF-R2 and that VEGF can protect CLL B cells from cell death, a VEGF-based autocrine pathway appears to exist in CLL B cells. EGCG not only has RTK inhibitory function,38 but recently, it has been shown to decrease VEGF binding to VEGF receptors of human endothelial cells.18 In our studies, EGCG was very effective at inducing more apoptosis/cell death and in the down-regulation of VEGF-R1 and VEGF-R2 phosphorylation levels of CLL B cells. PARP cleavage and decreases in caspase-3 were seen with exposure to EGCG, suggesting that the mechanism of cell death in the CLL B cells was related to the apoptosis process. In addition, we found decreases in Mcl-1 and XIAP with EGCG exposure, indicating that the same molecules up-regulated by VEGF were decreased with exposure to EGCG. The use of EGCG did suggest to us that other known RTK inhibitors could be used to alter the VEGF-based autocrine loop in CLL B cells. Therefore, we have tested 2 small molecules, SU5416 and SU11657,39 for their impact on VEGF-R1 and VEGF-R2 phosphorylation as well as CLL B-cell apoptosis. While we did find that both of these RTK inhibitors down-regulate VEGF receptor phosphorylation and induce in vitro apoptosis in CLL B cells (data not shown), they do not have sufficient activity in vitro to assume they would work in clinical trials for CLL patients. However, their activity underscores the potential specificity of the EGCG effect on CLL B cells. We believe that EGCG may be disrupting an autocrine VEGF pathway in CLL B cells linked to apoptosis resistance. It is of interest to us that the levels we used for our in vitro studies are consistent with those achievable in vivo using green tea.21 This point is relevant since EGCG, a flavonoid from green tea, possesses chemopreventive activity in experimental and epidemiologic studies.40 In the CLL patients used for these studies, we did assess the CD38 status and whether the patients were mutated or nonmutated for the heavy chain variable region of their B-cell clones. This was done to determine if the more unfavorable clinical prognosis known for CD38+ and/or nonmutated type CLL B-cell clones would have more evidence for a VEGF-based autocrine pathway than CD38– and/or mutated type CLL B-cell clones. There was no convincing evidence in these studies that these parameters selected out for CLL B-cell clones that were more sensitive to VEGF (data not shown). Specifically, we did not see higher levels of VEGF, greater expression of VEGF receptor phosphorylation, or a differential sensitivity to EGCG. Currently, we believe that all CLL B-cell clones use this VEGF-based autocrine pathway to a greater or lesser extent. Of note, we have previously published that nonmutated and mutated type CLL B-cell clones have equivalent expression of mRNA for both VEGF-R1 and VEGF-R2.12 However, we are continuing to examine this aspect as we refine and evaluate the exact mechanism operative in the VEGF pathway.
Tea, especially green tea and its polyphenols, has also been found to inhibit experimental tumorigenesis and tumor growth in animals.41 The most abundant tea catechin compound is EGCG, and humans who drink moderate amounts of green tea can achieve serum levels of EGCG in the range of 0.1 to 0.3 μM.42 Individuals who drink green tea have been reported to have lower incidences of esophageal and breast cancer,21 suggesting it may have a role in chemoprevention. The feasibility of using purified reagent is evident since a recent study of oral EGCG in healthy controls found that this type of administration is nontoxic, can be rapidly absorbed, and serum levels up to 3.4 mg/mL can be reached.43
While the mechanism of action for EGCG in reducing human cancer incidence has been unclear, one mechanism of action that has been recently explored is that of altering angiogenesis. One study has shown that green tea polyphenols are active as oral angiogenesis inhibitors based on inhibition of neovascularization.21 The antiangiogenic mechanisms described to date for EGCG include inhibition of matrix metalloproteinases 2 and 9, urokinase plasminogen activator, decreased tumor cell VEGF synthesis, and repression of activator protein-1 (AP-1), nuclear factor κB (NFκB), and STAT-1 transcription factor pathways.44,45 EGCG has been shown to not only modulate endothelial cell function but it can inhibit the phosphoinositide 3-kinase (PI3 kinase) and mitogen-activated protein kinase (MAP) kinase p42/p44 pathways in vascular smooth muscle cells.42 This latter finding is of interest in the therapy of CLL since activation of PI3 kinase pathways are commonly found in CLL B cells and may be related to their apoptosis resistance.46,47 Future work defining the nature of EGCG induction of apoptosis of CLL B cells is necessary, however, we would propose that our studies and others discussed above raise the exciting possibility of using this agent for both prevention and therapy of B-CLL. For example, the application of this type of relatively nontoxic agent could be tested in early-stage high-risk individuals with B-CLL.
Prepublished online as Blood First Edition Paper, March 2, 2004; DOI 10.1182/blood-2003-08-2763.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very appreciative for the intellectual guidance and support of Scott H. Kaufmann, MD, PhD.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal