Abstract
The TEL-PDGFRB fusion oncogene is associated with chronic myelomonocytic leukemia (CMML) and results in the expression of a constitutively active tyrosine kinase. SU11657 is a multitargeted selective inhibitor of class III/V receptor tyrosine kinases, including the platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) receptors KIT and FLT3. SU11657 inhibited TEL/PDGFβR kinase activity at nanomolar concentrations and inhibited TELPDGFRB-mediated factor-independent growth in myeloblastic 32D cells. Daily oral administration of SU11657 at 40 mg/kg suppressed myeloproliferation and significantly prolonged survival in TELPDGFRB mice treated prior to disease development, as well as in those with large tumor burdens. Our findings suggest that SU11657 or similar agents may have therapeutic potential in humans with hematologic malignancies expressing PDGFR fusion oncogenes. (Blood. 2004;104:561-564)
Introduction
The TEL/PDGFβR fusion protein is expressed as a result of the t(5;12) (q33;p13) chromosomal translocation and is found in a subset of patients with chronic myelomonocytic leukemia (CMML).1,2 Fusion of TEL (ETV6), an ETS-like transcription factor, to the C-terminal kinase region of the platelet-derived growth factor receptor (PDGFR) enables oligomerization of the mutant protein and its constitutive activation.3,4 A murine bone marrow transplantation model of TEL-PDGFRB-induced disease exhibits a rapidly fatal myeloproliferative disorder (MPD) marked by leukocytosis, splenomegaly, and extramedullary hematopoiesis.5
SU11657 is a multitargeted inhibitor of class III/V receptor tyrosine kinases6 (RTKs) with potency and selectivity similar to SU11248, which has been studied extensively in vitro, in preclinical animal models, and in humans.7-10 SU11657 has been tested in a preclinical mouse model of acute promyelocytic leukemia (APL) induced by the coexpression of promyelocytic leukemia-retinoic acid receptor α (PML-RARα) and activated FLT3 oncogenes. In these mice, treatment with SU11657 alone had modest effect, but demonstrated powerful synergy when combined with administration of all-trans-retinoic acid.6 We investigated the potential of SU11657 to pharmacologically inhibit TEL/PDGFβR signaling in a murine myeloid cell line and in a mouse model of TEL-PDGFRB-mediated disease.
Study design
Animal studies
The marrow of B6 × 129SF1 mice (Taconic Farms, Germantown, NY) was transduced with retroviral supernatants containing TEL-PDGFRB internal ribosome entry site (IRES) green fluorescent protein (GFP) and injected into lethally irradiated syngeneic recipients, as previously described.5 Mice were treated with SU11657 (oral gavage, 100 μL/dose) at 40 mg/kg or with vehicle (carboxymethylcellulose suspension, as described6 ) once daily beginning 14 days after transplantation unless otherwise stated. SU11657 dosage was empirically determined based on previous studies.6 Blood samples were collected from the saphenous vein at time points indicated and analyzed by a HemaVet 3700 hematology analyzer (CDC Technologies, Oxford, CT).
Immunoprecipitation
Protein lysates were made from TEL-PDGFRB-transfected 32D cells grown in the presence of inhibitor or vehicle at 37° for 1 hour. TEL/PDGFβR was immunoprecipitated from whole-cell lysates using anti-PDGFRA/B (Upstate Biotechnology, Lake Placid, NY) and protein A/G agarose beads (Oncogene, La Jolla, CA). Immunoblotting was performed using 4G10 antiphosphotyrosine antibody (Upstate Biotechnology) and anti-PDGFRB (BD Biosciences, San Diego, CA).
Immunohistochemical staining
Spleen sections were incubated with anti-pPDGFR (Cell Signaling, Beverly, MA) and stained using VECTASTAIN ABC and DAB reagent kits (Vector Laboratories, Burlingame, CA). Images were acquired using a Nikon 40 ×/0.70 objective with a Nikon Microphot-SA microscope (Nikon, Melville, NY). The ColorView II digital camera and analySIS (both from Soft Imaging Systems, Lakewood, CO) were used to capture images.
Statistical analysis
Kaplan-Meier graph and statistical analyses were generated with StatView (SAS Institute, Cary, NC).
Results and discussion
SU11657 inhibits TEL/PDGFβR kinase activity and reverses TEL-PDGFRB-induced growth factor independence in 32D cells
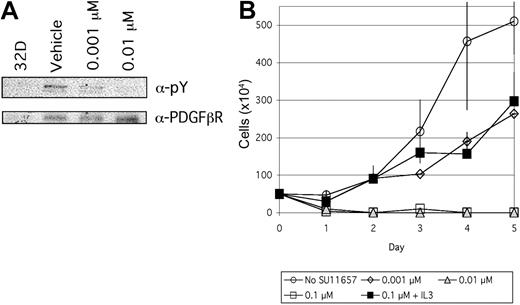
SU11657 is a selective inhibitor of class III/V RTKs6,11 with biochemical and cellular activities similar to those previously described for SU11248.7-9,12,13 Selected biochemical and cellular screening data for SU11657, summarized in Table 1, were determined using previously published methods.7 To determine the ability of SU11657 to inhibit TEL/PDGFβR kinase activity in hematopoietic cells, TEL/PDGFRB-transfected 32D cells were incubated in the presence of vehicle (dimethyl sulfoxide [DMSO]) or 1 nM to 1 μM inhibitor. SU11657 inhibited TEL/PDGFβR tyrosine kinase activity in a dose-dependent fashion (Figure 1A). Changes in global tyrosine phosphorylation were not detected on immunoblotting whole cell lysates with antiphosphotyrosine (data not shown), indicating that SU11657 does not broadly inhibit tyrosine phosphorylation in nontargeted proteins. To evaluate the ability of SU11657 to inhibit TEL/PDGFβR-mediated factor-independent growth, TELPDGFRB-transfected 32D cells were grown in the presence of vehicle or 1 nM to 1 μM SU11657. SU11657 (1 nM) inhibited TEL-PDGFRB-mediated interleukin 3 (IL-3)-independent, but not untransfected control IL-3-dependent, 32D cell growth (Figure 1B).
SU11657 target profiles
. | Biochemical IC50, μM . | Cell kinase assay cellular IC50, μM . | Ligand-dependent synthesis cellular IC50, μM . |
|---|---|---|---|
| PDGFβR | 0.001 | 0.01-0.1 | 0.01 |
| Flk-1/KDR | 0.01 | 0.005-0.05 | — |
| Flt3-ITD | — | 0.05 | — |
| c-Kit | — | 0.01-0.05 | — |
| EGFR | > 20 | — | 23 |
. | Biochemical IC50, μM . | Cell kinase assay cellular IC50, μM . | Ligand-dependent synthesis cellular IC50, μM . |
|---|---|---|---|
| PDGFβR | 0.001 | 0.01-0.1 | 0.01 |
| Flk-1/KDR | 0.01 | 0.005-0.05 | — |
| Flt3-ITD | — | 0.05 | — |
| c-Kit | — | 0.01-0.05 | — |
| EGFR | > 20 | — | 23 |
IC50 values for SU11657 in selected biochemical and cellular assays are shown. Assays were performed as previously described.7 IC50 indicates concentration that inhibits 50%;—, not determined; and EGFR, epidermal growth factor receptor.
SU11657 inhibits TEL/PDGFβR kinase activity. (A) SU11657 inhibits TEL/PDGFβR tyrosine autophosphorylation. TEL-PDGFRB-transfected 32D cells were incubated in the presence of SU11657 or vehicle for 1 hour and protein lysates were harvested. TEL/PDGFβR was present in α-PDGFβR immunoprecipitates from TEL-PDGFRB-transfected 32D, but not untransfected control 32D cell protein lysates (left lane). Immunoprecipitates were immunoblotted using an antiphosphotyrosine antibody to determine the phosphorylation status of TEL-PDGFβR in SU11657- and vehicle-treated cells. (B) SU11657 inhibits TEL-PDGFRB-mediated factor-independent cell growth; 105 cells/mL TELPDGFRB-transfected 32D cells were grown in 0.1% SU11657 or vehicle (DMSO). Cells were plated in RPMI supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin in the presence or absence of 1 ng/mL IL-3. Viable cells were detected via trypan blue exclusion and counted at 24-hour intervals. Experiments were performed in triplicate. Mean cell counts and SDs are shown.
SU11657 inhibits TEL/PDGFβR kinase activity. (A) SU11657 inhibits TEL/PDGFβR tyrosine autophosphorylation. TEL-PDGFRB-transfected 32D cells were incubated in the presence of SU11657 or vehicle for 1 hour and protein lysates were harvested. TEL/PDGFβR was present in α-PDGFβR immunoprecipitates from TEL-PDGFRB-transfected 32D, but not untransfected control 32D cell protein lysates (left lane). Immunoprecipitates were immunoblotted using an antiphosphotyrosine antibody to determine the phosphorylation status of TEL-PDGFβR in SU11657- and vehicle-treated cells. (B) SU11657 inhibits TEL-PDGFRB-mediated factor-independent cell growth; 105 cells/mL TELPDGFRB-transfected 32D cells were grown in 0.1% SU11657 or vehicle (DMSO). Cells were plated in RPMI supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin in the presence or absence of 1 ng/mL IL-3. Viable cells were detected via trypan blue exclusion and counted at 24-hour intervals. Experiments were performed in triplicate. Mean cell counts and SDs are shown.
SU11657 suppresses TEL-PDGFRB-induced disease in mice
To test whether SU11657 could affect TEL-PDGFRB-mediated hematopoietic malignancy, we evaluated this compound in a TEL-PDGFRB murine bone marrow transplantation model. Whole bone marrow of wild-type mice was transduced with TELPDGFRB retroviral supernatants and injected into lethally irradiated syngeneic recipient mice. These mice uniformly develop a fatal MPD, as previously described.5
Mice reconstituted with TEL-PDGFRB marrow were randomly selected after transplantation for treatment with SU11657 (40 mg/kg) or vehicle to be administered daily by oral gavage beginning 14 days after transplantation. TEL/PDGFβR tyrosine phosphorylation was detected in vehicle-treated, but not SU11657-treated, animals by immunohistochemical staining of spleen tissue (Figure 2A). TEL/PDGFβR protein was detected in both vehicle- and SU11657-treated animals (data not shown). A cohort of animals (n = 5) was treated with SU11657 for 10 weeks and another (n = 5) for 14 weeks. A control group of TEL-PDGFRB animals was administered vehicle until the animals were killed due to the development of MPD (n = 5). All vehicle-treated mice developed rapidly fatal MPD characterized by leukocytosis and splenomegaly, identical to that described previously5 with a median survival of 33 ± 14.2 days (Figure 2B-D). In contrast, all SU11657-treated TEL-PDGFRB mice (n = 10) survived without evidence of disease for the duration of treatment (Figure 2B-D). Mice treated 10 weeks survived a median 116 ± 22.5 days after transplantation (P = .0035, Mantel-Cox log-rank test), whereas mice treated for 14 weeks had a median survival of 137 ± 18.6 days (P = .0018).
SU11657 inhibits TEL-PDGFRB-induced MPD. (A) SU11657 inhibits TEL/PDGFβR autophosphorylation in animals that received a TEL-PDGFRB transplant. TEL-PDGFRB recipient mice were killed after treatment with vehicle or SU11657 for 7 days beginning 14 days after transplantation. Spleen sections of killed animals were stained for phosphorylated PDGFR by immunohistochemistry and counterstained with methyl green. (B) Splenomegaly is inhibited in TEL-PDGFRB mice treated with SU11657. Spleens of inhibitor-treated (left) and vehicle-treated (right) animals killed at day 33 after transplantation (19 days of treatment). Average spleen weight for vehicle-treated animals was 0.83 ± 0.2 g at time of death due to MPD (range, 31-61 days after transplantation; n = 5), whereas spleens of SU11657-treated animals averaged 0.04 ± 0.01 g at time of censor (33 and 94 days after transplantation after 19 and 80 days of treatment, respectively; n = 2). (C) SU11657 prolongs survival in a murine bone marrow transplantation model of TEL-PDGFRB-mediated disease. Recipient mice were treated with vehicle or inhibitor for 10 or 14 weeks, beginning 14 days after transplantation. Vehicle mice (squares) died of rapidly fatal MPD, whereas mice treated 10 weeks (triangles) and 14 weeks (diamonds) survived disease free for treatment duration. TEL-PDGFRB mice whose treatment began following the establishment of MPD and continued until 74 days after transplantation, labeled rescue (circles), also survived disease free for the duration of treatment. Censored mice, shown as open shapes, were killed to monitor disease development or GFP expression at given time points regardless of disease phenotype. Diseased animals were found dead or were killed on appearing sick (ruffled fur, weight loss, cachexia). Mice were classified with primary disease as follows: MPD (WBC count > 50 × 109/L [> 50 000/μL], spleen > 45 mg; shown as filled shapes), acidophilic macrophage pneumonia (perivascular cuffing and infiltration of macrophages in the lungs, with minimal or no extramedullary hematopoiesis in liver or spleen; shown as vertical half-filled shapes), or undetermined (insufficient data; shown as horizontal half-filled shapes). *Three animals from the group treated on days 25 to 74 were killed at day 112 because of anemia (red blood cell count < 6 × 1012/L [< 6 million per μL]), but 1 of 3 mice had evidence of pneumonia by histopathologic analysis. (D) SU11657 suppresses TEL-PDGFRB-induced leukocytosis for the duration of treatment. Blood was harvested from SU11657- and vehicle-treated mice at the time points indicated and differentials were acquired from a HemaVet hematology analyzer. Median WBC counts and interquartile range are shown, with the treatment period indicated by a bar in the upper left-hand corner of the graph. Fine red horizontal line represents normal WBC count range.
SU11657 inhibits TEL-PDGFRB-induced MPD. (A) SU11657 inhibits TEL/PDGFβR autophosphorylation in animals that received a TEL-PDGFRB transplant. TEL-PDGFRB recipient mice were killed after treatment with vehicle or SU11657 for 7 days beginning 14 days after transplantation. Spleen sections of killed animals were stained for phosphorylated PDGFR by immunohistochemistry and counterstained with methyl green. (B) Splenomegaly is inhibited in TEL-PDGFRB mice treated with SU11657. Spleens of inhibitor-treated (left) and vehicle-treated (right) animals killed at day 33 after transplantation (19 days of treatment). Average spleen weight for vehicle-treated animals was 0.83 ± 0.2 g at time of death due to MPD (range, 31-61 days after transplantation; n = 5), whereas spleens of SU11657-treated animals averaged 0.04 ± 0.01 g at time of censor (33 and 94 days after transplantation after 19 and 80 days of treatment, respectively; n = 2). (C) SU11657 prolongs survival in a murine bone marrow transplantation model of TEL-PDGFRB-mediated disease. Recipient mice were treated with vehicle or inhibitor for 10 or 14 weeks, beginning 14 days after transplantation. Vehicle mice (squares) died of rapidly fatal MPD, whereas mice treated 10 weeks (triangles) and 14 weeks (diamonds) survived disease free for treatment duration. TEL-PDGFRB mice whose treatment began following the establishment of MPD and continued until 74 days after transplantation, labeled rescue (circles), also survived disease free for the duration of treatment. Censored mice, shown as open shapes, were killed to monitor disease development or GFP expression at given time points regardless of disease phenotype. Diseased animals were found dead or were killed on appearing sick (ruffled fur, weight loss, cachexia). Mice were classified with primary disease as follows: MPD (WBC count > 50 × 109/L [> 50 000/μL], spleen > 45 mg; shown as filled shapes), acidophilic macrophage pneumonia (perivascular cuffing and infiltration of macrophages in the lungs, with minimal or no extramedullary hematopoiesis in liver or spleen; shown as vertical half-filled shapes), or undetermined (insufficient data; shown as horizontal half-filled shapes). *Three animals from the group treated on days 25 to 74 were killed at day 112 because of anemia (red blood cell count < 6 × 1012/L [< 6 million per μL]), but 1 of 3 mice had evidence of pneumonia by histopathologic analysis. (D) SU11657 suppresses TEL-PDGFRB-induced leukocytosis for the duration of treatment. Blood was harvested from SU11657- and vehicle-treated mice at the time points indicated and differentials were acquired from a HemaVet hematology analyzer. Median WBC counts and interquartile range are shown, with the treatment period indicated by a bar in the upper left-hand corner of the graph. Fine red horizontal line represents normal WBC count range.
To determine the effect of SU11657 treatment in animals with high tumor burdens, a cohort of TEL-PDGFRB mice (a total of 15 in 2 trials) was followed after transplantation and treatment was begun with 40 mg/kg daily only after splenomegaly and leukocytosis had developed (25 days after transplantation). White blood cell (WBC) counts returned to normal levels following treatment initiation, shown in Figure 2D. Animals whose treatment did not begin until MPD was established were treated with SU11657 for 49 days and survived a median of 104 ± 2.4 days (P < .0001). No disease was observed when secondary transplantations were performed using splenocytes from these mice after treatment (n = 5, data not shown). Because TEL-PDGFRB-mediated MPD is not transplantable,5 these data suggest that no additional mutations occurred during SU11657 treatment to contribute to the development of secondary disease.
Despite the significant impact of SU11657 on TEL-PDGFRB-mediated disease, most animals eventually died of disease. During treatment, GFP+ cells were detected in the marrow and spleen (data not shown) and a “rebound” leukocytosis was seen in most animals following the withdrawal of treatment. However, the WBC count had fallen in nearly all animals by the date of death (14 of 18 animals). All animals tested after treatment (n = 12) had splenomegaly, but we frequently attributed the cause of death to acidophilic macrophage pneumonia rather than MPD due to the relatively modest leukocytosis and pathologic changes seen in the lungs of these mice (data not shown).
In summary, we have demonstrated that SU11657 inhibited TEL/PDGFβR kinase activity in a murine myeloid cell line and inhibited TEL-PDGFRB-induced factor-independent growth. Furthermore, SU11657 inhibited MPD associated with the TEL/PDGFβR oncogenic fusion protein in mice. These data indicate that administration of SU11657 results in lowered WBC counts and prolonged survival of mice given transplants with TEL-PDGFRB. No evidence for drug resistance was observed for the duration of treatment.
The success of imatinib mesylate (Gleevec) in the treatment of BCR/ABL-induced chronic myelogenous leukemia (CML) and KIT-driven gastrointestinal stromal tumors has provided powerful validation for the concept of using selective tyrosine kinase inhibitors in target-appropriate indications.14-16 Despite the success of imatinib mesylate in the treatment of BCR/ABL+ CML and TEL/PDGFRB+CMML,2,17 not all patients can tolerate that drug. Furthermore, resistance to imatinib mesylate may ultimately arise in some CMML patients in whom TEL/PDGFRB is expressed, as it is in BCR/ABL+ CML patients. These results demonstrate that SU11657 is a highly effective, orally bioavailable small molecule inhibitor with in vivo activity against disease induced by TEL/PDGFβR.
Prepublished online as Blood First Edition Paper, March 25, 2004; DOI 10.1182/blood-2003-11-3801.
Supported by the American Cancer Society grant IRG-58-010-44 and National Institutes of Health grant CA81197-04 (M.H.T.).
A.D.L. was employed by SUGEN Inc, whose product (SU11657) was studied in the present work. SUGEN was acquired by Pfizer Inc and no longer exists. SU11657 is not commercially available.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Marie La Regina for her expert pathologic analysis.

![Figure 2. SU11657 inhibits TEL-PDGFRB-induced MPD. (A) SU11657 inhibits TEL/PDGFβR autophosphorylation in animals that received a TEL-PDGFRB transplant. TEL-PDGFRB recipient mice were killed after treatment with vehicle or SU11657 for 7 days beginning 14 days after transplantation. Spleen sections of killed animals were stained for phosphorylated PDGFR by immunohistochemistry and counterstained with methyl green. (B) Splenomegaly is inhibited in TEL-PDGFRB mice treated with SU11657. Spleens of inhibitor-treated (left) and vehicle-treated (right) animals killed at day 33 after transplantation (19 days of treatment). Average spleen weight for vehicle-treated animals was 0.83 ± 0.2 g at time of death due to MPD (range, 31-61 days after transplantation; n = 5), whereas spleens of SU11657-treated animals averaged 0.04 ± 0.01 g at time of censor (33 and 94 days after transplantation after 19 and 80 days of treatment, respectively; n = 2). (C) SU11657 prolongs survival in a murine bone marrow transplantation model of TEL-PDGFRB-mediated disease. Recipient mice were treated with vehicle or inhibitor for 10 or 14 weeks, beginning 14 days after transplantation. Vehicle mice (squares) died of rapidly fatal MPD, whereas mice treated 10 weeks (triangles) and 14 weeks (diamonds) survived disease free for treatment duration. TEL-PDGFRB mice whose treatment began following the establishment of MPD and continued until 74 days after transplantation, labeled rescue (circles), also survived disease free for the duration of treatment. Censored mice, shown as open shapes, were killed to monitor disease development or GFP expression at given time points regardless of disease phenotype. Diseased animals were found dead or were killed on appearing sick (ruffled fur, weight loss, cachexia). Mice were classified with primary disease as follows: MPD (WBC count > 50 × 109/L [> 50 000/μL], spleen > 45 mg; shown as filled shapes), acidophilic macrophage pneumonia (perivascular cuffing and infiltration of macrophages in the lungs, with minimal or no extramedullary hematopoiesis in liver or spleen; shown as vertical half-filled shapes), or undetermined (insufficient data; shown as horizontal half-filled shapes). *Three animals from the group treated on days 25 to 74 were killed at day 112 because of anemia (red blood cell count < 6 × 1012/L [< 6 million per μL]), but 1 of 3 mice had evidence of pneumonia by histopathologic analysis. (D) SU11657 suppresses TEL-PDGFRB-induced leukocytosis for the duration of treatment. Blood was harvested from SU11657- and vehicle-treated mice at the time points indicated and differentials were acquired from a HemaVet hematology analyzer. Median WBC counts and interquartile range are shown, with the treatment period indicated by a bar in the upper left-hand corner of the graph. Fine red horizontal line represents normal WBC count range.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2003-11-3801/6/m_zh80140464160002.jpeg?Expires=1769234307&Signature=W6RTPRwS5MARzaIkdyd5mGLW7Dk8JyeGotabwjiDT6~n4k3txnyXGwp5m2qdE3v5BE-jsG5nC3e6YjhEEtoOG8ktx974asTYsU8hI9bhJAgfgdqyXvl~xMfaNDedBLkTh~HkL92ncvZNCfvg38e3MNYpKxYPWEQ3S18v9H8J931hW0rQmSaxmMGxct8RqPGhcRkAYqnpccqpmhjkb35NLI8r0NAF5KPvllJ14Uht9Nyl6yWcmzFv1buU7dmkCd0wI3fj8J5pL5MdqnTpTeI6TLBP0xhcVv8eGJJxzyEqgWkY5lUO4FSRX1EcOJpT9TiSMpAGjlMi~HIi9GUuuV09Yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal