Abstract
Cyclophosphamide, doxorubicin, vincristine, and prednisone, given every 3 weeks (CHOP-21), is standard chemotherapy for aggressive lymphomas. To determine whether biweekly CHOP (CHOP-14) with or without etoposide is more effective than CHOP-21, 689 patients ages 61 to 75 years were randomized to 6 cycles of CHOP-21, CHOP-14, CHOEP-21 (CHOP plus etoposide 100 mg/m2 days 1-3), or CHOEP-14. Patients in the 2-weekly regimens received granulocyte colony-stimulating factor (G-CSF) starting from day 4. Patients received radiotherapy (36 Gy) to sites of initial bulky disease and extranodal disease. Complete remission rates were 60.1% (CHOP-21), 70.0% (CHOEP-21), 76.1% (CHOP-14), and 71.6% (CHOEP-14). Five-year event-free and overall survival rates were 32.5% and 40.6%, respectively, for CHOP-21 and 43.8% and 53.3%, respectively, for CHOP-14. In a multivariate analysis, the relative risk reduction was 0.66 (P = .003) for event-free and 0.58 (P < .001) for overall survival after CHOP-14 compared with CHOP-21. Toxicity of CHOP-14 and CHOP-21 was similar, but CHOEP-21 and in particular CHOEP-14 were more toxic. Due to its favorable efficacy and toxicity profile, CHOP-14 should be considered the new standard chemotherapy regimen for patients ages 60 or older with aggressive lymphoma.
Introduction
More than half of the patients with newly diagnosed aggressive lymphomas are older than 60 years. These patients have a worse prognosis than younger patients.1 The CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen2 is standard care for aggressive lymphoma, but 5 years after treatment only one third of the patients older than 60 years are alive and free of disease. More aggressive chemotherapy regimens have not been successful in aggressive lymphoma3 partly because they cannot be administered to elderly patients at the prescribed time and dosage.4
After the Intergroup Study3 had confirmed CHOP as the standard regimen for aggressive lymphomas, the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) decided to investigate whether the reduction of treatment intervals from 3 to 2 weeks (CHOP-14), the addition of etoposide (a potent cytotoxic agent) to CHOP (CHOEP-215 ), or a combination of both (CHOEP-14) would improve outcome after chemotherapy.
Having demonstrated the feasibility and safety of CHOEP-14 using granulocyte colony-stimulating factor (G-CSF),6 the DSHNHL initiated a randomized 4-arm trial in a 2 × 2 factorial design to investigate if shortening of the intervals and/or adding etoposide could improve the outcome of patients older than 60 years with aggressive lymphomas.
Patients and methods
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the ethics review committee of each participating center. All patients gave written informed consent. Patients were eligible if they had previously untreated, biopsy-confirmed, aggressive non-Hodgkin lymphoma according to the Revised European-American Lymphoma Classification7 (translated into the World Health Organization [WHO] classification8 ) and were between 61 and 75 years old. Patients were excluded if the diagnosis of aggressive or very aggressive lymphoma was not confirmed (ie, no pathology review was available) or if the diagnosis had to be changed into indolent lymphoma or no lymphoma at all by a panel of 5 expert hematopathologists who conducted a blinded central pathology review. Patients with previous treatment (chemotherapy or radiotherapy); lymphoma associated with the acquired immunodeficiency syndrome; a diagnosis or history of indolent lymphoma or other neoplasms; marked impairment of cardiac, pulmonary, hepatic, or renal function; WHO performance status 4; bone marrow involvement with more than 25% lymphoma cells; initial white blood cell count (WBC) less than 3 × 109/L; initial platelet count less than 100 × 109/L; or inability to comply with study requirements were excluded. The patients had mandatory baseline examinations that included clinical examination, laboratory tests, chest radiograph, abdominal sonography, computed tomography of chest and abdomen, and a bone marrow biopsy.
Between September 1993 and June 2000, 831 patients were randomized by 121 institutions after a telephone interview. Randomization was performed at a 1:1:1:1 ratio using the minimization algorithm by Pocock9 after stratification for centers, elevated lactate dehydrogenase (LDH), advanced disease (stage III/IV), and bulky disease. All patients for whom the eligibility criteria were not confirmed after randomization were withdrawn and the balances were appropriately adjusted in the randomization program.
Treatment protocol
A “prephase” treatment consisting of a single injection of 1 mg vincristine (intravenously) and 100 mg prednisone (orally) for 5 to 7 days was recommended to improve the performance status of the patients and to ameliorate the side effects of the first chemotherapy cycle. The CHOP regimen2 consisted of cyclophosphamide (750 mg/m2 intravenously), doxorubicin (50 mg/m2 intravenously), and vincristine (2 mg intravenously) on day 1 and prednisone (100 mg orally) given on days 1 to 5. CHOEP was identical to CHOP with etoposide (100 mg/m2 intravenously) added on days 1 to 3. CHOP-21 and CHOEP-21 were recycled every 3 weeks, and CHOP-14 and CHOEP-14 were recycled every 2 weeks, with patients receiving recombinant human G-CSF (filgrastim) from days 4 to 13 at a dosage of 300 μg/d or 480 μg/d for patients whose body weight was less than 75 kg, or 75 kg or greater, respectively. G-CSF administration in the 3-week regimens was at the treating physician's discretion. The next chemotherapy cycle was scheduled for day 15 or 22, respectively, after recovery of WBC (> 2.5 × 109/L) and platelet count (> 80 × 109/L). If recovery was not achieved, blood counts were repeated 3 to 4 days and, if necessary, 7 days later. The dosages of myelosuppressive drugs were reduced by 25% if WBC and platelet count recovery exceeded one week, or by 50% if the delay was longer than 2 weeks. Planned treatment consisted of 6 cycles of the assigned regimen. Treatment was stopped if lymphoma progressed, if the patient declined to continue with the protocol, or at the discretion of the treating physician in cases of intercurrent illness or adverse events. Patients with initial bulky disease (defined as lymphoma masses or conglomerates with a diameter ≥ 7.5 cm) received radiotherapy (36 Gy) to these areas irrespective of the result of chemotherapy. Radiotherapy was recommended at the same dose to extranodal sites of disease whenever feasible. Central nervous system (CNS) prophylaxis was recommended only for patients with lymphoblastic disease and consisted of 15 mg methotrexate given intrathecally on days 1 and 4 of the first chemotherapy cycle and a whole-brain radiotherapy with 25.2 Gy given after the end of chemotherapy.
All patients underwent restaging after 3 cycles of therapy and 4 weeks after the end of chemotherapy. Patients who received radiotherapy had an additional restaging 6 to 8 weeks after the end of radiotherapy. Restaging included the examination of all involved sites by appropriate methods. Tumor responses were classified as complete remission (CR), unconfirmed complete remission (CRu), partial remission (PR), stable disease, or progression under therapy according to the International Workshop criteria10 with the modification that CR and CRu had to be confirmed by the first follow-up examination 2 months after restaging. Death without progression during treatment or within 4 weeks after the end of therapy from causes other than lymphoma was designated as therapy-related death.
Adverse events reported by the patient or observed by the treating physician were coded on the case report forms according to WHO grades. An adverse event was defined as any adverse change from the patient's baseline condition after the initiation of therapy, whether or not it was considered related to treatment. The WHO grades for hematotoxicity were assessed from blood counts within treatment-specific nadir windows. For estimating the treatment duration, dose intensity, and dose erosion the technique of Kaplan-Meier estimators were used as described elsewhere.11
Statistical analysis
The trial was planned in a 2 × 2 factorial design. Hence, 2 independent comparisons were subjected to significance testing: interval reduction (comparing all patients randomized to 2-weekly regimens with those in 3-weekly regimens) and addition of etoposide (comparing all patients randomized to CHOP regimens with all patients randomized to CHOEP). The NHL-B2 trial was powered to reveal an improvement of 12% in the primary end point of 2-year event-free survival (EFS; baseline 46%) with a power of 80% and a significance level of 5% in a 2-sided log-rank test for each of the 2 comparisons. Taking a sequential stopping procedure into account, we calculated a sample size of at least 676 informative patients (truncated probability ratio test12 ). The 689 eligible patients were analyzed according to the intent to treat (ie, as allocated by the randomization procedure).
The primary end point was EFS. EFS was defined as the time from the beginning of therapy to either disease progression; initiation of salvage therapy; or additional (off-protocol) treatment, relapse, or death. Secondary end points investigated were overall survival (defined as time from the beginning of therapy to death for any cause), the rate of CR, and the rate of progression. Rates of CR and progression under treatment were defined as percentage of patients with CR/CRu or progression under treatment, respectively, among all eligible patients. Progression under treatment was defined as treatment failure during the treatment period or the time between the end of treatment and the first follow-up, which was performed 2 months after the final restaging. Complete remission was defined as disappearance of all disease symptoms for at least 2 months after the final restaging. Any such patient receiving additional off-protocol treatment was not considered to have achieved CR/CRu. EFS and overall survival were estimated according to Kaplan and Meier. The estimators at 3 and 5 years for event-free and overall survival are given with the 95% confidence limits.
The final analysis presented here proceeded as planned in the protocol. In a first step we checked for violations of the assumptions made in the factorial study design (ie, independence, interaction). A formal test using the proportional hazard model with the 2 comparisons and an interaction term revealed that the interaction term was significant (EFS, relative risk [RR] = 1.50; P = .041). Consequently, to evaluate the effect of each one of the 3 intensified regimens, CHOP-14, CHOEP-21, and CHOEP-14, the treatment effects were modeled using 3 indicator variables in all multivariate models. CHOP-21 was considered the baseline cohort and binary indicator variables were coded for each of the 3 other treatment arms. Proportional hazard models were used for the primary end point EFS and for overall survival. Logistic regression was used for secondary binary end points (ie, rate of complete remission and rate of progression under treatment). In all these models we adjusted for the stratification variables LDH, stage, and bulky disease.
Formal interim analyses were conducted, but criteria of premature stopping of the trial were never met. All tests for significance were 2-sided and were not adjusted for multiple comparisons. Patient characteristics were compared by chi-square tests. WHO toxicities and therapeutic interventions between treatment arms were compared by chi-square tests and, if required, by Fisher exact tests.
Results
Patient characteristics
Between September 1993 and June 2000, 831 patients were randomized by 121 institutions. Forty-nine patients (5.9%) had to be excluded because no pathology review was available, and 40 patients (4.8%) were excluded because after pathology review the original diagnosis of aggressive lymphoma had to be changed into indolent lymphoma or no lymphoma at all. Other reasons for exclusion were missing informed consent (n = 19), concomitant other neoplastic disease (n = 8), previous treatment of lymphoma (n = 7), serious other concomitant disease (n = 6), bone marrow involvement more than 25% (n = 4), no information about initiation of treatment (n = 4), primary CNS lymphoma (n = 1), and other (n = 4, including 2 patients with initial platelet count < 100 × 109/L; 1 patient with initial WBC < 3 × 109/L; and 1 patient with retraction of informed consent after randomization). There were no significant differences in exclusion rates between the treatment arms.
Of the 689 patients eligible for the trial, 178 were randomized to CHOP-21, 172 to CHOP-14, 170 to CHOEP-21, and 169 to CHOEP-14. Patients with more than 1 extranodal site of involvement were more frequent in the CHOP-21 cohort, and the CHOP-14 cohort had more patients older than 70 years of age; otherwise, the 4 cohorts were well-balanced in clinical or pathologic characteristics (Tables 1, 2).
Characteristics of patients included in the NHL-B2 trial
. | All, % . | CHOP-21, % . | CHOP-14, % . | CHOP-21, % . | CHOEP-14, % . | P . |
|---|---|---|---|---|---|---|
| Age, y | .004 | |||||
| 61-65 | 40.6 | 40.4 | 37.8 | 51.2 | 33.1 | |
| 66-70 | 37.0 | 42.1 | 38.4 | 25.3 | 42.0 | |
| 71-75 | 22.4 | 17.4 | 23.8 | 23.5 | 24.9 | |
| Sex | .368 | |||||
| Male | 50.9 | 51.7 | 46.5 | 55.9 | 49.7 | |
| Female | 49.1 | 48.3 | 53.5 | 44.1 | 50.3 | |
| LDH greater than normal | 45.9 | 46.1 | 45.9 | 44.7 | 46.7 | .986 |
| Performance status | ||||||
| ECOG | .400 | |||||
| 0 | 44.0 | 42.1 | 48.3 | 48.2 | 37.3 | |
| 1 | 37.9 | 38.8 | 33.1 | 38.2 | 41.4 | |
| 2 | 14.1 | 13.5 | 15.1 | 10.6 | 17.2 | |
| 3 | 4.1 | 5.6 | 3.5 | 2.9 | 4.1 | |
| More than 1 | 18.1 | 19.1 | 18.6 | 13.5 | 21.3 | .295 |
| Extranodal sites | .233 | |||||
| No | 43.4 | 41.6 | 47.7 | 46.5 | 37.9 | |
| Yes | 56.6 | 58.4 | 52.3 | 53.5 | 62.1 | |
| More than 1 | 25.0 | 30.9 | 18.6 | 22.4 | 27.8 | .038 |
| Stage | .154 | |||||
| I | 16.4 | 10.1 | 22.7 | 16.5 | 16.6 | |
| II | 32.8 | 36.5 | 27.9 | 34.1 | 32.5 | |
| III | 26.3 | 25.8 | 29.1 | 24.7 | 25.4 | |
| IV | 24.5 | 27.5 | 20.3 | 24.7 | 25.4 | |
| III/IV | 50.8 | 53.4 | 49.4 | 49.4 | 50.9 | .865 |
| Bulky disease, 7.5 cm or larger | 39.2 | 39.9 | 37.8 | 38.8 | 40.2 | .966 |
| Bone marrow involvement | 11.6 | 12.9 | 11.6 | 10.0 | 11.8 | .865 |
| B symptoms | 36.7 | 42.1 | 40.7 | 32.9 | 30.8 | .071 |
| IPI | .556 | |||||
| 1 | 29.2 | 28.7 | 30.8 | 30.6 | 26.6 | |
| 2 | 27.3 | 24.2 | 27.3 | 30.6 | 27.2 | |
| 3 | 23.4 | 21.9 | 25.6 | 22.4 | 23.7 | |
| 4, 5 | 20.2 | 25.3 | 16.3 | 16.5 | 22.5 |
. | All, % . | CHOP-21, % . | CHOP-14, % . | CHOP-21, % . | CHOEP-14, % . | P . |
|---|---|---|---|---|---|---|
| Age, y | .004 | |||||
| 61-65 | 40.6 | 40.4 | 37.8 | 51.2 | 33.1 | |
| 66-70 | 37.0 | 42.1 | 38.4 | 25.3 | 42.0 | |
| 71-75 | 22.4 | 17.4 | 23.8 | 23.5 | 24.9 | |
| Sex | .368 | |||||
| Male | 50.9 | 51.7 | 46.5 | 55.9 | 49.7 | |
| Female | 49.1 | 48.3 | 53.5 | 44.1 | 50.3 | |
| LDH greater than normal | 45.9 | 46.1 | 45.9 | 44.7 | 46.7 | .986 |
| Performance status | ||||||
| ECOG | .400 | |||||
| 0 | 44.0 | 42.1 | 48.3 | 48.2 | 37.3 | |
| 1 | 37.9 | 38.8 | 33.1 | 38.2 | 41.4 | |
| 2 | 14.1 | 13.5 | 15.1 | 10.6 | 17.2 | |
| 3 | 4.1 | 5.6 | 3.5 | 2.9 | 4.1 | |
| More than 1 | 18.1 | 19.1 | 18.6 | 13.5 | 21.3 | .295 |
| Extranodal sites | .233 | |||||
| No | 43.4 | 41.6 | 47.7 | 46.5 | 37.9 | |
| Yes | 56.6 | 58.4 | 52.3 | 53.5 | 62.1 | |
| More than 1 | 25.0 | 30.9 | 18.6 | 22.4 | 27.8 | .038 |
| Stage | .154 | |||||
| I | 16.4 | 10.1 | 22.7 | 16.5 | 16.6 | |
| II | 32.8 | 36.5 | 27.9 | 34.1 | 32.5 | |
| III | 26.3 | 25.8 | 29.1 | 24.7 | 25.4 | |
| IV | 24.5 | 27.5 | 20.3 | 24.7 | 25.4 | |
| III/IV | 50.8 | 53.4 | 49.4 | 49.4 | 50.9 | .865 |
| Bulky disease, 7.5 cm or larger | 39.2 | 39.9 | 37.8 | 38.8 | 40.2 | .966 |
| Bone marrow involvement | 11.6 | 12.9 | 11.6 | 10.0 | 11.8 | .865 |
| B symptoms | 36.7 | 42.1 | 40.7 | 32.9 | 30.8 | .071 |
| IPI | .556 | |||||
| 1 | 29.2 | 28.7 | 30.8 | 30.6 | 26.6 | |
| 2 | 27.3 | 24.2 | 27.3 | 30.6 | 27.2 | |
| 3 | 23.4 | 21.9 | 25.6 | 22.4 | 23.7 | |
| 4, 5 | 20.2 | 25.3 | 16.3 | 16.5 | 22.5 |
Patient populations are as follows: all, N = 689; CHOP-21, n = 178; CHOP-14, n = 172; CHOEP-21, n = 170; and CHOEP-14, n = 169.
Diagnosis of patients after histopathologic review
. | All, % . | CHOP-21, % . | CHOP-14, % . | CHOEP-21, % . | CHOEP-14, % . |
|---|---|---|---|---|---|
| B-cell | 94.2 | 91.6 | 94.7 | 93.6 | 96.0 |
| Diffuse large | 71.1 | 63.5 | 74.4 | 73.0 | 73.4 |
| Centroblastic, cb | 52.4 | 44.4 | 52.9 | 55.3 | 57.4 |
| Immunoblastic | 11.5 | 10.7 | 11.0 | 11.8 | 12.4 |
| Anaplastic | 1.6 | 1.1 | 3.5 | 1.2 | 0.6 |
| T-cell rich | 1.2 | 0.6 | 3.5 | 0.0 | 0.6 |
| Not otherwise specified | 4.4 | 6.7 | 3.5 | 4.7 | 2.4 |
| Mediastinal B-cell | 0.6 | 0.0 | 0.6 | 1.8 | 0.0 |
| Follicular grade 3b | 6.1 | 4.5 | 6.9 | 7.0 | 6.0 |
| Burkitt lymphoma | 3.7 | 6.7 | 1.8 | 2.4 | 3.6 |
| Lymphoblastic | 0.3 | 0.6 | 0.6 | 0.0 | 0.0 |
| Unspecified for technical reasons* | 5.2 | 9.0 | 2.9 | 2.9 | 5.9 |
| Not otherwise specified | 7.2 | 7.3 | 7.5 | 6.5 | 7.1 |
| T-cell | 5.8 | 8.5 | 5.2 | 6.0 | 4.2 |
| Anaplastic large cell | 3.5 | 3.9 | 3.4 | 3.6 | 3.0 |
| Peripheral T, unspecified | 1.8 | 3.4 | 1.8 | 1.2 | 1.2 |
| Angioimmunoblastic | 0.3 | 0.6 | 0.0 | 0.6 | 0.0 |
| Extranodal NK/T, nasal type | 0.1 | 0.0 | 0.0 | 0.6 | 0.0 |
| Unspecified for technical reasons* | 0.1 | 0.6 | 0.0 | 0.0 | 0.0 |
| Lymphoblastic, NOS | 0.1 | 0.0 | 0.0 | 0.6 | 0.0 |
. | All, % . | CHOP-21, % . | CHOP-14, % . | CHOEP-21, % . | CHOEP-14, % . |
|---|---|---|---|---|---|
| B-cell | 94.2 | 91.6 | 94.7 | 93.6 | 96.0 |
| Diffuse large | 71.1 | 63.5 | 74.4 | 73.0 | 73.4 |
| Centroblastic, cb | 52.4 | 44.4 | 52.9 | 55.3 | 57.4 |
| Immunoblastic | 11.5 | 10.7 | 11.0 | 11.8 | 12.4 |
| Anaplastic | 1.6 | 1.1 | 3.5 | 1.2 | 0.6 |
| T-cell rich | 1.2 | 0.6 | 3.5 | 0.0 | 0.6 |
| Not otherwise specified | 4.4 | 6.7 | 3.5 | 4.7 | 2.4 |
| Mediastinal B-cell | 0.6 | 0.0 | 0.6 | 1.8 | 0.0 |
| Follicular grade 3b | 6.1 | 4.5 | 6.9 | 7.0 | 6.0 |
| Burkitt lymphoma | 3.7 | 6.7 | 1.8 | 2.4 | 3.6 |
| Lymphoblastic | 0.3 | 0.6 | 0.6 | 0.0 | 0.0 |
| Unspecified for technical reasons* | 5.2 | 9.0 | 2.9 | 2.9 | 5.9 |
| Not otherwise specified | 7.2 | 7.3 | 7.5 | 6.5 | 7.1 |
| T-cell | 5.8 | 8.5 | 5.2 | 6.0 | 4.2 |
| Anaplastic large cell | 3.5 | 3.9 | 3.4 | 3.6 | 3.0 |
| Peripheral T, unspecified | 1.8 | 3.4 | 1.8 | 1.2 | 1.2 |
| Angioimmunoblastic | 0.3 | 0.6 | 0.0 | 0.6 | 0.0 |
| Extranodal NK/T, nasal type | 0.1 | 0.0 | 0.0 | 0.6 | 0.0 |
| Unspecified for technical reasons* | 0.1 | 0.6 | 0.0 | 0.0 | 0.0 |
| Lymphoblastic, NOS | 0.1 | 0.0 | 0.0 | 0.6 | 0.0 |
Patient populations are as follows: all, N = 689; CHOP-21, n = 178; CHOP-14, n = 172; CHOEP-21, n = 170; and CHOEP-14, n = 169. NK indicates natural killer; and NOS, not otherwise specified.
Diagnosis of aggressive B- or T-cell lymphoma was confirmed upon pathology review; however, due to quality of quantity of the biopsy material, a further subclassification was not possible.
Treatment
While the planned total treatment duration for 6 chemotherapy cycles (without the oral application of prednisone) was 71 days for CHOP-14 and 73 days for CHOEP-14, the observed treatment duration was 76 days in the CHOP-14 arm and 80 days in the CHOEP-14 arm. The 3-weekly regimens CHOP-21 and CHOEP-21 could be given without delays (ie, the total treatment duration was 106 days for CHOP-21 and 108 days for CHOEP-21).
Dosage reduction was permitted only if a treatment cycle had to be delayed by more than one week. This strategy resulted in high median relative dose intensities13 : the median relative dose intensities for the myelosuppressive drugs cyclophosphamide, doxorubicin, and etoposide were 97% for CHOP-21, 93% for CHOP-14, and 96% for CHOEP-21, but only 83% for the double-intensive CHOEP-14 regimen. More details on dose erosion are given elsewhere.11
Of the 170 patients with bulky disease who completed therapy, all but 24 received radiotherapy (36 Gy) to the initial site of bulky involvement (in 14 cases the bulk was removed surgically, in 1 patient radiotherapy was not possible for medical reasons, and in 9 cases the protocol was violated without any obvious reasons). In contrast, 8 patients received radiotherapy to their largest site of involvement even though the definition of initial bulky disease (≥ 7.5 cm) was not fulfilled. These 8 patients were counted as events at the time of initiation of radiotherapy, even though all these patients had been evaluated clinically as CR or CRu after 6 cycles of chemotherapy. The distribution of the patients who received additional treatment was not significantly different between the treatment arms.
Treatment results
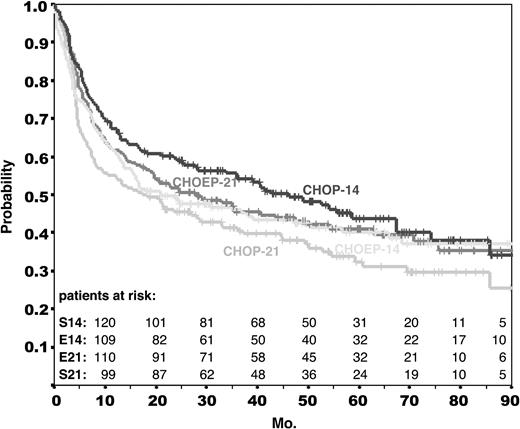
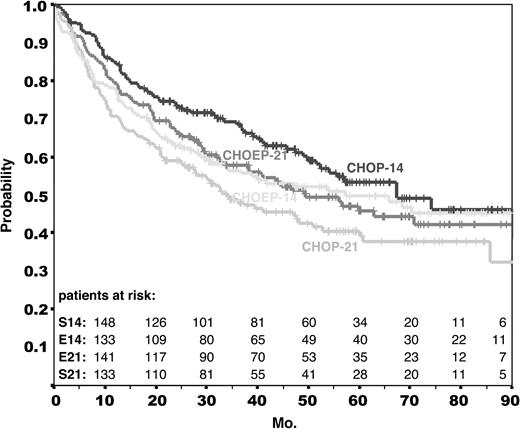
The primary end point of the trial was EFS. Overall survival and the rates of complete remission and progression under treatment were secondary end points. Median time of observation for EFS and overall survival is 58 months for all patients. Figures 1 and 2 provide the Kaplan-Meier estimates for EFS and OS for each of the treatment arms. Table 3 contains the estimates for the EFS rates after 3 and 5 years. It should be noted that among the 25 deaths occurring after a median time of observation of longer than 50 months, only 9 were lymphoma related; 3 were related to second neoplasms and 2 to treatment. This reflects that causes of death other than lymphoma are prevalent in this elderly population.
Event-free survival in the NHL-B2 trial. Event-free survival of all 689 eligible patients assigned to CHOP-21 (S21; n = 178), CHOP-14 (S14; n = 172), CHOEP-21 (E21; n = 170), and CHOEP-14 (E14; n = 169). Median time of observation for all patients was 58 months.
Event-free survival in the NHL-B2 trial. Event-free survival of all 689 eligible patients assigned to CHOP-21 (S21; n = 178), CHOP-14 (S14; n = 172), CHOEP-21 (E21; n = 170), and CHOEP-14 (E14; n = 169). Median time of observation for all patients was 58 months.
Overall survival in the NHL-B2 trial. Overall survival of all 689 eligible patients assigned to CHOP-21 (n = 178), CHOP-14 (n = 172), CHOEP-21 (n = 170), and CHOEP-14 (n = 169). Median time of observation for all patients was 58 months.
Overall survival in the NHL-B2 trial. Overall survival of all 689 eligible patients assigned to CHOP-21 (n = 178), CHOP-14 (n = 172), CHOEP-21 (n = 170), and CHOEP-14 (n = 169). Median time of observation for all patients was 58 months.
Response to treatment with CHOP-21, CHOP-14, CHOEP-21, and CHOEP-14
. | CHOP-21 . | CHOP-14 . | CHOEP-21 . | CHOEP-14 . |
|---|---|---|---|---|
| Complete response | ||||
| n (%) | 107 (60.1) | 131 (76.1) | 119 (70.0) | 121 (71.6) |
| 95% Cl, % (lower limit; upper limit) | (52.5; 67.4) | (69.1; 82.3) | (62.5; 76.8) | (64.2; 78.3) |
| Partial response | ||||
| n (%) | 5 (2.8) | 11 (6.4) | 10 (5.9) | 11 (6.5) |
| 95% Cl, % (lower limit; upper limit) | (0.9; 6.4) | (3.2; 11.2) | (2.9; 10.6) | (3.3; 11.4) |
| Stable disease | ||||
| n (%) | 2 (1.1) | 1 (0.6) | 2 (1.2) | 1 (0.6) |
| 95% Cl, % (lower limit; upper limit) | (0.1; 4.0) | (0.0; 3.2) | (0.1; 4.2) | (0.0; 3.2) |
| Progression under treatment* | ||||
| n (%) | 52 (29.2) | 20 (11.6) | 25 (14.7) | 16 (9.5) |
| 95% Cl, % (lower limit; upper limit) | (22.6; 36.5) | (7.2; 17.4) | (9.8; 20.9) | (5.5; 14.9) |
| Therapy-associated deaths without progression | ||||
| n (%) | 6 (3.4) | 5 (2.9) | 9 (5.3) | 13 (7.7) |
| 95% Cl, % (lower limit; upper limit) | (1.2; 7.2) | (1.0; 6.6) | (2.4; 9.8) | (4.2; 12.8) |
| Unknown | ||||
| n (%) | 0 (0.0) | 1 (0.6) | 2 (1.2) | 4 (2.4) |
| 95% Cl, % (lower limit; upper limit) | (0.0; 2.0) | (0.0; 3.2) | (0.1; 4.2) | (0.6; 6.0) |
| Additional therapy† | ||||
| n (%) | 6 (3.4) | 3 (1.8) | 3 (1.8) | 3 (1.8) |
| 95% Cl, % (lower limit; upper limit) | (1.2; 7.2) | (0.4; 5.0) | (0.4; 5.1) | (0.4; 5.1) |
| 3-year EFS | ||||
| % patients | 41.3 | 54.2 | 45.5 | 46.0 |
| 95% Cl, % (lower limit; upper limit) | (33.9; 48.6) | (46.6; 61.8) | (37.9; 53.2) | (38.3; 53.6) |
| 5-year EFS‡ | ||||
| % patients | 32.5 | 43.8 | 41.1 | 40.2 |
| 95% Cl, % (lower limit; upper limit) | (24.7; 40.3) | (35.4; 52.1) | (33.2; 48.9) | (32.2; 48.2) |
| 3-year overall survival | ||||
| % patients | 48.8 | 68.5 | 57.7 | 56.4 |
| 95% Cl, % (lower limit; upper limit) | (41.1; 56.4) | (61.3; 75.6) | (50.1; 65.3) | (48.6; 64.1) |
| 5-year overall survival‡ | ||||
| % patients | 40.6 | 53.3 | 45.8 | 49.8 |
| 95% Cl, % (lower limit; upper limit) | (32.5; 48.6) | (44.6; 62.1) | (37.4; 54.2) | (41.5; 58.0) |
. | CHOP-21 . | CHOP-14 . | CHOEP-21 . | CHOEP-14 . |
|---|---|---|---|---|
| Complete response | ||||
| n (%) | 107 (60.1) | 131 (76.1) | 119 (70.0) | 121 (71.6) |
| 95% Cl, % (lower limit; upper limit) | (52.5; 67.4) | (69.1; 82.3) | (62.5; 76.8) | (64.2; 78.3) |
| Partial response | ||||
| n (%) | 5 (2.8) | 11 (6.4) | 10 (5.9) | 11 (6.5) |
| 95% Cl, % (lower limit; upper limit) | (0.9; 6.4) | (3.2; 11.2) | (2.9; 10.6) | (3.3; 11.4) |
| Stable disease | ||||
| n (%) | 2 (1.1) | 1 (0.6) | 2 (1.2) | 1 (0.6) |
| 95% Cl, % (lower limit; upper limit) | (0.1; 4.0) | (0.0; 3.2) | (0.1; 4.2) | (0.0; 3.2) |
| Progression under treatment* | ||||
| n (%) | 52 (29.2) | 20 (11.6) | 25 (14.7) | 16 (9.5) |
| 95% Cl, % (lower limit; upper limit) | (22.6; 36.5) | (7.2; 17.4) | (9.8; 20.9) | (5.5; 14.9) |
| Therapy-associated deaths without progression | ||||
| n (%) | 6 (3.4) | 5 (2.9) | 9 (5.3) | 13 (7.7) |
| 95% Cl, % (lower limit; upper limit) | (1.2; 7.2) | (1.0; 6.6) | (2.4; 9.8) | (4.2; 12.8) |
| Unknown | ||||
| n (%) | 0 (0.0) | 1 (0.6) | 2 (1.2) | 4 (2.4) |
| 95% Cl, % (lower limit; upper limit) | (0.0; 2.0) | (0.0; 3.2) | (0.1; 4.2) | (0.6; 6.0) |
| Additional therapy† | ||||
| n (%) | 6 (3.4) | 3 (1.8) | 3 (1.8) | 3 (1.8) |
| 95% Cl, % (lower limit; upper limit) | (1.2; 7.2) | (0.4; 5.0) | (0.4; 5.1) | (0.4; 5.1) |
| 3-year EFS | ||||
| % patients | 41.3 | 54.2 | 45.5 | 46.0 |
| 95% Cl, % (lower limit; upper limit) | (33.9; 48.6) | (46.6; 61.8) | (37.9; 53.2) | (38.3; 53.6) |
| 5-year EFS‡ | ||||
| % patients | 32.5 | 43.8 | 41.1 | 40.2 |
| 95% Cl, % (lower limit; upper limit) | (24.7; 40.3) | (35.4; 52.1) | (33.2; 48.9) | (32.2; 48.2) |
| 3-year overall survival | ||||
| % patients | 48.8 | 68.5 | 57.7 | 56.4 |
| 95% Cl, % (lower limit; upper limit) | (41.1; 56.4) | (61.3; 75.6) | (50.1; 65.3) | (48.6; 64.1) |
| 5-year overall survival‡ | ||||
| % patients | 40.6 | 53.3 | 45.8 | 49.8 |
| 95% Cl, % (lower limit; upper limit) | (32.5; 48.6) | (44.6; 62.1) | (37.4; 54.2) | (41.5; 58.0) |
Patient populations are as follows: CHOP-21, n = 178; CHOP-14, n = 172; CHOEP-21, n = 170; and CHOEP-14, n = 169. 95% Cl indicates 95% confidence interval.
Progression under treatment was defined as progressive disease during the treatment period or the time between the end of treatment until the first follow-up assessment after restaging, which was performed 2 months after the final restaging.
Patients in CR/CRu, but after receiving (additional off-protocol) treatment (eg, radiotherapy in the absence of bulky disease, more than 6 cycles of chemotherapy, or alternative treatment).
Estimated after a median time of observation of 58 months.
Modeling the originally planned 2 × 2 factorial analysis (ie, the 2 primary comparisons: interval reduction and addition of etoposide), the relative risk of an event would have been 0.97 (P = .742) with respect to event-free survival and 0.97 (P = .776) with respect to overall survival if the CHOP regimens were compared with the CHOEP regimens; if the 2-weekly regimens were compared with the 3-weekly regimens, the relative risk for an event would have been 0.85 (P = .109) with respect to EFS and 0.79 (P = .03) with respect to overall survival in favor of the 2-weekly regimens. However, the 2 contrasting treatment factors (time effect of 2- versus 3-weekly treatments, addition of etoposide) were not independent of each other, as demonstrated by a statistically significant interaction term (RR = 1.50, P = .041; proportional hazard model for EFS). Therefore, the 2 primary comparisons (interval reduction and addition of etoposide) must be disregarded. The Kaplan-Meier curves of all 4 treatment arms in Figures 1 and 2 provide a clear insight of this interaction: while the CHOEP-21 curves are above the CHOP-21 curves, the CHOEP-14 curves are not above the CHOP-14 curves.
As a consequence of the significant interaction in the 2 × 2 factorial analysis, the treatment effect of each of the 3 intensified regimens, CHOP-14, CHOEP-21, and CHOEP-14, was modeled using 3 indicator variables in all multivariate models. CHOP-21 was considered the baseline cohort and binary variables were coded for each of the 3 other treatments. All models were adjusted for the stratification variables LDH, stage, and bulky disease (Table 4). Using a Cox proportional hazard model for the primary treatment end point EFS, only CHOP-14, but not CHOEP-21 or CHOEP-14, significantly reduced the risk of an event compared with CHOP-21 (relative risk, 0.66; P = .003).
Results of multivariate modeling
. | No complete remission*† . | . | . | Progression under therapy* . | . | . | EFS event‡§ . | . | . | Death‡ . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | OR . | 95% Cl, upper limit; lower limit . | P∥ . | OR . | 95% Cl, upper limit; lower limit . | P∥ . | RR . | 95% Cl, upper limit; lower limit . | P∥ . | RR . | 95% Cl, upper limit; lower limit . | P∥ . | ||||||||
| CHOP-14 vs CHOP-21 | 0.45 | 0.28;0.73 | .001 | 0.34 | 0.19;0.61 | < .001 | 0.66 | 0.50;0.87 | .003 | 0.58 | 0.43;0.79 | < .001 | ||||||||
| CHOEP-21 vs CHOP-21 | 0.64 | 0.40;1.02 | .064 | 0.41 | 0.23;0.73 | .002 | 0.82 | 0.63;1.07 | .145 | 0.79 | 0.59;1.05 | .109 | ||||||||
| CHOEP-14 vs CHOP-21 | 0.57 | 0.36;0.92 | .020 | 0.24 | 0.13;0.46 | < .001 | 0.77 | 0.59;1.02 | .064 | 0.73 | 0.54;0.98 | .035 | ||||||||
| LDH greater than normal | 2.07 | 1.43;2.99 | < .001 | 2.23 | 1.37;3.63 | .001 | 1.75 | 1.40;2.17 | < .001 | 2.16 | 1.70;2.74 | < .001 | ||||||||
| Stage III/IV | 1.83 | 1.27;2.64 | .001 | 1.62 | 1.00;2.61 | .051 | 1.94 | 1.56;2.42 | < .001 | 1.72 | 1.36;2.18 | < .001 | ||||||||
| Bulky disease | 1.89 | 1.33;2.68 | < .001 | 2.07 | 1.32;3.24 | .002 | 1.10 | 0.90;1.36 | .353 | 1.15 | 0.92;1.44 | .227 | ||||||||
. | No complete remission*† . | . | . | Progression under therapy* . | . | . | EFS event‡§ . | . | . | Death‡ . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | OR . | 95% Cl, upper limit; lower limit . | P∥ . | OR . | 95% Cl, upper limit; lower limit . | P∥ . | RR . | 95% Cl, upper limit; lower limit . | P∥ . | RR . | 95% Cl, upper limit; lower limit . | P∥ . | ||||||||
| CHOP-14 vs CHOP-21 | 0.45 | 0.28;0.73 | .001 | 0.34 | 0.19;0.61 | < .001 | 0.66 | 0.50;0.87 | .003 | 0.58 | 0.43;0.79 | < .001 | ||||||||
| CHOEP-21 vs CHOP-21 | 0.64 | 0.40;1.02 | .064 | 0.41 | 0.23;0.73 | .002 | 0.82 | 0.63;1.07 | .145 | 0.79 | 0.59;1.05 | .109 | ||||||||
| CHOEP-14 vs CHOP-21 | 0.57 | 0.36;0.92 | .020 | 0.24 | 0.13;0.46 | < .001 | 0.77 | 0.59;1.02 | .064 | 0.73 | 0.54;0.98 | .035 | ||||||||
| LDH greater than normal | 2.07 | 1.43;2.99 | < .001 | 2.23 | 1.37;3.63 | .001 | 1.75 | 1.40;2.17 | < .001 | 2.16 | 1.70;2.74 | < .001 | ||||||||
| Stage III/IV | 1.83 | 1.27;2.64 | .001 | 1.62 | 1.00;2.61 | .051 | 1.94 | 1.56;2.42 | < .001 | 1.72 | 1.36;2.18 | < .001 | ||||||||
| Bulky disease | 1.89 | 1.33;2.68 | < .001 | 2.07 | 1.32;3.24 | .002 | 1.10 | 0.90;1.36 | .353 | 1.15 | 0.92;1.44 | .227 | ||||||||
OR indicates odds ratio; and RR, relative risk.
Logistic regression.
No complete remission: partial response, stable disease, progression under therapy, therapy-associated deaths, unknown response, additional therapy.
Cox proportional models.
EFS events: progression, no complete remission at the end of treatment, relapse after complete remission, death, unplanned additional treatment or change of treatment, what ever comes first.
Due to multiple comparisons in this analysis, only P values less than .016 (0.05:3) should be considered significant.
With respect to the secondary end point overall survival, the proportional hazard analysis showed that (compared with CHOP-21) the risk to die was significantly reduced by CHOP-14 (relative risk, 0.58; P < .001), while CHOEP-14 (relative risk, 0.73; P = .035) and CHOEP-21 (relative risk, 0.79; P = .109; Table 4) were not significantly better than CHOP-21, if a P value of .016 (0.05:3) is considered the cutoff point due to the multiple comparisons in this analysis.
As a further secondary end point we assessed response to therapy. The complete remission rates ranged between 60.1% in the CHOP-21 and 76.1% in the CHOP-14 cohort (Table 3). In the multivariate analysis using logistic regression, CHOP-14 and CHOEP-14 had a significantly higher complete remission rate (RR = 0.45 and RR = 0.57, respectively; Table 4), and all 3 intensified treatment arms had a lower rate of progression under therapy (Table 4).
In the multivariate analyses for event-free and overall survival times we adjusted for the stratification variables (ie, elevated LDH, stage III/IV, bulky disease). The elevated LDH was identified as a negative prognostic factor in terms of both event-free survival (relative risk, 1.75; P < .001) and overall survival (relative risk, 2.16; P < .001), as was a stage III/IV (relative risk for event-free survival, 1.94, P < .001; relative risk for overall survival, 1.72, P < .001). However, bulky disease was not found to be an adverse prognostic factor.
Sensitivity analysis
In an additional exploratory sensitivity analysis we investigated whether the slight imbalance in the cohort with regard to the number of extranodal involvement and age might have an impact on treatment outcome. Multivariate modeling adjusting for the stratification variables (elevated LDH, bulky disease, and advanced stage) and for more than one extranodal involvement or the age groups, showed no relevant contribution (RR of CHOP-14 vs CHOP-21 for EFS was 0.66 after adjusting for extranodal involvement and 0.64 after adjusting for the age groups, respectively). Likewise, an adjustment for the risk factors according to the age-adjusted international prognostic index (IPI; LDH, Eastern Cooperative Oncology Group [ECOG], > 1 extranodal involvement, advanced stage) did not change the results of the model regarding treatments (ie, RR of CHOP-14 vs CHOP-21 for EFS after adjustment for IPI was 0.64).
In a further analysis we investigated whether inclusion of the 49 patients excluded because of missing histopathology review would have changed the results. All these patients were treated according to the protocol and equally allocated to the treatment arms. As about 6% of all reviews did not confirm the inclusion criteria, we estimate that about 3 of 49 patients were not eligible for the trial. The sensitivity analysis of all primary and secondary end points provided almost identical results to the primary analysis. Moreover, we investigated whether the slight imbalance in the frequency of diffuse large B-cell lymphoma (DLBCL) in the 4 treatment arms was relevant. However, the adjustment for DLBCL had only a minor effect on the comparison of CHOP-14 versus CHOP-21. The adjustment for DLBCL also had only a minor effect on the comparison of treatment results after CHOP-14 and CHOP-21 (RR for EFS, 0.66). In summary, all these sensitivity analyses showed that the results are robust.
Safety
Safety was assessed by reports of adverse events (Table 5). Due to the use of G-CSF in the 2-weekly regimens, leukocytopenia of grades 3 and 4 did not occur more frequently in the CHOP-14 than in the CHOP-21 cohort. The neutrophil nadirs occurred on days 10 to 12 of the cycle in 3-week regimens and on days 8 to 10 in the 2-week regimens. Besides leukocytopenia, anemia and thrombocytopenia were the most frequent adverse events. There was a tendency for cumulative thrombocytopenia in the regimens containing etoposide. Anemia increased with treatment duration. Each toxicity was tested separately. The interpretation of the toxicity analyses remained valid if multiple testing is applied. The rates of red blood cell transfusion, platelet transfusion, and intravenous administration of antibiotics are shown in Table 6.
WHO grade 3 and grade 4 events observed in patients treated with CHOP-21, CHOP-14, CHOEP-21, and CHOEP-14
. | CHOP-21, % . | CHOP-14, % . | CHOEP-21, % . | CHOEP-14, % . | P . |
|---|---|---|---|---|---|
| Leukocytopenia | 72.1 | 70.1 | 94.4 | 92.4 | < .001 |
| Thrombocytopenia | 4.7 | 15.1 | 28.4 | 50.8 | < .001 |
| Anemia | 12.5 | 19.5 | 28.7 | 45.1 | < .001 |
| Infection | 8.0 | 10.6 | 13.2 | 24.1 | < .001 |
| Mucositis | 0.0 | 7.1 | 4.9 | 14.3 | < .001 |
| Cardiac toxicity | 3.4 | 4.7 | 3.7 | 5.0 | .871 |
| Neurologic toxicity | 3.4 | 3.6 | 6.1 | 4.3 | .617 |
| Renal toxicity | 1.1 | 0.0 | 0.6 | 0.6 | .756 |
| Lung toxicity | 4.0 | 4.7 | 3.0 | 6.2 | .560 |
| Nausea or vomiting | 8.0 | 13.5 | 9.7 | 15.4 | .126 |
| Alopecia | 62.5 | 58.3 | 58.2 | 52.7 | .328 |
. | CHOP-21, % . | CHOP-14, % . | CHOEP-21, % . | CHOEP-14, % . | P . |
|---|---|---|---|---|---|
| Leukocytopenia | 72.1 | 70.1 | 94.4 | 92.4 | < .001 |
| Thrombocytopenia | 4.7 | 15.1 | 28.4 | 50.8 | < .001 |
| Anemia | 12.5 | 19.5 | 28.7 | 45.1 | < .001 |
| Infection | 8.0 | 10.6 | 13.2 | 24.1 | < .001 |
| Mucositis | 0.0 | 7.1 | 4.9 | 14.3 | < .001 |
| Cardiac toxicity | 3.4 | 4.7 | 3.7 | 5.0 | .871 |
| Neurologic toxicity | 3.4 | 3.6 | 6.1 | 4.3 | .617 |
| Renal toxicity | 1.1 | 0.0 | 0.6 | 0.6 | .756 |
| Lung toxicity | 4.0 | 4.7 | 3.0 | 6.2 | .560 |
| Nausea or vomiting | 8.0 | 13.5 | 9.7 | 15.4 | .126 |
| Alopecia | 62.5 | 58.3 | 58.2 | 52.7 | .328 |
Values presented represent the percentage of patients who experienced the respective grade 3 and 4 event.
Therapeutic interventions in the different treatment groups
. | CHOP-21, % . | CHOP-14, % . | CHOEP-21, % . | CHOEP-14, % . | P . |
|---|---|---|---|---|---|
| Red blood cell transfusions | |||||
| Per patient | 24.6 | 40.2 | 39.2 | 64.3 | < .001 |
| Per cycle | 8.7 | 12.0 | 16.9 | 29.4 | < .001 |
| Platelet transfusions | |||||
| Per patient | 1.7 | 3.6 | 9.0 | 15.5 | < .001 |
| Per cycle | 0.3 | 0.6 | 2.4 | 4.5 | < .001 |
| Antibiotics, intravenous | |||||
| Per patient | 37.9 | 48.2 | 60.6 | 62.5 | < .001 |
| Per cycle | 13.5 | 15.3 | 26.6 | 26.4 | < .001 |
. | CHOP-21, % . | CHOP-14, % . | CHOEP-21, % . | CHOEP-14, % . | P . |
|---|---|---|---|---|---|
| Red blood cell transfusions | |||||
| Per patient | 24.6 | 40.2 | 39.2 | 64.3 | < .001 |
| Per cycle | 8.7 | 12.0 | 16.9 | 29.4 | < .001 |
| Platelet transfusions | |||||
| Per patient | 1.7 | 3.6 | 9.0 | 15.5 | < .001 |
| Per cycle | 0.3 | 0.6 | 2.4 | 4.5 | < .001 |
| Antibiotics, intravenous | |||||
| Per patient | 37.9 | 48.2 | 60.6 | 62.5 | < .001 |
| Per cycle | 13.5 | 15.3 | 26.6 | 26.4 | < .001 |
Of the nonhematologic toxicities, neurologic side effects were not significantly different in the CHOP-14 compared with the CHOP-21 cohort. This demonstrates that the 2-week interval did not increase the rate of vincristine-associated polyneuropathies (Table 5).
Except for therapy-related deaths (which are shown in Table 3), the causes of death other than from lymphoma were not different in the 4 treatment arms. After a median time of observation of 58 months, 20 secondary neoplasms have occurred: 2 acute myeloid leukemias, 16 solid tumors, one Hodgkin lymphoma, and one non-Hodgkin lymphoma of the T-cell type after a primary diffuse large B-cell lymphoma. The incidence of secondary tumors was not correlated with any particular regimen, and of the 2 cases of acute myeloid leukemia (AML), one each was observed after CHOEP-21 and CHOEP-14.
Discussion
The originally planned 2 × 2 factorial design analysis was not possible because an interaction term between the 2 factors (interval reduction and addition of etoposide) was relevant. Therefore, according to the protocol, the 3 intensified treatment arms, CHOP-14, CHOEP-21, and CHOEP-14, each had to be compared with the standard CHOP-21 regimen. As can be seen from Table 4 and Figures 1 and 2, of the 3 intensified arms, only CHOP-14 improved both the primary end point event-free survival as well as the secondary end points overall survival and rates of complete remissions and progressions. As one of the risk factors according to the International Prognostic Index,1 involvement of more than 1 extranodal site, did not evolve as a risk factor in this study, the imbalance in the different groups (fewer patients with multiple extranodal sites in the CHOP-14 group) cannot explain the better results obtained with CHOP-14. This was also confirmed by the sensitivity analysis that showed that after adjustment for extranodal involvement, for the different age groups, or for the number of risk factors according to IPI (LDH, ECOG, > 1 extranodal involvement), the improvement of results obtained with CHOP-14 compared with CHOP-21 remained significant. Similarly, the advantage of CHOP-14 over CHOP-21 remained highly significant after adjusting for multiple comparisons. In contrast to CHOP-14, the advantage of CHOEP-14 over CHOP-21 was less significant (Table 4). Clinically as important, of the 3 intensified treatment arms, CHOP-14 had the least side effects and necessitated the least therapeutic interventions (Table 5). Moreover, the shorter total treatment duration of the biweekly regimens not only allows for a significantly higher dose intensity,13 but for the patients it also affords cessation of therapy one month earlier and thus, contributes an important gain in quality of life for these elderly patients, for whom prolonged treatment protocols are particularly arduous. In summary, while there is no formal statistical proof that the efficacy of CHOP-14 is superior to that of CHOEP-14 or CHOEP-21, the favorable toxicity profile of CHOP-14 together with its significantly improved efficacy over the classical CHOP-21 qualifies CHOP-14 as the new standard chemotherapy regimen for elderly patients. A statistical comparison between the 3 intensified treatment arms was not done because it had not been planned in the protocol and the trial had not been powered for this analysis.
A prephase treatment consisting of a single injection of vincristine and 100 mg prednisone for 5 to 7 days was recommended before the application of the first chemotherapy cycle to improve the performance status of the patients and ameliorate the side effects of the first chemotherapy cycle. However, since the prephase treatment was not regularly documented, no statistical data quantifying this clinical experience can be provided. Six cycles of CHOP and CHOEP were given in this trial. In the original CHOP protocol,2 CHOP was given 3 cycles beyond achieving complete remission, resulting in 5 to 8 cycles for most patients. While we cannot exclude the possibility that 8 cycles of CHOP might be better than 6, there is no evidence from randomized trials to support this assumption; indeed, this question is currently being addressed in the ongoing RICOVER-60 trial of the DSHNHL, where elderly patients are randomized to 6 or 8 cycles of CHOP-14 with or without rituximab.
The role of etoposide in elderly patients is more difficult to determine. While the addition of etoposide in the CHOEP-21 regimen significantly reduced the risk of progression under therapy compared with CHOP-21, it failed to improve the results with respect to the other end points. Moreover, when added to the 2-weekly CHOP-14, the positive effect of the 2-weekly regimen was even partially offset by the increased toxicity of the double-intensified regimen because CHOEP-14 caused more therapy-associated deaths and frequent treatment delays. This suggests that dose intensification beyond a certain limit might be counterproductive in elderly patients.
According to Hryniuk and colleagues,13 CHOP-14 has a relative dose intensity of 150% compared with CHOP-21 and this is entirely due to its higher “dose density” (ie, the same total doses of cytotoxic drugs are given in a shorter period of time). This suggests that dose density is the factor responsible for the success of CHOP-14, since chemotherapy regimens trying to increase dose intensity by means other than dose density have failed to improve outcome in aggressive lymphoma.3
This trial for patients older than 60 years of age was open for aggressive and also very aggressive lymphomas, in order to provide the option of a randomized trial for elderly patients with Burkitt and lymphoblastic lymphoma without significant bone marrow involvement for whom acute lymphoblastic leukemia (ALL)–type chemotherapy might not be justified. On the other hand, patients with more than 25% bone marrow involvement were excluded because in Germany these patients are usually treated with age-adapted ALL-like chemotherapy regimens.
In order to assure that treatment results were not diluted by more favorable histologies, patients were excluded after randomization if no pathology review was available or if the diagnosis of aggressive or very aggressive lymphoma had to be changed into indolent lymphoma or no lymphoma at all upon pathology review. All other lymphomas, as diagnosed upon histology review by an expert panel of 5 hematopathologists and listed in Table 2, were not excluded. The adjustment for DLBCL had only a minor effect on the comparison of treatment results after CHOP-14 and CHOP-21 (RR for EFS, 0.66).
Our trial shows for the first time that long-term treatment results in elderly patients with aggressive lymphomas with CHOP-21, which has been considered the standard chemotherapy regimen for all aggressive lymphomas for 25 years,3 can be improved using “dose-densified” CHOP.
Recently, GELA, the French cooperative lymphoma study group, reported similar improvements in patients older than 60 years of age with stage II to IV diffuse large B-cell lymphoma by adding rituximab, a chimeric anti-CD20 immunoglobulin G1 (IgG1) monoclonal antibody, to the classical CHOP-2114 ; however, the median time of observation (2 years) of the GELA study is still short and further follow-up is necessary before definitive conclusions can be drawn. Whether the combination of both approaches (ie, the combination of CHOP-14 with rituximab) will further improve results in elderly patients with diffuse large B-cell lymphomas will be answered by the current RICOVER-60 trial of the DSHNHL, which compares 6 and 8 cycles of CHOP-14 each with and without rituximab.
Appendix
The membership of the DSHNHL is composed of all of the individuals who participated in the study. The following is a list of study participants. Pathologic review committee: A. C. Feller, M. L. Hansmann, H.-K. Müller-Hermelink, P. Moeller, R. Parwaresch, H. Stein. Coordinating physicians: R. Schmits, F. Hartmann, L. Trümper. Reference radiotherapist: K. Schnabel, C. Rübe. Biometry: M. Loeffler, D. Hasenclever, M. Kloess. Data management team: B. Mann, U. Schönwiese, A. Schöler, L. Martin Montanez, W. Beck, V. Barnstorf, G. Held, H. Maintz. Database: M. Kunert, B. Wicklein. Institutions recruiting patients: Carl-Thiem-Klinikum, Cottbus: Ch. Rudolph, H. Steinhauer; Universitätsklinik, Köln: V. Diehl, A. Engert, M. Reiser; Med Universitätsklinik, Homburg: F. Hartmann, M. Pfreundschuh, R. Schmits; Universitätskrankenhaus Eppendorf, Hamburg: D. K. Hossfeld; St-Josef-/St-Marien-Hospital, Hagen: H. Eimermacher; Krankenhaus Küchwald, Chemnitz: F. Fiedler, A. Thiel; Klinikum der Friedrich-Schiller-Universität, Jena: K. Höffken; Ruprecht-Karls-Universität, Heidelberg: M. Baudis, A. D. Ho, A. Krämer; Krankenhaus Maria-Hilf/Franziskushaus, Mönchengladbach: D. Kohl, H. E. Reis; Med Universitätsklinik, Münster: W. E. Berdel, R. Mesters, P. Koch; Klinikum, Minden: H. Bodenstein, J. Fleeth, D. Nischik; Städt Klinikum, Oldenburg: H. J. Illiger, B. Metzner; Universitätsklinik, Rostock: M. Freund; Medizinische Akademie, Magdeburg: A. Franke; Krankenhaus der Barmherzigen Brüder, Trier: H. Kirchen, C. B. Kölbel, S. Nispel; Zentralklinikum, Augsburg: G. Schlimok; Universitätsklinikum, Marburg: A. Lorsch, U. Kaiser, A. Neubauer; Klinikum der Universität, Regensburg: R. Andresen; Katharinenhospital, Stuttgart: H. G. Mergenthaler, D. Assmann; Universitätsklinikum, Essen: U. Dührsen; Kreiskrankenhaus, Aurich: T. Langenbuch, F. Püschel; Med Universitätsklinik, Ulm: H. Döhner, S. Wessendorf; Med Universitätsund Poliklinik, Bonn: E. Ortiz, H. Vetter; Klinikum der Stadt, Ludwigshafen: H. Brass, M. Hoffmann, M. Uppenkamp; Leopoldina-Krankenhaus, Schweinfurt: W. Koch, M. Lutz; Städt Kliniken, Darmstadt: D. Fritze, H. Schuppert; Städt Krankenhaus, Kiel: M. Kneba; Medizinische Universität, Lübeck: T. Wagner; Krankenhaus Mutterhaus der Borromäerinnen, Trier: M. Clemens; Dr-Horst-Schmidt-Kliniken, Wiesbaden: N. Frickhofen; Evangl Diakonie-Krankenhaus, Bremen: K. H. Pflüger; Kreiskrankenhaus, Waldbröl: H. J. Bias, L. Labedzki; Klinikum der Stadt, Mannheim: R. Hehlmann, F. Schlegel; Krankenhaus Altstadt, Magdeburg: E. Kettner; Universitätsklinikum Charité, Berlin: B. Dörken; Zentrum für Innere Medizin, Gießen: H. Pralle; Evangl Krankenhaus, Hamm: L. Balleisen; Städt Krankenhaus Martha-Maria, Halle: U. Neef, W. Schütt; Georg-August-Universität, Göttingen: G. Brittinger, R. B. Kühn, L. Trümper; Bürgerhospital, Stuttgart: H. Ch. Benöhr, W. Grimminger; St Vincenz-Krankenhaus, Limburg: K. Schalk; Robert-Bosch-Krankenhaus, Stuttgart: W. E. Aulitzky; Caritasklinik St Theresia, Saarbrücken: J. Preiß, P. Schmidt; Westpfalz-Klinikum, Kaiserslautern: F. G. Hagmann, H. Link, Ch. Wollermann; Ernst-Moritz-Arndt-Universität, Greifswald: D. Dölken, U. Hutzschenreuter, Ch. Sucker, M. Schwenke; Klinikum Ernst von Bergmann, Potsdam: R. Pasold; Allgemeines Krankenhaus Altona, Hamburg: D. Braumann; Städt Krankenhaus Schwabing, München: Ch. Nerl, R. Schulz; St-Antonius-Hospital, Eschweiler: R. Fuchs, S. Schäfer; Diakonissenkrankenhaus, Stuttgart: E. Heidemann; Allgemeines Krankenhaus, Hagen: T. Scholten; Klinikum, Aschaffenburg: W. Fischbach; Kreiskrankenhaus, Bad Hersfeld: R. Paliege; Kreiskrankenhaus Am Plattenwald, Bad Friedrichshall: P. Keller, C. Wojatschek; Gemeinschaftspraxis fürHämat & Intern Onkologie, Köln: St Schmitz, T. Steinmetz; Klinikum Großhadern, München: W. Hiddemann, Ch. Nickenig, C. Warmuth-Lembcke; Med Hochschule, Hannover: J. Atzpodien, A. Ganser, G. Röhrig; Klinikum Kreis Herford: U. Schmitz-Hübner; Städt Krankenanstalten, Krefeld: K. Becker, T. Frieling, M. Planker; Städt Klinikum, Karlsruhe: Th. Fischer; Caritas-Krankenhaus, Lebach: D. Hufnagel; Universität, Würzburg: T. Wässa, K. Wilms; Med Universitätsklinik, Bochum: U. Greven, W. Schmiegel; Universitätsspital, Zürich: R. Stahel; St Johannes-Hospital, Duisburg: C. Aul; Lukaskrankenhaus, Neuss: P. Czygan; St-Johannes-Hospital, Dortmund: V. Hagen, H. Pielken; Martin-Luther-Krankenhaus, Schleswig: M. Schöttler; Klinikum, Frankfurt/Oder: H. Burchardt; Kreiskrankenhaus, Neumarkt: F. Tympner; Universitätsklinikum, Dresden: G. Ehninger, C. Schimming; Marienhospital, Herne: R. Voigtmann, E. Schilling; Evangl Krankenhaus, Mülheim/Ruhr: J. Freise; Allgemeines Krankenhaus, Celle: J. Hotz; Klinikum, Bernburg: F. Walther, E. Winter; Evangl Krankenhaus, Essen-Werden: W. Heit; Evangl Stift St Martin, Koblenz: F. Kersting, C. van Roye; Klinikum Lippe, Lemgo: H. Lohrmann; St-Lukas-Klinik, Solingen: K. H. Beckers, F. Koller, F. Mader; Kreiskrankenhaus, Heidenheim: F. Klumpp; Universität, Leipzig: D. Niederwieser; Heinrich-Braun-KH/Städt Klinikum, Zwickau: G. Schott; Klinikum Südstadt, Rostock: M. Kaysser; Kliniken St Antonius, Wuppertal: M. Sandmann; Kreiskrankenhaus, Mayen: R. Schubortz; Kreiskrankenhaus, Offenburg: F. Hirsch; St Elisabethen-Krankenhaus, Ravensburg: G. Meuret; St Marien-Hospital, Mühlheim/Ruhr: T. Grävinghoff, H. König, H. Lukas; Onkologische Schwerpunktpraxis, Harrislee: W. Grimm; Thoraxklinik, Heidelberg: H. Bischoff, P. Drings; Kreiskrankenhaus, Münchberg: A. Fuchs; Krankenhaus Bad Cannstatt, Stuttgart: U. v. Gaisberg; St-Agnes-Hosptial, Bocholt: E. Horst; Ev Krankenhaus Bethesda, Mönchengladbach: H. Drost; Hämatologische Praxis, Trier: M. Grundheber; Krankenhaus, Neunkirchen: W. Maurer; Kreiskrankenhaus, Kronach: W. Bachmann; Krankenhaus, Wetzlar: D. Heinrich; St Elisabeth-Krankenhaus, Thuine: J. P. Schwiedessen; Evangl Krankenhaus, Witten: H. Gallenkamp; Hans-Susemihl-Krankenhaus, Emden: H. Becker; Gemeinschaftspraxis, Hamburg: W. Zeller, K. Veerpoort; Krankenhaus, Bietigheim: S. Walker; Franz-Hospital, Dülmen: G. Dresemann; Klinikum Siloah, Hannover: H. Kirchner; Gemeinschaftspraxis, Jena: S. Hahnfeld, K. Ruffert; Universitätsklinikum Kröllwitz, Halle: H. J. Schmoll, H. H. Wolf; Rotes Kreuz Krankenhaus, Kassel: Ch. Löser, H. Urbanke-Siebert; Evangl Jung-Stilling-Krankenhaus, Siegen: E. Jaehde; Schwerpunktpraxis für Onkologie, Aschaffenburg: M. Klausmann, G. Welslau; Onkologische Gemeinschaftspraxis, München: W. Abenhardt, L. Böning, D. Bosse, F. J. Tigges; St Marienhospital, Vechta: J. Diers; Robert-Koch-Krankenhaus, Gehrden: B. Ullmann; Städt Klinikum, Pforzheim: L. Theilmann; Dreifaltigkeitshospital, Lippstadt: K. A. Jost; Städt Klinikum, Fulda: M. Arland, C. Hofmann; St Josef-Hosptial, Bochum: W. E. Schmidt; Städt Klinikum St Georg, Leipzig: L. Mantovani, B. Matthé; Klinikum Nord, Heidberg, Hamburg: M. Waschewski; Praxis für Onkologie, Wendlingen: T. Kamp; Knappschaftskrankenhaus, Bottrop: G. Trenn.
Prepublished online as Blood First Edition Paper, March 11, 2004; DOI 10.1182/blood-2003-06-2095.
A complete list of the DSHNHL participants appears in the “Appendix.”
Supported by a grant from Deutsche Krebshilfe e.V. and unrestricted grants from Amgen and Bristol-Myers.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal